Effect of Storage Time on the Nutritional Value of Sugarcane Genotypes Treated with Calcium Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Chemical Analysis
2.3. Statistical Analysis
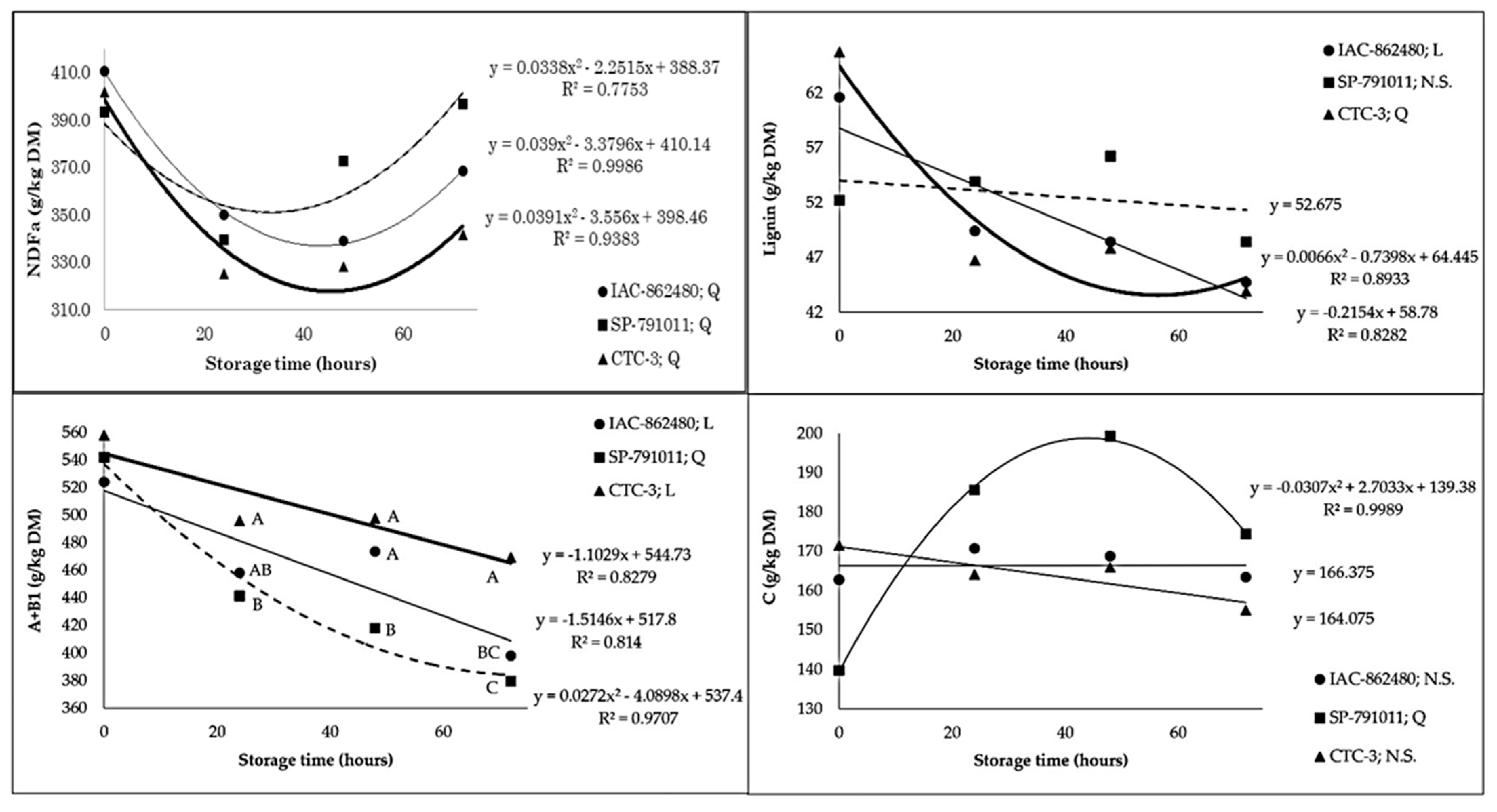
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pereira, J.O.; Oliveira, D.; Faustino, M.; Vidigal, S.S.; Pereira, A.M.; Ferreira, C.M.H.; Oliveira, A.S.; Durão, J.; Rodríguez-Alcalá, L.M.; Pintado, M.E.; et al. Use of Various Sugarcane Byproducts to Produce Lipid Extracts with Bioactive Properties: Physicochemical and Biological Characterization. Biomolecules 2024, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Sanches, G.M.; Bordonal, R.O.; Magalhães, P.S.G.; Otto, R.; Chagas, M.F.; Cardoso, T.F.; Luciano, A.C.S. Towards greater sustainability of sugarcane production by precision agriculture to meet ethanol demands in south-central Brazil based on a life cycle assessment. Biosyst. Eng. 2023, 229, 57–68. [Google Scholar] [CrossRef]
- Moura, R.R.; Camilo, M.G.; Processi, E.F.; Fernandes, A.M.; Silva, I.N.D.; Aniceto, E.S.; Oliveira, T.S.D. Evaluation of sugarcane rind on the nutritional value of ruminant feeding. Sci. Agric. 2024, 81, e20220245. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Y.R. Climate change and sugarcane production: Potential impact and mitigation strategies. Int. J. Agron. 2015, 2015, 547386. [Google Scholar] [CrossRef]
- Da Cruz, T.V.; Machado, R.L. Measuring climate change’s impact on different sugarcane varieties production in the South of Goiás. Sci. Rep. 2023, 13, 11637. [Google Scholar] [CrossRef]
- Molavian, M.; Ghorbani, G.R.; Rafiee, H.; Beauchemin, K.A. Substitution of wheat straw with sugarcane bagasse in low-forage diets fed to mid-lactation dairy cows: Milk production, digestibility, and chewing behavior. J. Dairy Sci. 2020, 103, 8034–8047. [Google Scholar] [CrossRef]
- Almeida, G.A.P.; Andrade Ferreira, M.; de Lima Silva, J.; Chagas, J.C.C.; Véras, A.S.C.; de Barros, L.J.A.; de Almeida, G.L.P. Sugarcane bagasse as exclusive roughage for dairy cows in smallholder livestock system. Asian-Australas. J. Anim. Sci. 2018, 31, 379. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Anannya, F.R. Sugarcane bagasse-A source of cellulosic fiber for diverse applications. Heliyon 2021, 7, e07771. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Romão, C.O.; Tosto, M.S.L.; Santos, S.A.; Pires, A.J.V.; Ribeiro, O.L.; Maranhão, C.M.A.; Rufino, L.M.A.; Correia, G.S.; Alba, H.D.R.; de Carvalho, G.G.P. Nutritive profile, digestibility, and carbohydrate fractionation of three sugarcane genotypes treated with calcium oxide. Agronomy 2023, 13, 733. [Google Scholar] [CrossRef]
