Exogenous Hydrogen Sulfide Alleviates the Toxicity of Cu2+ Stress on the Growth Physiology and Quality Components in Camellia sinensis L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Cu Content Determination
2.3. Biomass and Root Activity Determination
2.4. Ultrastructural Observation of Leaf Cells
2.5. Chlorophyll Content and Photosynthetic Index Determination
2.6. Antioxidant-System-Associated Indicator Determination
2.7. LCD Activity Determination
2.8. Component Determination
2.9. Statistical Analysis
3. Results
3.1. The Cu Contents
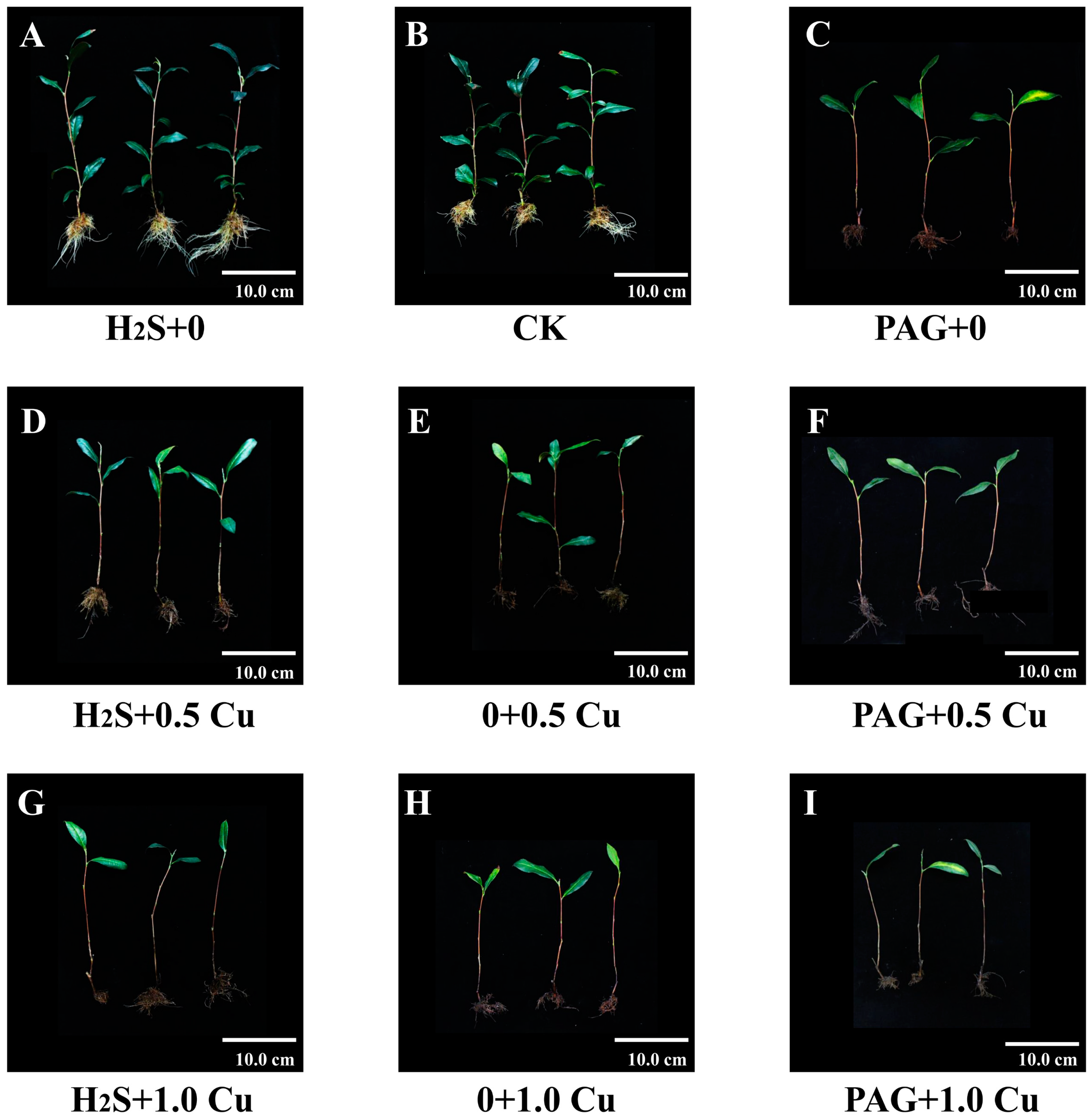
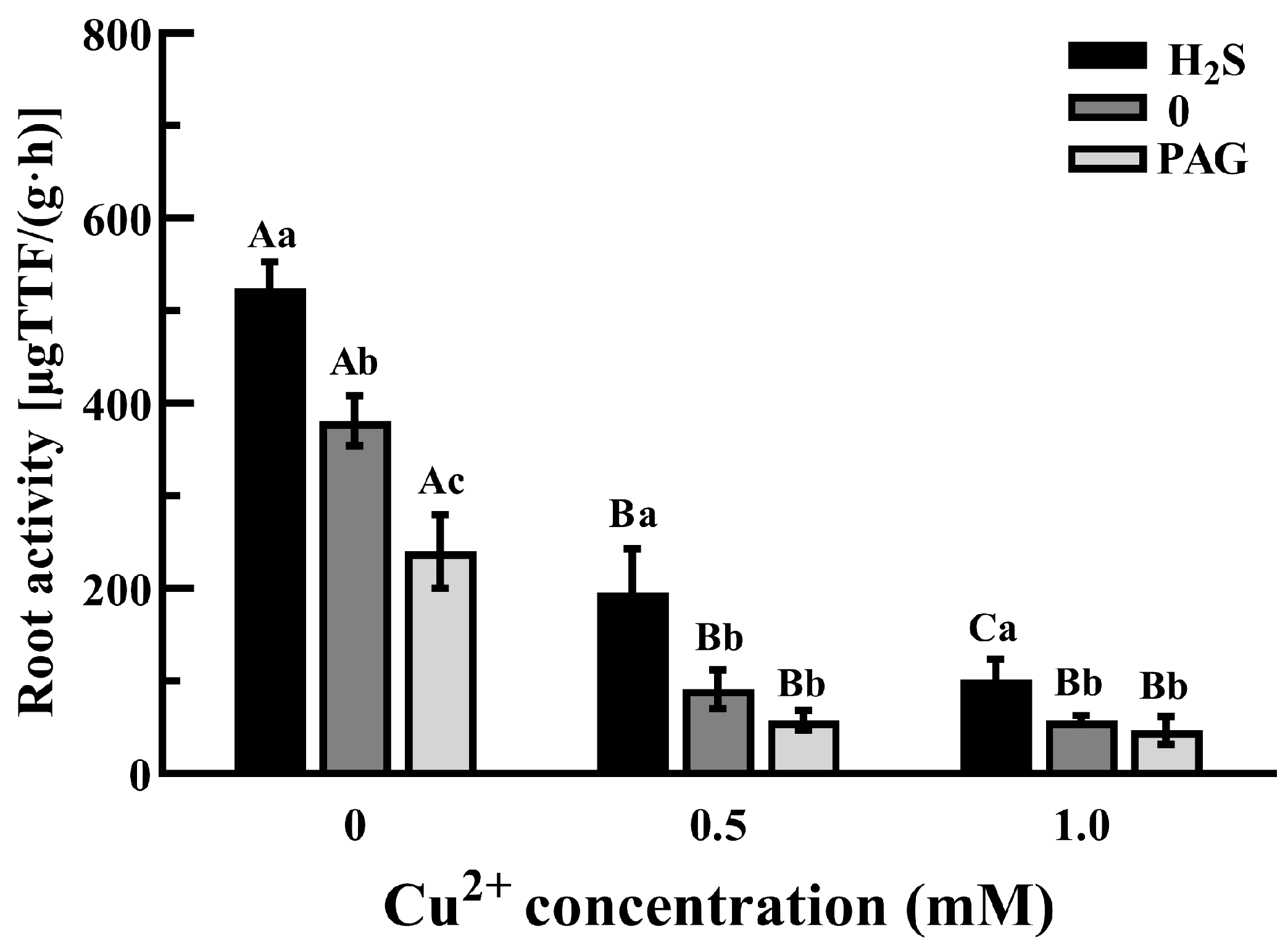
3.2. The Growth and Root Activities of Tea Plants
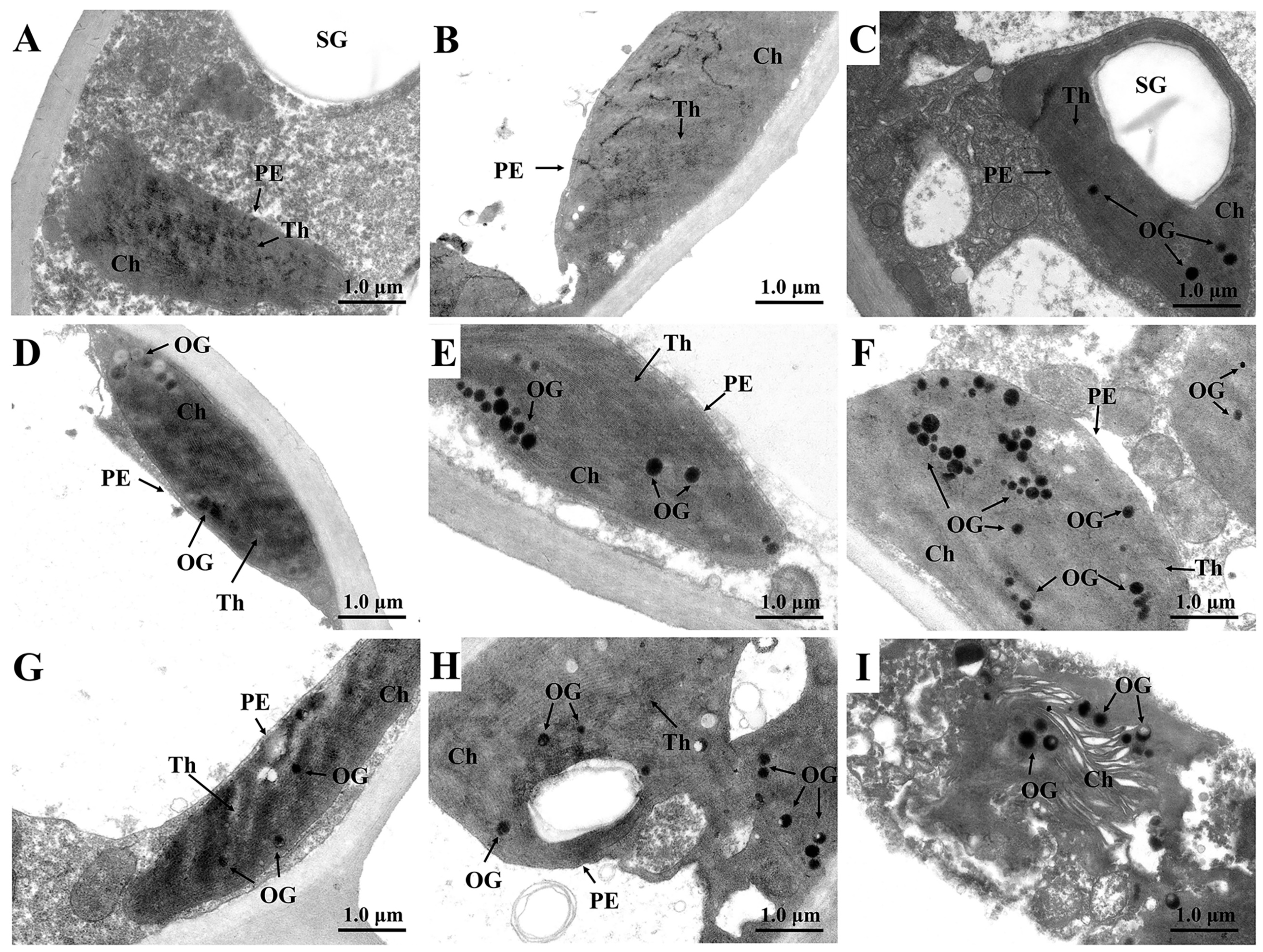
3.3. The Ultrastructural Changes in Leaf Cells
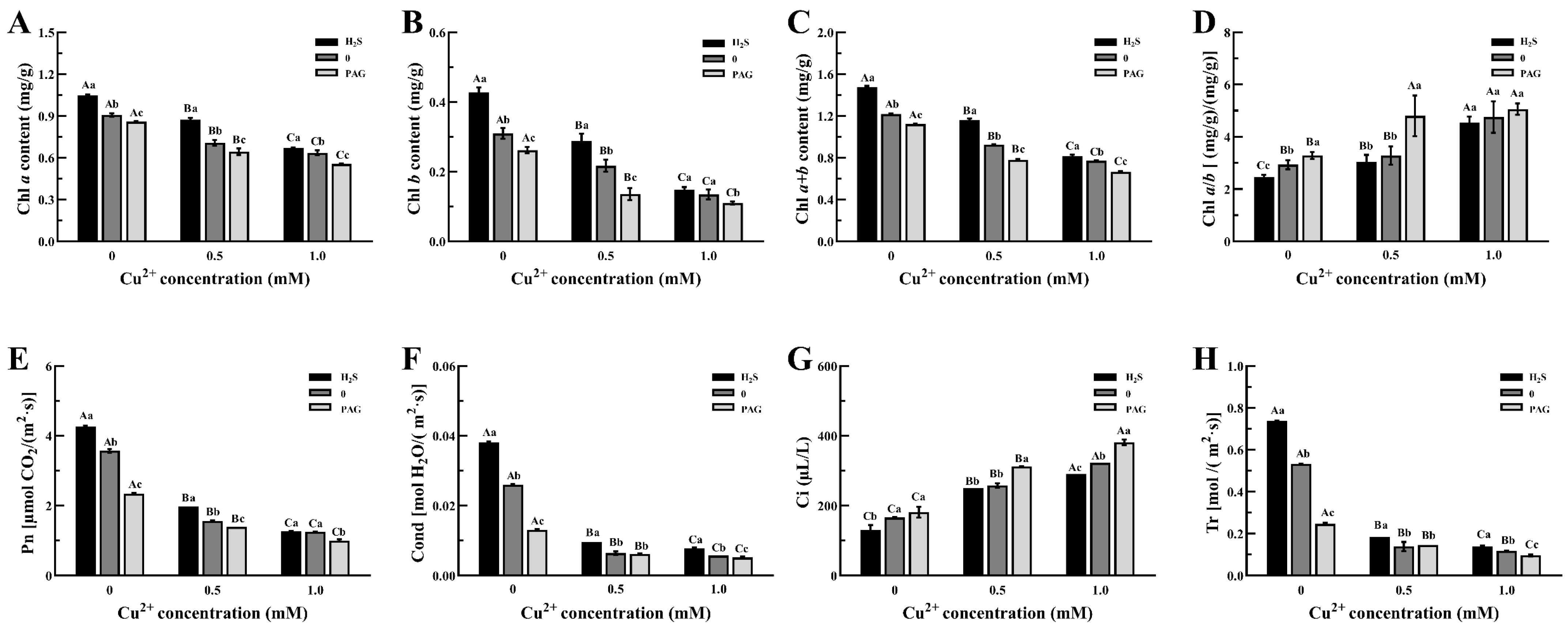
3.4. The Changes in the Photosynthetic System in Tea Plants
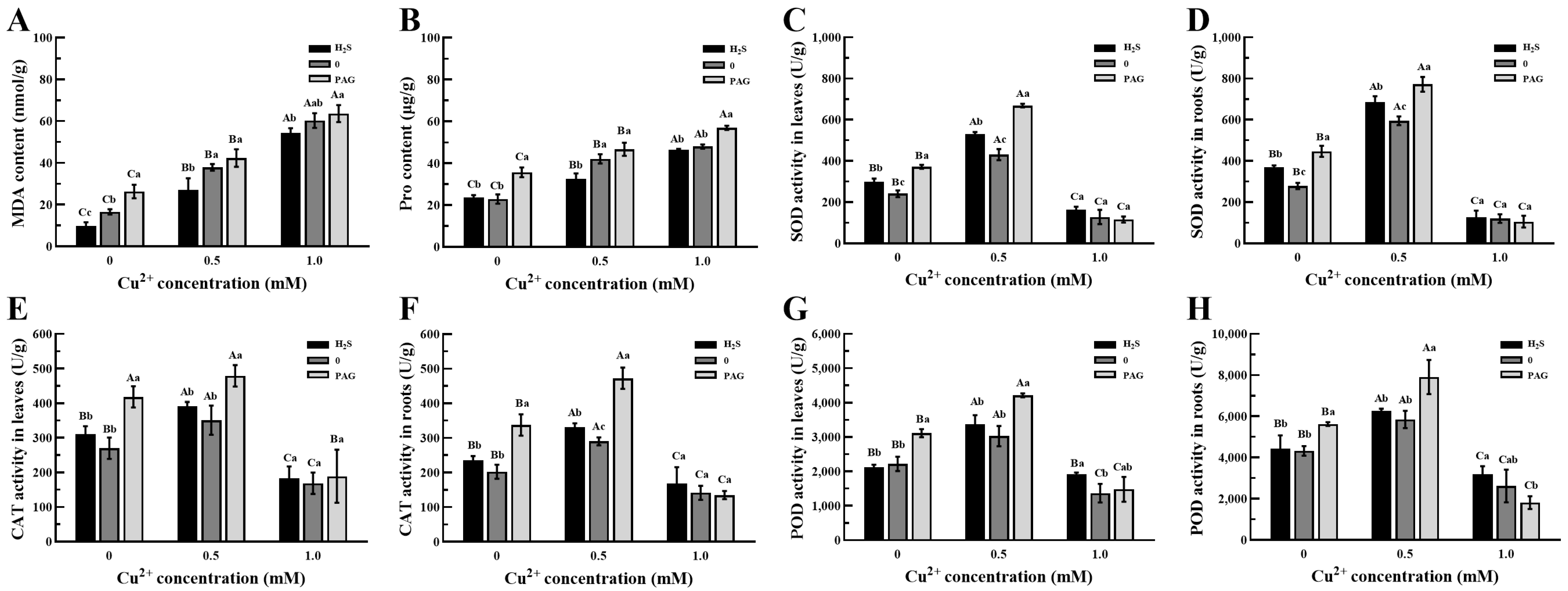
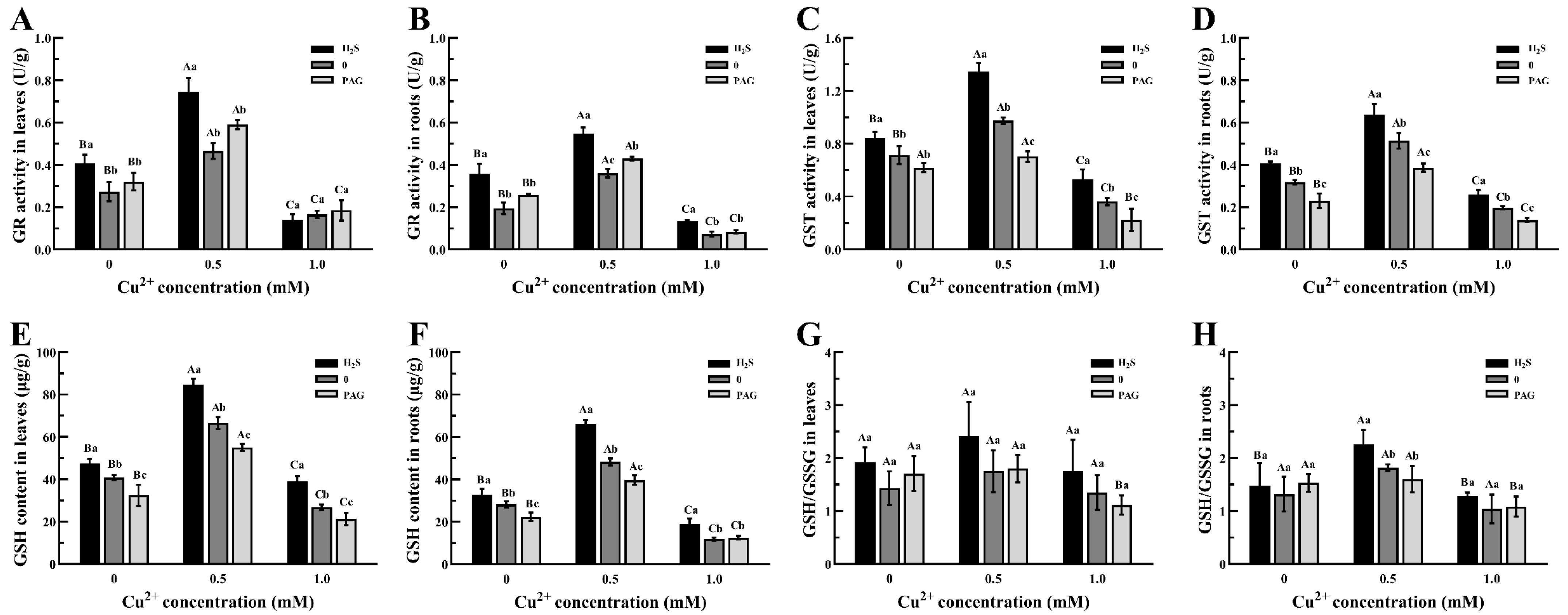
3.5. The Changes in the Antioxidant System in Tea Plants
3.6. The Changes in LCD Activities in Tea Plants
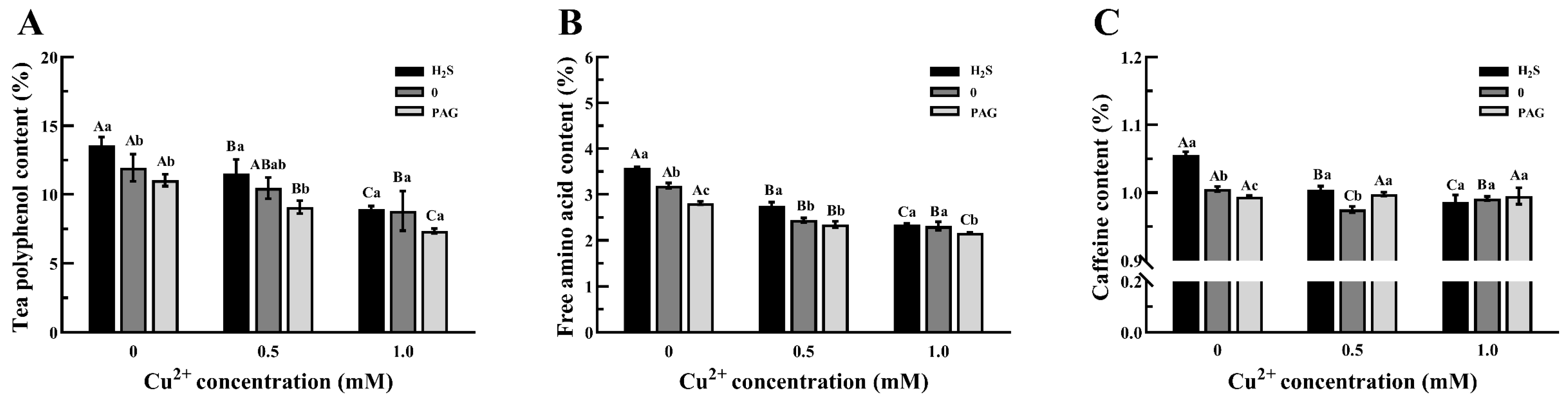
3.7. The Changes in Main Components in Tea Plants
4. Discussion
4.1. H2S Pretreatment Reduces Cu Accumulation in Tea Plants Under Cu2+ Stress
4.2. H2S Pretreatment Alleviates the Toxicities of Cu2+ Stress on the Growth Physiology of Tea Plants
4.3. H2S Pretreatment Enhances Antioxidant Stress Activation in Tea Plants Under Cu2+ Stress
4.4. H2S Pretreatment Compensates for the Quality Loss of Tea Plants Caused by Cu2+ Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, M.Q.; Huang, J.H.; Chen, J.Y.; Wang, Q.; Xu, Z.R. Research progress on copper homeostasis in plants: A review. Jiangsu Agric. Sci. 2019, 47, 305–311. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. BioMetals 2021, 34, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.L.; Shen, Z.G. The mechanisms of heavy metal uptake and accumulation in plants. Chin. Bull. Bot. 2003, 20, 59–66. [Google Scholar] [CrossRef]
