Viability of Cyperus esculentus Seeds and Tubers After Ensiling, Digestion by Cattle, and Manure Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ensiling Experiment
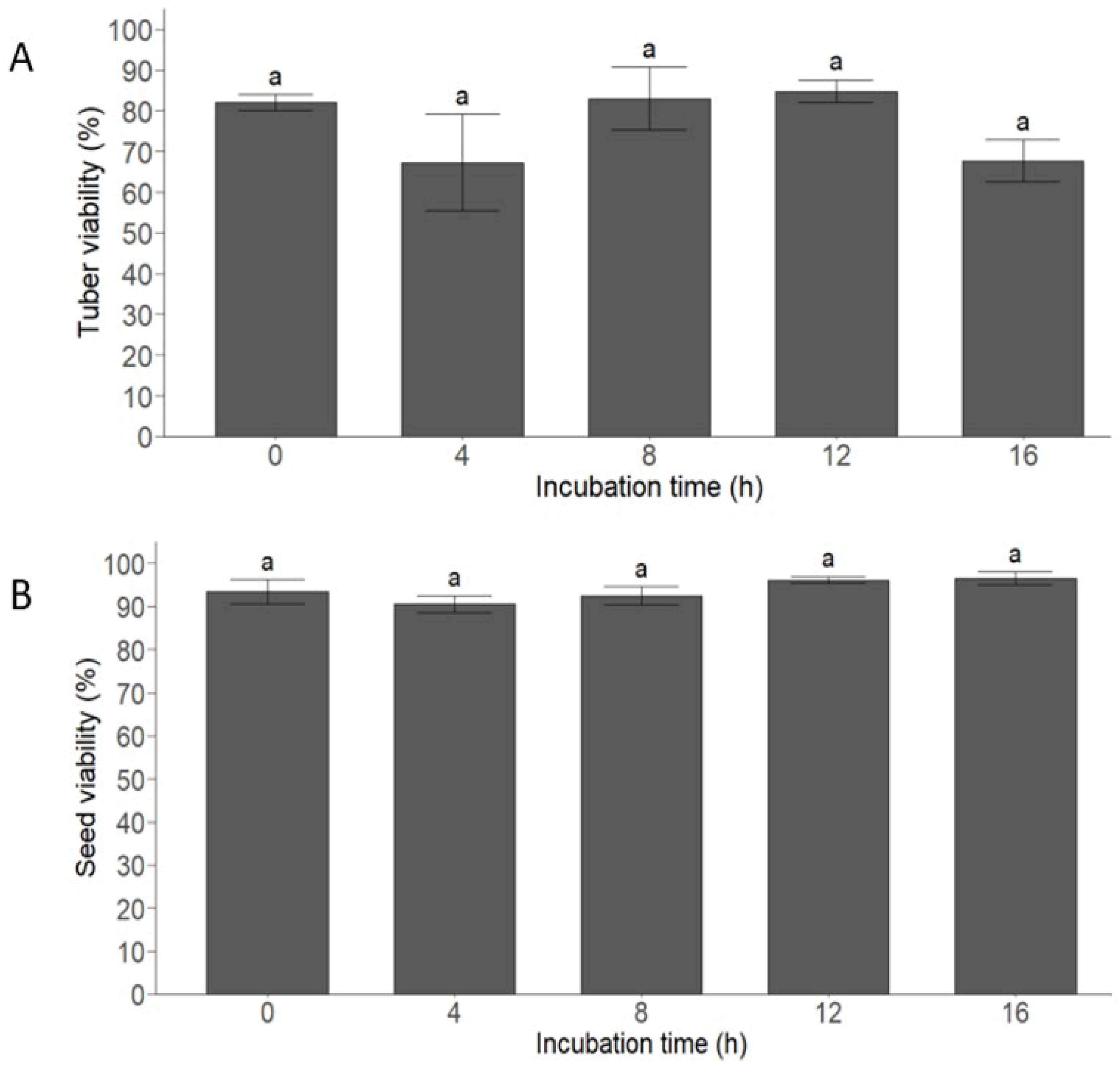
2.3. Digestion Experiment
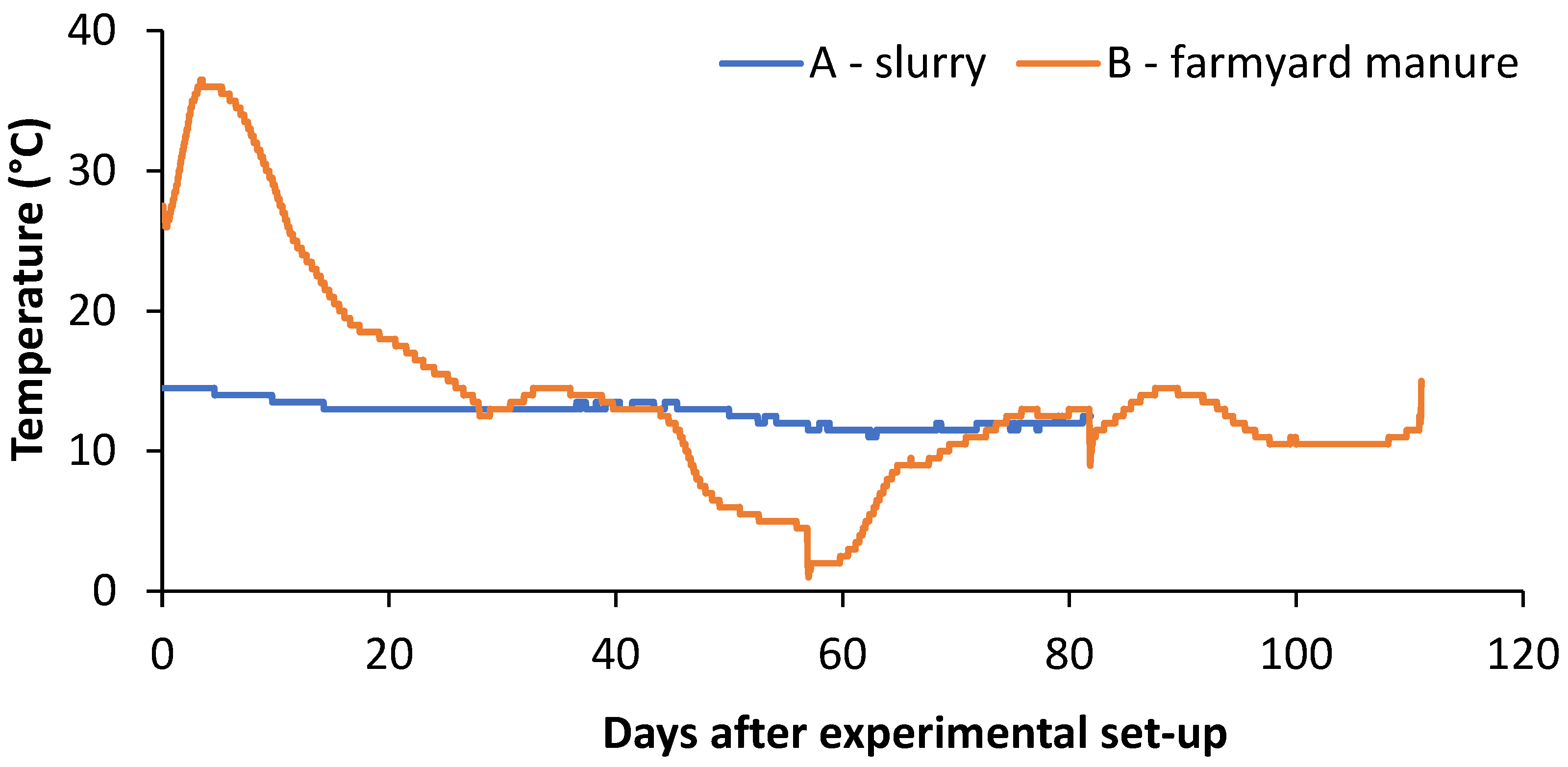
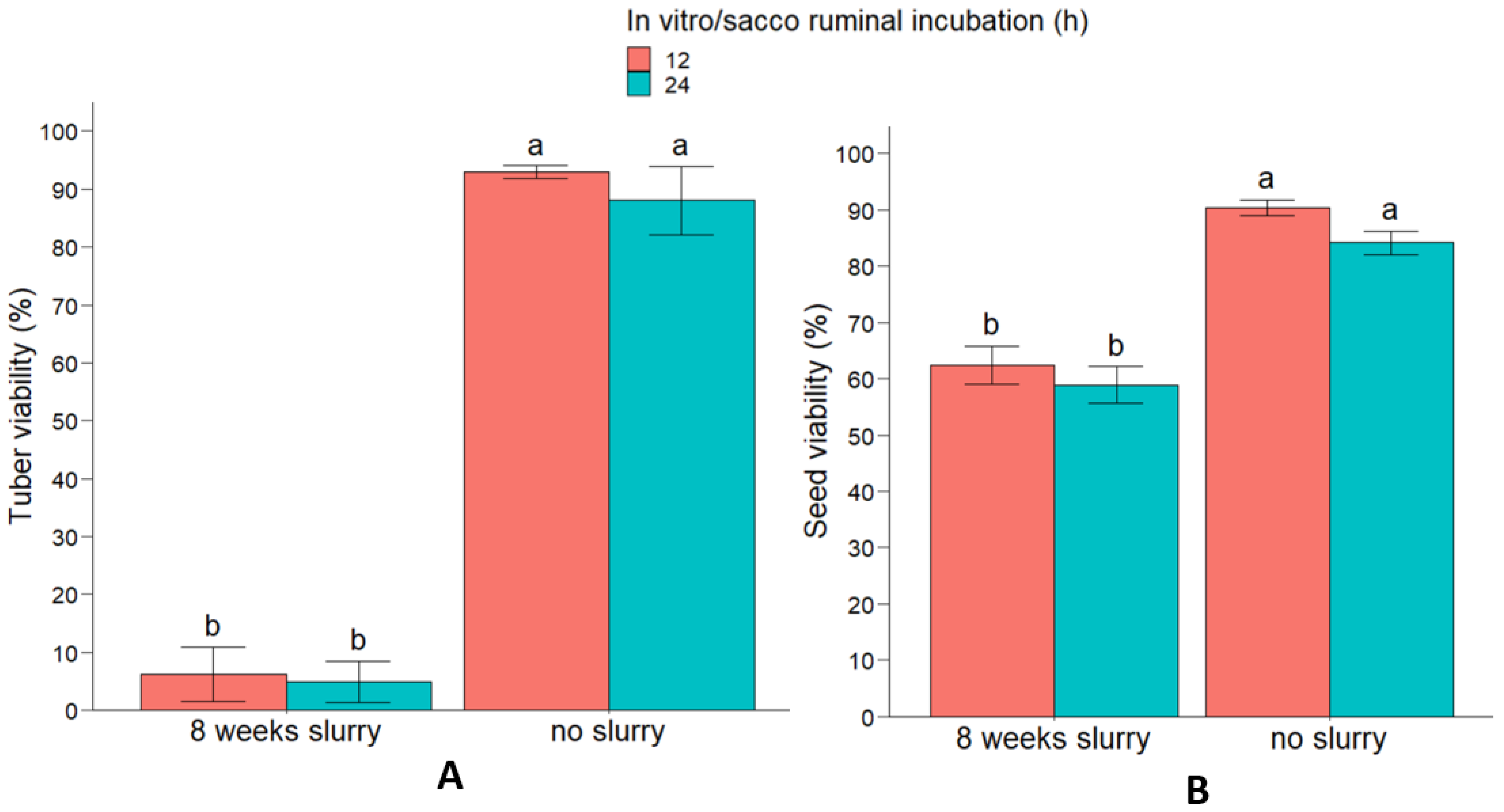
2.4. Slurry and Farmyard Manure Experiment
2.5. Combined Exposures Experiment
2.6. Evaluation of Germination and Viability
2.7. Statistical Analysis
3. Results
3.1. Ensiling Experiment
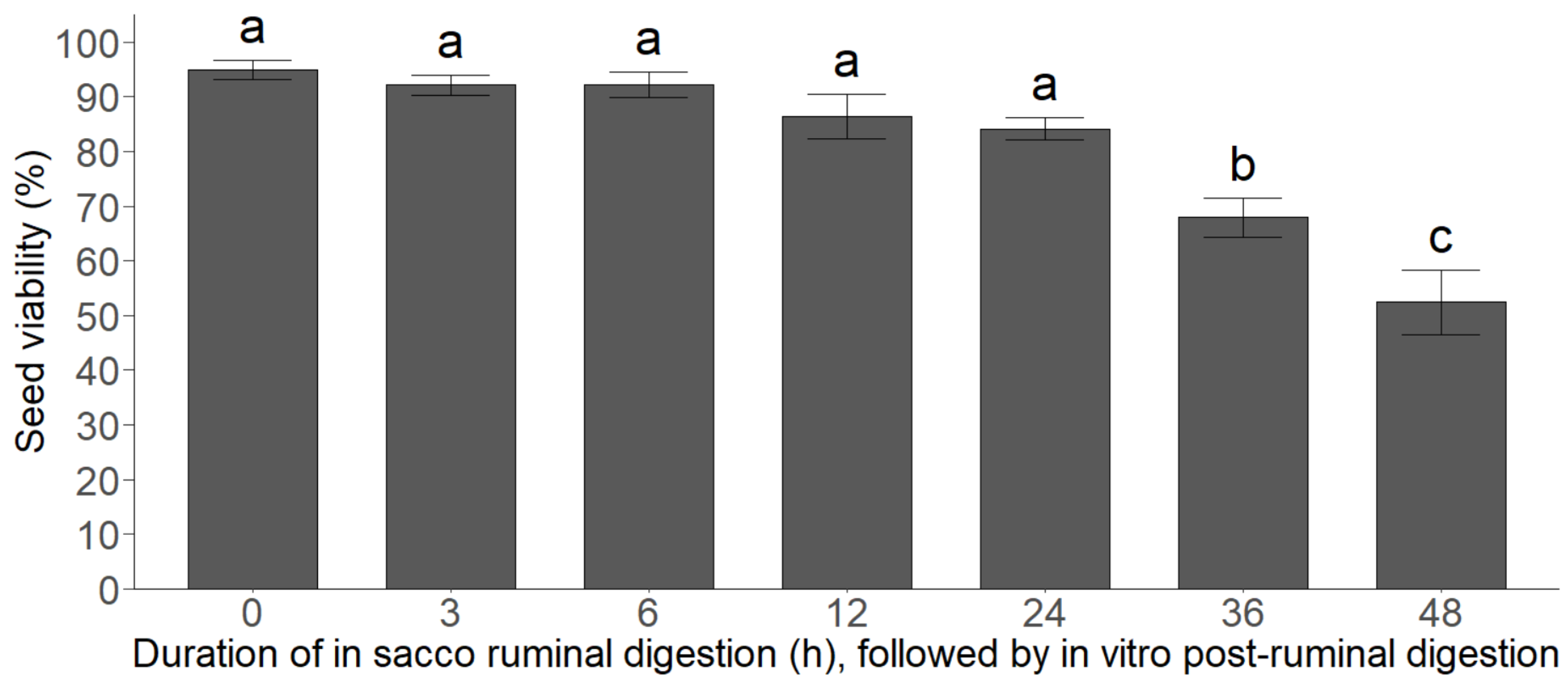
3.2. Digestion Experiment
3.3. Slurry and Farmyard Manure Experiment
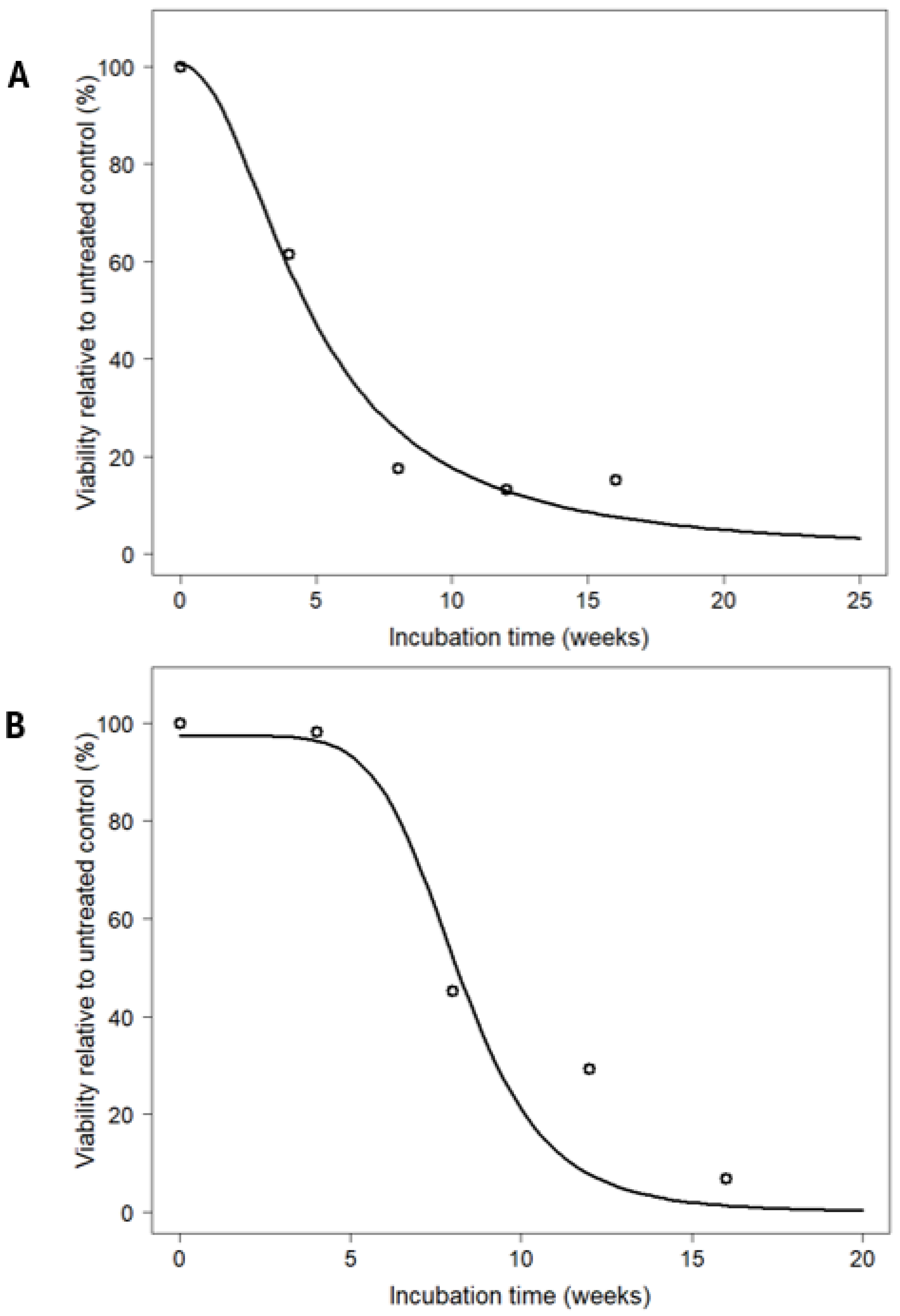
3.4. Combined Exposures Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds; Kriegar Publishing: Malabar, FL, USA, 1991; ISBN 0894644157. [Google Scholar]
- EPPO List of Invasive Alien Plants. Available online: https://www.eppo.int/ACTIVITIES/invasive_alien_plants/iap_lists (accessed on 18 February 2025).
- Bohren, C.; Wirth, J. The spreading of yellow nutsedge grass (Cyperus esculentus L.) applies to all. Agrarforsch. Schweiz+ 2015, 6, 384–391. [Google Scholar]
- De Cauwer, B.; De Ryck, S.; Claerhout, S.; Biesemans, N.; Reheul, D. Differences in growth and herbicide sensitivity among Cyperus esculentus clones found in Belgian maize fields. Weed Res. 2017, 57, 234–246. [Google Scholar]
