Transcriptome Analysis of the Effects of Selenium Form and Concentration on Rice Growth and Metabolism at the Seedling Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Experimental Materials
2.2. Determination of Contamination and Germination Rates
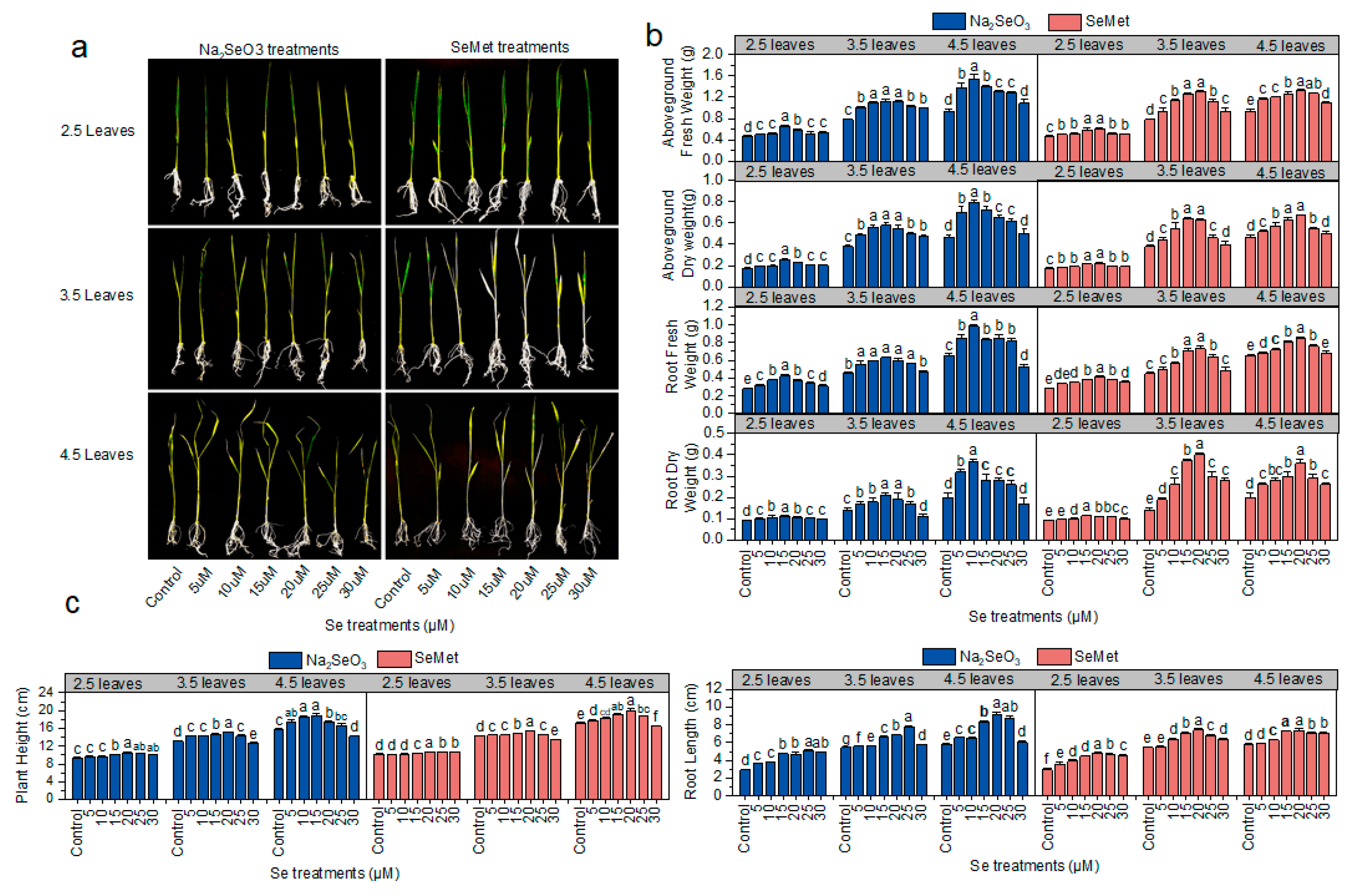
2.3. Plant Biomass and Growth Parameters
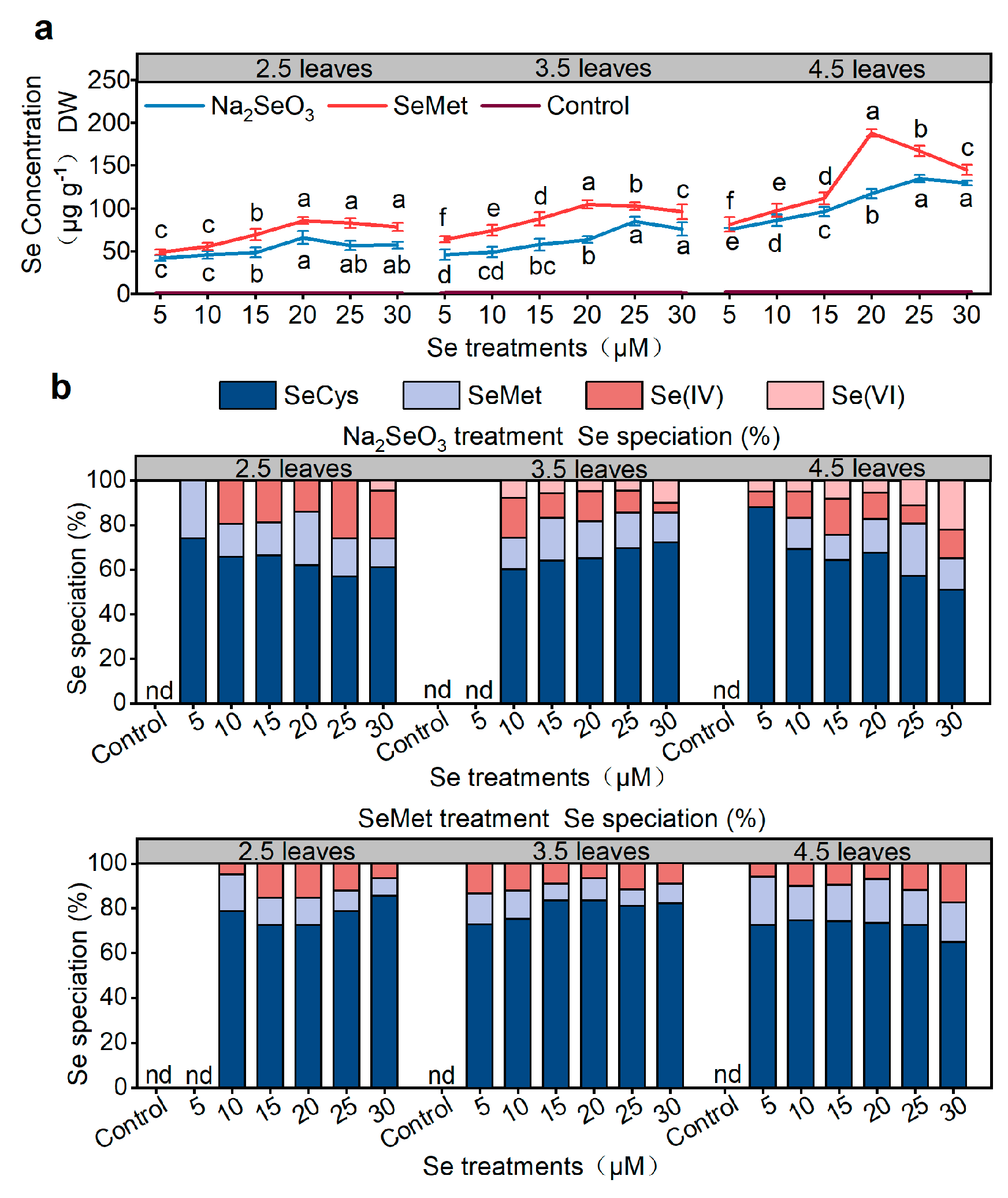
2.4. Determination of Se Concentration in Rice
2.5. Determination of Se Species in Rice
2.6. Transcriptome Sequencing and Analysis
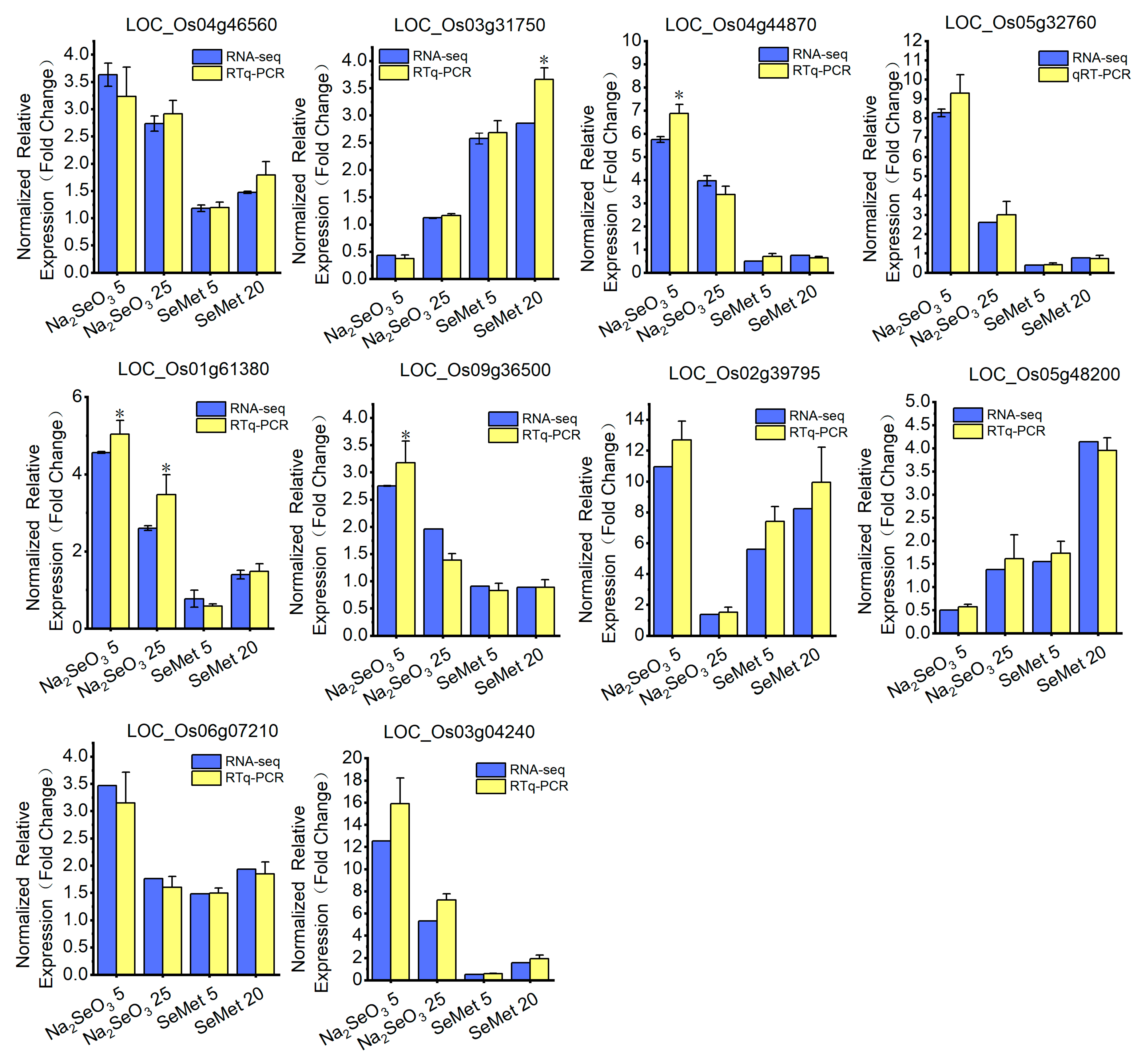
2.7. Validation of RNA-seq by RT-qPCR
2.8. Statistical Analysis
3. Results
3.1. Screening of Seed Disinfection Methods for Hydroponic Experiments
3.2. Effects of Different Se Application Rates on Rice Growth
3.3. Effects of Different Forms of Se on Se Concentration and Speciation in Rice
3.4. Transcriptome Analysis
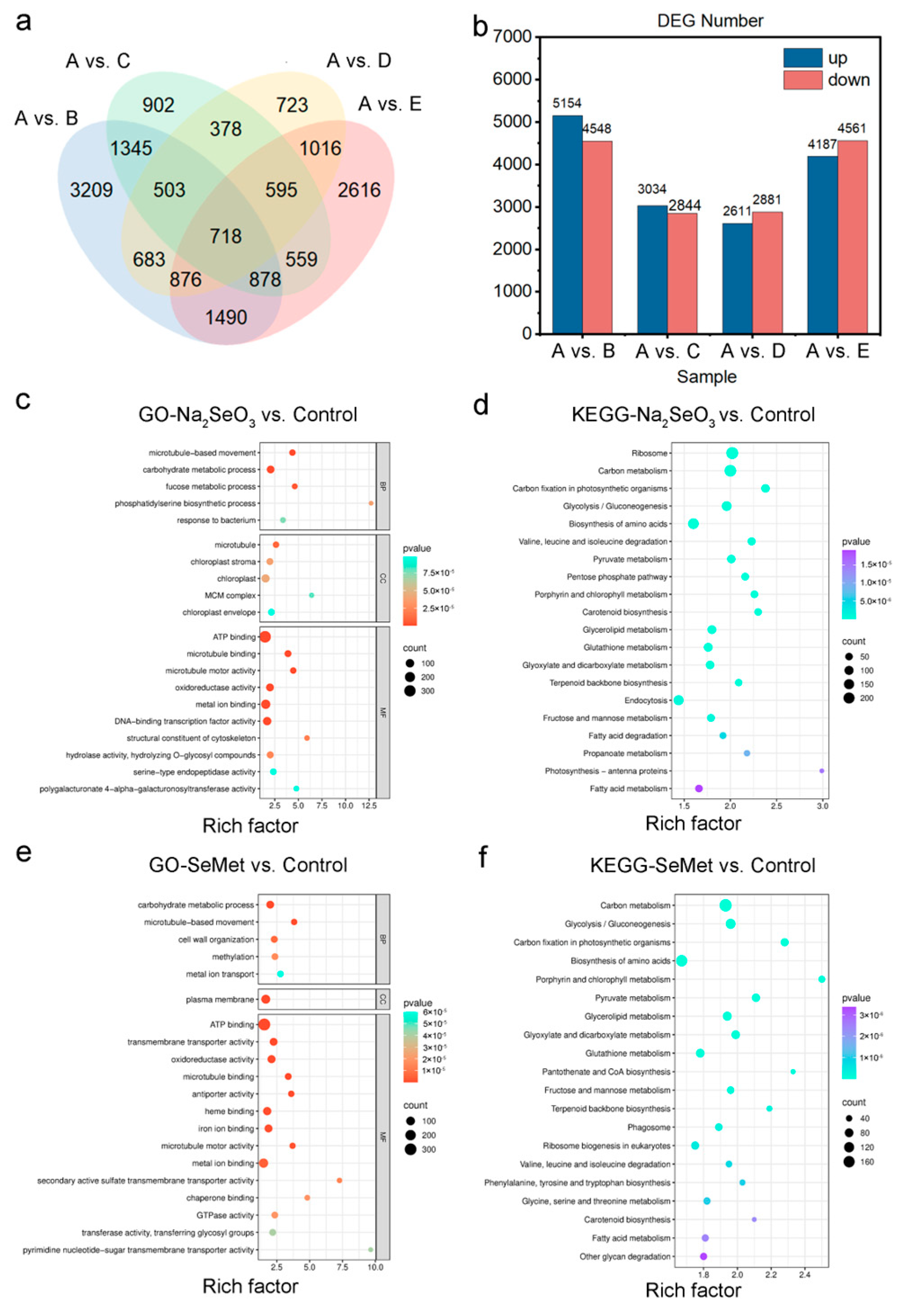
3.4.1. Transcriptome Sequencing and Differentially Expressed Gene (DEG) Identification
3.4.2. GO Enrichment and KEGG Pathway Analysis
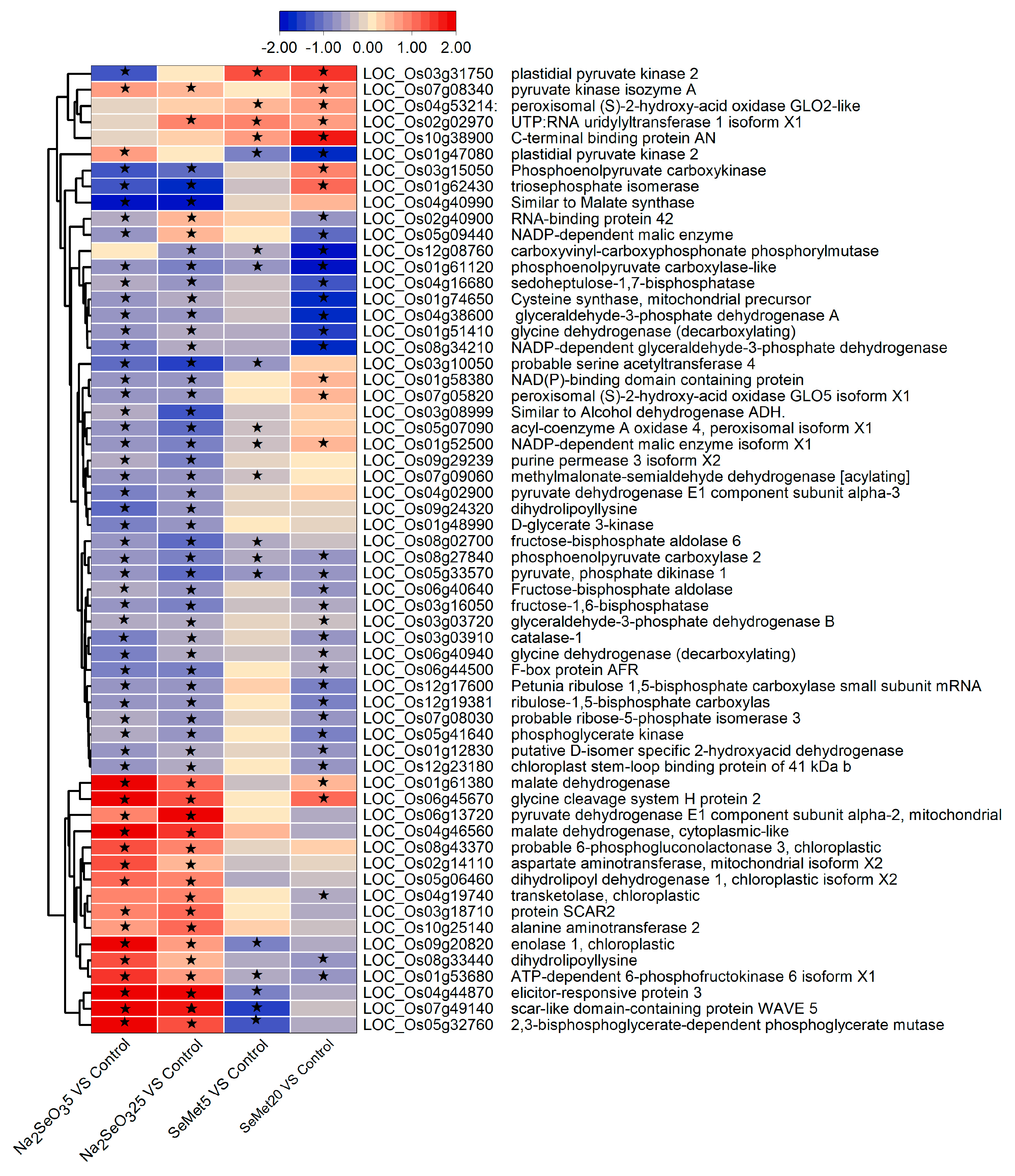
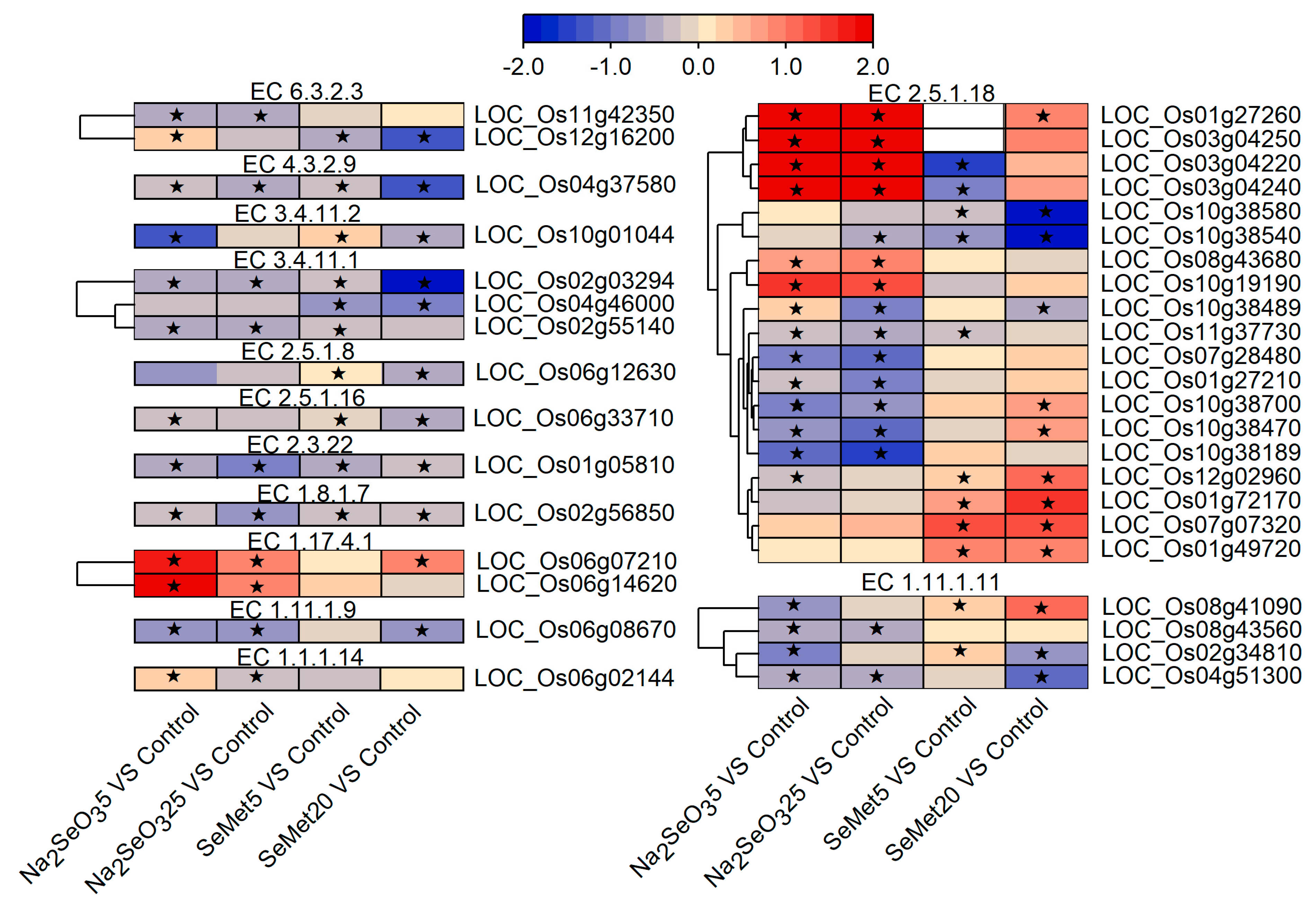
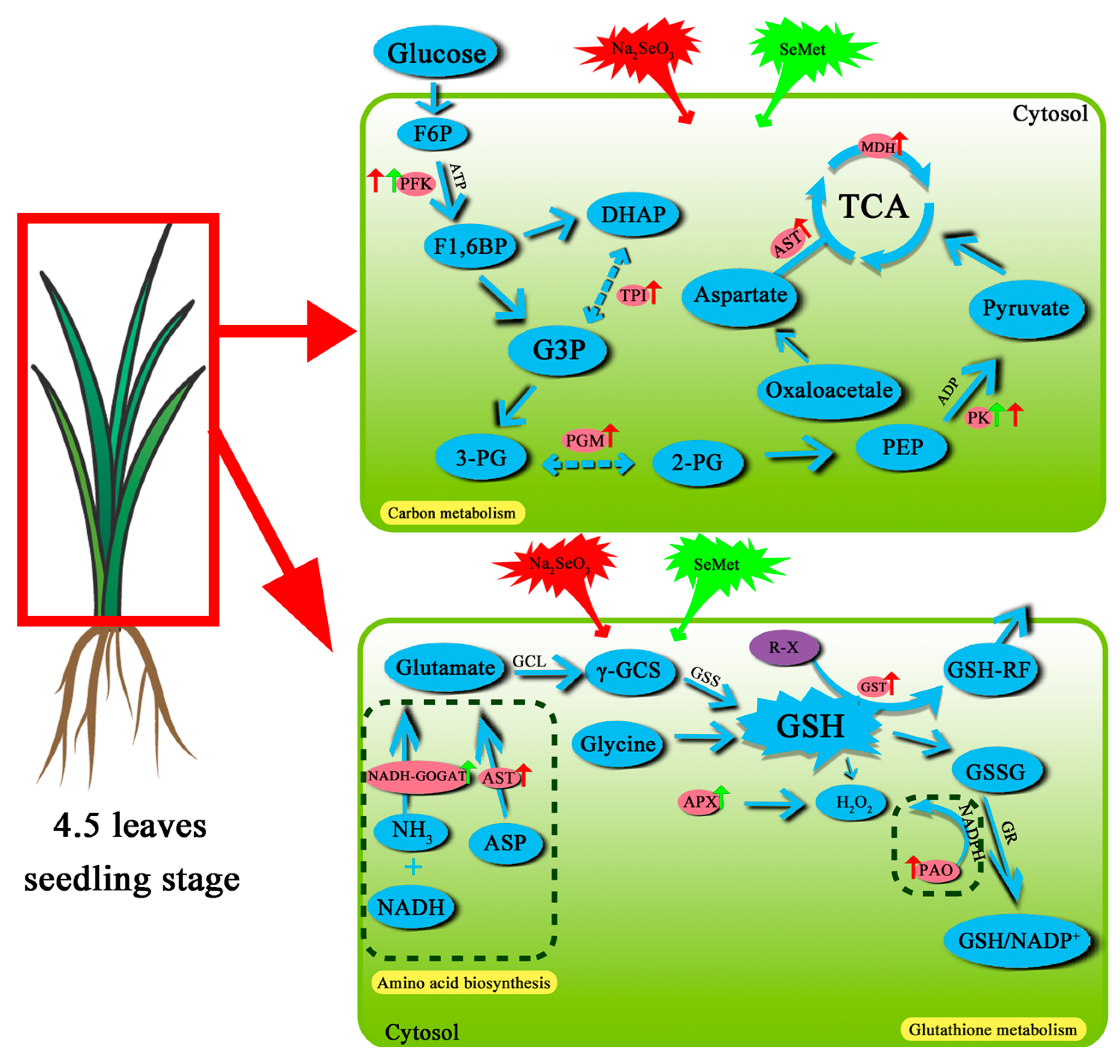
3.4.3. Key Metabolic Pathways and DEGs Involved in Se Assimilation and Metabolism
- DEGs related to carbon metabolism
- DEGs related to amino acid biosynthesis and metabolism
- DEGs related to glutathione metabolism
3.4.4. Validation of DEGs Through Reverse-Transcription–Quantitative PCR (RT-qPCR)
4. Discussion
4.1. Effect of Different Types of Se on Rice Seedling Growth and Se Absorption
4.2. The Effect of Different Forms of Exogenous Se on the Se Speciation Changes in Rice Seedlings
4.3. Key Se Metabolic Pathways in Rice Seedlings Under Se Treatment
4.4. Metabolic Pathways and Differentially Expressed Genes for Se Bioaugmentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Chu, C. Selenium Uptake, Transport, Metabolism, Reutilization, and Biofortification in Rice. Rice 2022, 15, 30. [Google Scholar] [CrossRef]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium Transport and Metabolism in Plants: Phytoremediation and Biofortification Implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium−fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.Y.; Cheng, S.R.; Lu, R.H.; Lai, R.F.; Zheng, A.X.; Ashraf, U.; Fan, P.S.; Du, B.; Luo, H.W.; Tang, X.R. Effect of Foliar Sodium Selenate on Leaf Senescence of Fragrant Rice in South China. Appl. Ecol. Environ. Res. 2019, 17, 3343–3351. [Google Scholar] [CrossRef]
