Stem Coloration in Alfalfa: Anthocyanin Accumulation Patterns and Nutrient Profiles of Red- and Green-Stemmed Variants
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Methods
2.2.1. Evaluation of Agronomic Traits and Nutritional Indicators in Alfalfa at Initial Flowering Period
2.2.2. Microstructure Analysis of Alfalfa Stem Tissue by Frozen Sectioning at Initial Flowering Period
2.2.3. Anthocyanin and Proanthocyanidin Quantification in Alfalfa Stems and Leaves at Various Growth Stages
2.2.4. ICP-MS Analysis of Mineral Elements in Alfalfa at Initial Flowering Period
2.2.5. LC-MS/MS Metabolomics of Alfalfa Stems During Initial Flowering Period
2.3. Statistical Analysis and Software
3. Results
3.1. Development of Segregated Populations for Stem Color and Height, with Genetic Characterization of Stem Color at the Initial Flowering Stage
3.2. Comparative Analysis of Total Anthocyanins and Proanthocyanidins in Two Alfalfa Varieties Across Growth Stages
3.3. Anthocyanin Deposition Predominantly in the Cambium of Alfalfa Stems at Initial Flowering Stage
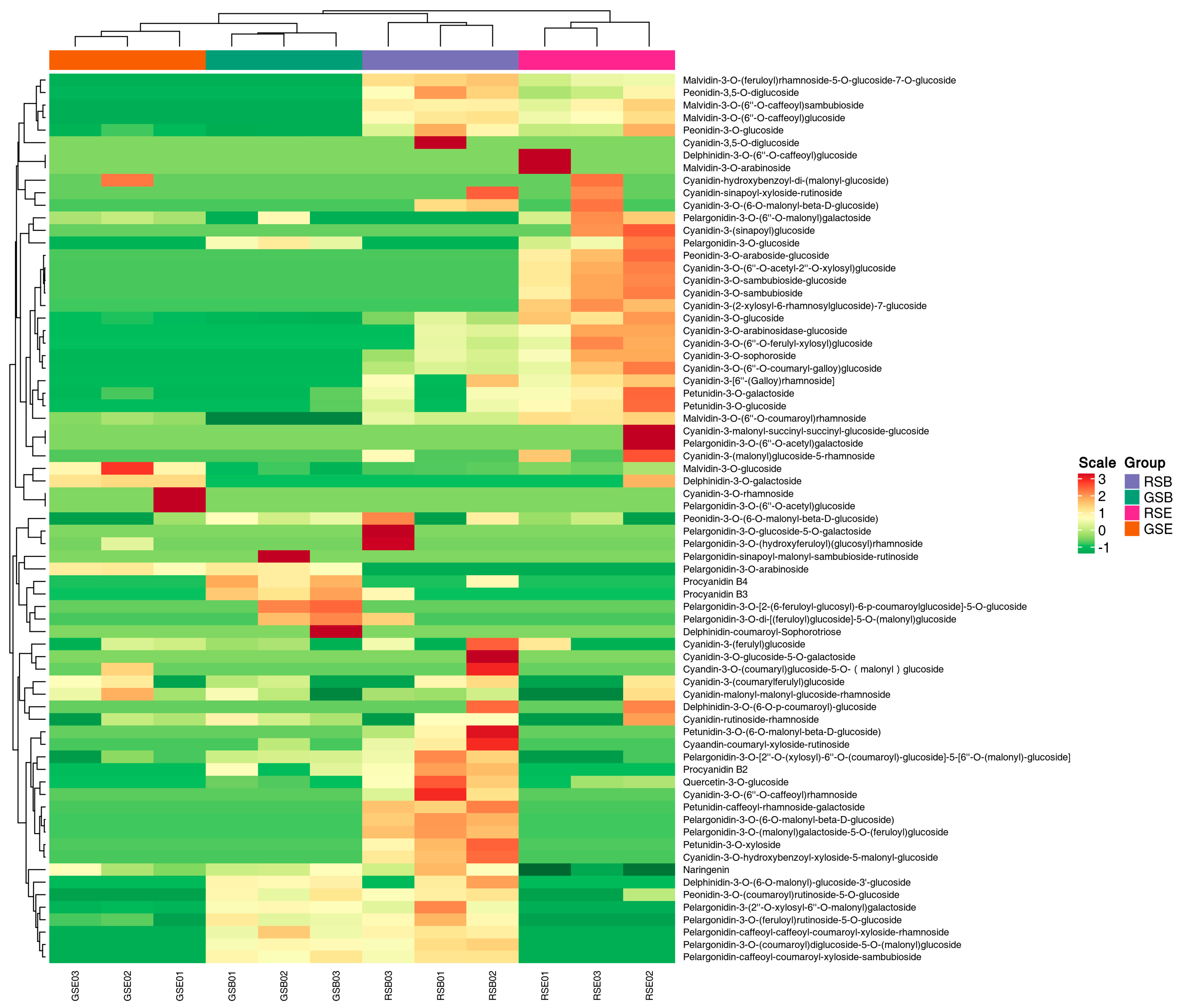
3.4. Predominance of Cyanidin-3-O-Glucoside (Cy3G) in Red-Stemmed Alfalfa at Initial Flowering Stage
3.5. Nutritional Differences Between Red- and Green-Stemmed Alfalfa at Initial Flowering Stage
3.6. Comparative Analysis of Mineral Elements in Alfalfa Stems at Initial Flowering Stage
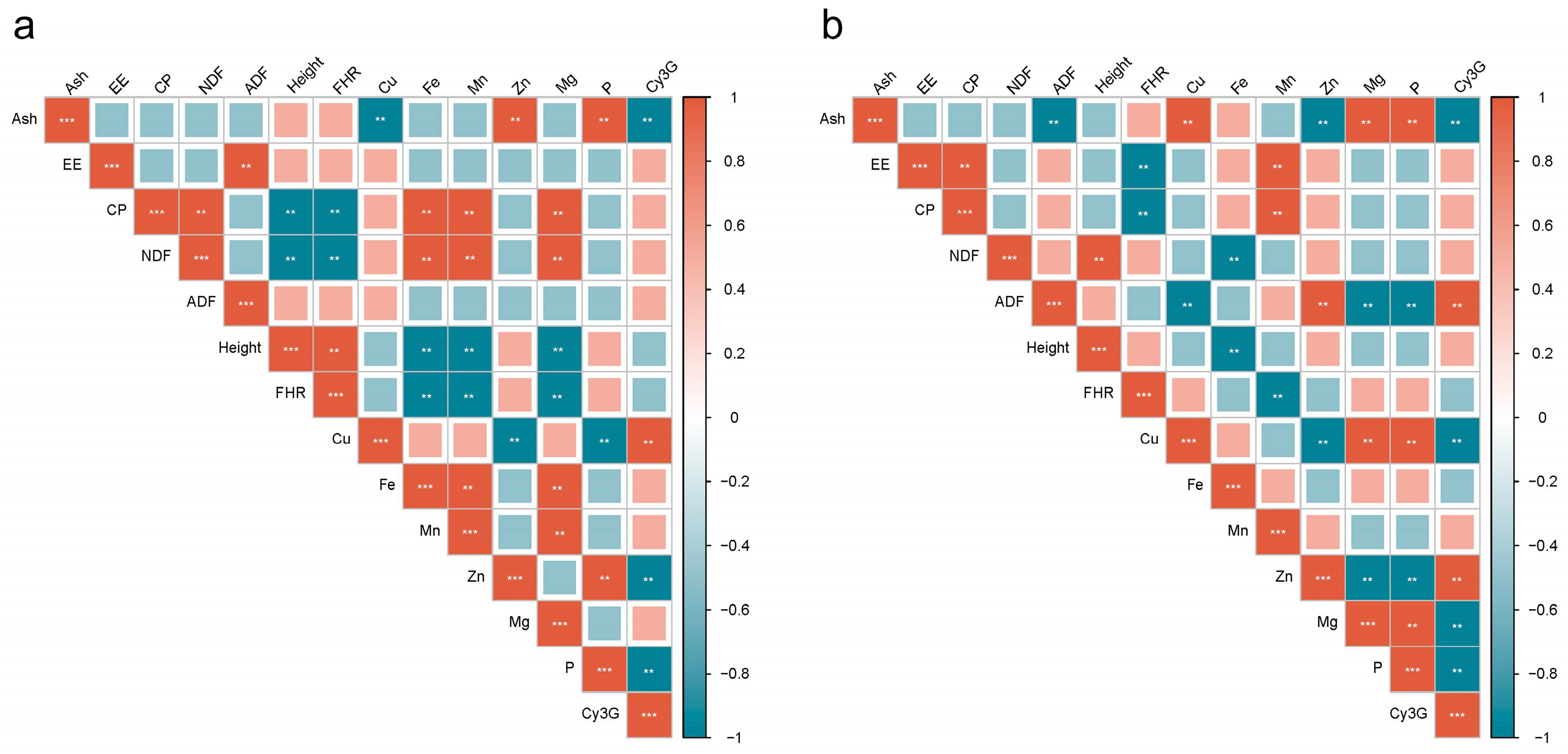