- Demeyere, A. Praktijkgids Gewasbescherming. Module IPM Akkerbouw. Departement Landbouw En Visserij. Available online: https://www.vlaanderen.be/publicaties/praktijkgids-gewasbescherming-module-ipm-akkerbouw (accessed on 18 February 2025).
- Follak, S.; Belz, R.; Bohren, C.; De Castro, O.; Del Guacchio, E.; Pascual-Seva, N.; Schwarz, M.; Verloove, F.; Essl, F. Biological flora of Central Europe: Cyperus esculentus L. Perspect. Plant Ecol. Evol. Syst. 2016, 23, 33–51. [Google Scholar]
- Neuweiler, R.; Total, R. Mit vereinten Kräften gegen das Erdmandelgras. Gemüsebau 2012, 7, 1–2. [Google Scholar]
- Keller, M.; Eppler, L.; Collet, L.; Wirth, J.; Total, R. Beim Erdmandelgras auf nummer sicher gehen: Auch blütenbildung und abblühen verhindern! Gemüsebau Info 2015, 22, 7–9. [Google Scholar]
- Total, R. Eviter le moindre risque avec le souchet comestible en l’empêchant impérativement de fleurir et de fructifier! Agroscope Ext. Gemüsebau 2015, 22, 4–6. [Google Scholar]
- De Ryck, S. Towards an integrated approach for effective Cyperus esculentus control. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2024. [Google Scholar]