- Gao, H.-H.; Chen, M.-X.; Hu, X.-Q.; Chai, S.-S.; Qin, M.-L.; Cao, Z.-Y. Separation of Selenium Species and Their Sensitive Determination in Rice Samples by Ion-Pairing Reversed-Phase Liquid Chromatography with Inductively Coupled Plasma Tandem Mass Spectrometry. J. Sep. Sci. 2018, 41, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The Importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Song, T.; Su, X.; He, J.; Liang, Y.; Zhou, T.; Liu, C. Selenium (Se) Uptake and Dynamic Changes of Se Content in Soil–Plant Systems. Environ. Sci. Pollut. Res. 2018, 25, 34343–34350. [Google Scholar] [CrossRef]
- D’Amato, R.; Fontanella, M.C.; Falcinelli, B.; Beone, G.M.; Bravi, E.; Marconi, O.; Benincasa, P.; Businelli, D. Selenium Biofortification in Rice (Oryza sativa L.) Sprouting: Effects on Se Yield and Nutritional Traits with Focus on Phenolic Acid Profile. J. Agric. Food Chem. 2018, 66, 4082–4090. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the Health-Promoting Effect of Adequate Selenium Status. Front. Nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, F.; Xing, H.; Mao, K.; Chen, G.; Guo, Q.; Chen, J. Correlation Analysis of Lignin Accumulation and Expression of Key Genes Involved in Lignin Biosynthesis of Ramie (Boehmeria nivea). Genes 2019, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, Y.; Dun, X.; Wang, X.; Wang, H. Research Progress of Selenium-Enriched Foods. Nutrients 2023, 15, 4189. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Zou, Q.; Wu, B.; Bao, H.; Zheng, J.; Yu, F. Investigation of a Cascading Core-Offset in-Line Fiber Mach-Zehnder Interferometer for Refractive Index Measurement. In Advanced Sensor Systems and Applications X; SPIE: Bellingham, WA, USA, 2020; Volume 11554, pp. 155–162. [Google Scholar]
- Lanza, M.G.D.B.; Silva, V.M.; Montanha, G.S.; Lavres, J.; Pereira de Carvalho, H.W.; Reis, A.R.D. Assessment of Selenium Spatial Distribution Using μ-XFR in Cowpea (Vigna unguiculata (L.) Walp.) Plants: Integration of Physiological and Biochemical Responses. Ecotoxicol. Environ. Saf. 2021, 207, 111216. [Google Scholar] [CrossRef]
