The Effect of Intercropping with Eucommia ulmoides on the Growth and Quality of Abelmoschus manihot and Its Rhizosphere Microbial Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Environmental Conditions
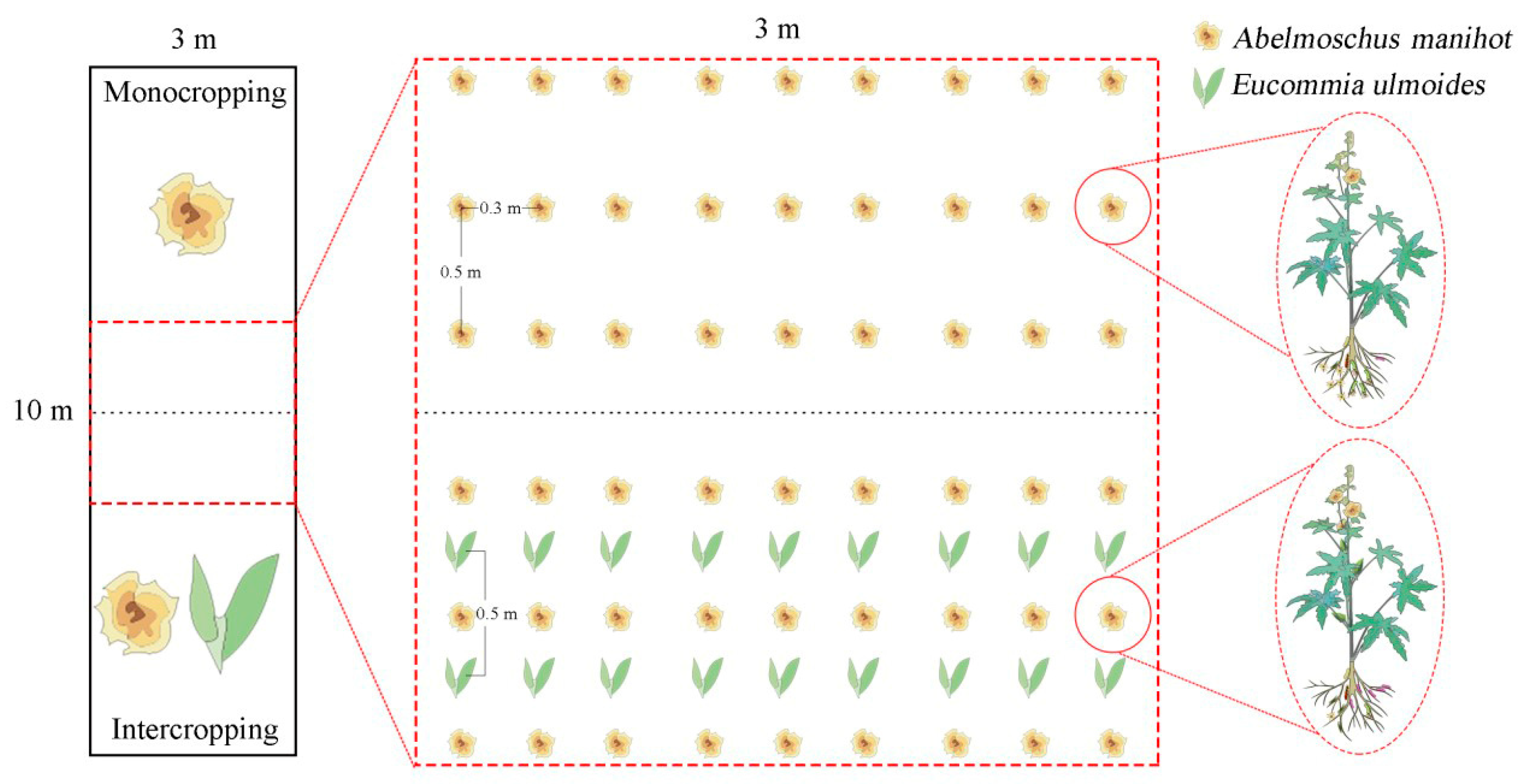
2.2. Experimental Design
2.3. Plant Materials
2.4. Soil Sampling
2.5. Reagents
2.6. Data Collection
2.6.1. Observations Recorded
2.6.2. Determination of Total Flavonoid Content (TFC)
2.6.3. Determination of Total Polyphenol Content (TPC)
2.6.4. UPLC-Q-Exactive Orbitrap MS Analysis
2.6.5. Soil Microbial Determination
2.7. Statistical Analysis
3. Results
3.1. Response of Biomass and Morphological Characteristics of A. manihot to Intercropping
3.2. Analysis of Secondary Metabolites and Differences in A. manihot Flowers
3.2.1. Total Flavonoid Content and Total Polyphenol Content of A. manihot Flowers
3.2.2. Difference Analysis of Secondary Metabolites in A. manihot Flowers
3.3. The Soil Physical and Chemical Properties of the Intercropping and Monocropping Systems of A. manihot
3.4. The Difference in Alpha and Beta Diversity Indices of Rhizosphere Bacteria and Fungi Between the Intercropping and Monocropping Systems of A. manihot
3.5. Venn Diagram of Bacterial and Fungal Communities Between Intercropping and Monocropping
3.6. Phylum-Level Community Composition of Rhizosphere Bacteria and Fungi Between Intercropping and Monocropping
3.7. Genus-Level Community Composition of Rhizosphere Bacteria and Fungi Between Intercropping and Monocropping
3.8. Predictive Analysis of Rhizosphere Bacterial and Fungal Functions Between Intercropping and Monocropping
4. Discussion
4.1. The Effect of Intercropping on the Growth of A. manihot
4.2. The Effect of Intercropping on Secondary Metabolites in A. manihot Flowers
4.3. The Effect of Intercropping on the Rhizosphere Microorganisms of A. manihot
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | arbuscular mycorrhiza fungi |
| LC-MS | liquid chromatography–mass spectrometry |
| TFC | total flavonoid content |
| TPC | total polyphenol content |
| CE | catechin equivalent |
| GAE | gallic acid equivalent |
| PCA | principal component analysis |
| PCoA | principal co-ordinatest analysis |
References
- Schutz, L.; Saharan, K.; Mader, P.; Boller, T.; Mathimaran, N. Rate of hyphal spread of arbuscular mycorrhizal fungi from pigeon pea to finger millet and their contribution to plant growth and nutrient uptake in experimental microcosms. Appl. Soil Ecol. 2022, 169, 104156. [Google Scholar]
