Saline–Alkali Tolerance Evaluation of Giant Reed (Arundo donax) Genotypes Under Saline–Alkali Stress at Seedling Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Chemicals
2.3. Morphological Measurements
2.4. Determination of Photosynthetic Parameters
2.5. Determination of the Relative Electrical Conductivity
2.6. Measurement of Malondialdehyde (MDA) Levels
2.7. Measurement of Osmotic Adjustment Compounds
2.8. Measurement of Antioxidant Enzyme Activities
2.9. Salt Tolerance Evaluation
2.10. Statistical Analysis
3. Result
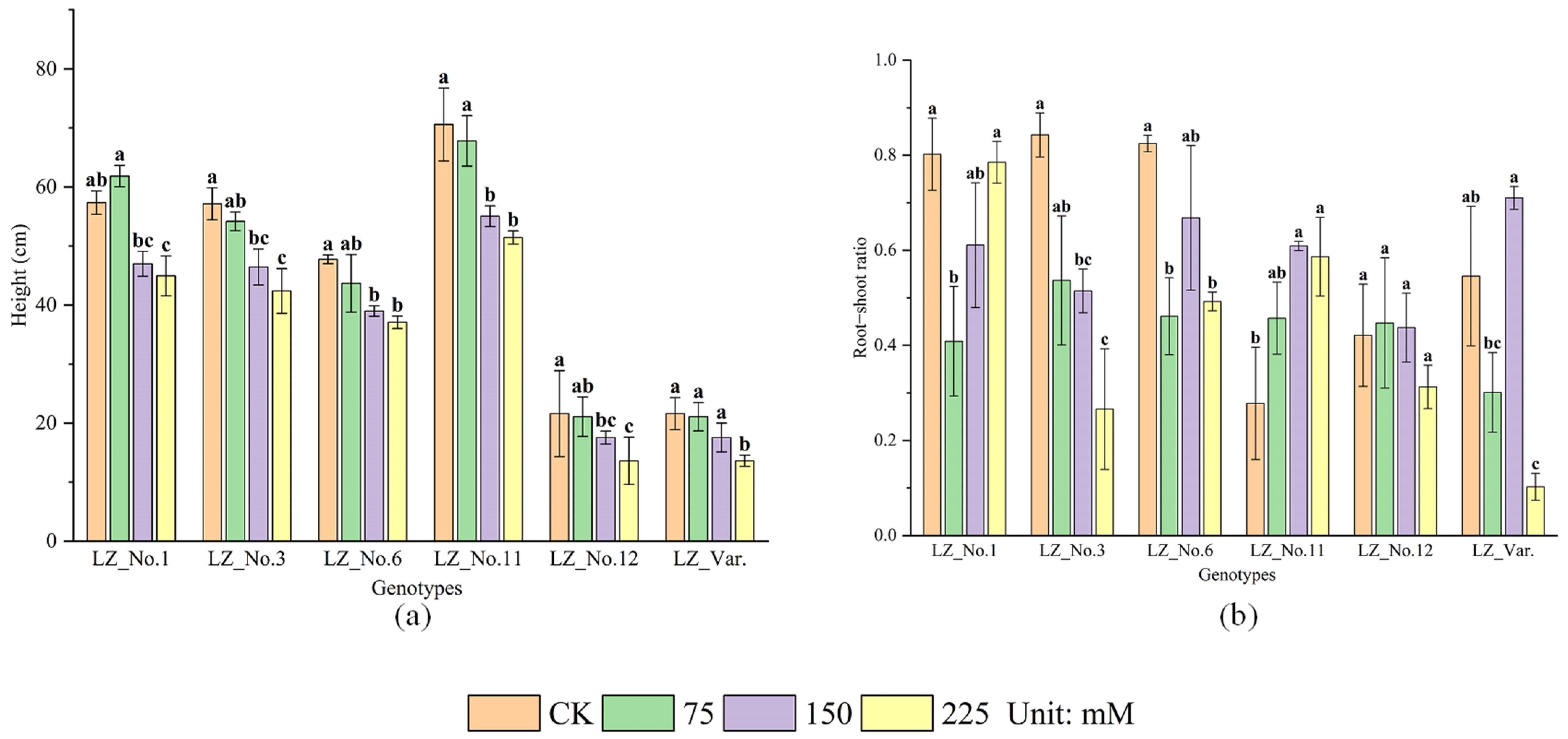
3.1. Impact of Varying Salinity Levels on Plant Height and Root–Shoot Ratio Across Different Genotypes of A. donax at Seedling Stage
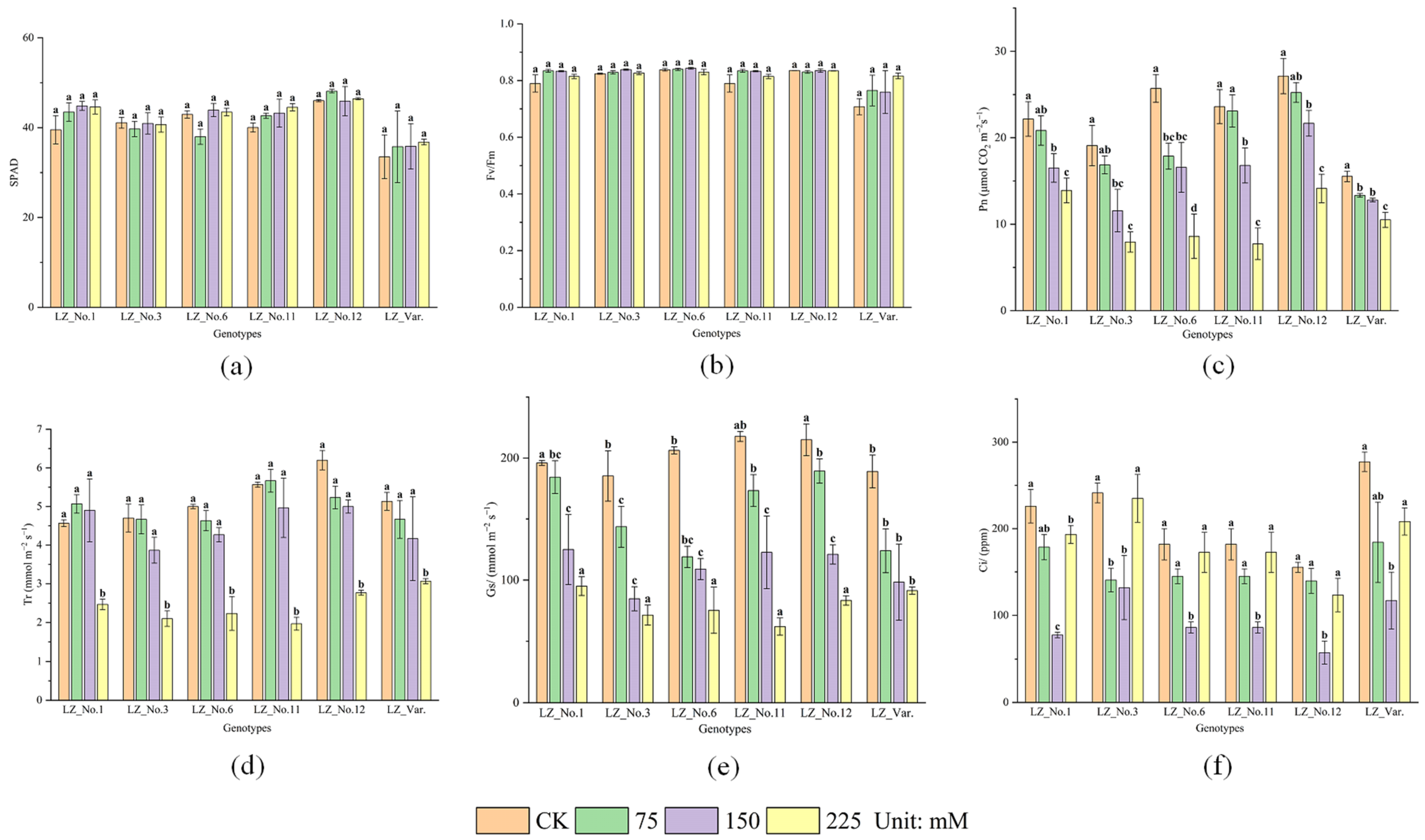
3.2. Effects of Saline–Alkali Stress on Photosynthesis Parameters
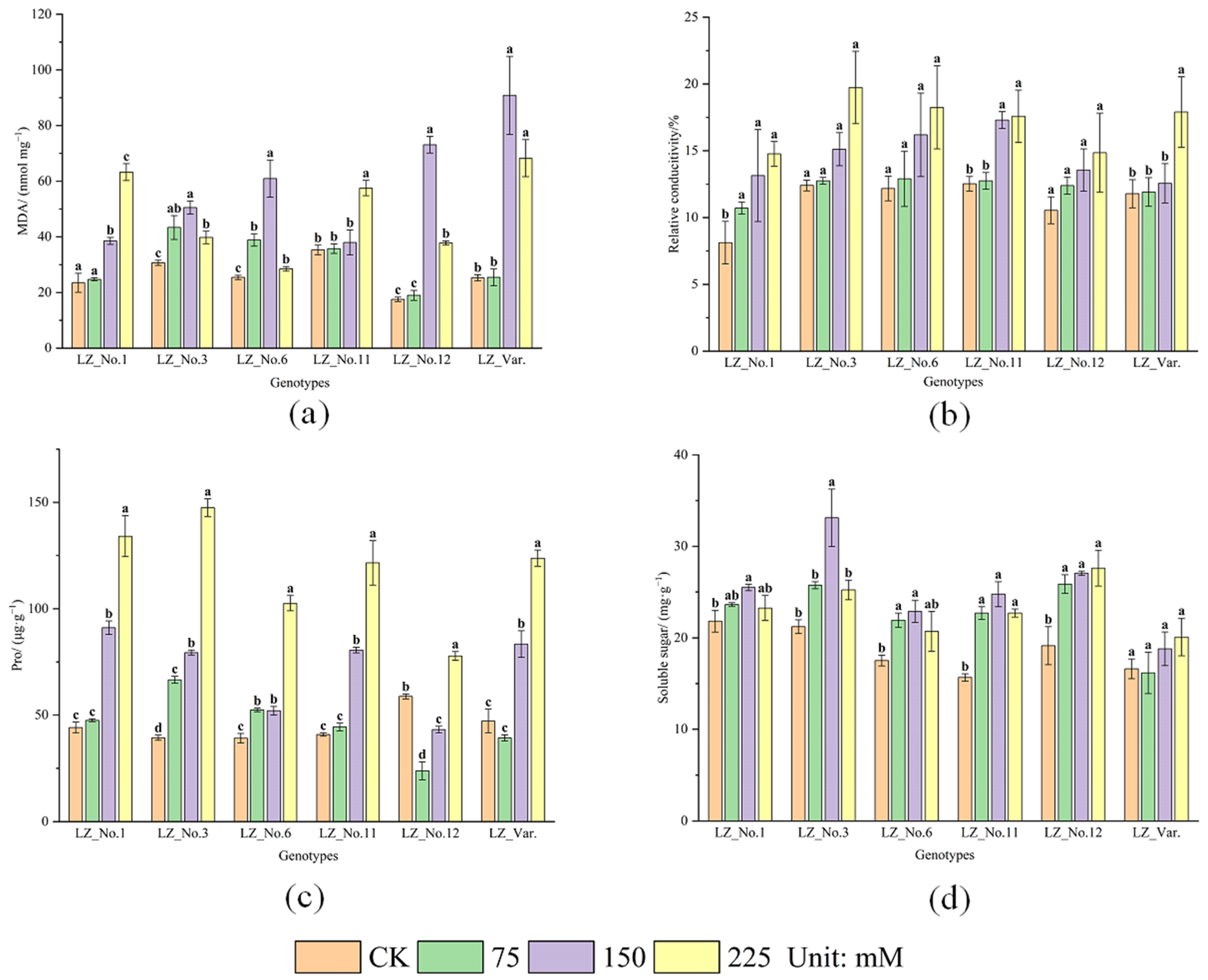
3.3. Effects of Saline–Alkali Stress on MDA Content and REC
3.4. Effects of Saline–Alkali Stress on Osmotic Adjustment Substance
3.5. Impact of Saline–Alkali Stress on Antioxidant Enzyme Activity
3.6. Comprehensive Evaluation of Salt Tolerance
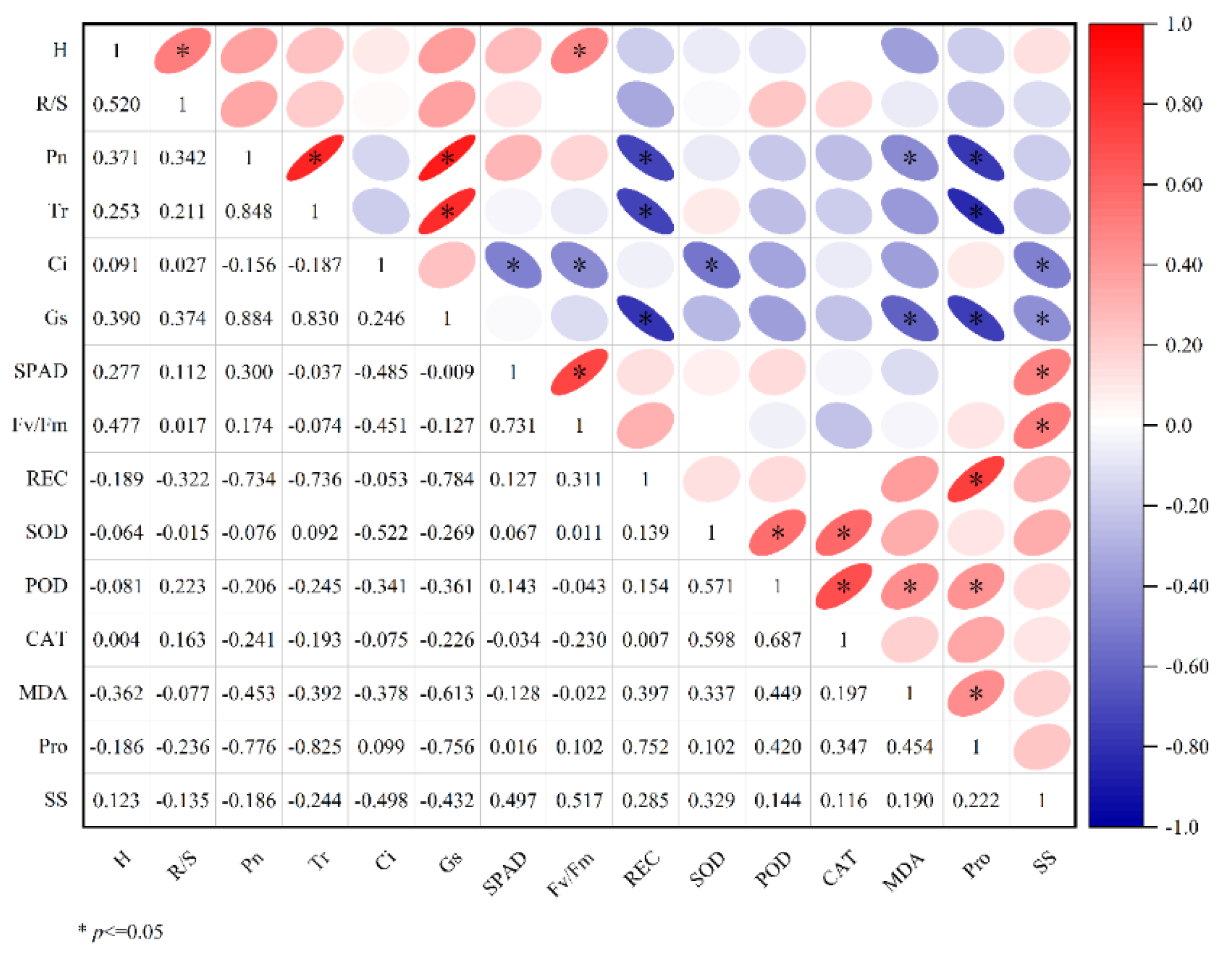
3.6.1. Correlation Analysis
3.6.2. Principal Component Analysis
3.6.3. Membership Function Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil science. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef]
- Wang, Y.; Jie, W.; Peng, X.; Hua, X.; Yan, X.; Zhou, Z.; Lin, J. Physiological Adaptive Strategies of Oil Seed Crop Ricinus communis Early Seedlings (Cotyledon vs. True Leaf) Under Salt and Alkali Stresses: From the Growth, Photosynthesis and Chlorophyll Fluorescence. Front. Plant Sci. 2018, 9, 1939. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. PPB 2022, 178, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Tarchoun, N.; Saadaoui, W.; Mezghani, N.; Pavli, O.I.; Falleh, H.; Petropoulos, S.A. The Effects of Salt Stress on Germination, Seedling Growth and Biochemical Responses of Tunisian Squash (Cucurbita maxima Duchesne) Germplasm. Plants 2022, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Huang, Z.; Zhao, G.; Zhai, R.; Ye, J.; Wu, M.; Yu, F.; Zhu, G.; Zhang, X. Differential Physiological Responses to Salt Stress between Salt-Sensitive and Salt-Tolerant japonica Rice Cultivars at the Post-Germination and Seedling Stages. Plants 2021, 10, 2433. [Google Scholar] [CrossRef] [PubMed]
- Jam, B.J.; Shekari, F.; Andalibi, B.; Fotovat, R.; Jafarian, V.; Najafi, J.; Uberti, D.; Mastinu, A. A Impact of Silicon Foliar Application on the Growth and Physiological Traits of Arundo donax L. Exposed to Salt Stress. Silicon 2023, 15, 1235–1245. [Google Scholar] [CrossRef]
- Pachepsky, Y.; Yakirevich, A.; Ponizovsky, A.A.; Gummatov, N. The osmotic potential of soil solutions in salt tolerance studies: Following M. Th. van Genuchten’s innovation. Vadose Zone J. 2024, 23, e20299. [Google Scholar] [CrossRef]