- Gong, R.; Ai, C.; Zhang, B.; Cheng, X. Effect of Selenite on Organic Selenium Speciation and Selenium Bio accessibility in Rice Grains of Two Se-Enriched Rice Cultivars. Food Chem. 2018, 264, 443–448. [Google Scholar] [CrossRef]
- Deng, X.-F.; Zhao, Z.-Q.; Han, Z.-Y.; Huang, L.-Q.; Lv, C.-H.; Zhang, Z.-H.; Zhang, H.-Q.; Liu, X.-W. Selenium Uptake and Fruit Quality of Pear (Pyrus communis L.) Treated with Foliar Se Application. J. Plant Nutr. Soil Sci. 2019, 182, 637–646. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, L.; Huang, Q.; Li, H.; Wan, Y. Uptake and Translocation Mechanisms of Different Forms of Organic Selenium in Rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 970480. [Google Scholar] [CrossRef]
- Zhao, C.; Li, J.; Chen, N.; Bai, H.; Qu, L.; Yang, Y.; Ye, H.; Xiao, H.; Yan, H.; Zhang, T. Antioxidant Activity and Transcriptomic Analysis of Se-Enriched Golden Oyster Mushroom Pleurotus citrinopileatus (Agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Sun, X.; Tang, S.; Xu, P.; Pang, Y.; Huang, X.; Huang, Q.; Huang, J.; Zhang, M. Comparation of Se Accumulation and Distribution of Two Rice (Oryza sativa L.) Cultivars with High- and Low- Se Efficiency as Affected by Exogenous Application of Selenite. J. Cereal Sci. 2022, 105, 103475. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Zhang, M.; Wilson, L.; Xing, G.; Jiang, L.; Tang, S. Optimizing Root Architecture and Increasing Transporter Gene Expression Are Strategies to Promote Selenium Uptake by High-Se Accumulating Rice Cultivar. Plant Soil 2020, 447, 319–332. [Google Scholar] [CrossRef]
- Zhang, M.; Pang, Y.; Yi, Q.; Huang, J.; Huang, X.; Huang, Q.; Xu, P.; Tang, S. Comparative Effectiveness of Se Translocation between Low-Se and High-Se Rice Cultivars under Se Fertilization. Ecotoxicol. Environ. Saf. 2020, 205, 111372. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a Phosphate Transporter, Is Involved in the Active Uptake of Selenite in Rice. New Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Y.; Ma, L.; Jin, X.; Guo, G.; Tan, R.; Liu, Z.; Zheng, L.; Ye, F.; Liu, W. Transcriptome Analysis of Differentially Expressed Genes Involved in Selenium Accumulation in Tea Plant (Camellia sinensis). PLoS ONE 2018, 13, e0197506. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Shi, J.; Pan, Y.; Yang, S.; Xue, Y. Combined Metabolome and Transcriptome Analysis Reveals the Key Pathways Involved in the Responses of Soybean Plants to High Se Stress. Ecotoxicol. Environ. Saf. 2024, 287, 117262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, B.; Zhao, C.-R.; Sun, J.; Lai, Q.; Yu, C. Comparative de Novo Transcriptomics and Untargeted Metabolomic Analyses Elucidate Complicated Mechanisms Regulating Celery (Apium graveolens L.) Responses to Selenium Stimuli. PLoS ONE 2019, 14, e0226752. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, G.; Qin, H.; Guan, Y.; Wang, T.; Ni, W.; Xie, H.; Xing, Y.; Tian, G.; Lyu, M.; et al. Metabolomics Combined with Physiology and Transcriptomics Reveal Key Metabolic Pathway Responses in Apple Plants Exposure to Different Selenium Concentrations. J. Hazard. Mater. 2024, 464, 132953. [Google Scholar] [CrossRef]
- Li, L.; Wu, S.; Wang, S.; Shi, X.; Cheng, S.; Cheng, H. Molecular Mechanism of Exogenous Selenium Affecting the Nutritional Quality, Species and Content of Organic Selenium in Mustard. Agronomy 2023, 13, 1425. [Google Scholar] [CrossRef]
- Han, D.; Li, X.; Xiong, S.; Tu, S.; Chen, Z.; Li, J.; Xie, Z. Selenium Uptake, Speciation and Stressed Response of Nicotiana tabacum L. Environ. Exp. Bot. 2013, 95, 6–14. [Google Scholar] [CrossRef]
- Hu, J.; Huang, L.; Chen, G.; Liu, H.; Zhang, Y.; Zhang, R.; Zhang, S.; Liu, J.; Hu, Q.; Hu, F.; et al. The Elite Alleles of OsSPL4 Regulate Grain Size and Increase Grain Yield in Rice. Rice 2021, 14, 90. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ahsan, M.Z.; Li, Z.; Panhwar, F.H.; Zhang, Y.; Luo, D.; Su, Y.; Jia, X.; Ye, X.; Shen, C.; et al. Transcriptome Analysis of High- and Low-Selenium Genotypes Identifies Genes Responsible for Selenium Absorption, Translocation, and Accumulation. Front. Plant Sci. 2024, 15, 1413549. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Salem, M.; Jahed, K.R.; Artlip, T.S.; Sherif, S.M. From Endodormancy to Ecodormancy: The Transcriptional Landscape of Apple Floral Buds. Front. Plant Sci. 2023, 14, 1194244. [Google Scholar] [CrossRef]