- Zhai, F.Q.; Wang, X.L.; Hua, J.M.; Si, J.Y.; Feng, K. Copper toxicity on seedlings of wheat and the detoxification of calcium. J. Agro-Environ. Sci. 2007, 2, 694–698. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Zhang, Y.X.; Zhang, X.W.; Li, P. Effects of exogenous Pb and Cu stress on eco-physiological characteristics on foxtail millet seedlings of different genotypes. Acta Ecol. Sin. 2012, 32, 889–897. [Google Scholar] [CrossRef]
- Lidon, F.C.; Henriques, F.S. Effects of copper toxicity on growth and the uptake and translocation of metals in rice plants. J. Plant Nutr. 1993, 16, 1449–1464. [Google Scholar] [CrossRef]
- Tian, S.K.; Li, T.X.; Peng, H.Y.; Yang, X.E.; Li, T.Q. Influence of Cu toxcity on root morphology and Cu accumulation of Elsholtzia splendens and Elsholtzia argyi. J. Soil Water Conserv. 2005, 19, 97–100+183. [Google Scholar] [CrossRef]
- Ni, C.Y.; Chen, Y.X.; Luo, Y.M. Change of superficial shape and cell microstructure of milk-vetch (Astragalus L.) root under simulated Cu pollution in a red soil. Environ. Sci. 2003, 24, 116–121. [Google Scholar] [CrossRef]
- Zhang, D.J.; Ma, J.H.; Yang, S.F.; Chen, H.T.; Liu, P.; Wang, W.F.; Li, C.X. Effects of silicon on the ultrastructures of wheat radical cells under copper stress. Chin. J. Appl. Ecol. 2014, 25, 2385–2389. [Google Scholar] [CrossRef]
- Zheng, B.J.; Xu, S.C.; Tu, Y.H.; Zhou, F.; Liang, D.D.; Wang, H.; Mo, Y.W. Research progress on crops toxicity of copper. J. Shaoxing Univ. 2022, 42, 58–64. [Google Scholar] [CrossRef]