- Qin, Y.; Bai, J.; Wang, Y.; Liu, J.; Hu, Y.; Dong, Z.; Ji, L. Comparative effects of salt and alkali stress on photosynthesis and root physiology of oat at anthesis. Arch. Biol. Sci. 2018, 70, 329–338. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, F.; He, Z.; Liu, Y.; Chen, Z.; Ottosen, C.O.; Mittler, R.; Wu, Z.; Zhou, R. Higher Intensity of Salt Stress Accompanied by Heat Inhibits Stomatal Conductance and Induces ROS Accumulation in Tomato Plants. Antioxidants 2024, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liu, Y.; Duo, T.; Zhao, B.; Cui, G.; Ji, J.; Kuang, X.; Ervin, E.H.; Zhang, X. Antioxidant metabolism variation associated with alkali-salt tolerance in thirty switchgrass (Panicum virgatum) lines. PLoS ONE 2018, 13, e0199681. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Peng, L.; Wang, J.; Liu, J.L.; Jia, J.J.; Tang, L.P. Transcriptome analyses reveal the effects of mixed saline-alkali stress on indoleacetic acid and cytokinins in Malus hupehensis Rehd. leaves. Physiol. Mol. Biol. Plants 2023, 29, 11–22. [Google Scholar] [CrossRef]
- Sivakumar, J.; Sridhar Reddy, M.; Sergeant, K.; Hausman, J.F.; ShaValli Khan, P.S.; Osman Basha, P. Principal component analysis-assisted screening and selection of salt-tolerant tomato genotypes. Plant Physiol. Rep. 2023, 28, 272–288. [Google Scholar] [CrossRef]
- Abid, M.; Zhang, Y.J.; Li, Z.; Bai, D.F.; Zhong, Y.P.; Fang, J.B. Effect of Salt stress on growth, physiological and biochemical characters of Four kiwifruit genotypes. Sci. Hortic. 2020, 271, 109473. [Google Scholar] [CrossRef]
- Chen, T.; Niu, Y.; Yang, C.; Liang, Y.; Xu, J. Screening of Rice (Oryza sativa L.) Genotypes for Salinity Tolerance and Dissecting Determinants of Tolerance Mechanism. Plants 2024, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Abdel-Haleem, M.; Khedr, E.H. Harnessing GABA Pathways to Improve Plant Resilience Against Salt Stress. Horticulturae 2024, 10, 1296. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, H.; Wang, B.; Yuan, F. Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops. Int. J. Mol. Sci. 2022, 23, 7036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chang, L.; Li, G.; Li, Y. Advances and future research in ecological stoichiometry under saline-alkali stress. Environ. Sci. Pollut. Res. Int. 2023, 30, 5475–5486. [Google Scholar] [CrossRef]
- Lino, G.; Espigul, P.; Nogues, S.; Serrat, X. Arundo donax L. growth potential under different abiotic stress. Heliyon 2023, 9, e15521. [Google Scholar] [CrossRef] [PubMed]
- Ngernsaengsaruay, C.; Puangsin, B.; Leksungnoen, N.; Khantayanuwong, S.; Chanton, P.; Thaepthup, T.; Wessapak, P.; Meeboonya, R.; Yimlamai, P.; Wanitpinyo, K.; et al. Morphology, Taxonomy, Culm Internode and Leaf Anatomy, and Palynology of the Giant Reed (Arundo donax L.), Poaceae, Growing in Thailand. Plants 2023, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Verónica, C.; Alejandra, M.; Estela, S. Thermal Behaviour and Emission Characteristics of Arundo donax L. as Potential Biofuel. BioEnergy Res. 2023, 16, 1618–1628. [Google Scholar] [CrossRef]