- Di, X.; Jing, R.; Qin, X.; Liang, X.; Wang, L.; Xu, Y.; Sun, Y.; Huang, Q. The Role and Transcriptomic Mechanism of Cell Wall in the Mutual Antagonized Effects between Selenium Nanoparticles and Cadmium in Wheat. J. Hazard. Mater. 2024, 472, 134549. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, L.; Zhang, Y.; Wang, R.; Lu, B.; Liu, X. Transcriptome Analysis Provides New Insight into the Distribution and Transport of Selenium and Its Associated Metals in Selenium-Rich Rice. Environ. Pollut. 2022, 301, 118980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xing, G.; Tang, S.; Pang, Y.; Yi, Q.; Huang, Q.; Huang, X.; Huang, J.; Li, P.; Fu, H. Improving Soil Selenium Availability as a Strategy to Promote Selenium Uptake by High-Se Rice Cultivar. Environ. Exp. Bot. 2019, 163, 45–54. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar Spraying with Silicon and Selenium Reduces Cadmium Uptake and Mitigates Cadmium Toxicity in Rice. Sci. Total Environ. 2018, 631–632, 1100–1108. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, M.; Xu, J.; Xu, F.; Zhang, W. The Application of Organic Selenium (SeMet) Improve the Photosynthetic Characteristics, Yield and Quality of Hybrid Rice. Plant Physiol. Biochem. 2024, 208, 108457. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S. Influence of Selenite and Selenate on Growth, Leaf Physiology and Antioxidant Defense System in Wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 5700–5710. [Google Scholar] [CrossRef]
- Hu, Z.; Cheng, Y.; Suzuki, N.; Guo, X.; Xiong, H.; Ogra, Y. Speciation of Selenium in Brown Rice Fertilized with Selenite and Effects of Selenium Fertilization on Rice Proteins. Int. J. Mol. Sci. 2018, 19, 3494. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, A.K.; Singh, D.P. Regulation of Oxidative Stress and Mineral Nutrient Status by Selenium in Arsenic Treated Crop Plant Oryza sativa. Ecotoxicol. Environ. Saf. 2018, 148, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Rahman, M.M.; Siddiqui, M.N.; Fujita, M.; Tran, L.-S.P. Salicylic Acid Antagonizes Selenium Phytotoxicity in Rice: Selenium Homeostasis, Oxidative Stress Metabolism and Methylglyoxal Detoxification. J. Hazard. Mater. 2020, 394, 122572. [Google Scholar] [CrossRef]
- Kong, W.; Huo, R.; Lu, Y.; Fan, Z.; Yue, R.; Ren, A.; Li, L.; Ding, P.; Ren, Y.; Gao, Z.; et al. Nitrogen Application Can Optimize Form of Selenium in Soil in Selenium-Rich Areas to Affect Selenium Absorption and Accumulation in Black Wheat. Plants 2023, 12, 4160. [Google Scholar] [CrossRef]