- Zhang, G.; Weng, Y.; Li, D.X.; Sun, N.; Yang, R.Q.; Wang, X.B.; Wang, Z.J.; Wang, D.L. Growth and physiology responses of Perennial Ryegrass to Cu2+ stress. J. Northeast Norm. Univ. (Nat. Sci. Ed.) 2019, 51, 119–124. [Google Scholar] [CrossRef]
- Zhang, G.J.; Jiang, H.; Zheng, L.Q.; Chen, J.; Qiu, D.L.; Liu, X.H. Effect of copper stress on photosynthesis of navel orange seedlings. Chin. J. Eco-Agric. 2009, 17, 130–134. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Li, G.Z.; Kang, G.Z. Physiological response and expression of genes encoding ascorbate-glutathione synthesis enzymes to Cu2+ stress in wheat. J. Agric. Sci. Technol. 2021, 23, 21–29. [Google Scholar] [CrossRef]
- Li, Y.; Huang, F.Y.; Tao, Y.; Zhou, Y.; Bai, A.M.; Yu, Z.H.; Xiao, D.; Zhang, C.W.; Liu, T.K.; Hou, X.L.; et al. BcGR1.1, a cytoplasmic localized glutathione reductase, enhanced tolerance to copper stress in Arabidopsis thaliana. Antioxidants 2022, 11, 389. [Google Scholar] [CrossRef]
- Jain, M.; Ghanashyam, C.; Bhattacharjee, A. Comprehensive expression analysis suggests overlapping and specific roles of rice glutathione S-transferase genes during development and stress responses. BMC Genom. 2010, 11, 73. [Google Scholar] [CrossRef]
- Al-Khateeb, W.; Al-Qwasemeh, H. Cadmium, copper and zinc toxicity effects on growth, proline content and genetic stability of Solanum nigrum L., a crop wild relative for tomato; comparative study. Physiol. Mol. Biol. Plants 2014, 20, 31–39. [Google Scholar] [CrossRef]
- Wang, Y.C. Effect of copper stress on photosynthesis and quality index of tea trees. Mod. Agric. Sci. Technol. 2021, 14, 9–12+14. [Google Scholar] [CrossRef]
- Dey, S.; Mazumder, P.B.; Paul, S.B. Accumulation of Cu at different concentration in tea plant (Camellia sinensis (L.) O. Kuntze). IOSR J. Agric. Vet. Sci. 2014, 7, 39–43. [Google Scholar] [CrossRef]