3.7. Correlation Analysis Between Cy3G and Agronomic Traits, Nutritional Components in Red- and Green-Stemmed Alfalfa at Initial Flowering Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Blum, J.A.; Ma, F.; Wang, Y.; Borejsza-Wysocka, E.; Ma, F.; Cheng, L.; Li, P. Anthocyanin Accumulation Provides Protection against High Light Stress While Reducing Photosynthesis in Apple Leaves. Int. J. Mol. Sci. 2022, 23, 12616. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.N.; McNabb, W.C. The Implications of Condensed Tannins on the Nutritive Value of Temperate Forages Fed to Ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lu, Q. Anthocyanins in Dairy Cow Nutrition: A Review. Agriculture 2022, 12, 1806. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.-L.; Rémésy, C. Anthocyanins Are Efficiently Absorbed from the Small Intestine in Rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef]
- Ray, H.; Yu, M.; Auser, P.; Blahut-Beatty, L.; McKersie, B.; Bowley, S.; Westcott, N.; Coulman, B.; Lloyd, A.; Gruber, M.Y. Expression of Anthocyanins and Proanthocyanidins after Transformation of Alfalfa with Maize Lc. Plant Physiol. 2003, 132, 1448–1463. [Google Scholar] [CrossRef]
- Duan, H.-R.; Wang, L.-R.; Cui, G.-X.; Zhou, X.-H.; Duan, X.-R.; Yang, H.-S. Identification of the Regulatory Networks and Hub Genes Controlling Alfalfa Floral Pigmentation Variation Using RNA-Sequencing Analysis. BMC Plant Biol. 2020, 20, 110. [Google Scholar] [CrossRef]
- Gu, Z.; Zhou, X.; Li, S.; Pang, Y.; Xu, Y.; Zhang, X.; Zhang, J.; Jiang, H.; Lu, Z.; Wang, H.; et al. The HD-ZIP IV Transcription Factor GLABRA2 Acts as an Activator for Proanthocyanidin Biosynthesis in Medicago Truncatula Seed Coat. Plant J. 2024, 119, 2303–2315. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, W.; Li, M.; Liu, B.; Zhao, S.; Hu, M.; Li, J.; Li, D.; Shi, Y.; Sun, H.; et al. Comprehensive evaluation of the nutritional value and contaminants of alfalfa (Medicago sativa L.) in China. Front. Nutr. 2025, 12, 1539462. [Google Scholar] [CrossRef]
- Yin, H.; Wang, L.; Wu, Y.; Xi, Z. Roles of Non-Visible Light and Temperature in the Regulation of Anthocyanin Synthesis in Fruits and Vegetables. Food Front. 2024, 5, 1968–1983. [Google Scholar] [CrossRef]
- An, J.-P.; Liu, Y.-J.; Zhang, X.-W.; Bi, S.-Q.; Wang, X.-F.; You, C.-X.; Hao, Y.-J. Dynamic Regulation of Anthocyanin Biosynthesis at Different Light Intensities by the BT2-TCP46-MYB1 Module in Apple. J. Exp. Bot. 2020, 71, 3094–3109. [Google Scholar] [CrossRef] [PubMed]
- Gao-Takai, M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. A Low Temperature Promotes Anthocyanin Biosynthesis but Does Not Accelerate Endogenous Abscisic Acid Accumulation in Red-Skinned Grapes. Plant Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, M.; Xin, H.; Zhang, H.; Cong, R.; Huang, D. Anthocyanin Biosynthesis and Regulate Mechanisms in Plants: A Review. Chin. Agric. Sci. Bull. 2021, 37, 41–51. (In Chinese) [Google Scholar] [CrossRef]
- Lu, Z.; Wang, X.; Lin, X.; Mostafa, S.; Zou, H.; Wang, L.; Jin, B. Plant Anthocyanins: Classification, Biosynthesis, Regulation, Bioactivity, and Health Benefits. Plant Physiol. Biochem. 2024, 217, 109268. [Google Scholar] [CrossRef]
- GB/T 6438-2007; Animal Feeding Stuffs—Determination of Crude Ash. Standards Press of China: Beijing, China, 2007.