- Williams, M.C.; Mayland, H.F. Selenium Absorption by Two-Grooved Milkvetch and Western Wheatgrass from Selenomethionine, Selenocystine, and Selenite. J. Range Manag. 1992, 45, 374–378. [Google Scholar] [CrossRef]
- Whanger, P.D. Selenium and Its Relationship to Cancer: An Update. Br. J. Nutr. 2004, 91, 11–28. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Yang, X.; Xia, Z. Effects of Sodium Selenite and Germination on the Sprouting of Chickpeas (Cicer arietinum L.) and Its Content of Selenium, Formononetin and Biochanin a in the Sprouts. Biol. Trace Elem. Res. 2012, 146, 376–380. [Google Scholar] [CrossRef]
- Yeasmin, M.; Lamb, D.; Choppala, G.; Rahman, M.M. Impact of Sulfur on Biofortification and Speciation of Selenium in Wheat Grain Grown in Selenium-Deficient Soils. J. Soil Sci. Plant Nutr. 2022, 22, 3243–3253. [Google Scholar] [CrossRef]
- Sun, N.; Wang, H.; Zhang, W.; Zhang, Y.; Zhao, Y.; He, B.; Zhang, H.; Zhang, B. Research Progress on the Sources and Enrichment of Selenium and Selenium-Rich Dietary Resources. Food Sci. 2024, 45, 299. [Google Scholar] [CrossRef]
- Funes-Collado, V.; Morell-Garcia, A.; Rubio, R.; López-Sánchez, J.F. Selenium Uptake by Edible Plants from Enriched Peat. Sci. Hortic. 2013, 164, 428–433. [Google Scholar] [CrossRef]
- Németh, A.; Dernovics, M. Effective Selenium Detoxification in the Seed Proteins of a Hyperaccumulator Plant: The Analysis of Selenium-Containing Proteins of Monkeypot Nut (Lecythis minor) Seeds. J. Biol. Inorg. Chem. 2015, 20, 23–33. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, H.; Yang, H.; Wu, Y.; Fan, S.; Wu, W.; Lyu, L.; Li, W. Integrative Physiological, Metabolomic and Transcriptomic Analysis Reveals Nitrogen Preference and Carbon and Nitrogen Metabolism in Blackberry Plants. J. Plant Physiol. 2023, 280, 153888. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Sui, F.; Li, S.; Liu, Y.; Lu, X.; Lu, Y.; Fan, Y. Transcriptome Analysis of the Effects of Different Carbon Dioxide Concentrations on Paramylon Accumulation in Euglena gracilis Z. Bioresour. Technol. 2024, 393, 130114. [Google Scholar] [CrossRef]
- Lone, S.N.; Maqbool, R.; Parray, F.Q.; Ul Hussain, M. Triose-Phosphate Isomerase Is a Novel Target of miR-22 and miR-28, with Implications in Tumorigenesis. J. Cell. Physiol. 2018, 233, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Kamrad, S.; Grossbach, J.; Rodríguez-López, M.; Mülleder, M.; Townsend, S.; Cappelletti, V.; Stojanovski, G.; Correia-Melo, C.; Picotti, P.; Beyer, A.; et al. Pyruvate Kinase Variant of Fission Yeast Tunes Carbon Metabolism, Cell Regulation, Growth and Stress Resistance. Mol. Syst. Biol. 2020, 16, e9270. [Google Scholar] [CrossRef]