- Wang, H.B.; Ye, J.H.; Kong, X.H.; Ding, L.; Qiu, F.Y.; Jia, X.L. Comparative study on biological responses and Cu speciation in tissues of two different tea trees after Cu exposure. Asian J. Ecotoxicol. 2016, 11, 216–225. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, W.F.; Ma, J.Y.; Zhu, C.M. Effects of Cu on the physiological response of large-leaf tea seedling. J. Yunnan Agric. Univ. (Nat. Sci.) 2016, 31, 368–371. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mohanpuria, P. Responses of Camellia sinensis cultivars to Cu and Al stress. Biol. Plant. 2009, 53, 737–740. [Google Scholar] [CrossRef]
- Jin, Z.P.; Qiao, Z.J.; Zhang, L.P.; Pei, Y.X. Hydrogen sulfide alleviates cadmium toxicity by restricting Cd2+ influx in Arabidopsis. Chin. J. Biochem. Mol. Biol. 2020, 36, 61–70. [Google Scholar] [CrossRef]
- Pei, Y.X. Gasotransmitter hydrogen sulfide in plants: Stinking to high heaven, but refreshing to fine life. Chin. J. Biochem. Mol. Biol. 2016, 32, 721–733. [Google Scholar] [CrossRef]
- Gao, X.L.; Sun, M.X.; Wu, X.L.; Xiao, Y.S.; Peng, F.T. Effects of exogenous hydrogen sulfide on root growth of mature peach trees. Shandong Agric. Sci. 2022, 54, 76–80. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.H.; Wang, W.H.; Zheng, C.J.; Lin, G.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef]
- Luo, S.L.; Calderón-Urrea, A.; Yu, J.H.; Liao, W.B.; Xie, J.M.; Lv, J.; Feng, Z.; Tang, Z.Q. The role of hydrogen sulfide in plant alleviates heavy metal stress. Plant Soil 2020, 449, 1–10. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhang, J.H.; Sun, L.M.; Zhu, L.F.; Abliz, B.; Hu, W.J.; Zhong, C.; Bai, Z.G.; Sajid, H.; Cao, X.C.; et al. Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast Al contents in Rice. Front. Plant Sci. 2018, 9, 294. [Google Scholar] [CrossRef]