- Prakhyath, K.M.; Yogendra, N.D.; Prakash, T.A.; Kumar, D.; Anandakumar, T.M. Resource use efficiency and system productivity of patchouli (Pogostemon cablin (Blanco) Benth) intercropping with food crops. Ind. Crops Prod. 2024, 222, 119868. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.; Li, H.; Zhang, F.; van der Werf, W. Syndromes of production in intercropping impact yield gains. Nat Plants 2020, 6, 653–660. [Google Scholar]
- Andersen, I.K.L.; Fomsgaard, I.S.; Rasmussen, J. Intercropping of Narrow-Leafed Lupin (Lupinus angustifolius L.) and Barley (Hordeum vulgare L.) Affects the Flavonoid Composition of Both Crops. J. Agric. Food Chem. 2023, 72, 108–115. [Google Scholar] [CrossRef]
- Duan, Y.; Shang, X.W.; Liu, G.D.; Zou, Z.W.; Zhu, X.J.; Ma, Y.C.; Li, F.; Fang, W.P. The effects of tea plants-soybean intercropping on the secondary metabolites of tea plants by metabolomics analysis. BMC Plant Biol. 2021, 21, 482. [Google Scholar]
- Zhang, C.F.; Tayyab, M.; Abubakar, A.Y.; Yang, Z.Q.; Pang, Z.Q.; Islam, W.; Lin, Z.L.; Li, S.Y.; Luo, J.; Fan, X.L.; et al. Bacteria with Different Assemblages in the Soil Profile Drive the Diverse Nutrient Cycles in the Sugarcane Straw Retention Ecosystem. Diversity 2019, 11, 194. [Google Scholar] [CrossRef]
- Karmakar, K.; Krishna, S.; Majumdar, S.; Nath, U.; Nataraj, K.N.; Prakash, N.B.; Chakravortty, D. Co-cultivation of Beta vulgaris limits the pre-harvest colonization of foodborne pathogen (Salmonella spp.) on tomato. Int. J. Food Microbiol. 2020, 332, 108768. [Google Scholar]
- Wang, B.Y.; Xia, Q.; Lin, Y.L.; Wei, F.G.; Yang, S.Z.; Dai, C.C.; Huang, X.Q.; Zhang, J.B.; Cai, Z.C.; Zhao, J. Root rot induces a core assemblage of bacterial microbiome to prevent disease infection in Sanqi ginseng. Appl. Soil Ecol. 2024, 198, 105371. [Google Scholar]
- Munar, A.; Sembiring, M.; Tantawi, A.R.; Sabrina, T. Isolation and identification of phosphate solubilizing microbes in the rhizosphere of maize by sound exposure. Emir. J. Food Agric. 2023, 35, 964–970. [Google Scholar] [CrossRef]
- Hu, H.Y.; Li, H.; Hao, M.M.; Ren, Y.N.; Zhang, M.K.; Liu, R.Y.; Zhang, Y.; Li, G.; Chen, J.S.; Ning, T.Y.; et al. Nitrogen fixation and crop productivity enhancements co-driven by intercrop root exudates and key rhizosphere bacteria. J. Appl. Ecol. 2021, 58, 2243–2255. [Google Scholar]
- Xie, W.; Zhang, K.; Wang, X.Y.; Zou, X.X.; Zhang, X.J.; Yu, X.N.; Wang, Y.F.; Si, T. Peanut and cotton intercropping increases productivity and economic returns through regulating plant nutrient accumulation and soil microbial communities. BMC Plant Biol. 2022, 22, 121. [Google Scholar] [CrossRef]