- Justice, O.; Whitehead, M. Seed production, viability and dormancy in the nutgrasses, Cyperus rotundus and Cyperus esculentus. J. Agric. Res. 1946, 73, 303–318. [Google Scholar]
- Stoller, E.W.; Wax, L.M.; Slife, F.W. Yellow nutsedge (Cyperus esculentus) competition and control in corn (Zea mays). Weed Sci. 1979, 27, 32–37. [Google Scholar] [CrossRef]
- Woolford, M.K. The Silage Fermentation; Marcel Dekker: New York, NY, USA, 1984. [Google Scholar]
- Seglar, B. Fermentation analysis and silage quality testing. In Proceedings of the Minnesota Dairy Health Conference, University of Minnesota. Minneapolis, MN, USA, 20 May 2003. [Google Scholar]
- Aper, J.; De Cauwer, B.; Lourenco, M.; Fievez, V.; Bulcke, R.; Reheul, D. Seed germination and viability of herbicide resistant and susceptible Chenopodium album populations after ensiling, digestion by cattle and manure storage. Weed Res. 2014, 54, 169–177. [Google Scholar]
- Simard, M.J.; Lambert-Beaudet, C. Weed seed survival in experimental mini-silos of corn and alfalfa. Can. J. Plant Sci. 2016, 96, 448–454. [Google Scholar]
- Blackshaw, R.E.; Rode, L.M. Effect of ensiling and rumen digestion by cattle on weed seed viability. Weed Sci. 1991, 39, 104–108. [Google Scholar]
