Rhamnolipid-Stabilized Essential Oils Nanoemulsions: Sustainable Biopesticides and Biostimulants with Potential for Crop Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spectroscopic Characterization of Essential Oils
2.3. Design and Production of RL-NEs
2.4. Particle Analysis
2.5. Contact Angle Analysis
2.6. Plant Growth and Treatment with NEs. A. thaliana
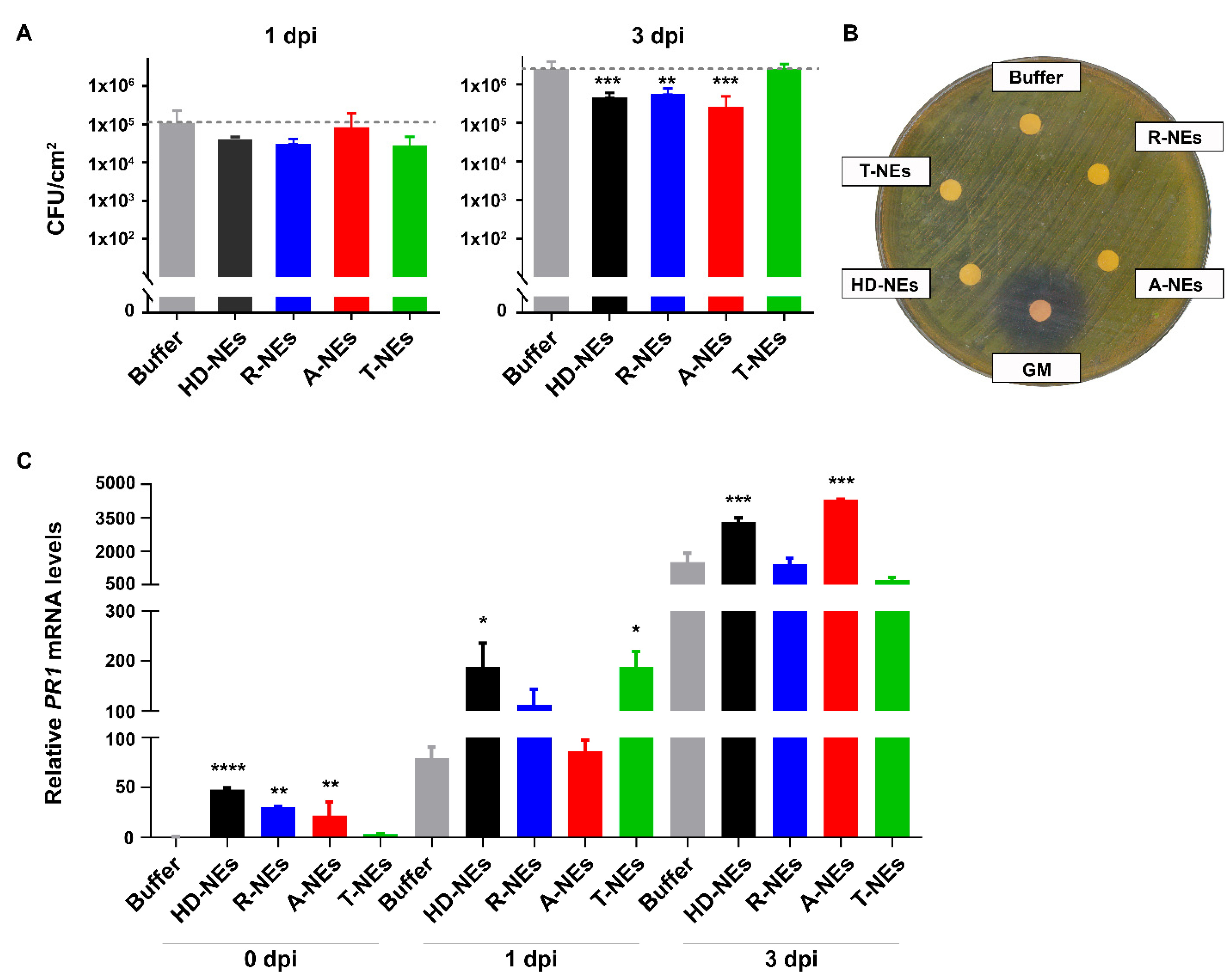
2.7. Effect of NEs on the Growth of Beneficial Soil Microorganisms and the Pathogenic Bacterial Strain Pseudomonas Syringae pv Tomato DC3000 (Pst) In Vitro
2.8. Analysis of NEs Effect on Bacterial Growth in Planta
2.9. Evaluation of the Effect of NEs on Arabidopsis thaliana Defense Gene Expression
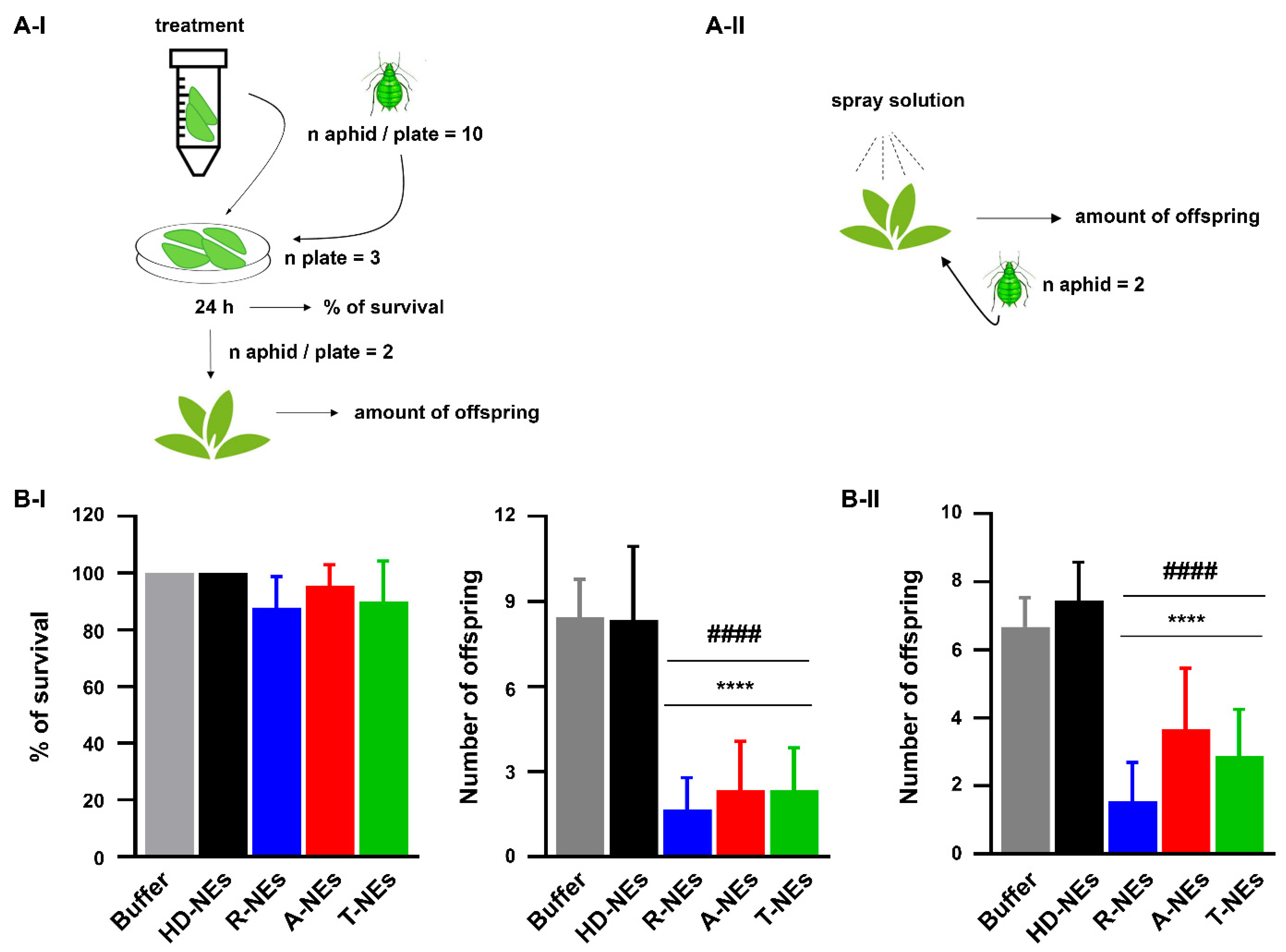
2.10. Effects of NEs Against the Aphid Myzus Persicae
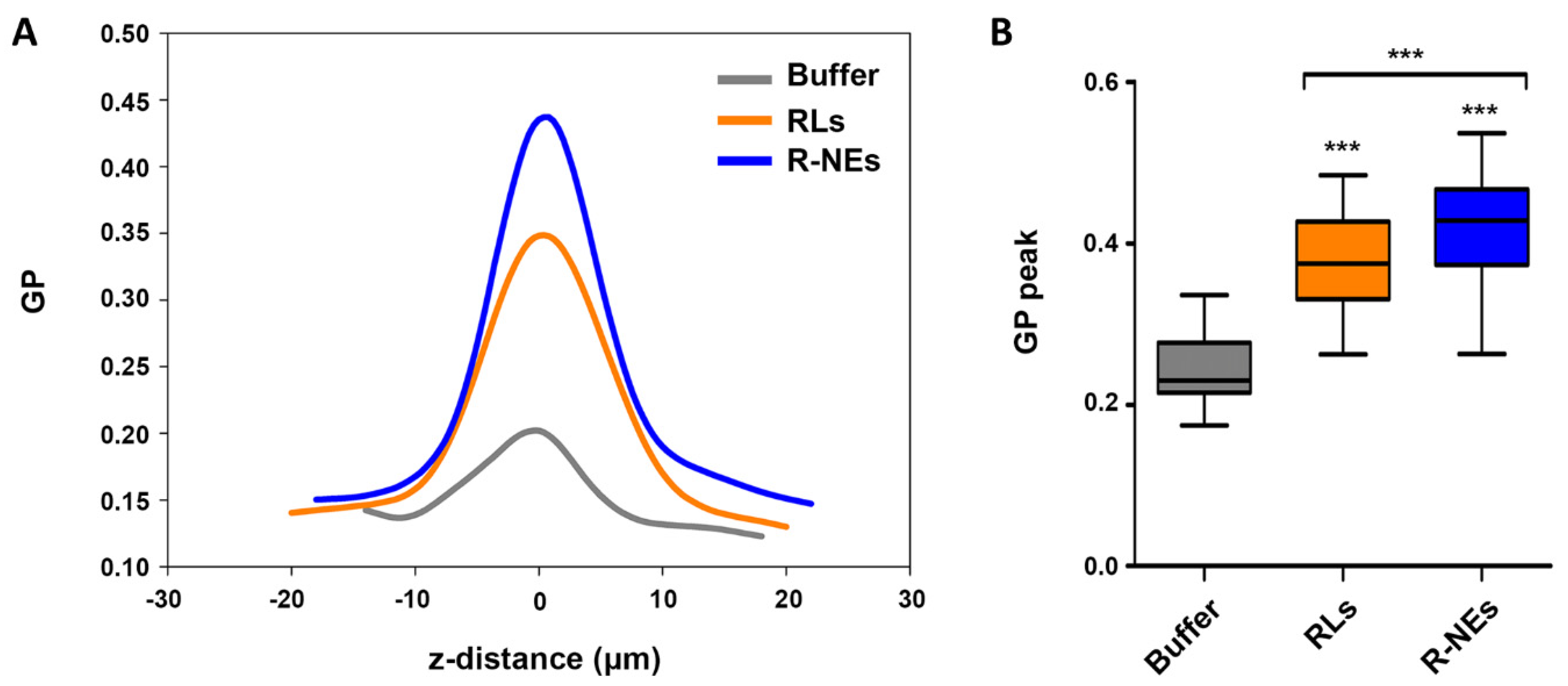
2.11. Regulation of Plant Leaf Cuticle’s Molecular Environment Dynamics by NE
2.12. Statistical Analysis
3. Results
3.1. Development and Stability of RL-Stabilized EO-NEs
3.2. Wettability of NEs on the Surface of Plant Leaves
3.3. Evaluating the Efficacy of NEs in Protecting Plants Against Bacterial Pathogens
3.4. Assessing the Efficacy of NEs in Protecting Plants from the Aphid Myzus Persicae
3.5. Evaluation of NE Activity on Natural Soil Microbiota and Bioinoculants of Interest for Agroindustry
3.6. Regulation of Environmental Dipolar Relaxation of Plant Leaf Cuticle by RLs and RL-NEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RLs | Rhamnolipid |
| NEs | Nanoemulsions |
| EOs | Essential oils |
| HD | n-hexadecane |
| Pst | Pseudomonas syringae pv tomato DC3000 |
| LAURDAN | 1-[6-(Dimethylamino)naphthalen-2-yl]dodecan-1-one |
| GP | Generalized Polarization of LAURDAN |
| T-NEs | NEs containing thyme EO |
| R-NEs | NEs containing rue EO |
| A-NEs | NEs containing tea tree EO |
| HD-NEs | NEs containing n-hexadecane |
| PR1 | Pathogenesis-related 1 gene |
| PDF1.2 | Plant defensin 1.2 gene |
References