- Jámbor, A.; Török, Á. The Economics of Arundo donax—A Systematic Literature Review. Sustainability 2019, 11, 4225. [Google Scholar] [CrossRef]
- Giertl, T.; Pauková, Z.; Hauptvogl, M.; Prcík, M.; Gadus, J. Evaluation of the Biomass of Arundo donax L. in the Context of Regional Bioenergetics. Pol. J. Environ. Stud. 2022, 31, 3043–3049. [Google Scholar] [CrossRef]
- Vitrone, F.; Brinker, S.; Ramos, D.; Ferrando, F.; Salvadó, J.; Mai, C. Approaching Self-Bonded Medium Density Fiberboards Made by Mixing Steam Exploded Arundo donax L.and Wood Fibers: A Comparison with pMDI-Bonded Fiberboards on the Primary Properties of the Boards. Materials 2023, 16, 4343. [Google Scholar] [CrossRef] [PubMed]
- Molari, L.; Coppolino, F.S.; García, J.J. Arundo donax: A widespread plant with great potential as sustainable structural material. Constr. Build. Mater. 2021, 268, 121143. [Google Scholar] [CrossRef]
- Mirza, N.; Pervez, A.; Mahmood, Q.; Shah, M.M.; Shafqat, M.N. Ecological restoration of arsenic contaminated soil by Arundo donax L. Ecol. Eng. 2011, 37, 1949–1956. [Google Scholar] [CrossRef]
- Fagnano, M.; Impagliazzo, A.; Mori, M.; Fiorentino, N. Agronomic and Environmental Impacts of Giant Reed (Arundo donax L.): Results from a Long-Term Field Experiment in Hilly Areas Subject to Soil Erosion. BioEnergy Res. 2015, 8, 415–422. [Google Scholar] [CrossRef]
- Webster, R.J.; Driever, S.M.; Kromdijk, J.; McGrath, J.; Leakey, A.D.B.; Siebke, K.; Demetriades-Shah, T.; Bonnage, S.; Peloe, T.; Lawson, T.; et al. High C3 photosynthetic capacity and high intrinsic water use efficiency underlies the high productivity of the bioenergy grass. Sci. Rep. 2016, 6, 20694. [Google Scholar] [CrossRef]
- Nawaz, A.; Kumar, P. Elucidating the bioenergy potential of raw, hydrothermally carbonized and torrefied waste biomass in terms of physicochemical characterization, kinetic and thermodynamic parameters. Renew. Energy 2022, 187, 844–856. [Google Scholar] [CrossRef]
- Soleimani, T.; Sordes, F.; Techer, I.; Junqua, G.; Hayek, M.; Salgues, M.; Souche, J.C. Comparative environmental and economic life cycle assessment of phytoremediation of dredged sediment using Arundo donax, integrated with biomass to bioenergy valorization chain. Sci. Total Environ. 2023, 903, 166160. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.P.; Li, Y.; Akhtar, M.; Liu, C.X.; Ma, T.L.; Min, W.F.; Bai, X.R.; She, Y.M.F.; Chen, L.; Tian, L.; et al. A DUF966 gene family member OsDSR3 positively regulates alkali stress tolerance in rice. Plant Sci. 2024, 343, 112072. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.W.; Liu, J.; Zhu, Q.A.; Li, Q.A.; Liu, J.Y.; Cui, Y.H.; Mu, Y.G.; Rasheed, A. Effect of Six Single Salt Stresses on Germination of Alfalfa. Appl. Ecol. Environ. Res. 2023, 21, 5771–5783. [Google Scholar] [CrossRef]
- Cai, Y.X.; Lin, D.M.; Cao, X.M.; Liu, B.; Lin, H.; Luo, H.L.; Liu, F.S.; Lin, Z.X. Salt tolerance evaluation of Arundo at axillary bud germination stage. J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2024, 53, 541–548. [Google Scholar] [CrossRef]