- Mayer, F.; Albrecht, H.; Pfadenhauer, J. The influence of digestion and storage in silage and organic manure on the germinative ability of six weed species (Papaver argemone, P. dubium, Legousia speculum-veneris, Centaurea cyanus, Spergula arvensis, Trifolium arvense). Z. Pflanzenk. Pflanz. 2000, 17, 47–54. [Google Scholar]
- Westerman, P.R.; Hildebrandt, F.; Gerowitt, B. Weed seed survival following ensiling and mesophilic anaerobic digestion in batch reactors. Weed Res. 2012, 52, 286–295. [Google Scholar]
- Piltz, J.W.; Stanton, R.A.; Wu, H. Effect of ensiling and in sacco digestion on the viability of seeds of selected weed species. Weed Res. 2017, 57, 382–389. [Google Scholar]
- Hahn, J.; Mol, F.; Müller, J. Ensiling reduces seed viability: Implications for weed management. Front. Agron. 2021, 3, 708851. [Google Scholar]
- Elema, A.G.; Scheepens, P.C. Verspreiding van onkruiden en planteziekten met dierlijke mest. Proefstation voor de Akkerbouw en de Groententeelt in de Vollegrond, Lelystad, The Netherlands. Cent. Landbouwcatalogus 1992, 62. Available online: https://edepot.wur.nl/278456 (accessed on 20 February 2025).
- Gardener, C.J.; McIvor, J.G.; Jansen, A. Survival of seeds of tropical grassland species subjected to bovine digestion. J. Appl. Ecol. 1993, 30, 75–85. [Google Scholar]
- Stanton, R.; Piltz, J.; Pratley, J.; Kaiser, A.; Hudson, D.; Dill, G. Annual ryegrass (Lolium rigidum) seed survival and digestibility in cattle and sheep. Aust. J. Exp. Agric. 2002, 42, 111–115. [Google Scholar]