- GB/T 6433-2006; Determination of Crude Fat in Feeds. Standards Press of China: Beijing, China, 2006.
- GB/T 6432-2018; Determination of Crude Protein in Feeds—Kjeldahl Method. Standards Press of China: Beijing, China, 2018.
- NY/T 1459-2022; Determination of Acid Detergent Fiber in Feeds. Standards Press of China: Beijing, China, 2022.
- GB/T 20806-2022; Determination of Neutral Detergent Fiber in Feeds. Standards Press of China: Beijing, China, 2022.
- Zeng, H.; Wang, J.; Shen, P.; Zhao, Y.; Li, Y.; Liu, Y.; Bi, Z.; Liu, Z. Determination of 33 kinds of metallic elements in food aquatic products and animal tissues by microwave digestion and ICP-MS method. J. Food Saf. Qual. 2015, 6, 953–961. [Google Scholar] [CrossRef]
- McCubbin, T.J.; Braun, D.M. Phloem Anatomy and Function as Shaped by the Cell Wall. J. Plant Physiol. 2021, 266, 153526. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple Functional Roles of Anthocyanins in Plant-Environment Interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Cai, M.; Huang, J.; Chen, M.; Chen, L.; Zhang, X.; Chen, M.; Wu, J.; Pan, Y.; Peng, C. The Role and Synthesis Mechanism of Anthocyanins in Sphagneticola Trilobata Stems under Low Temperature. Biol. Invasions 2024, 26, 2851–2867. [Google Scholar] [CrossRef]
- Yoon, H.S.; Yang, H.C.; Kang, H.M.; Jang, D.C. Mechanical Stress-Induced Anthocyanin Regulatory Genes Involved in Anthocyanin Accumulation in Tomato Plants. Hortic. Environ. Biotechnol. 2024, 65, 283–291. [Google Scholar] [CrossRef]
- Ballaré, C.L. Stress under the Sun: Spotlight on Ultraviolet-B Responses. Plant Physiol. 2003, 132, 1725–1727. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Mishiba, K.; Kubota, A.; Abe, Y.; Yamamura, S.; Nakamura, N.; Tanaka, Y.; Nishihara, M. Genetic Engineering of Novel Flower Colour by Suppression of Anthocyanin Modification Genes in Gentian. J. Plant Physiol. 2010, 167, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Julian McClements, D.; Chen, L.; Peng, X.; Xu, Z.; Meng, M.; Ji, H.; Zhi, C.; Ye, L.; Zhao, J.; et al. Progress on Molecular Modification and Functional Applications of Anthocyanins. Crit. Rev. Food Sci. Nutr. 2024, 64, 11409–11427. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; van der Kooi, C.J. Coloration of Flowers by Flavonoids and Consequences of pH Dependent Absorption. Front. Plant Sci. 2020, 11, 600124. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Sun, B.; Yang, Y.; Wang, S.; Feng, Z.; Li, J. The Structure of Anthocyanins and the Copigmentation by Common Micromolecular Copigments: A Review. Food Res. Int. 2024, 176, 113837. [Google Scholar] [CrossRef]
- Song, S.; Yu, Y.; Song, S.; Zhang, X.; Zhang, W. Effect of Co-Pigments on Anthocyanins of Wild Cranberry and Investigation of Interaction Mechanisms. Food Chem. 2025, 466, 142212. [Google Scholar] [CrossRef]
- Xie, C.; Hu, C.; Deng, X.; Shao, W.; Gao, Y.; Huang, W.; Song, X. Relationship between Flower Color and Cellular Physicochemical Factors in Bletilla Striata. Horticulturae 2023, 9, 426. [Google Scholar] [CrossRef]