- Melo, V.M.; Ferreira, G.F.; Fregolente, L.V. Sustainable catalysts for biodiesel production: The potential of CaO supported on sugarcane bagasse biochar. Renew. Sustain. Energy Rev. 2024, 189, 114042. [Google Scholar] [CrossRef]
- Jacovaci, F.A.; Jobim, C.C.; Schmidt, P.; Nussio, L.G.; Daniel, J.L.P. A data-analysis on the conservation and nutritive value of sugarcane silage treated with calcium oxide. Anim. Feed Sci. Technol. 2017, 225, 1–7. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, M.; Sufyan, A.; Saeed, A.; Sun, L.; Wang, S.; Nazar, M.; Tan, Z.; Liu, Y.; Tang, S. Biotechnological processing of sugarcane bagasse through solid-state fermentation with white rot fungi into nutritionally rich and digestible ruminant feed. Fermentation 2024, 10, 181. [Google Scholar] [CrossRef]
- Ishikawa, C.; Date, Y.; Umeda, M.; Tarumoto, Y.; Okubo, M.; Morimitsu, Y.; Tamura, Y.; Nishiba, Y.; Ono, H. A Data-Driven Approach to Sugarcane Breeding Programs with Agronomic Characteristics and Amino Acid Constituent Profiling. Metabolites 2024, 14, 243. [Google Scholar] [CrossRef]
- Carvalho, M.V.; Rodrigues, P.H.M.; Lima, M.L.P.; dos ANJOS, I.A.; de Andrade Landell, M.G.; dos Santos, M.V.; Prada, L.F. Chemical composition and digestibility of sugarcane harvested at two periods of the year. Braz. J. Vet. Res. Anim. Sci. 2010, 47, 298–306. [Google Scholar]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists Inc.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis; Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1970. [Google Scholar]
- Sniffen, C.J.; O’connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- NRC—National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academic Press: Washington, DC, USA, 2001. [Google Scholar]
- Mori, A.S.; Cornelissen, J.H.C.; Fujii, S.; Okada, K.I.; Isbell, F. A meta-analysis on decomposition quantifies afterlife effects of plant diversity as a global change driver. Nat. Commun. 2020, 11, 4547. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, K.; Tang, Y.; Huang, H.; Peng, G.; Wang, D. Research Progress on the Decomposition Process of Plant Litter in Wetlands: A Review. Water 2023, 15, 3246. [Google Scholar] [CrossRef]
- Le, Q.; Price, G.W. A review of the influence of heat drying, alkaline treatment, and composting on biosolids characteristics and their impacts on nitrogen dynamics in biosolids-amended soils. Waste Manag. 2024, 176, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Ma, Z.; Tian, Y.; Zhang, X.; Shan, H. Effects of maize straw treated with various levels of CaO and moisture on composition, structure, and digestion by in vitro gas production. Anim. Biosci. 2021, 34, 1940. [Google Scholar] [CrossRef]