- Zhu, S.B.; Wuerguli, T.; Tang, G.M.; Zhang, Y.S.; Xu, W.L.; Ma, H.G. Research Progress on Saline-Alkali Land Changes and Its Treatment Measures in Xinjiang. Shandong Agric. Sci. 2023, 55, 158–165. [Google Scholar] [CrossRef]
- Li, Y.Y.; Pang, H.C.; Zhang, Z.Z.; Liu, S.P.; Zhang, C.P.; Wei, Y.Q. Characteristics and Countermeasures of Improvement Zonning of Saline-Alkali Soil in Hetao Plain of Inner Mongolia. Chin. J. Agric. Resour. Reg. Plan. 2020, 41, 115–121. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Wang, N. Study on the harm of saline alkali land and its improvement technology in china. IOP Conf. Ser. Earth Environ. Sci. 2020, 692, 042053. [Google Scholar] [CrossRef]
- Ghalati, R.E.; Shamili, M.; Homaei, A. Effect of putrescine on biochemical and physiological characteristics of guava (Arundo donax L.) seedlings under salt stress. Sci. Hortic. 2020, 261, 108961. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Liu, H.H.; Sun, C.; Ma, Q.B.; Bu, H.Y.; Chong, K.; Xu, Y.Y. A C2H2 zinc-finger protein OsZFP213 interacts with OsMAPK3 to enhance salt tolerance in rice. J. Plant Physiol. 2018, 229, 100–110. [Google Scholar] [CrossRef]
- Wang, S.G. Experimental Course in Plant Physiology; Science Press: Beijing, China, 2017. [Google Scholar]
- Sikder, R.K.; Wang, X.; Jin, D.; Zhang, H.; Gui, H.; Dong, Q.; Pang, N.; Zhang, X.; Song, M. Screening and evaluation of reliable traits of upland cotton (Gossypium hirsutum L.) genotypes for salt tolerance at the seedling growth stage. J. Cotton Res. 2020, 3, 11. [Google Scholar] [CrossRef]
- Li, L.; Du, L.; Cao, Q.; Yang, Z.; Liu, Y.; Yang, H.; Duan, X.; Meng, Z. Salt Tolerance Evaluation of Cucumber Germplasm under Sodium Chloride Stress. Plants 2023, 12, 2927. [Google Scholar] [CrossRef]
- Ji, X.; Tang, J.; Fan, W.; Li, B.; Bai, Y.; He, J.; Pei, D.; Zhang, J. Phenotypic Differences and Physiological Responses of Salt Resistance of Walnut with Four Rootstock Types. Plants 2022, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.C.; Dai, L.X.; Ding, H.; Ci, D.W.; Ning, T.Y.; Yang, J.S.; Zhao, X.H.; Yu, H.Q.; Zhang, Z.M. Response and adaptation to the accumulation and distribution of photosynthetic product in peanut under salt stress. J. Integr. Agric. 2020, 19, 690–699. [Google Scholar] [CrossRef]
- Zhou, H.P.; Shi, H.F.; Yang, Y.Q.; Feng, X.X.; Chen, X.; Xiao, F.; Lin, H.H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2023, 51, 16–34. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Ganapati, R.K.; Naveed, S.A.; Zafar, S.; Wang, W.; Xu, J. Saline-Alkali Tolerance in Rice: Physiological Response, Molecular Mechanism, and QTL Identification and Application to Breeding. Rice Sci. 2022, 29, 412–434. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, Y.; Guo, W.; Xue, Y.; Yu, L. Metabolic and Physiological Changes in the Roots of Two Oat Cultivars in Response to Complex Saline-Alkali Stress. Front. Plant Sci. 2022, 13, 835414. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, J.; Lu, Z.; Liu, R.; Hong, Y.; Su, B.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; et al. Melatonin-Mediated Enhancement of Photosynthetic Capacity and Photoprotection Improves Salt Tolerance in Wheat. Plants 2023, 12, 3984. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Z.H.; Gao, S.; Lv, Y.; Chen, Z.J.; Cao, B.L.; Xu, K. Different responses of two Chinese cabbage (Brassicarapa L. ssp. pekinensis) cultivars in photosynthetic characteristics and chloroplast ultrastructure to salt and alkali stress. Planta 2021, 254, 102. [Google Scholar] [CrossRef]
- Khan, W.U.D.; Aziz, T.; Hussain, I.; Ramzani, P.M.A.; Reichenauer, T.G. Silicon: A beneficial nutrient for maize crop to enhance photochemical efficiency of photosystem II under salt stress. Arch. Agron. Soil Sci. 2017, 63, 599–611. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, S.S.; Yang, A.; Akhtar, J.; Jacobsen, S.E. Antioxidative Response of Quinoa Exposed to Iso-Osmotic, Ionic and Non-Ionic Salt Stress. J. Agron. Crop Sci. 2015, 201, 452–460. [Google Scholar] [CrossRef]
- De Stefano, R.; Cappetta, E.; Guida, G.; Mistretta, C.; Caruso, G.; Giorio, P.; Albrizio, R.; Tucci, M. Screening of giant reed (Arundo donax L.) ecotypes for biomass production under salt stress. Plant Biosyst. 2018, 152, 911–917. [Google Scholar] [CrossRef]
- Li, X.Y.; Peng, X.Y.; Du, Z.X.; Li, S.X.; Lin, J.X. Biomass, Gas Exchange and Chlorophyll Fluorescence in Wheat Seedlings under Salt and Alkali Stress. Int. J. Agric. Biol. 2020, 23, 751–756. [Google Scholar] [CrossRef]
- Pei, L.; Wang, Z.; Zhang, J.; Li, W. The influence of soil salt content on the photosynthetic characteristics of spring wheat with trickle irrigation. BMC Plant Biol. 2016, 16, 83. [Google Scholar] [CrossRef][Green Version]
- Zhu, S.P.; Nong, J.F.; Luo, G.T.; Li, Q.P.; Wang, F.S.; Jiang, D.; Zhao, X.C. Varied tolerance and different responses of five citrus rootstocks to acid stress by principle component analysis and orthogonal analysis. Sci. Hortic. 2021, 278, 109853. [Google Scholar] [CrossRef]
- Mohsin, S.M.; Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Bhuyan, M.; Parvin, K.; Fujita, M. Tebuconazole and trifloxystrobin regulate the physiology, antioxidant defense and methylglyoxal detoxification systems in conferring salt stress tolerance in Triticum aestivum L. Physiol. Mol. Biol. Plants 2020, 26, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.J.; Zhao, Y.X.; Weisong, W.S.; Zhang, R.Y.; Su, A.G.; Li, C.H.; Wang, X.P.; Xing, J.F.; Shi, Z.; Zhao, J.R. Effect of saline stress on the physiology and growth of maize hybrids and their related inbred lines. Maydica 2017, 62, 11. [Google Scholar]
- Ding, T.; Yang, Z.; Wei, X.; Yuan, F.; Yin, S.; Wang, B. Evaluation of salt-tolerant germplasm and screening of the salt-tolerance traits of sweet sorghum in the germination stage. Funct. Plant Biol. 2018, 45, 1073–1081. [Google Scholar] [CrossRef]

| Genotype’s Name | Code Number | Location | Source and Country Name |