- Ploenlap, P.; Pattanagul, W. Effects of Exogenous Abscisic Acid on Foliar Anthocyanin Accumulation and Drought Tolerance in Purple Rice. Biologia 2015, 70, 915–921. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The Effect of Condensed Tannins on the Nutrition and Health of Ruminants Fed Fresh Temperate Forages: A Review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Gámez, A.L.; Vatter, T.; Santesteban, L.G.; Araus, J.L.; Aranjuelo, I. Onfield Estimation of Quality Parameters in Alfalfa through Hyperspectral Spectrometer Data. Comput. Electron. Agric. 2024, 216, 108463. [Google Scholar] [CrossRef]
- Liu, J.; Fu, S.; Gao, J.; Feng, S.; Miao, C.; Li, Y.; Wu, C.; Feng, Q.; Liang, T. Estimating Alfalfa Fiber Components Using Machine Learning Algorithms Based on in Situ Hyperspectral and Sentinel-2 Data in the Hexi Corridor Region. Comput. Electron. Agric. 2024, 226, 109394. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Wang, J.; Fan, Z.; Shen, J.; Chen, Y.; Shao, Q.; Zheng, Y. Effect of Flowering Stages on the Content of Active Ingredients and Antioxidant Capability of Bletilla Striata Flowers. Chem. Biodivers. 2023, 20, e202200773. [Google Scholar] [CrossRef]
- Ullah, M.Z.; Biswas, P.; Islam, M.S. Genetic Analysis of Agronomic Traits and Grain Anthocyanin and Micronutrient (Zinc and Iron) Content in Rice (Oryza sativa L.). Trends Agric. Sci. 2023, 2, 288–297. [Google Scholar] [CrossRef]
- Utasee, S.; Jamjod, S.; Lordkaew, S.; Prom-U-Thai, C. Improve Anthocyanin and Zinc Concentration in Purple Rice by Nitrogen and Zinc Fertilizer Application. Rice Sci. 2022, 29, 435–450. [Google Scholar] [CrossRef]
- Shaked-Sachray, L.; Weiss, D.; Reuveni, M.; Nissim-Levi, A.; Oren-Shamir, M. Increased Anthocyanin Accumulation in Aster Flowers at Elevated Temperatures Due to Magnesium Treatment. Physiol. Plant 2002, 114, 559–565. [Google Scholar] [CrossRef]
- Nissim-Levi, A.; Ovadia, R.; Forer, I.; Oren-Shamir, M. Increased Anthocyanin Accumulation in Ornamental Plants Due to Magnesium Treatment. J. Hortic. Sci. Biotechnol. 2007, 82, 481–487. [Google Scholar] [CrossRef]
- Tian, G.; Qin, H.; Liu, C.; Xing, Y.; Feng, Z.; Xu, X.; Liu, J.; Lyu, M.; Jiang, H.; Zhu, Z.; et al. Magnesium Improved Fruit Quality by Regulating Photosynthetic Nitrogen Use Efficiency, Carbon–Nitrogen Metabolism, and Anthocyanin Biosynthesis in ‘Red Fuji’ Apple. Front. Plant Sci. 2023, 14, 1136179. [Google Scholar] [CrossRef]
- Xie, K.; Pan, Y.; Meng, X.; Wang, M.; Guo, S. Critical Leaf Magnesium Thresholds for Growth, Chlorophyll, Leaf Area, and Photosynthesis in Rice (Oryza sativa L.) and Cucumber (Cucumis sativus L.). Agronomy 2024, 14, 1508. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Niu, D.; Liu, X. Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 2024, 342, 112030. [Google Scholar] [CrossRef]
- Chen, X.; Pu, H.; Fang, Y.; Wang, X.; Zhao, S.; Lin, Y.; Zhang, M.; Dai, H.-E.; Gong, W.; Liu, L. Crystal structure of the catalytic subunit of magnesium chelatase. Nat. Plants 2015, 1, 15125. [Google Scholar] [CrossRef]