- Oliveira, M.D.S.; Rêgo, A.C.D.; Sforcini, M.P.R.; Freitas Júnior, J.E.; Santos, J.D.; Carvalho, M.V.D. Bromatological characteristics and in vitro digestibility of four sugarcane varieties subjected or not to the application of quicklime. Acta Sci. Anim. Sci. 2012, 34, 355–361. [Google Scholar] [CrossRef][Green Version]
- Andrade Junior, A.S.; Bastos, E.A.; Ribeiro, V.Q.; Athayde, C.; Silva, P.H.D. Stalk yield of sugarcane cultivars under different water regimes by subsurface drip irrigation. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 169–174. [Google Scholar] [CrossRef][Green Version]
- Romão, C.O.; de Carvalho, G.G.P.; Tosto, M.S.L.; Santos, S.A.; Pires, A.J.V.; Maranhão, C.M.A.; Rufino, L.M.A.; Correia, G.S.; Oliveira, P.A. Nutritional profiles of three genotypes of sugarcane silage associated with calcium oxide. Grassl. Sci. 2018, 64, 16–28. [Google Scholar] [CrossRef]
- Melo, L.J.O.T.D.; Oliveira, F.J.D.; Bastos, G.Q.; Anunciação Filho, C.J.D.; Reis, O.V.D. Sugarcane genotype x harvest cycles interaction in Zona da Mata Norte of Pernambuco. Bragantia 2006, 65, 197–205. [Google Scholar] [CrossRef]
- Mota, D.A.; Oliveira, M.D.S.D.; Domingues, F.N.; Manzi, G.M.; Ferreira, D.D.S.; Santos, J.D. Hydrolysis of cane sugar with lime or hydrated lime. Rev. Bras. Zootec. 2010, 39, 1186–1190. [Google Scholar] [CrossRef]
- Balieiro Neto, G.; Siqueira, G.R.; Reis, R.A.; Nogueira, J.R.; Roth, M.D.T.P.; Roth, A.P.D.T.P. Calcium oxide as additive on the sugarcane ensilage. Rev. Bras. Zootec. 2007, 36, 1231–1239. [Google Scholar] [CrossRef]
- Liang, X.; Dai, R.; Chang, S.; Wei, Y.; Zhang, B. Antibacterial mechanism of biogenic calcium oxide and antibacterial activity of calcium oxide/polypropylene composites. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129446. [Google Scholar] [CrossRef]
- Rezende, A.V.; Rabelo, C.H.S.; Andrade, L.P.; Rabelo, F.H.S.; Santos, W.B. Characteristics of sugar cane in natura and hydrolyzed with lime in different storage times. Rev. Caatinga 2013, 26, 107–116. [Google Scholar]
- Amaral, R.C.; Pires, A.V.; Susin, I.; Nussio, L.G.; Ferreira, E.M.; Gentil, R.S. Fresh sugarcane or ensiled without and with chemical additives: Aerobic stability of forage and diets. Rev. Bras. Zootec. 2009, 38, 1857–1864. [Google Scholar] [CrossRef]
- Carvalho, G.G.P.; Cavali, J.; Fernandes, F.E.P.; Rosa, L.O.; Olivindo, C.S.; Porto, M.O.; Pires, A.J.V.; Garcia, R. Chemical composition and dry matter digestibility of sugarcane bagasse treated with calcium oxide. Arq. Bras. Med. Vet. Zoo. 2009, 61, 1346–1352. [Google Scholar] [CrossRef]