- Stanton, R.A.; Piltz, J.W.; Rodham, C.; Wu, H. Silage for managing weed seeds. In Proceedings of the 18th Australasian Weeds Conference: Developing Solutions to Evolving Weed Problems, Melbourne, Australia, 8–11 October 2012. [Google Scholar]
- Edwards, A.R.; Younger, A. The dispersal of traditionally managed hay meadow plants via farmyard manure application. Seed Sci. Res. 2006, 16, 137–147. [Google Scholar]
- Haidar, M.A.; Gharib, C.; Sleiman, F.T. Survival of weed seeds subjected to sheep rumen digestion. Weed Res. 2010, 50, 467–471. [Google Scholar]
- Piltz, J.W.; Kaiser, A.G. Principles of silage conservation. In Successful Silage; Kaiser, A.G., Piltz, J.W., Burns, H.M., Griffiths, N.W., Eds.; NSW Department of Primary Industries and Dairy Australia: Orange, Australia, 2004. [Google Scholar]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Ohmomo, S.; Tanaka, O.; Kitamoto, H. Analysis of organic acids in silage by HPLC. Souchi Shikenjo Kenkyu Houkoku (Bull. Natl. Grassl. Inst.) 1993, 48, 51–55. [Google Scholar]
- Tilley, J.M.A.; Terry, R.A. A Two-stage technique for in vitro digestion of forage crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Vincent, E.M.; Roberts, E.H. The interaction of light nitrate and alternating temperatures in promoting the germination of dormant seeds of common weed species. Seed Sci. Technol. 1977, 5, 659–670. [Google Scholar]
- Bouwmeester, H.J.; Karssen, C.M. Annual changes in dormancy and germination in seeds of Sisymbrium officinale (L.) Scop. New Phytol. 1993, 124, 179–191. [Google Scholar] [CrossRef]
- Hampton, J.G.; Tekrony, D.M. Handbook of Vigour Test Methods; The International Seed Testing Association: Zurich, Switzerland, 1995. [Google Scholar]
- Sawma, J.T.; Mohler, C.L. Evaluating seed viability by an unimbibed seed crush test in comparison with the tetrazolium test. Weed Technol. 2002, 16, 781–786. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 20 February 2025).
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R software package for dose-response studies: The concept and data analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- Ritz, C.; Streibig, J.C. Bioassay analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef]
- Van Saun, R.J. Nutritional Requirements of Dairy Cattle. MSD Veterinary Manual. 2022. Available online: https://www.msdvetmanual.com/management-and-nutrition/nutrition-dairy-cattle/nutritional-requirements-of-dairy-cattle (accessed on 20 February 2025).