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kashif, A.; Rehman, R.; Fuwad, A.; Shahid, M.K.; Dayarathne, H.N.P.; Jamal, A.; Aftab, M.N.; Mainali, B.; Choi, Y. Current Advances in the Classification, Production, Properties and Applications of Microbial Biosurfactants—A Critical Review. Adv. Colloid Interface Sci. 2022, 306, 102718. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Exploring the World of Rhamnolipids: A Critical Review of Their Production, Interfacial Properties, and Potential Application. Curr. Opin. Colloid Interface Sci. 2024, 69, 101780. [Google Scholar] [CrossRef]
- Suh, S.-J.; Invally, K.; Ju, L.-K. Rhamnolipids: Pathways, Productivities, and Potential. In Biobased Surfactants; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; AOCS Press: Champaign, IL, USA, 2019; pp. 169–203. ISBN 9780128127056. [Google Scholar]
- De Kesel, J.; Conrath, U.; Flors, V.; Luna, E.; Mageroy, M.H.; Mauch-Mani, B.; Pastor, V.; Pozo, M.J.; Pieterse, C.M.J.; Ton, J.; et al. The Induced Resistance Lexicon: Do’s and Don’ts. Trends Plant Sci. 2021, 26, 685–691. [Google Scholar] [CrossRef]
- Sanchez, L.; Courteaux, B.; Hubert, J.; Kauffmann, S.; Renault, J.-H.; Clément, C.; Baillieul, F.; Dorey, S. Rhamnolipids Elicit Defense Responses and Induce Disease Resistance against Biotrophic, Hemibiotrophic, and Necrotrophic Pathogens That Require Different Signaling Pathways in Arabidopsis and Highlight a Central Role for Salicylic Acid. Plant Physiol. 2012, 160, 1630–1641. [Google Scholar] [CrossRef]
- Schellenberger, R.; Crouzet, J.; Nickzad, A.; Shu, L.-J.; Kutschera, A.; Gerster, T.; Borie, N.; Dawid, C.; Cloutier, M.; Villaume, S.; et al. Bacterial Rhamnolipids and Their 3-Hydroxyalkanoate Precursors Activate Arabidopsis Innate Immunity through Two Independent Mechanisms. Proc. Natl. Acad. Sci. USA 2021, 118, e2101366118. [Google Scholar] [CrossRef]
- Mottola, M.; Bertolino, M.C.; Kourdova, L.T.; Valdivia Pérez, J.A.; Bogino, M.F.; Nocelli, N.E.; Chaveriat, L.; Martin, P.; Vico, R.V.; Fabro, G.; et al. Nanoemulsions of Synthetic Rhamnolipids Act as Plant Resistance Inducers without Damaging Plant Tissues or Affecting Soil Microbiota. Front. Plant Sci. 2023, 14, 1195718. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. How Do Plants Achieve Immunity? Defence without Specialized Immune Cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Grandellis, C.; Garavaglia, B.S.; Gottig, N.; Lonez, C.; Ruysschaert, J.M.; Ottado, J. DOTAP, a Lipidic Transfection Reagent, Triggers Arabidopsis Plant Defense Responses. Planta 2019, 249, 469–480. [Google Scholar] [CrossRef]