- Papenbrock, J.; Reimenschneider, A.; Kamp, A.; Schulz-Vogt, H.N.; Schmidt, A. Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants—From the field to the test tube and back. Plant Biol. 2007, 9, 582–588. [Google Scholar] [CrossRef]
- Jin, Z.P.; Shen, J.J.; Qiao, Z.J.; Yang, G.D.; Wang, R.; Pei, Y.X. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2011, 414, 481–486. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Huo, C.H.; Xie, Y.H. Research progress on hydrogen sulfide donors. Chin. J. Med. Chem. 2020, 30, 107–123. [Google Scholar] [CrossRef]
- Shen, X.X.; Zhang, Y.H.; Huang, Z.J. Research advances in hydrogen sulfide donors. J. China Pharm. Univ. 2019, 50, 265–273. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, Q.; Tian, S.; Xu, L.; Shen, G. Research progress of a new gas signal molecule H2S in plants. Shandong Agric. Sci. 2016, 48, 151–156+161. [Google Scholar] [CrossRef]
- Li, Q.R.; Wang, Y.M. Trace element copper and human health. Stud. Trace Elem. Health 2007, 3, 61–63. [Google Scholar] [CrossRef]
- Subbiah, S.; Natarajan, M.; Nair Muraleedharan, N.; Rajagopal, S. Heavy metal content of black teas from south India. Food Control 2008, 19, 746–749. [Google Scholar] [CrossRef]
- Wang, H.B.; Ye, J.H.; Kong, X.H.; Ding, L.; Zhang, Q.X.; Ma, X.M.; Jia, X.L. Physiological responses and subcellular copper distribution characteristics of different tea plants under copper stress. Jiangsu Agric. Sci. 2016, 44, 219–223. [Google Scholar] [CrossRef]
- Karak, T.; Paul, R.K.; Kutu, F.R.; Mehra, A.; Khare, P.; Dutta, A.K.; Bora, K.; Boruah, R.K. Comparative assessment of copper, iron, and zinc contents in selected Indian (Assam) and South African (Thohoyandou) tea (Camellia sinensis L.) samples and their infusion: A quest for health risks to consumer. Biol. Trace Elem. Res. 2017, 175, 475–487. [Google Scholar] [CrossRef]