|---|---|---|---|
| Lvzhou_No.1 | LZ_No.1 | Latitude 26.056579° N, longitude 119.180505° E, | Fujian Province, China (Asia) |
| Lvzhou_No.3 | LZ_No.3 | Latitude 25.870612° N, longitude 118.927801° E, | Fujian Province, China (Asia) |
| Lvzhou_No.6 | LZ_No.6 | Latitude 29.651045° N, longitude 94.360232° E, | Tibet Autonomous Region, China (Asia) |
| Lvzhou_No.11 | LZ_No.11 | Latitude 32.061551° S, Longitude 118.791562° E, | Jiangsu Province, China (Asia) |
| Lvzhou_No.12 | LZ_No.12 | Latitude 1.9706° S, Longitude 30.1044° E, | Kigali, Rwanda (Africa) |
| A. donax var. Versicolor | LZ_Var. | Latitude 26.082995° N, longitude 119.237382° E, | Fujian Province, China (Asia) |
| Name of Chemicals | Cas No. | Manufacturer | Country |
|---|---|---|---|
| Potassium permanganate | 7722-64-7 | Sinopharm Chemical Reagent Co., Ltd | Shanghai China |
| Sodium chloride | 7647-14-5 | Shanghai Macklin Biochemical Technology Co., Ltd. | Shanghai China |
| Sodium sulfite anhydrous | 7757-83-7 | Shanghai Macklin Biochemical Technology Co., Ltd. | Shanghai China |
| Sodium bicarbonate | 144-55-8 | Shanghai Macklin Biochemical Technology Co., Ltd. | Shanghai China |
| Sodium carbonate anhydrous | 497-19-8 | Shanghai Macklin Biochemical Technology Co., Ltd. | Shanghai China |
| Parameters | Salinity (S) | Genotypes (G) | S × G Interaction | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| H | 26.505 | *** | 26.505 | *** | 0.701 | ns |
| R/S | 3.544 | * | 4.571 | ** | 3.338 | ** |
| Pn | 45.821 | *** | 9.666 | *** | 1.070 | ns |
| Tr | 76.942 | *** | 5.075 | ** | 1.126 | ns |
| Ci | 24.694 | *** | 5.783 | *** | 1.245 | ns |
| Gs | 78.733 | *** | 3.927 | ** | 1.254 | ns |
| SPAD | 1.689 | ns | 6.638 | *** | 1.149 | ns |
| Fv/Fm | 3.174 | * | 3.318 | * | 1.176 | ns |
| REC | 5.945 | ** | 1.593 | ns | 0.147 | ns |
| SOD | 53.762 | *** | 13.909 | *** | 12.484 | *** |
| POD | 58.358 | *** | 31.378 | *** | 19.153 | *** |
| CAT | 12.769 | *** | 42.907 | *** | 6.603 | *** |
| MDA | 94.051 | *** | 1.951 | ns | 25.091 | *** |
| Pro | 378.064 | *** | 47.002 | *** | 17.493 | *** |
| SS | 29.127 | *** | 12.557 | *** | 1.989 | ns |
| Index | 75 mM | 150 mM | 225 mM | Mean | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | |
| H | 0.95 | 0.147 | −0.232 | 0.145 | −0.39 | 0.794 | −0.392 | −0.246 | −0.285 | 0.817 | 0.418 | −0.225 | 0.092 | 0.586 | −0.069 | −0.109 |
| R/S | −0.483 | 0.833 | −0.001 | 0.026 | 0.528 | −0.618 | −0.288 | 0.28 | 0.169 | 0.477 | 0.798 | 0.189 | 0.071 | 0.231 | 0.170 | 0.165 |
| Pn | 0.567 | 0.631 | 0.512 | 0.05 | 0.719 | −0.307 | −0.551 | 0.292 | 0.962 | −0.255 | 0.027 | −0.034 | 0.749 | 0.023 | −0.004 | 0.103 |
| Tr | 0.766 | −0.3 | 0.191 | 0.502 | 0.572 | 0.769 | 0.206 | −0.196 | 0.912 | −0.113 | −0.306 | −0.23 | 0.750 | 0.119 | 0.030 | 0.025 |
| Ci | −0.186 | 0.661 | −0.624 | 0.169 | −0.769 | 0.03 | 0.439 | 0.391 | −0.917 | 0.166 | −0.227 | 0.249 | −0.624 | 0.286 | −0.137 | 0.270 |