- Lovaglio, R.B.; dos Santos, F.J.; Jafelicci, M.; Contiero, J. Rhamnolipid Emulsifying Activity and Emulsion Stability: PH Rules. Colloids Surfaces B Biointerfaces 2011, 85, 301–305. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.C.I.; Dinu-Pirvu, C.E.; Fierascu, I.C.I.; Paunescu, A. The Application of Essential Oils as a Next-Generation of Pesticides: Recent Developments and Future Perspectives. Z. Naturforsch. C 2020, 75, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, K.; Gupta, S.S.; Ranjan, K.R.; Mishra, V.; Mukherjee, M. Das Essential Oil-Based Biopesticides. In Essential Oils; Wiley: Hoboken, NJ, USA, 2023; pp. 443–463. [Google Scholar]
- Sharma, A.; Gumber, K.; Gohain, A.; Bhatia, T.; Sohal, H.S.; Mutreja, V.; Bhardwaj, G. Importance of Essential Oils and Current Trends in Use of Essential Oils (Aroma Therapy, Agrofood, and Medicinal Usage). In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 53–83. [Google Scholar]
- Napoli, E.; Di Vito, M. Toward a New Future for Essential Oils. Antibiotics 2021, 10, 207. [Google Scholar] [CrossRef]
- Parashar, P.; Pathak, K. Augmented Stability and Efficacy of Essential Oils Through Encapsulation Approach. In Essential Oils; Wiley: Hoboken, NJ, USA, 2023; pp. 269–289. [Google Scholar]
- Bezerra, F.W.F.; Freitas, L.C.; Cunha, V.M.B.; Aires, G.C.M.; Pinto, R.H.H.; de Carvalho Junior, R.N. Encapsulated Essential Oils. In Essential Oils; Wiley: Hoboken, NJ, USA, 2023; pp. 619–634. [Google Scholar]
- Kha, T.C.; Le, P.H. Encapsulation Technologies of Essential Oils for Various Industrial Applications. In Essential Oils; Wiley: Hoboken, NJ, USA, 2023; pp. 635–670. [Google Scholar]
- Haba, E.; Bouhdid, S.; Torrego-Solana, N.; Marqués, A.M.; Espuny, M.J.; García-Celma, M.J.; Manresa, A. Rhamnolipids as Emulsifying Agents for Essential Oil Formulations: Antimicrobial Effect against Candida albicans and Methicillin-Resistant Staphylococcus aureus. Int. J. Pharm. 2014, 476, 134–141. [Google Scholar] [CrossRef]