- Ghanati, F.; Morita, A.; Yokota, H. Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil 2005, 276, 133–141. [Google Scholar] [CrossRef]
- Liu, F.Z. Practical Handbook for Agricultural Environmental Monitoring; China Agriculture Press: Beijing, China, 2001; p. 500. [Google Scholar]
- Gao, J.F. Guidance for Plant Physiology Experiments; Higher Education Press: Beijing, China, 2006; pp. 57–59. [Google Scholar]
- Xiang, F.; Li, W.; Liu, H.; Zhou, L.; Ding, D.; Zeng, Z. Comparisonon methods of chlorophyll extraction in Camellia sinensis. J. Tea Commun. 2016, 43, 37–40. [Google Scholar] [CrossRef]
- Xiong, Q.E. Experimental Course of Plant Physiology; Sichuan Science and Technology Press: Chengdu, China, 2003; pp. 126–127. [Google Scholar]
- Bates, L.S.; Walderen, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1975, 39, 205–207. [Google Scholar] [CrossRef]
- ISO 1572:1980; Tea-Preparation of Ground Sample of Known Dry Matter Content. Standards Press of China: Beijing, China, 1980.
- ISO 14502–1:2005; Determination of Substances Characteristic of Green and Black Tea-Part 1: Content of Total Polyphenols in Tea—Colorimetric Method Using Folin-Ciocalteu Reagent. Standards Press of China: Beijing, China, 2005.
- ISO 14502–2:2005; Determination of Substances Characteristic of Green and Black Tea-Part 2: Content of Catechins in Green Tea—Method Using High-Performance Liquid Chromatography. Standards Press of China: Beijing, China, 2005.
- GB/T 8314–2013; Tea-Determination of Free Amino Acids Content. Standards Press of China: Beijing, China, 2013.
- ISO 10727:2002; Tea and Instant Tea in Solid Form-Determination of Caffeine Content- Method Using High-Performance Liquid Chromatography. Standards Press of China: Beijing, China, 2002.