| Gs | 0.568 | 0.669 | 0.068 | 0.417 | 0.923 | 0.274 | 0.094 | −0.182 | 0.95 | 0.005 | −0.114 | −0.29 | 0.814 | 0.316 | 0.016 | −0.018 |
| SPAD | 0.769 | 0.554 | 0.158 | −0.278 | 0.605 | 0.726 | −0.116 | 0.203 | 0.525 | 0.202 | 0.001 | 0.815 | 0.633 | 0.494 | 0.014 | 0.247 |
| Fv/Fm | 0.732 | −0.253 | −0.215 | −0.594 | 0.242 | 0.56 | −0.697 | 0.36 | 0.681 | −0.406 | −0.545 | 0.274 | 0.552 | −0.033 | −0.486 | 0.013 |
| REC | 0.532 | 0.416 | −0.584 | 0.45 | 0.565 | 0.51 | 0.523 | −0.381 | 0.669 | 0.711 | 0.033 | −0.165 | 0.589 | 0.546 | −0.009 | −0.032 |
| SOD | −0.545 | 0.786 | 0.233 | −0.019 | 0.777 | −0.382 | 0.41 | 0.033 | 0.207 | −0.24 | 0.801 | −0.005 | 0.146 | 0.055 | 0.481 | 0.003 |
| POD | −0.21 | 0.075 | 0.958 | −0.129 | 0.69 | 0.078 | 0.295 | 0.593 | 0.505 | 0.717 | 0.358 | 0.308 | 0.328 | 0.290 | 0.537 | 0.257 |
| CAT | −0.793 | 0.338 | −0.487 | −0.123 | 0.771 | −0.333 | 0.523 | −0.112 | 0.159 | −0.415 | 0.868 | −0.214 | 0.046 | −0.137 | 0.301 | −0.150 |
| MDA | −0.575 | −0.595 | −0.287 | 0.421 | 0.195 | −0.717 | −0.629 | −0.158 | 0.908 | −0.246 | 0.199 | 0.275 | 0.176 | −0.519 | −0.239 | 0.179 |
| Pro | −0.012 | −0.825 | 0.298 | 0.463 | −0.178 | 0.864 | 0.103 | 0.457 | −0.051 | 0.69 | −0.554 | 0.116 | −0.080 | 0.243 | −0.051 | 0.345 |
| SS | −0.495 | 0.451 | 0.492 | 0.446 | −0.378 | −0.312 | 0.825 | 0.278 | −0.567 | −0.626 | 0.366 | 0.383 | −0.480 | −0.162 | 0.561 | 0.369 |
| Eigenvalues | 5.358 | 4.607 | 2.783 | 1.735 | 5.349 | 4.496 | 3.174 | 1.441 | 6.269 | 3.385 | 3.270 | 1.429 | 5.659 | 4.163 | 3.076 | 1.535 |
| Contribution/% | 35.720 | 30.712 | 18.552 | 11.569 | 35.658 | 29.974 | 21.161 | 9.606 | 41.796 | 22.565 | 21.797 | 9.529 | 37.725 | 27.750 | 20.503 | 10.234 |
| Cumulative Contribution/% | 35.720 | 66.431 | 84.983 | 96.552 | 35.658 | 65.632 | 86.793 | 96.399 | 41.796 | 64.361 | 86.158 | 95.687 | 37.725 | 65.475 | 85.978 | 96.213 |
| Index | 75 mM | 150 mM | 225 mM | Mean | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | |
| H | 0.177 | 0.032 | −0.084 | 0.083 | −0.073 | 0.177 | −0.123 | −0.171 | −0.045 | 0.241 | 0.128 | −0.158 | 0.020 | 0.150 | −0.026 | −0.082 |
| R/S | −0.090 | 0.181 | 0.000 | 0.015 | 0.099 | −0.138 | −0.091 | 0.194 | 0.027 | 0.141 | 0.244 | 0.132 | 0.012 | 0.061 | 0.051 | 0.114 |
| Pn | 0.106 | 0.137 | 0.184 | 0.029 | 0.134 | −0.068 | −0.174 | 0.202 | 0.153 | −0.075 | 0.008 | −0.024 | 0.131 | −0.002 | 0.006 | 0.069 |
| Tr | 0.143 | −0.065 | 0.069 | 0.289 | 0.107 | 0.171 | 0.065 | −0.136 | 0.146 | −0.033 | −0.094 | −0.161 | 0.132 | 0.024 | 0.013 | −0.003 |
| Ci | −0.035 | 0.144 | −0.224 | 0.097 | −0.144 | 0.007 | 0.138 | 0.271 | −0.146 | 0.049 | −0.069 | 0.174 | −0.108 | 0.067 | −0.052 | 0.181 |
| Gs | 0.106 | 0.145 | 0.025 | 0.240 | 0.173 | 0.061 | 0.030 | −0.126 | 0.152 | 0.001 | −0.035 | −0.203 | 0.144 | 0.069 | 0.007 | −0.030 |
| SPAD | 0.143 | 0.120 | 0.057 | −0.160 | 0.113 | 0.161 | −0.036 | 0.141 | 0.084 | 0.060 | 0.000 | 0.570 | 0.113 | 0.114 | 0.007 | 0.184 |
| Fv/Fm | 0.137 | −0.055 | −0.077 | −0.343 | 0.045 | 0.124 | −0.220 | 0.250 | 0.109 | −0.120 | −0.167 | 0.191 | 0.097 | −0.017 | −0.155 | 0.033 |