- Napiórkowska, A.; Kurek, M. Coacervation as a Novel Method of Microencapsulation of Essential Oils—A Review. Molecules 2022, 27, 5142. [Google Scholar] [CrossRef] [PubMed]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in Essential Oils Encapsulation: Development, Characterization and Release Mechanisms. Polym. Bull. 2024, 81, 3837–3882. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef]
- Dunan, L.; Malanga, T.; Benhamou, S.; Papaiconomou, N.; Desneux, N.; Lavoir, A.-V.; Michel, T. Effects of Essential Oil-Based Formulation on Biopesticide Activity. Ind. Crops Prod. 2023, 202, 117006. [Google Scholar] [CrossRef]
- Hebishy, E.; Collette, L.; Iheozor-Ejiofor, P.; Onarinde, B.A. Stability and Antimicrobial Activity of Lemongrass Essential Oil in Nanoemulsions Produced by High-intensity Ultrasounds and Stabilized by Soy Lecithin, Hydrolyzed Whey Proteins, Gum Arabic, or Their Ternary Admixture. J. Food Process. Preserv. 2022, 46, e16840. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical Characterization and Antimicrobial Activity of Food-Grade Emulsions and Nanoemulsions Incorporating Essential Oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Iahtisham-Ul-Haq; Khan, S.; Sohail, M.; Iqbal, M.J.; Awan, K.A.; Nayik, G.A. Tea Tree Essential Oil. In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 479–500. [Google Scholar]
- Saeed, F.; Afzaal, M.; Raza, M.A.; Rasheed, A.; Hussain, M.; Nayik, G.A.; Ansari, M.J. Lavender Essential Oil: Nutritional, Compositional, and Therapeutic Insights. In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 85–101. [Google Scholar]
- Kaur, G.; Kaur, K.; Saluja, P. Citrus Essential Oil (Grapefruit, Orange, Lemon). In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 179–215. [Google Scholar]
- Inanoglu, S.; Goksen, G.; Nayik, G.A.; Mozaffari Nejad, A.S. Essential Oils from Lamiaceae Family (Rosemary, Thyme, Mint, Basil). In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 309–324. [Google Scholar]