- Arafat, Y.; Tayyab, M.; Khan, M.U.; Chen, T.; Amjad, H.; Awais, S.; Lin, X.M.; Lin, W.X.; Lin, S. Long-Term Monoculture Negatively Regulates Fungal Community Composition and Abundance of Tea Orchards. Agronomy 2019, 9, 466. [Google Scholar] [CrossRef]
- Zhong, J.; Pan, W.Z.; Jiang, S.L.; Hu, Y.X.; Yang, G.Y.; Zhang, K.; Xia, Z.Y.; Chen, B. Flue-cured tobacco intercropping with insectary floral plants improves rhizosphere soil microbial communities and chemical properties of flue-cured tobacco. BMC Microbiol. 2024, 24, 446. [Google Scholar]
- Chi, B.J.; Liu, J.; Dai, J.L.; Li, Z.H.; Zhang, D.M.; Xu, S.Z.; Nie, J.J.; Wan, S.M.; Li, C.D.; Dong, H.Z. Alternate intercropping of cotton and peanut increases productivity by increasing canopy photosynthesis and nutrient uptake under the influence of rhizobacteria. Field Crops Res. 2023, 302, 109059. [Google Scholar]
- Gao, D.M.; Zhou, X.G.; Duan, Y.D.; Fu, X.P.; Wu, F.Z. Wheat cover crop promoted cucumber seedling growth through regulating soil nutrient resources or soil microbial communities? Plant Soil 2017, 418, 459–475. [Google Scholar]
- Zha, L.; Yang, C.Q.; Fang, G.W.; Zhi, M.L.; Chen, B.L.; Zhou, Z.G.; Meng, Y.L. Crop residue return reduces cotton Verticillium wilt by altering potassium nutrition and root exudates. Appl. Soil Ecol. 2022, 177, 104545. [Google Scholar]
- Jin, Q.; Jiang, Q.Y.; Zhao, L.; Su, C.Z.; Li, S.S.; Si, F.Y.; Li, S.S.; Zhou, C.H.; Mu, Y.L.; Xiao, M. Complete genome sequence of Bacillus velezensis S3-1, a potential biological pesticide with plant pathogen inhibiting and plant promoting capabilities. J. Biotechnol. 2017, 259, 199–203. [Google Scholar]
- Hazrati, H.; Fomsgaard, I.S.; Ding, L.; Kudsk, P. Mass spectrometry-based metabolomics unravel the transfer of bioactive compounds between rye and neighbouring plants. Plant Cell Environ. 2021, 44, 3492–3501. [Google Scholar]
- Bauermeister, A.; Mannochio-Russo, H.; Costa-Lotufo, L.V.; Jarmusch, A.K.; Dorrestein, P.C. Mass spectrometry-based metabolomics in microbiome investigations. Nat. Rev. Microbiol. 2022, 20, 143–160. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, Y.X.; Wang, X.Y.; Dang, P.P.; Malacrinò, A.; Zhang, J.Y.; Li, Z.; Rensing, C.; Zhang, Z.Y.; Lin, W.X.; et al. Pseudostellaria heterophylla cultivar mixtures driven changes in rhizosphere metabolites to suppress soil-borne Fusarium disease. Agric. Ecosyst. Environ. 2025, 380, 109409. [Google Scholar]
- Jiang, L.Y.; Zhang, J.; Zhu, B.J.; Bao, X.M.; Tian, J.Z.; Li, Y.S.; Zhang, G.R.; Wang, L.; Zhang, W.L.; Tang, Y.N.; et al. The aqueous extract of Reynoutria japonica ameliorates damp-heat ulcerative colitis in mice by modulating gut microbiota and metabolism. J. Ethnopharmacol. 2025, 338, 119042. [Google Scholar] [PubMed]