| REC | 0.099 | 0.090 | −0.210 | 0.260 | 0.106 | 0.113 | 0.165 | −0.265 | 0.107 | 0.210 | 0.010 | −0.116 | 0.104 | 0.138 | −0.012 | −0.040 |
| SOD | −0.102 | 0.171 | 0.084 | −0.011 | 0.145 | −0.085 | 0.129 | 0.023 | 0.033 | −0.071 | 0.245 | −0.004 | 0.025 | 0.005 | 0.153 | 0.003 |
| POD | −0.039 | 0.016 | 0.344 | −0.074 | 0.129 | 0.017 | 0.093 | 0.412 | 0.081 | 0.212 | 0.109 | 0.216 | 0.057 | 0.082 | 0.182 | 0.185 |
| CAT | −0.148 | 0.073 | −0.175 | −0.071 | 0.144 | −0.074 | 0.165 | −0.078 | 0.025 | −0.122 | 0.265 | −0.149 | 0.007 | −0.041 | 0.085 | −0.099 |
| MDA | −0.107 | −0.129 | −0.103 | 0.243 | 0.037 | −0.159 | −0.198 | −0.109 | 0.145 | −0.073 | 0.061 | 0.192 | 0.025 | −0.120 | −0.080 | 0.109 |
| Pro | −0.002 | −0.179 | 0.107 | 0.267 | −0.033 | 0.192 | 0.032 | 0.317 | −0.008 | 0.204 | −0.169 | 0.081 | −0.014 | 0.072 | −0.010 | 0.222 |
| SS | −0.092 | 0.098 | 0.177 | 0.257 | −0.071 | −0.069 | 0.260 | 0.193 | −0.091 | −0.185 | 0.112 | 0.268 | −0.085 | −0.052 | 0.183 | 0.239 |
| Total scoring formula | F = 0.374F1 + 0.318F2 + 0.192F3 + 0.120F4 | F = 0.370F1 + 0.311F2 + 0.220F3 + 0.100F4 | F = 0.437F1 + 0.236F2 + 0.228F3 + 0.100F4 | F = 0.394F1 + 0.288F2 + 0.213F3 + 0.106F4 | ||||||||||||
| No. | 75 mM | 150 mM | 225 mM | Mean | ||||
|---|---|---|---|---|---|---|---|---|
| Score | Ranking | Score | Ranking | Score | Ranking | Score | Ranking | |
| LZ_No.1 | 0.622 | 1 | 0.852 | 1 | 0.884 | 1 | 0.786 | 1 |
| LZ_No.3 | 0.396 | 4 | 0.432 | 5 | 0.308 | 5 | 0.379 | 5 |
| LZ_No.6 | 0.102 | 6 | 0.377 | 6 | 0.274 | 6 | 0.251 | 6 |
| LZ_No.11 | 0.610 | 2 | 0.724 | 2 | 0.340 | 4 | 0.558 | 2 |
| LZ_No.12 | 0.490 | 3 | 0.502 | 3 | 0.402 | 3 | 0.465 | 3 |
| LZ_Var. | 0.323 | 5 | 0.438 | 4 | 0.459 | 2 | 0.407 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Cao, X.; Liu, B.; Lin, H.; Luo, H.; Liu, F.; Su, D.; Lv, S.; Lin, Z.; Lin, D. Saline–Alkali Tolerance Evaluation of Giant Reed (Arundo donax) Genotypes Under Saline–Alkali Stress at Seedling Stage. Agronomy 2025, 15, 463. https://doi.org/10.3390/agronomy15020463
Cai Y, Cao X, Liu B, Lin H, Luo H, Liu F, Su D, Lv S, Lin Z, Lin D. Saline–Alkali Tolerance Evaluation of Giant Reed (Arundo donax) Genotypes Under Saline–Alkali Stress at Seedling Stage. Agronomy. 2025; 15(2):463. https://doi.org/10.3390/agronomy15020463
Chicago/Turabian StyleCai, Yangxing, Xiuming Cao, Bin Liu, Hui Lin, Hailing Luo, Fengshan Liu, Dewei Su, Shi Lv, Zhanxi Lin, and Dongmei Lin. 2025. "Saline–Alkali Tolerance Evaluation of Giant Reed (Arundo donax) Genotypes Under Saline–Alkali Stress at Seedling Stage" Agronomy 15, no. 2: 463. https://doi.org/10.3390/agronomy15020463
APA StyleCai, Y., Cao, X., Liu, B., Lin, H., Luo, H., Liu, F., Su, D., Lv, S., Lin, Z., & Lin, D. (2025). Saline–Alkali Tolerance Evaluation of Giant Reed (Arundo donax) Genotypes Under Saline–Alkali Stress at Seedling Stage. Agronomy, 15(2), 463. https://doi.org/10.3390/agronomy15020463