- Liaqat, A.; Ahsan, S.; Fayyaz, M.S.; Ali, A.; Ashfaq, S.A.; Khan, S.; Khan, M.A.; Mehmood, T.; Khaliq, A.; Chughtai, M.F.J.; et al. Cinnamon Essential Oil. In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 377–390. [Google Scholar]
- Said-, H.A.H.; Ahl, A.; Hikal, W.; Tkachenko, K.; Said-Al, H.A.H.; Said-Al Ahl, H.A.H.; Hikal, W.M.; Tkachenko, K.G. Essential Oils with Potential as Insecticidal Agents: A Review. Int. J. Environ. Plan. Manag. 2017, 3, 23–33. [Google Scholar]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent Activity of Essential Oils: A Review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Altalhi, T.; Neves Cruz, J.; Inamuddin, I. Essential Oils. Extraction Methods and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; ISBN 9781119829355. [Google Scholar]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-Bond Axisymmetric Drop Shape Analysis for Surface Tension and Contact Angle Measurements of Sessile Drops. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Fabro, G.; Rizzi, Y.S.; Alvarez, M.E. Arabidopsis Proline Dehydrogenase Contributes to Flagellin-Mediated PAMP-Triggered Immunity by Affecting RBOHD. Mol. Plant-Microbe Interact. 2016, 29, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Moritz, L.; Pieterse, C.M.J.; Van Wees, S.C.M. Impact of Hormonal Crosstalk on Plant Resistance and Fitness under Multi-Attacker Conditions. Front. Plant Sci. 2015, 6, 639. [Google Scholar] [CrossRef]
- Van der Does, D.; Leon-Reyes, A.; Koornneef, A.; Van Verk, M.C.; Rodenburg, N.; Pauwels, L.; Goossens, A.; Körbes, A.P.; Memelink, J.; Ritsema, T.; et al. Salicylic Acid Suppresses Jasmonic Acid Signaling Downstream of SCFCOI1-JAZ by Targeting GCC Promoter Motifs via Transcription Factor ORA59. Plant Cell 2013, 25, 744–761. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Silva-Sanzana, C.; Zavala, D.; Moraga, F.; Herrera-Vásquez, A.; Blanco-Herrera, F. Oligogalacturonides Enhance Resistance against Aphids through Pattern-Triggered Immunity and Activation of Salicylic Acid Signaling. Int. J. Mol. Sci. 2022, 23, 9753. [Google Scholar] [CrossRef]