- Warner, D.; Dijkstra, J.; Tamminga, S.; Pellikaan, W. Passage kinetics of concentrates in dairy cows measured with carbon stable isotopes. Animal 2013, 7, 1935–1943. [Google Scholar] [CrossRef]
- Hummel, J.; Südekum, K.H.; Bayer, D.; Ortmann, S.; Streich, W.J.; Hatt, J.M.; Clauss, M. Physical characteristics of reticuloruminal contents of oxen in relation to forage type and time after feeding. J. Anim. Physiol. An. N. 2009, 93, 209–220. [Google Scholar] [CrossRef]
- Van Renterghem, B.; Carlier, L.; Huysman, F.; Dendooven, L. Milieuhygiënische implicaties. In Mengmest: Implikaties voor de Landbouw en het Milieu; Van Renterghem, B., Verstraete, W., Eds.; IWONL: Brussels, Belgium, 1991. [Google Scholar]
- Chaudhry, A.S.; Mohamed, R.A. Using fistulated sheep to compare in sacco and in vitro rumen degradation of selected feeds. Anim. Prod. Sci. 2011, 51, 1015–1024. [Google Scholar]
- Rupende, E.; Chivinge, O.A.; Mariga, I.K. Effect of storage time on weed seedling emergence and nutrient release in cattle manure. Exp. Agric. 1998, 34, 277–285. [Google Scholar]

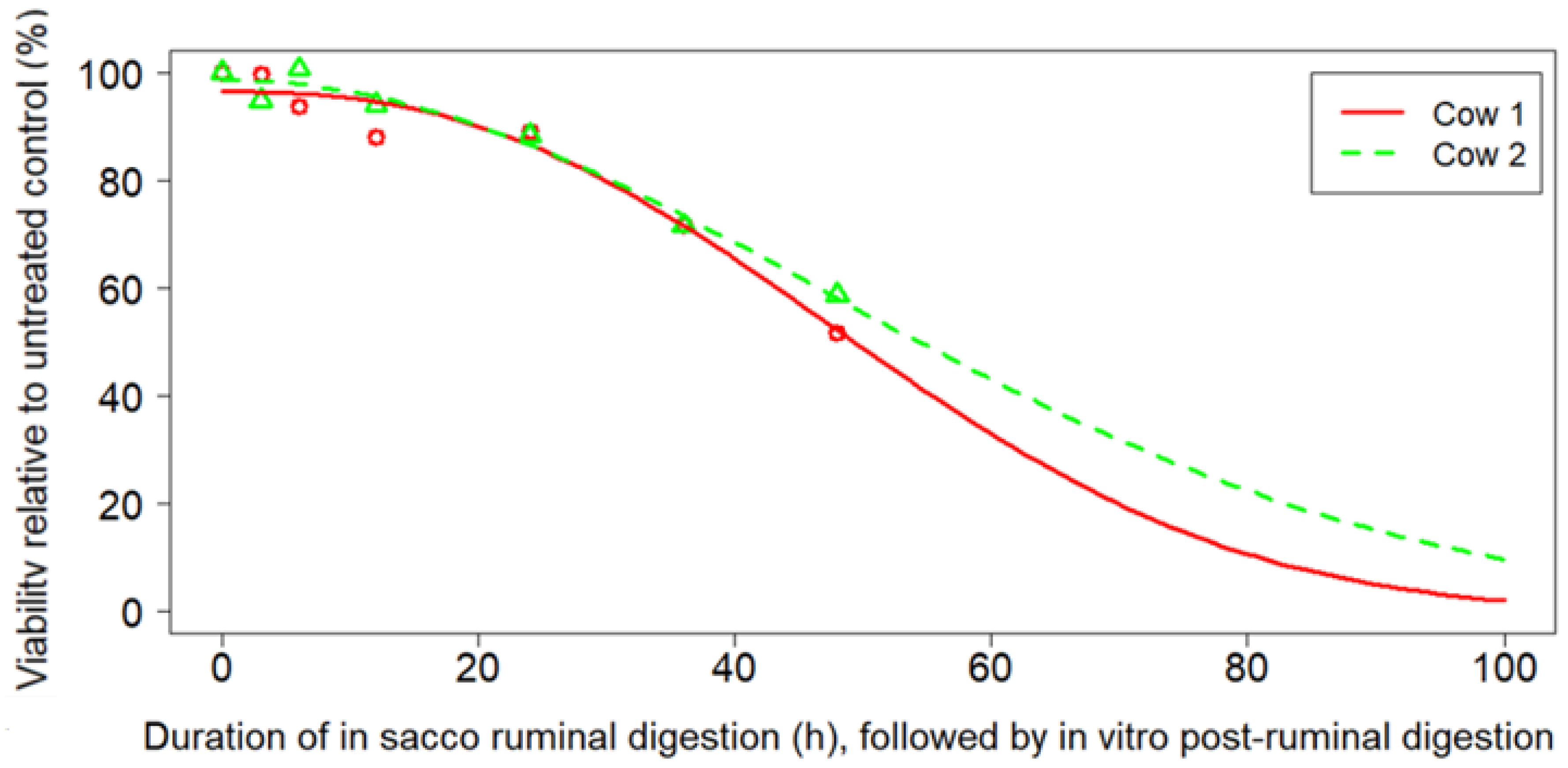
| Cow | Parameter | Estimated Value ± Standard Error | p-Value |
|---|---|---|---|
| 1 | b | 2.50 ± 0.89 | 0.006733 |
| d | 91.45 ± 2.80 | <2.2 × 10−16 | |
| e | 58.32 ± 6.44 | 4.145 × 10−12 | |
| 2 | b | 2.02 ± 0.66 | 0.003304 |
| d | 93.48 ± 2.86 | <2.2 × 10−16 | |
| e | 65.82 ± 9.71 | 1.340 × 10−8 |
| Propagule | Parameter | Estimated Value ± Standard Error | p-Value |
|---|---|---|---|
| Tubers | b | 2.05 ± 0.48 | 0.0006394 |
| d | 82.36 ± 4.94 | 4.268 × 10−11 | |
| e | 4.71 ± 0.60 | 1.104 × 10−6 | |
| Seeds | b | 3.31 ± 0.84 | 0.001315 |
| d | 95.81 ± 6.83 | 4.950 × 10−10 | |
| e | 8.26 ± 0.96 | 3.404 × 10−7 |
| Propagule | Factor | Df | Sum sq | Mean sq | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Tubers | Type of exposure | 1 | 28,884 | 28,884 | 402.980 | 1.340 × 10−10 |