- Chen, G.; Li, J.; Han, H.M.; Du, R.Y.; Wang, X. Physiological and molecular mechanisms of plant responses to copper stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Yang, Z.F.; Wang, X.Y.; Feng, J.R.; Zhu, S.H. Biological functions of hydrogen sulfide in plants. Int. J. Mol. Sci. 2022, 23, 15107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Cao, H.; Yang, Y.; Xie, L. Effects of hydrogen sulfide and nitric oxide on resistance of Malus hupehensis Rehd. seedlings against cadmium stress. J. Northwest A F Univ. (Nat. Sci. Ed.) 2021, 49, 120–128. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Lin, H.R.; Cui, H.M. Effect of exogenous hydrogen sulfide on the physiological and biochemical characteristics of processing tomato seedlings under NaCl stress. Acta Agric. Boreali-Sin. 2017, 32, 208–214. [Google Scholar] [CrossRef]
- Qian, P.; Sun, R.; Ali, B.; Gill, R.A.; Xu, L.; Zhou, W.J. Effects of hydrogen sulfide on growth, antioxidative capacity, and ultrastructural changes in Oilseed rape seedlings under aluminum toxicity. J. Plant Growth Regul. 2014, 33, 526–538. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- Nomani, L.; Zehra, A.; Choudhary, S.; Wani, K.I.; Naeem, M.; Siddiqui, M.H.; Khan, M.M.A.; Aftab, T.; Singh, V. Exogenous hydrogen sulphide alleviates copper stress impacts in Artemisia annua L.: Growth, antioxidant metabolism, glandular trichome development and artemisinin biosynthesis. Plant Biol. 2022, 24, 642–651. [Google Scholar] [CrossRef]
- Shi, H.T.; Ye, T.T.; Chan, Z.L. Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass [Cynodon dactylon (L). Pers.]. Plant Physiol. Biochem. 2013, 71, 226–234. [Google Scholar] [CrossRef]
- Shan, C.; Liu, H.; Zhao, L.; Wang, X. Effects of exogenous hydrogen sulfide on the redox states of ascorbate and glutathione in maize leaves under salt stress. Biol. Plant. 2014, 58, 169–173. [Google Scholar] [CrossRef]
- Guo, L.; Ling, L.; Wang, X.Q.; Cheng, T.; Wang, H.Y.; Ruan, Y.A. Exogenous hydrogen sulfide and methylglyoxal alleviate cadmium-induced oxidative stress in Salix matsudana Koidz by regulating glutathione metabolism. BMC Plant Biol. 2023, 23, 73. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Zhou, M.J.; Zhou, H.; Cui, B.M.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Liu, J.X.; Liu, R.R.; Liu, X.L.; Jia, H.Y.; Bu, T.; Li, N. Effects of spraving NaHS at different growth stages on osmotic adjustment substance and antioxidant activity in leaves of naked oat under saline-alkali stress. Chin. J. Ecol. 2021, 40, 3620–3632. [Google Scholar] [CrossRef]
- Wan, X.C. Tea Biochemistry; China Agriculture Press: Beijing, China, 2003; pp. 68–116, 319. [Google Scholar]
- Wang, Q.Z.; Liu, Q.; Gao, Y.N.; Liu, X. Review on the mechanisms of the response to salinity-alkalinity stress in plants. Acta Ecol. Sin. 2017, 37, 5565–5577. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.X. Variations of main quality components of tea genetic resources [Camellia sinensis (L.) O. Kuntze] preserved in the China National Germplasm Tea Repository. Plant Foods Hum. Nutr. 2005, 60, 31–35. [Google Scholar] [CrossRef] [PubMed]


| Abbreviation | Treatment |
|---|---|
| CK (0 + 0) | Untreated culture solution (0) pretreatment for 15 days, followed by untreated culture solution (0) treatment for 15 days. |
| H2S + 0 | 100 μM Na2S·9H2O (H2S) pretreatment for 15 days, followed by untreated culture solution (0) treatment for 15 days. |
| PAG + 0 | 1.0 mM PAG (PAG) pretreatment for 15 days, followed by untreated culture solution (0) treatment for 15 days. |
| 0 + 0.5 Cu | Untreated culture solution (0) pretreatment for 15 days, followed by 0.5 mM CuSO4·5H2O (0.5 Cu) treatment for 15 days. |
| H2S + 0.5 Cu | 100 μM Na2S·9H2O (H2S) pretreatment for 15 days, followed by 0.5 mM CuSO4·5H2O (0.5 Cu) treatment for 15 days. |
| PAG + 0.5 Cu | 1.0 mM PAG (PAG) pretreatment for 15 days, followed by 0.5 mM CuSO4·5H2O (0.5 Cu) treatment for 15 days. |
| 0 + 1.0 Cu | Untreated culture solution (0) pretreatment for 15 days, followed by 1.0 mM CuSO4·5H2O (1.0 Cu) treatment for 15 days. |
| H2S + 1.0 Cu | 100 μM Na2S·9H2O (H2S) pretreatment for 15 days, followed by 1.0 mM CuSO4·5H2O (1.0 Cu) treatment for 15 days. |
| PAG + 1.0 Cu | 1.0 mM PAG (PAG) pretreatment for 15 days, followed by 1.0 mM CuSO4·5H2O (1.0 Cu) treatment for 15 days. |
| Treatment | Young Leaf | Mature Leaf | Stem | Root | ||||
|---|---|---|---|---|---|---|---|---|