- Socas, L.B.P.; Valdivia-Pérez, J.A.; Fanani, M.L.; Ambroggio, E.E. Multidimensional Spectral Phasors of LAURDAN’s Excitation–Emission Matrices: The Ultimate Sensor for Lipid Phases? J. Am. Chem. Soc. 2024, 146, 17230–17239. [Google Scholar] [CrossRef]
- Kumar, M.; Poonam; Ahmad, S.; Singh, R.P. Plant Growth Promoting Microbes: Diverse Roles for Sustainable and Ecofriendly Agriculture. Energy Nexus 2022, 7, 100133. [Google Scholar] [CrossRef]
- Evans, F.D.; Wennerstrom, H. The Colloidal Domain. Where Physics, Chemistry, Biology an Thechnology Meet, 2nd ed.; Wiley-VCH: New York, NY, USA, 1999; ISBN 0-471-24247-0. [Google Scholar]
- Landfester, K. Synthesis of Colloidal Particles in Miniemulsions. Annu. Rev. Mater. Res. 2006, 36, 231–279. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Bai, L.; McClements, D.J. Formation and Stabilization of Nanoemulsions Using Biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79. [Google Scholar] [CrossRef]
- Khan, M.A.; Ullah, K.; Rahman, N.u.; Mahmood, A.; Müllertz, A.; Mannan, A.; Murtaza, G.; Khan, S.A. Formulation, Characterization and in-Vitro Evaluation of Self-Nanoemulsifying Drug Delivery System Containing Rhamnolipid Biosurfactant. J. Drug Deliv. Sci. Technol. 2022, 75, 103673. [Google Scholar] [CrossRef]
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical Characterization and Antimicrobial Properties of Rhamnolipids Produced by Pseudomonas Aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322. [Google Scholar] [CrossRef]
- Liu, H.; Shao, B.; Long, X.; Yao, Y.; Meng, Q. Foliar Penetration Enhanced by Biosurfactant Rhamnolipid. Colloids Surfaces B Biointerfaces 2016, 145, 548–554. [Google Scholar] [CrossRef]
- Soffe, R.; Bernach, M.; Remus-Emsermann, M.N.P.; Nock, V. Replicating Arabidopsis Model Leaf Surfaces for Phyllosphere Microbiology. Sci. Rep. 2019, 9, 14420. [Google Scholar] [CrossRef]
- Davies, J.T.; Rideal, E.K. Interfacial Fenomena, 2nd ed.; Academic Press: London, UK, 1963. [Google Scholar]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Valenzuela, I.; Hoffmann, A.A. Effects of Aphid Feeding and Associated Virus Injury on Grain Crops in Australia. Austral Entomol. 2015, 54, 292–305. [Google Scholar] [CrossRef]