- Chen, C.; Huang, L.; Xia, N.; Teng, J.; Zhang, Q.; Zhu, P.; Wang, H.; Deng, H. Combining non-targeted and targeted metabolomics to study key bitter and astringent substances of Liupao tea. Food Chem. 2024, 467, 142289. [Google Scholar]
- Guo, X.N.; Hou, Z.N.; Wu, X.; Liu, W.Z.; Cai, J.J.; An, S.S. Long-term intercropping shaped soil bacterial microbiome composition and structure of maize fields in a semiarid region. Soil Tillage Res. 2025, 247, 106383. [Google Scholar] [CrossRef]
- Che, B.J.; Yang, W.C.; He, Q.Q.; Jiang, Y.; Zhang, B.C.; Chen, H.D. Soil microbial community composition and diversity in the rhizosphere of Alsophila spinulosa growing in different habitats within the Chishui Alsophila National Nature Reserve in Guizhou Province, China. Front. Microbiol. 2024, 15, 1445255. [Google Scholar] [CrossRef]
- Hu, B.J.; Zheng, Y.R.; Wang, D.S.; Guo, Y.T.; Dong, Y. Intercropping wheat and appropriate nitrogen supply can alleviate faba bean wilt disease by reshaping soil microbial community structure. Ind. Crops Prod. 2024, 222, 119538. [Google Scholar]
- Zhu, S.Q.; Wang, W.; Liu, X.; Yi, C.X.; Li, L.; Zhu, Z.H.; Guo, S.; Duan, J.A. Qualitative and quantitative analysis of major components in Abelmoschus manihot flowers treated with different drying methods using UHPLC Q-exactive MS and HPLC-PDA. J. Pharm. Biomed. Anal. 2025, 253, 116558. [Google Scholar]
- Diao, Z.P.; Yu, H.M.; Wu, Y.P.; Sun, Y.B.; Tang, H.T.; Wang, M.; Li, N.; Ge, H.T.; Sun, J.G.; Gu, H.F. Identification of the main flavonoids of Abelmoschus manihot (L.) medik and their metabolites in the treatment of diabetic nephropathy. Front. Pharmacol. 2024, 14, 1290868. [Google Scholar]
- Xue, C.; Zhang, X.; Ge, H.T.; Tang, Q.L.; Jeon, J.; Zhao, F.; Wang, Y.J.; Zhu, M.X.; Cao, Z.Y. Total flavone of flowers of Abelmoschus manihot (L.) Medic inhibits the expression of adhesion molecules in primary mesenteric arterial endothelial cells and ameliorates dextran sodium sulphate-induced ulcerative colitis in mice. Phytomedicine 2023, 112, 154713. [Google Scholar] [PubMed]
- Gao, Y.A.; Liang, Z.H.; Lv, N.Y.; Shan, J.J.; Zhou, H.H.; Zhang, J.F.; Shi, L.Y. Exploring the total flavones of Abelmoschus Manihot against IAV-induced lung inflammation by network pharmacology. BMC Complement. Med. Ther. 2022, 22, 36. [Google Scholar]