| Fresh Weight (g) | Dry Weight (g) | Fresh Weight (g) | Dry Weight (g) | Fresh Weight (g) | Dry Weight (g) | Fresh Weight (g) | Dry Weight (g) | |
| H2S + 0 | 0.53 ± 0.10 a | 0.20 ± 0.05 a | 1.4 ± 0.13 a | 0.50 ± 0.07 a | 1.3 ± 0.10 a | 0.53 ± 0.03 a | 1.38 ± 0.15 a | 0.23 ± 0.01 a |
| CK | 0.45 ± 0.07 bc | 0.19 ± 0.01 a | 1.04 ± 0.12 b | 0.39 ± 0.05 b | 0.99 ± 0.03 cde | 0.4 ± 0.02 bcd | 0.88 ± 0.24 b | 0.09 ± 0.03 bc |
| PAG + 0 | 0.28 ± 0.01 de | 0.11 ± 0.01 bc | 0.98 ± 0.21 b | 0.33 ± 0.06 b | 0.93 ± 0.11 de | 0.39 ± 0.03 bcd | 0.65 ± 0.04 bc | 0.08 ± 0.01 cd |
| H2 S + 0.5 Cu | 0.36 ± 0.07 cd | 0.13 ± 0.01 b | 0.54 ± 0.03 c | 0.2 ± 0.02 c | 1.14 ± 0.11 bc | 0.47 ± 0.05 ab | 0.84 ± 0.03 b | 0.12 ± 0.00 b |
| 0 + 0.5 Cu | 0.25 ± 0.05 de | 0.09 ± 0.02 bc | 0.44 ± 0.13 cd | 0.15 ± 0.06 cd | 1.05 ± 0.06 cd | 0.42 ± 0.02 bc | 0.70 ± 0.04 bc | 0.06 ± 0.00 cd |
| PAG + 0.5 Cu | 0.24 ± 0.06 de | 0.09 ± 0.01 bc | 0.33 ± 0.06 de | 0.13 ± 0.03 cde | 0.92 ± 0.15 de | 0.38 ± 0.04 cd | 0.48 ± 0.11 c | 0.04 ± 0.01 d |
| H2 S + 1.0 Cu | 0.27 ± 0.08 de | 0.09 ± 0.02 bc | 0.21 ± 0.01 ef | 0.06 ± 0.00 def | 1.06 ± 0.15 cd | 0.44 ± 0.08 bc | 0.71 ± 0.21 bc | 0.08 ± 0.03 cd |
| 0 + 1.0 Cu | 0.20 ± 0.06 e | 0.07 ± 0.02 c | 0.19 ± 0.04 ef | 0.06 ± 0.01 ef | 1.00 ± 0.08 cde | 0.41 ± 0.02 bcd | 0.55 ± 0.10 c | 0.07 ± 0.02 cd |
| PAG + 1.0 Cu | 0.17 ± 0.02 e | 0.06 ± 0.01 c | 0.06 ± 0.02 f | 0.02 ± 0.01 f | 0.82 ± 0.02 e | 0.33 ± 0.01 d | 0.50 ± 0.10 c | 0.05 ± 0.02 cd |
| Treatment | GC (Gallocatechin) (%) | EGC (Epigallocatechin) (%) | C (Catechin) (%) | EC (Epicatechin) (%) | EGCG (Epigallocatechin Gallate) (%) | ECG (Epicatechin Gallate) (%) | GCG (Gallocatechin Gallate) (%) | Ester Catechin (%) | Total Catechins (%) |
|---|---|---|---|---|---|---|---|---|---|
| H2S + 0 | 0.8 ± 0.01 abc | 2.91 ± 0.06 a | 0.25 ± 0 ab | 1.11 ± 0.04 a | 4.3 ± 0.17 a | 0.39 ± 0 a | 0.73 ± 0.01 a | 5.42 ± 0.18 a | 10.48 ± 0.16 a |
| CK | 0.81 ± 0.03 ab | 2.92 ± 0.41 a | 0.24 ± 0 cd | 0.93 ± 0.09 bc | 3.68 ± 0.42 bc | 0.38 ± 0.01 a | 0.62 ± 0.06 b | 4.68 ± 0.48 bc | 9.57 ± 1.02 ab |
| PAG + 0 | 0.82 ± 0.01 a | 2.44 ± 0.22 bc | 0.25 ± 0 a | 0.94 ± 0.05 bc | 3.95 ± 0.13 ab | 0.37 ± 0.01 ab | 0.59 ± 0.04 bc | 4.91 ± 0.13 b | 9.37 ± 0.23 b |
| H2 S + 0.5 Cu | 0.8 ± 0.02 abc | 2.98 ± 0.23 a | 0.23 ± 0 e | 1.09 ± 0.08 a | 3.04 ± 0.35 de | 0.39 ± 0.01 a | 0.68 ± 0.05 a | 4.11 ± 0.4 d | 9.22 ± 0.72 bc |
| 0 + 0.5 Cu | 0.79 ± 0.02 bc | 2.65 ± 0.18 ab | 0.24 ± 0 d | 1.04 ± 0.02 ab | 3.28 ± 0.14 cde | 0.36 ± 0.03 b | 0.7 ± 0.02 a | 4.34 ± 0.2 cd | 9.05 ± 0.41 bc |
| PAG + 0.5 Cu | 0.76 ± 0.01 c | 2.45 ± 0.03 bc | 0.24 ± 0 cd | 0.99 ± 0.02 abc | 3.43 ± 0.07 cd | 0.38 ± 0 ab | 0.59 ± 0.01 bc | 4.4 ± 0.08 bcd | 8.84 ± 0.07 bc |
| H2 S + 1.0 Cu | 0.78 ± 0.03 bc | 2.72 ± 0.06 ab | 0.23 ± 0 e | 1.06 ± 0.17 ab | 2.92 ± 0.12 e | 0.37 ± 0 ab | 0.68 ± 0.03 a | 3.97 ± 0.09 d | 8.76 ± 0.28 bc |
| 0 + 1.0 Cu | 0.76 ± 0.01 c | 2.42 ± 0.18 bc | 0.24 ± 0 d | 0.87 ± 0.02 c | 3.3 ± 0.25 cde | 0.38 ± 0.01 ab | 0.55 ± 0.02 c | 4.22 ± 0.27 cd | 8.52 ± 0.46 bc |
| PAG + 1.0 Cu | 0.77 ± 0.03 bc | 2.2 ± 0.27 c | 0.24 ± 0 bc | 0.88 ± 0.06 c | 3.26 ± 0.35 cde | 0.37 ± 0.01 ab | 0.56 ± 0.05 bc | 4.2 ± 0.4 cd | 8.29 ± 0.76 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Huang, P.; Zhu, J.; Wan, S.; Xing, A.; Shang, Y.; Zhao, Z.; Liu, S.; Chen, X.; Li, X.; et al. Exogenous Hydrogen Sulfide Alleviates the Toxicity of Cu2+ Stress on the Growth Physiology and Quality Components in Camellia sinensis L. Agronomy 2025, 15, 820. https://doi.org/10.3390/agronomy15040820
Wu Z, Huang P, Zhu J, Wan S, Xing A, Shang Y, Zhao Z, Liu S, Chen X, Li X, et al. Exogenous Hydrogen Sulfide Alleviates the Toxicity of Cu2+ Stress on the Growth Physiology and Quality Components in Camellia sinensis L. Agronomy. 2025; 15(4):820. https://doi.org/10.3390/agronomy15040820
Chicago/Turabian StyleWu, Zichen, Peifang Huang, Jiangyuan Zhu, Shuai Wan, Anqi Xing, Yuanbing Shang, Zhen Zhao, Shujing Liu, Xuan Chen, Xinghui Li, and et al. 2025. "Exogenous Hydrogen Sulfide Alleviates the Toxicity of Cu2+ Stress on the Growth Physiology and Quality Components in Camellia sinensis L." Agronomy 15, no. 4: 820. https://doi.org/10.3390/agronomy15040820
APA StyleWu, Z., Huang, P., Zhu, J., Wan, S., Xing, A., Shang, Y., Zhao, Z., Liu, S., Chen, X., Li, X., & Wang, Y. (2025). Exogenous Hydrogen Sulfide Alleviates the Toxicity of Cu2+ Stress on the Growth Physiology and Quality Components in Camellia sinensis L. Agronomy, 15(4), 820. https://doi.org/10.3390/agronomy15040820