- Heering, R.; Baumont, R.; Selje-Aßmann, N.; Dickhoefer, U. Effect of physically effective fibre on chewing behaviour, ruminal fermentation, digesta passage and protein metabolism of dairy cows. J. Agric. Sci. 2023, 161, 720–733. [Google Scholar] [CrossRef]
- Pate, F.M.; Alvarez, J.; Phillips, J.D.; Eiland, B.R. Sugarcane as a cattle feed: Production and utilization. Bulletin 2002, 844, 1–21. [Google Scholar]
- Rabelo, C.H.S.; Rezende, A.V.; Rabelo, F.H.S.; Nogueira, D.A.; Vieira, P.F. Chemical-bromatologic composition of hidrolyzed sugarcane with white-wash. Rev. Caatinga 2010, 23, 135–143. [Google Scholar]
- Carvalho, G.G.P.; Garcia, R.; Pires, A.J.V.; Pereira, O.G.; Fernandes, F.E.P.; Obeid, J.A.; Carvalho, B.M.A. Carbohydrate fractioning of elephantgrass silage wilted or enriched with cocoa meal. Rev. Bras. Zootec. 2007, 36, 1000–1005. [Google Scholar] [CrossRef]
- Singh, S.; Koli, P.; Ahmed, S.; Kumar, N.; Rana, M.; Singhal, R.; Indu; Choudhary, M.; Ren, Y. Exploring the genetic variability in yield, nutritional and digestibility traits in oat grains through ruminant nutrition. Heliyon 2024, 10, e31541. [Google Scholar] [CrossRef]
| Genotype | Storage Time (Hours) | ||
|---|---|---|---|
| 24 | 48 | 72 | |
| Temperature (°C) | |||
| IAC-862480 | 33.3 | 28.8 | 28.8 |
| SP-791011 | 32.6 | 29.1 | 28.3 |
| CTC-3 | 29.3 | 29.5 | 28.0 |
| Item | Genotypes | Storage Time | SEM 1 | p-Value 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IAC-862480 | SP-791011 | CTC-3 | 0 h | 24 h | 48 h | 72 h | Genotype (G) | Time (P) | G × P 3 | ||
| Dry matter 4 | 343.1 b | 360.5 a | 326.2 c | 302.9 | 356.2 | 360.0 | 353.8 | 3.23 | <0.01 | <0.01 | 1.00 |
| Organic matter | 908.0 | 911.9 | 918.6 | 964.1 | 898.7 | 890.1 | 898.3 | 3.27 | 0.05 | <0.01 | 0.11 |
| Crude protein | 46.7 a | 41.3 b | 42.1 b | 45.8 | 41.0 | 42.0 | 44.5 | 0.57 | 0.01 | 0.10 | 0.79 |
| Ether extract | 9.97 | 9.84 | 10.02 | 9.97 | 9.66 | 10.07 | 10.07 | 0.05 | 0.50 | 0.10 | 0.49 |
| NDFa 5 | 367.1 a | 375.5 a | 349.3 b | 401.9 | 338.2 | 346.7 | 368.9 | 3.24 | <0.01 | <0.01 | <0.01 |
| ADF 6 | 269.6 ab | 290.1 a | 256.7 b | 305.4 | 271.3 | 274.5 | 237.3 | 3.64 | <0.01 | <0.01 | 0.21 |
| Hemicellulose | 122.4 | 114.7 | 112.7 | 122.0 | 98.5 | 93.3 | 152.6 | 2.84 | 0.24 | <0.01 | 0.82 |
| Cellulose | 212.3 b | 233.1 a | 200.5 b | 236.4 | 216.1 | 219.3 | 189.3 | 2.91 | <0.01 | <0.01 | 0.64 |
| Lignin | 51.0 | 52.7 | 51.0 | 59.8 | 50.0 | 50.8 | 45.7 | 0.84 | 0.68 | <0.01 | 0.02 |
| NFC 7 | 345.6 b | 332.1 b | 378.4 a | 491.8 | 321.2 | 317.4 | 277.8 | 8.79 | <0.01 | <0.01 | 0.06 |