- Kavetsou, E.; Koutsoukos, S.; Daferera, D.; Polissiou, M.G.; Karagiannis, D.; Perdikis, D.C.; Detsi, A. Encapsulation of Mentha Pulegium Essential Oil in Yeast Cell Microcarriers: An Approach to Environmentally Friendly Pesticides. J. Agric. Food Chem. 2019, 67, 4746–4753. [Google Scholar] [CrossRef]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid Biosurfactants as New Players in Animal and Plant Defense against Microbes. Int. J. Mol. Sci. 2010, 11, 5095–5108. [Google Scholar] [CrossRef]
- Aragón, W.; Formey, D.; Aviles-Baltazar, N.Y.; Torres, M.; Serrano, M. Arabidopsis thaliana Cuticle Composition Contributes to Differential Defense Response to Botrytis Cinerea. Front. Plant Sci. 2021, 12, 738949. [Google Scholar] [CrossRef]
- Ju, S.; Go, Y.S.; Choi, H.J.; Park, J.M.; Suh, M.C. DEWAX Transcription Factor Is Involved in Resistance to Botrytis cinerea in Arabidopsis thaliana and Camelina sativa. Front. Plant Sci. 2017, 8, 1210. [Google Scholar] [CrossRef] [PubMed]
- von Wettstein-Knowles, P. Plant Waxes. In Encyclopedia of Life Sciences; Wiley: Chichester, UK, 2016; pp. 1–13. ISBN 9780470015902. [Google Scholar]
- Bagatolli, L.A. To see or not to see: Lateral organization of biological membranes and fluorescence microscopy. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 1541–1556. [Google Scholar] [CrossRef]
- Balci, M. (Ed.) Chemical Shift. In Basic 1H- and 13C-NMR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2005; pp. 25–85. [Google Scholar]
- Gunther, G.; Malacrida, L.; Jameson, D.M.; Gratton, E.; Sánchez, S.A. LAURDAN since Weber: The Quest for Visualizing Membrane Heterogeneity. Acc. Chem. Res. 2021, 54, 976–987. [Google Scholar] [CrossRef]
- Otaiza-González, S.; Cabadas, M.; Robert, G.; Stock, R.P.; Malacrida, L.; Lascano, R.; Bagatolli, L.A. The innards of the cell: Studies of water dipolar relaxation using the ACDAN fluorescent probe. Methods Appl. Fluoresc. 2022, 10, 044010. [Google Scholar] [CrossRef]