- Luan, F.; Wu, Q.H.; Yang, Y.; Lv, H.Z.; Liu, D.H.; Gan, Z.P.; Zeng, N. Traditional Uses, Chemical Constituents, Biological Properties, Clinical Settings, and Toxicities of Abelmoschus manihot L.: A Comprehensive Review. Front. Pharmacol. 2020, 11, 1068. [Google Scholar] [CrossRef]
- Sun, Z.H.; Liu, W.H.; Zhang, S.; Tian, S.G.; Aikemu, A. Optimization of Flavonoid Extraction from Abelmoschus manihot Flowers Using Ultrasonic Techniques: Predictive Modeling through Response Surface Methodology and Deep Neural Network and Biological Activity Assessment. Molecules 2024, 29, 2610. [Google Scholar] [CrossRef] [PubMed]
- Olasantan, F.O.; Bello, N.J. Optimum sowing dates for okra (Abelmoschus esculentus) in monoculture and mixture with cassava (Manihot esculenta) during the rainy season in the south-west of Nigeria. J. Agric. Sci. 2004, 142, 49–58. [Google Scholar]
- Huang, H.; Chang, Y.H.; Xu, J.; Ni, H.Y.; Zhao, H.; Zhai, B.W.; Efferth, T.; Gu, C.B.; Fu, Y.J. Aucubin as a natural potential anti-acute hepatitis candidate: Inhibitory potency and hepatoprotective mechanism. Phytomedicine 2022, 102, 154170. [Google Scholar] [PubMed]
- Xu, J.; Yang, H.; Nie, C.D.; Wang, T.; Qin, X.Y.; Yang, J.; Chang, Y.H.; Nie, S.M.; Fu, Y.J. Comprehensive phytochemical analysis of lingonberry (Vaccinium vitis-idaea L.) from different regions of China and their potential antioxidant and antiproliferative activities. RSC Adv. 2023, 13, 29438–29449. [Google Scholar] [PubMed]
- Bai, Z.Z.; Tang, J.M.; Ni, J.; Zheng, T.T.; Zhou, Y.; Sun, D.Y.; Li, G.N.; Liu, P.; Niu, L.X.; Zhang, Y.L. Comprehensive metabolite profile of multi-bioactive extract from tree peony (Paeonia ostii and Paeonia rockii) fruits based on MS/MS molecular networking. Food Res. Int. 2021, 148, 110609. [Google Scholar]
- Huang, Y.X.; Zhao, X.Y.; Zhou, D.W.; Luo, Y.Y.; Mao, W. Allometry of Corispermum macrocarpum in response to soil nutrient, water, and population density. Botany 2010, 88, 13–19. [Google Scholar] [CrossRef]
- Suter, M. Reproductive allocation of Carex flava reacts differently to competition and resources in a designed plant mixture of five species. Plant Ecol. 2009, 201, 481–489. [Google Scholar] [CrossRef]
- Bicho, M.C.; Correia, A.C.; Pinto, C.; Barcik, P.; David, J.S.; Silva, F.C.E. More than the climate: Reproductive and vegetative growth compete for resources in Quercus suber. Eur. J. For. Res. 2024, 143, 1853–1869. [Google Scholar] [CrossRef]
- Tao, Y.Y.; Qu, H.; Li, Q.J.; Gu, X.H.; Zhang, Y.N.; Liu, M.J.; Guo, L.; Liu, J.; Wei, J.J.; Wei, G.J.; et al. Potential to improve N uptake and grain yield in water saving Ground Cover Rice Production System. Field Crops Res. 2014, 168, 101–108. [Google Scholar] [CrossRef]