- Wu, J.-L.; Wu, Q.-P.; Huang, J.-M.; Chen, R.; Cai, M.; Tan, J.-B. Effects of L-Malate on Physical Stamina and Activities of Enzymes Related to the Malate-Aspartate Shuttle in Liver of Mice. Physiol. Res. 2007, 56, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium Forms and Methods of Application Differentially Modulate Plant Growth, Photosynthesis, Stress Tolerance, Selenium Content and Speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- de la Torre, F.; Santis, L.D.; Suárez, M.F.; Crespillo, R.; Cánovas, F.M. Identification and Functional Analysis of a Prokaryotic-Type Aspartate Aminotransferase: Implications for Plant Amino Acid Metabolism. Plant J. 2006, 46, 414–425. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, P.; Wang, W.; Yuan, F.; Zhu, D.; Wang, Z.; Ying, X. Molecular Evolution and Expression Divergence of HMT Gene Family in Plants. Int. J. Mol. Sci. 2018, 19, 1248. [Google Scholar] [CrossRef]
- Kojima, S.; Konishi, N.; Beier, M.P.; Ishiyama, K.; Maru, I.; Hayakawa, T.; Yamaya, T. NADH-Dependent Glutamate Synthase Participated in Ammonium Assimilation in Arabidopsis Root. Plant Signal. Behav. 2014, 9, e29402. [Google Scholar] [CrossRef]
- Rao, S.; Yu, T.; Cong, X.; Lai, X.; Xiang, J.; Cao, J.; Liao, X.; Gou, Y.; Chao, W.; Xue, H.; et al. Transcriptome, Proteome, and Metabolome Reveal the Mechanism of Tolerance to Selenate Toxicity in Cardamine Violifolia. J. Hazard. Mater. 2021, 406, 124283. [Google Scholar] [CrossRef]
- Yoshimura, K.; Ishikawa, T. Physiological Function and Regulation of Ascorbate Peroxidase Isoforms. J. Exp. Bot. 2024, 75, 2700–2715. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Zhang, Y.; Li, C.; Gong, S.; Yan, S.; Li, G.; Hu, G.; Ren, H.; Yang, J.; et al. Comparative Transcriptome Analysis of Salt-Sensitive and Salt-Tolerant Maize Reveals Potential Mechanisms to Enhance Salt Resistance. Genes Genom. 2019, 41, 781–801. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, Y.-S.; Xing, X.-J.; Peng, R.-H.; Zhu, B.; Gao, J.-J.; Yao, Q.-H. Over-Expression of AtGSTU19 Provides Tolerance to Salt, Drought and Methyl Viologen Stresses in Arabidopsis. Physiol. Plant. 2016, 156, 164–175. [Google Scholar] [CrossRef]