| Total carbohydrates | 737.6 | 733.8 | 742.7 | 908.3 | 691.0 | 685.2 | 667.6 | 10.10 | 0.31 | <0.01 | 0.24 |
| TDN 8 | 552.1 ab | 540.1 b | 557.9 a | 696.8 | 507.8 | 502.9 | 492.6 | 8.75 | 0.01 | <0.01 | 0.13 |
| IVDMD 9 | 305.1 ab | 335.2 a | 297.1 b | 335.1 | 301.9 | 288.5 | 324.4 | 4.64 | 0.02 | 0.03 | 0.16 |
| A + B1 10 | 463.3 b | 445.0 c | 505.0 a | 541.3 | 464.9 | 462.8 | 415.4 | 5.71 | <0.01 | <0.01 | <0.01 |
| B2 10 | 370.4 a | 380.2 a | 330.9 b | 300.8 | 361.7 | 359.3 | 420.3 | 5.20 | <0.01 | <0.01 | 0.47 |
| C 10 | 166.3 | 174.7 | 164.1 | 157.9 | 173.4 | 178.0 | 164.3 | 2.19 | 0.26 | 0.06 | 0.03 |
| Item | Regression Equation | Determination Coefficient |
|---|---|---|
| Dry matter | Ŷ = 304.88 + 2.5115X − 0.0258X2 | 0.96 |
| Organic matter | Ŷ = 962.1 − 3.1583X + 0.0319X2 | 0.98 |
| Acid detergent fiber | Ŷ = 302.29 − 0.8379X | 0.87 |
| Hemicellulose | Ŷ = 124.31 − 2.2267X + 0.0359X2 | 0.95 |
| Cellulose | Ŷ = 235.99 − 0.5754X | 0.84 |
| Non-fibrous carbohydrates | Ŷ = 481.67 − 6.7846X + 0.0569X2 | 0.92 |
| Total carbohydrates | Ŷ = 847.21 − 3.0329X | 0.68 |
| Total digestible nutrients | Ŷ = 642.65 − 2.5729X | 0.66 |
| In vitro dry matter digestibility | Ŷ = 336.58 − 2.349X + 0.03X2 | 0.91 |
| B2 | Ŷ = 307.11 + 1.4838X | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romão, C.d.O.; Carvalho, G.G.P.d.; Tosto, M.S.L.; Santos, S.A.; Pires, A.J.V.; Maranhão, C.M.A.; Rufino, L.M.A.; Correia, G.S.; Alba, H.D.R. Effect of Storage Time on the Nutritional Value of Sugarcane Genotypes Treated with Calcium Oxide. Agronomy 2025, 15, 882. https://doi.org/10.3390/agronomy15040882
Romão CdO, Carvalho GGPd, Tosto MSL, Santos SA, Pires AJV, Maranhão CMA, Rufino LMA, Correia GS, Alba HDR. Effect of Storage Time on the Nutritional Value of Sugarcane Genotypes Treated with Calcium Oxide. Agronomy. 2025; 15(4):882. https://doi.org/10.3390/agronomy15040882
Chicago/Turabian StyleRomão, Claudio de O., Gleidson G. P. de Carvalho, Manuela S. L. Tosto, Stefanie A. Santos, Aureliano J. V. Pires, Camila M. A. Maranhão, Luana M. A. Rufino, George S. Correia, and Henry D. R. Alba. 2025. "Effect of Storage Time on the Nutritional Value of Sugarcane Genotypes Treated with Calcium Oxide" Agronomy 15, no. 4: 882. https://doi.org/10.3390/agronomy15040882
APA StyleRomão, C. d. O., Carvalho, G. G. P. d., Tosto, M. S. L., Santos, S. A., Pires, A. J. V., Maranhão, C. M. A., Rufino, L. M. A., Correia, G. S., & Alba, H. D. R. (2025). Effect of Storage Time on the Nutritional Value of Sugarcane Genotypes Treated with Calcium Oxide. Agronomy, 15(4), 882. https://doi.org/10.3390/agronomy15040882