- Qu, H.; Tao, H.B.; Tao, Y.Y.; Liu, M.J.; Shen, K.R.; Lin, S. Ground Cover Rice Production System Increases Yield and Nitrogen Recovery Efficiency. Agron. J. 2012, 104, 1399–1407. [Google Scholar]
- Smith, M.R.; Hodecker, B.E.R.; Fuentes, D.; Merchant, A. Investigating Nutrient Supply Effects on Plant Growth and Seed Nutrient Content in Common Bean. Plants 2022, 11, 737. [Google Scholar] [CrossRef]
- Wang, C.Q.; Xue, L.; Dong, Y.H.; Jiao, R.Z. Effects of stand density on soil microbial community composition and enzyme activities in subtropical Cunninghamia lanceolate (Lamb.) Hook plantations. For. Ecol. Manag. 2021, 479, 118559. [Google Scholar]
- Zhang, Y.Y.; Zhao, F.Y.; Feng, C.; Bai, W.; Zhang, Z.; Cai, Q.; Sun, Z.X.; Feng, L.S. Effects of Maize/Peanut Intercropping and Nitrogen Fertilizer Application on Soil Fungal Community Structure. Agronomy 2024, 14, 14051053. [Google Scholar] [CrossRef]
- Li, L.F.; Jin, N. Effect of Nitrogen Concentration on the Growth and Fatty Acid Content of Mortierella alpina. Int. J. Agric. Biol. 2020, 24, 838–848. [Google Scholar]
- Martikainen, P.J. Heterotrophic nitrification-An eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 2022, 168, 108611. [Google Scholar]
- Gao, W.L.; Fan, C.H.; Zhang, W.; Li, N.; Liu, H.R.; Chen, M. Heterotrophic nitrification of organic nitrogen in soils: Process, regulation, and ecological significance. Biol. Fertil. Soils 2023, 59, 261–274. [Google Scholar]
- Han, M.; Yang, H.; Huang, H.; Du, J.; Zhang, S.; Fu, Y. Allelopathy and allelobiosis: Efficient and economical alternatives in agroecosystems. Plant Biol. 2024, 26, 11–27. [Google Scholar] [PubMed]
- Challacombe, J.F.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, L.; Porras-Alfaro, A.; Kuske, C.R. Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. Bmc Genom. 2019, 20, 976. [Google Scholar]
- Pertile, G.; Panek, J.; Oszust, K.; Siczek, A.; Oleszek, M.; Gryta, A.; Frac, M. Effect of different organic waste on cellulose-degrading enzymes secreted by Petriella setifera in the presence of cellobiose and glucose. Cellulose 2019, 26, 7905–7922. [Google Scholar]
- Miyamoto, T.; Koda, K.; Kawaguchi, A.; Uraki, Y. Ligninolytic Activity at 0 °C of Fungi on Oak Leaves Under Snow Cover in a Mixed Forest in Japan. Microb. Ecol. 2017, 74, 322–331. [Google Scholar]
- Jas, K.; Malolepsza, U. Underground communication—The new elements of signalling pathways of arbuscular mycorrhizal symbiosis. Postep. Mikrobiol. 2017, 56, 275–281. [Google Scholar]