- Yang, L. Analysis on the Characteristics of Thermo-Sensitive Albino Germplasm and the Expression of Photosynthesis-Related Genes in Alfalfa (Medicago sativa). Master’s Thesis, Yangzhou University, Yangzhou, China, 2024. [Google Scholar]




| Code | Line | Source | Origin | Leaf Type |
|---|---|---|---|---|
| R 1 | Huaiyin | National Animal Husbandry Station | China | Trifoliate |
| G 2 | PL34HQ | China—Australia Alfalfa Co-operation Projects (ASI/1998/026) | Australia | Hept-foliate |
| Type | Branches | F | p-Value |
|---|---|---|---|
| G | 12.44 ± 1.43 | 0.166 | <0.001 |
| R | 18.24 ± 1.72 |
| Class | RSB (μg g−1) | GSB (μg g−1) | RSE (μg g−1) | GSE (μg g−1) |
|---|---|---|---|---|
| Cyanidin glycosides | 1.9140 ± 0.7502 b | 0.1065 ± 0.0427 c | 4.6460 ± 0.7166 a | 0.3449 ± 0.1199 c |
| Peonidin glycosides | 1.3766 ± 0.4196 a | 0.0892 ± 0.0038 b | 1.1372 ± 0.5771 a | 0.1783 ± 0.0933 b |
| Flavonoid | 1.5153 ± 0.5066 a | 0.3815 ± 0.0520 b | 0.3292 ± 0.2296 b | 0.2644 ± 0.0564 b |
| Malvidin glycosides | 0.2150 ± 0.0048 a | 0.0083 ± 0.0072 c | 0.2264 ± 0.0345 a | 0.1494 ± 0.0532 b |
| Petunidin glycosides | 0.1205 ± 0.0728 a | 0.0076 ± 0.0130 b | 0.1458 ± 0.0542 a | 0.0028 ± 0.0049 b |
| Pelargonidin glycosides | 0.5450 ± 0.1545 a | 0.4959 ± 0.0620 a | 0.1204 ± 0.0489 b | 0.0853 ± 0.0202 b |
| Delphinidin glycosides | 0.0123 ± 0.0112 b | 0.0140 ± 0.0006 b | 0.0325 ± 0.0562 ab | 0.0800 ± 0.0032 a |
| Procyanidin | 0.1078 ± 0.0083 a | 0.1479 ± 0.0452 a | 0 | 0 |
| Elements | RS | GS | F | p |
|---|---|---|---|---|
| Cu (mg/kg) | 9.08 ± 0.02 | 8.52 ± 0.29 | 0.084 | 0.030 * |
| Fe (mg/kg) | 110.69 ± 6.03 | 94.35 ± 5.83 | 0.998 | 0.028 * |
| Mn (mg/kg) | 14.30 ± 2.07 | 12.23 ± 0.63 | 0.061 | 0.173 ns |
| Zn (mg/kg) | 25.36 ± 1.71 | 17.16 ± 1.84 | 0.901 | 0.004 ** |
| Mg (mg/g) | 1.82 ± 0.03 | 2.09 ± 0.09 | 0.169 | 0.008 ** |
| P (g/kg) | 2.08 ± 0.12 | 2.11 ± 0.35 | 0.073 | 0.882 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Li, J.; Wang, C.; Min, X.; Wei, Z. Stem Coloration in Alfalfa: Anthocyanin Accumulation Patterns and Nutrient Profiles of Red- and Green-Stemmed Variants. Agronomy 2025, 15, 862. https://doi.org/10.3390/agronomy15040862
Cao Z, Li J, Wang C, Min X, Wei Z. Stem Coloration in Alfalfa: Anthocyanin Accumulation Patterns and Nutrient Profiles of Red- and Green-Stemmed Variants. Agronomy. 2025; 15(4):862. https://doi.org/10.3390/agronomy15040862
Chicago/Turabian StyleCao, Zhengfeng, Jiaqing Li, Chuanjie Wang, Xueyang Min, and Zhenwu Wei. 2025. "Stem Coloration in Alfalfa: Anthocyanin Accumulation Patterns and Nutrient Profiles of Red- and Green-Stemmed Variants" Agronomy 15, no. 4: 862. https://doi.org/10.3390/agronomy15040862
APA StyleCao, Z., Li, J., Wang, C., Min, X., & Wei, Z. (2025). Stem Coloration in Alfalfa: Anthocyanin Accumulation Patterns and Nutrient Profiles of Red- and Green-Stemmed Variants. Agronomy, 15(4), 862. https://doi.org/10.3390/agronomy15040862