- Brentner, L.B.; Mukherji, S.T.; Merchie, K.M.; Yoon, J.M.; Schnoor, J.L.; Van Aken, B. Expression of Glutathione S-Transferases in Poplar Trees (Populus trichocarpa) Exposed to 2,4,6-Trinitrotoluene (TNT). Chemosphere 2008, 73, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction with Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium Metabolism in Plants. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Wu, W.-L.; Zhao, G.-S.; Guo, Y.-B. Progress of selenium biological transformation in animals, plants, and microorganisms. J. Agric. Resour. Environ. 2018, 35, 189–198. [Google Scholar] [CrossRef]

| Disinfection Method | Germination Rate (%) | Contamination Rate (%) |
|---|---|---|
| Control | 82.25 ± 1.9 d | 32.75 ± 3.74 a |
| a | 88.70 ± 1.2 bc | 31.20 ± 1.41 a |
| b | 85.80 ± 0.17 c | 19.20 ± 2.28 ab |
| c | 80.60 ± 1.13 d | 10.95 ± 3.9 b |
| d | 90.60 ± 1.13 ab | 7.70 ± 2.7 c |
| e | 93.65 ± 1.62 a | 5.50 ± 1.2 d |
| f | 85.55 ± 2.89 c | 3.60 ± 1.8 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Yu, H.; Yin, J.; Ullah, F.; Zhang, X.; Chen, D.; Li, S.; Zhao, H.; Jin, X. Transcriptome Analysis of the Effects of Selenium Form and Concentration on Rice Growth and Metabolism at the Seedling Stage. Agronomy 2025, 15, 867. https://doi.org/10.3390/agronomy15040867
Jiang X, Yu H, Yin J, Ullah F, Zhang X, Chen D, Li S, Zhao H, Jin X. Transcriptome Analysis of the Effects of Selenium Form and Concentration on Rice Growth and Metabolism at the Seedling Stage. Agronomy. 2025; 15(4):867. https://doi.org/10.3390/agronomy15040867
Chicago/Turabian StyleJiang, Xinbo, Hairu Yu, Jiamin Yin, Fazl Ullah, Xilu Zhang, Di Chen, Shixin Li, Hongyan Zhao, and Xijiu Jin. 2025. "Transcriptome Analysis of the Effects of Selenium Form and Concentration on Rice Growth and Metabolism at the Seedling Stage" Agronomy 15, no. 4: 867. https://doi.org/10.3390/agronomy15040867
APA StyleJiang, X., Yu, H., Yin, J., Ullah, F., Zhang, X., Chen, D., Li, S., Zhao, H., & Jin, X. (2025). Transcriptome Analysis of the Effects of Selenium Form and Concentration on Rice Growth and Metabolism at the Seedling Stage. Agronomy, 15(4), 867. https://doi.org/10.3390/agronomy15040867