- Bakar, N.A.; Chung, B.L.Y.; Smykla, J.; Karsani, S.A.; Alias, S.A. Proteomic characterization of Pseudogymnoascus spp. isolates from polar and temperate regions. Mycologia 2024, 116, 449–463. [Google Scholar]
- Coelho, L.D.; de Carvalho, C.R.; Rosa, C.A.; Rosa, L.H. Diversity; distribution, and xerophilic tolerance of cultivable fungi associated with the Antarctic angiosperms. Polar Biol. 2021, 44, 379–388. [Google Scholar]
- Huang, Y.Y.; Yan, Y.Y.; Ma, Y.; Zhang, X.; Zhao, Q.; Men, M.; Huang, Y.L.; Peng, Z.P. The effect of low-temperature straw-degrading microbes on winter wheat growth and soil improvement under straw return. Front. Microbiol. 2024, 15, 1391632. [Google Scholar]
- Johnson, J.M.; Ludwig, A.; Furch, A.C.U.; Mithöfer, A.; Scholz, S.; Reichelt, M.; Oelmüller, R. The Beneficial Root-Colonizing Fungus Mortierella hyalina Promotes the Aerial Growth of Arabidopsis and Activates Calcium-Dependent Responses That Restrict Alternaria brassicae—Induced Disease Development in Roots. Mol. Plant-Microbe Interact. 2019, 32, 351–363. [Google Scholar] [PubMed]
- Meents, A.K.; Furch, A.C.U.; Almeida-Trapp, M.; Özyürek, S.; Scholz, S.S.; Kirbis, A.; Lenser, T.; Theissen, G.; Grabe, V.; Hansson, B.; et al. Beneficial and Pathogenic Arabidopsis Root-Interacting Fungi Differently Affect Auxin Levels and Responsive Genes During Early Infection. Front. Microbiol. 2019, 10, 380. [Google Scholar]
- Ozimek, E.; Jaroszuk-Scisel, J.; Bohacz, J.; Kornillowicz-Kowalska, T.; Tyskiewicz, R.; Slomka, A.; Nowak, A.; Hanaka, A. Synthesis of Indoleacetic Acid, Gibberellic Acid and ACC-Deaminase by Mortierella Strains Promote Winter Wheat Seedlings Growth under Different Conditions. Int. J. Mol. Sci. 2018, 19, 3218. [Google Scholar] [CrossRef]
- Qiu, Z.J.; Wang, Z.B.; Wang, S.Y.; Fei, J.Y.; Qu, Z.M.; Wu, H.; Zhao, M.; Yang, H.Y. Protective role of Mortierella alpina-derived lipids in resisting root rot in Panax ginseng. Rhizosphere 2024, 32, 100994. [Google Scholar]
- Cui, R.F.; Geng, G.; Wang, G.; Stevanato, P.; Dong, Y.Z.; Li, T.; Yu, L.H.; Wang, Y.G. The response of sugar beet rhizosphere micro-ecological environment to continuous cropping. Front. Microbiol. 2022, 13, 956785. [Google Scholar]
- Li, X.; de Boer, W.; Zhang, Y.N.; Ding, C.; Zhang, T.; Wang, X. Suppression of soil-borne Fusarium pathogens of peanut by intercropping with the medicinal herb Atractylodes lancea. Soil Biol. Biochem. 2018, 116, 120–130. [Google Scholar]






| Primers | Primer Type | F-End Sequence | R-End Sequence | Length |
|---|---|---|---|---|
| 338F_806 | Bacteria 16S rRNA | ACTCCTACGGGAGGCAGCAG | GGACTACHVGGGTWTCTAAT | 468 |