| Incubation time | 1 | 38 | 38 | 0.533 | 0.479 | |
| Type of exposure Incubation time | 1 | 13 | 13 | 0.178 | 0.680 | |
| Seeds | Type of exposure | 1 | 5278 | 5278 | 91.955 | 2.37 × 10−9 |
| Incubation time | 1 | 72 | 72 | 1.257 | 0.274 | |
| Cow | 1 | 0 | 0 | 0.006 | 0.939 | |
| Type of exposure Incubation time | 1 | 13 | 13 | 0.222 | 0.642 | |
| Type of exposure: cow | 1 | 17 | 17 | 0.293 | 0.594 | |
| Incubation time: cow | 1 | 88 | 88 | 1.541 | 0.228 | |
| Type of exposure Incubation time: cow | 1 | 54 | 54 | 0.944 | 0.342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feys, J.; Welvaert, E.; De Meester, M.; Latré, J.; Wambacq, E.; Callens, D.; Clercx, S.; Van de Ven, G.; Reheul, D.; De Cauwer, B. Viability of Cyperus esculentus Seeds and Tubers After Ensiling, Digestion by Cattle, and Manure Storage. Agronomy 2025, 15, 844. https://doi.org/10.3390/agronomy15040844
Feys J, Welvaert E, De Meester M, Latré J, Wambacq E, Callens D, Clercx S, Van de Ven G, Reheul D, De Cauwer B. Viability of Cyperus esculentus Seeds and Tubers After Ensiling, Digestion by Cattle, and Manure Storage. Agronomy. 2025; 15(4):844. https://doi.org/10.3390/agronomy15040844
Chicago/Turabian StyleFeys, Jeroen, Emiel Welvaert, Mattie De Meester, Joos Latré, Eva Wambacq, Danny Callens, Shana Clercx, Gert Van de Ven, Dirk Reheul, and Benny De Cauwer. 2025. "Viability of Cyperus esculentus Seeds and Tubers After Ensiling, Digestion by Cattle, and Manure Storage" Agronomy 15, no. 4: 844. https://doi.org/10.3390/agronomy15040844
APA StyleFeys, J., Welvaert, E., De Meester, M., Latré, J., Wambacq, E., Callens, D., Clercx, S., Van de Ven, G., Reheul, D., & De Cauwer, B. (2025). Viability of Cyperus esculentus Seeds and Tubers After Ensiling, Digestion by Cattle, and Manure Storage. Agronomy, 15(4), 844. https://doi.org/10.3390/agronomy15040844