- Pinto, S.N.; Fernandes, F.; Fedorov, A.; Futerman, A.H.; Silva, L.C.; Prieto, M. A combined fluorescence spectroscopy, confocal and 2-photon microscopy approach to re-evaluate the properties of sphingolipid domains. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2099–2110. [Google Scholar] [CrossRef]
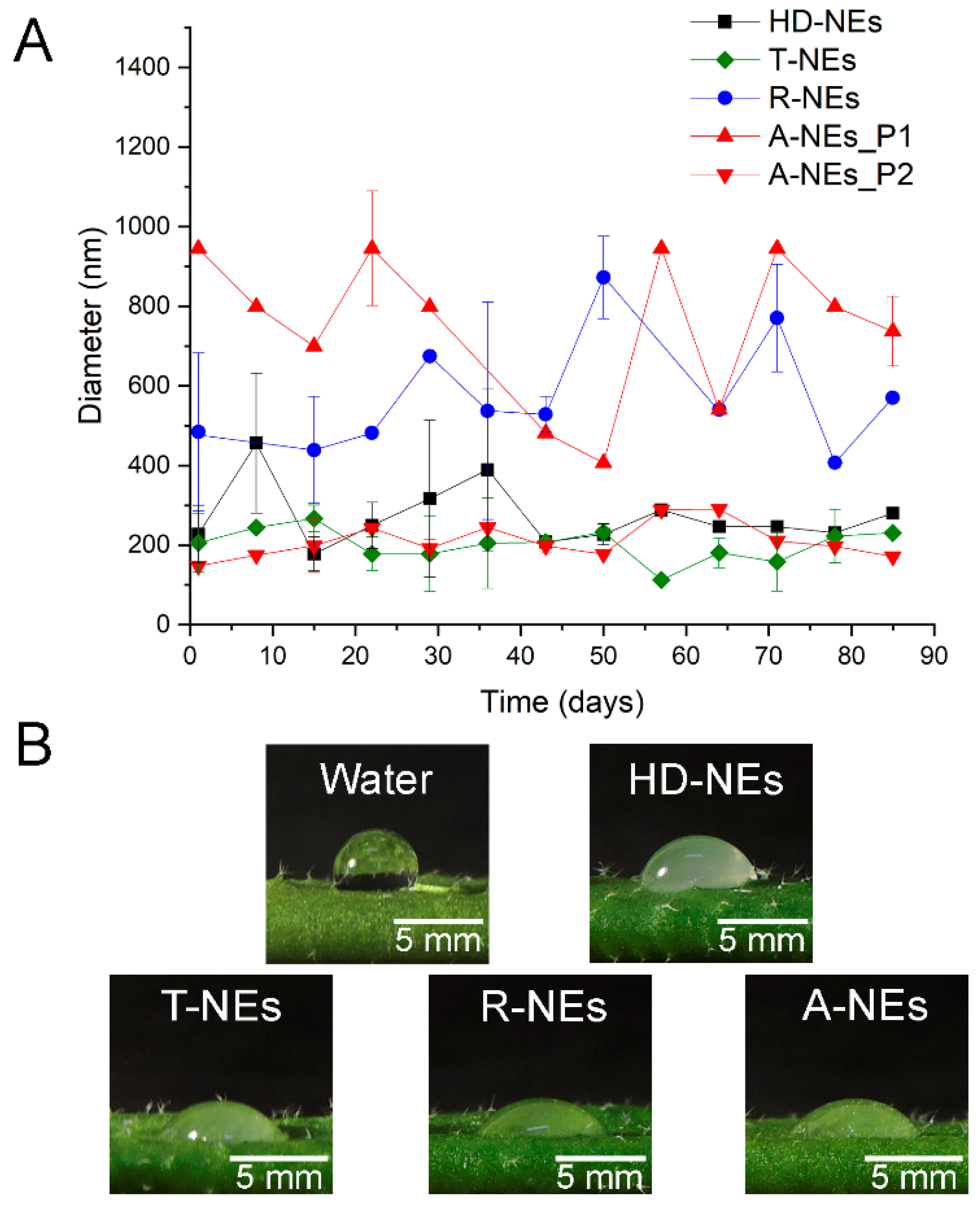
| Nanoemulsions (NEs) | Oil Phase | Diameter (nm) * | PDI ** | ζ-Potential ** (mV) | Contact Angle (°) *** | |
|---|---|---|---|---|---|---|
| P1 | P2 | |||||
| HD-NEs | Hexadecane | 241 ± 80 | - | 0.4 ± 0.1 | −55 ± 2 | 84 ± 11 |
| T-NEs | Thyme EO | 184 ± 61 | - | 0.7 ± 0.3 | −65 ± 2 | 57 ± 4 |
| R-NEs | Rue EO | 553 ± 149 | - | 1.1 ± 0.1 | −51 ± 3 | 60 ± 8 |
| A-NEs | Tea Tree EO | 196 ± 49 | 684 ± 203 | 1.6 ± 0.5 | −25 ± 4 | 61 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourdova, L.T.; Mottola, M.; Peppino Margutti, M.; Bogino, M.F.; Maritano, P.; Vico, R.V.; Blanco-Herrera, F.; Fanani, M.L.; Fabro, G. Rhamnolipid-Stabilized Essential Oils Nanoemulsions: Sustainable Biopesticides and Biostimulants with Potential for Crop Protection. Agronomy 2025, 15, 824. https://doi.org/10.3390/agronomy15040824
Kourdova LT, Mottola M, Peppino Margutti M, Bogino MF, Maritano P, Vico RV, Blanco-Herrera F, Fanani ML, Fabro G. Rhamnolipid-Stabilized Essential Oils Nanoemulsions: Sustainable Biopesticides and Biostimulants with Potential for Crop Protection. Agronomy. 2025; 15(4):824. https://doi.org/10.3390/agronomy15040824
Chicago/Turabian StyleKourdova, Lucille T., Milagro Mottola, Micaela Peppino Margutti, María Florencia Bogino, Paula Maritano, Raquel Viviana Vico, Francisca Blanco-Herrera, María Laura Fanani, and Georgina Fabro. 2025. "Rhamnolipid-Stabilized Essential Oils Nanoemulsions: Sustainable Biopesticides and Biostimulants with Potential for Crop Protection" Agronomy 15, no. 4: 824. https://doi.org/10.3390/agronomy15040824
APA StyleKourdova, L. T., Mottola, M., Peppino Margutti, M., Bogino, M. F., Maritano, P., Vico, R. V., Blanco-Herrera, F., Fanani, M. L., & Fabro, G. (2025). Rhamnolipid-Stabilized Essential Oils Nanoemulsions: Sustainable Biopesticides and Biostimulants with Potential for Crop Protection. Agronomy, 15(4), 824. https://doi.org/10.3390/agronomy15040824