| ITS1F_ITS2R | fungus ITS | CTTGGTCATTTAGAGGAAGTAA | GCTGCGTTCTTCATCGATGC | 350 |
| Abelmoschus manihot | Branches | Leaf Numbers | Ground Diameter (mm) | Height (cm) | Fruit Numbers | Above-Ground Biomass (g) | Underground Biomass (g) | Total Biomass (g) |
|---|---|---|---|---|---|---|---|---|
| Monocropping | 4 ± 3 | 20 ± 12 | 17.38 ± 1.67 | 1.56 ± 0.23 | 13 ± 2 ** | 64.68 ± 21.13 | 18.90 ± 5.87 | 83.58 ± 27.00 |
| Intercropping | 1 ± 1 | 24 ± 6 | 20.12 ± 1.78 | 1.77 ± 0.14 | 19 ± 2 | 82.62 ± 7.33 | 21.25 ± 4.16 | 103.87 ± 11.49 |
| Abelmoschus manihot | Branches | Leaf Numbers | Ground Diameter (mm) | Height (m) | Fruit Numbers | Flowers Numbers | Above-Ground Biomass (g) | Underground Biomass (g) | Total Biomass (g) |
|---|---|---|---|---|---|---|---|---|---|
| Monocropping | 0 ± 1 | 17 ± 4 | 20.09 ± 2.11 | 2.29 ± 0.08 | 9 ± 2 ** | 13 ± 2 ** | 64.56 ± 7.01 | 10.65 ± 2.09 | 75.21 ± 8.33 |
| Intercropping | 1 ± 1 | 17 ± 4 | 19.55 ± 1.53 | 2.12 ± 0.08 | 14 ± 2 | 20 ± 2 | 59.82 ± 8.46 | 12.02 ± 2.41 | 71.84 ± 10.86 |
| Soil Characteristics | Monocropping A. manihot | Intercropping A. manihot |
|---|---|---|
| Total N (g·kg−1) | 0.79 ± 0.11 ** | 0.26 ± 0.05 |
| Ammoniume N (mg·kg−1) | 12.99 ± 1.21 | 10.79 ± 1.39 |
| Nitrate N (mg·kg−1) | 48.64 ± 6.97 | 122.74 ± 23.58 ** |
| Available P (mg·kg−1) | 17.22 ± 2.39 | 25.89 ± 3.25 * |
| Available K (mg·kg−1) | 155.44 ± 9.26 | 161.81 ± 18.23 |
| SOC (g·kg−1) | 118.54 ± 29.20 | 94.31 ± 31.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Zhang, Z.; Yang, H.; Du, J.; Wu, X.; Fu, Y. The Effect of Intercropping with Eucommia ulmoides on the Growth and Quality of Abelmoschus manihot and Its Rhizosphere Microbial Community. Agronomy 2025, 15, 863. https://doi.org/10.3390/agronomy15040863
Han M, Zhang Z, Yang H, Du J, Wu X, Fu Y. The Effect of Intercropping with Eucommia ulmoides on the Growth and Quality of Abelmoschus manihot and Its Rhizosphere Microbial Community. Agronomy. 2025; 15(4):863. https://doi.org/10.3390/agronomy15040863
Chicago/Turabian StyleHan, Minghao, Ze Zhang, Han Yang, Jiyu Du, Xue Wu, and Yujie Fu. 2025. "The Effect of Intercropping with Eucommia ulmoides on the Growth and Quality of Abelmoschus manihot and Its Rhizosphere Microbial Community" Agronomy 15, no. 4: 863. https://doi.org/10.3390/agronomy15040863
APA StyleHan, M., Zhang, Z., Yang, H., Du, J., Wu, X., & Fu, Y. (2025). The Effect of Intercropping with Eucommia ulmoides on the Growth and Quality of Abelmoschus manihot and Its Rhizosphere Microbial Community. Agronomy, 15(4), 863. https://doi.org/10.3390/agronomy15040863




