Abstract
Members of Agave L. utilize CAM. To date, research on the physiological and morphological adaptations of Agave has analyzed only a few species. With the aim of understanding basic aspects of the physiological responses of polyploid (2n = 2x = 60 to 2n = 6x = 180) Agave accessions in association with CO2 fixation, we carried out genetic and physiological studies of A. tequilana Weber, A. fourcroydes Lem., and A. angustifolia Haw., which are adapted to two ecoregions in the Yucatán of Mexico: the Regional Roger Orellana-CICY Botanical Garden (RO-CICY) in the city of Merida and the Germplasm Bank of the Scientific-Technological Park of Yucatán (GB-PCTY) located in the Sierra Papacal. Differences in genetic variability in Agave spp. were assessed using AFLP markers. Monitoring of stomatal openings during the night showed differences in polyploid species of Agave. The highest expression levels of PEPC and PEPCK genes were observed at the largest suprastomatol cavity areas. All of the evaluated accessions showed a four-fold increase in internal CO2 during the transition from 23:00 h to 3:00 h, indicating a larger diurnal fixation of internal CO2. The results of PCA, including photosynthetic parameters during night–day, indicated differential physiological responses that suggest the occurrence of five groups within the two ecoregions. The physiological data we report here indicate thar polyploid accessions of Agave exhibited differences associated with CO2 exchange, suggesting that these polyploid taxa may be better adapted to climates with high CO2 concentrations and they could be used in atmospheric CO2 sequestration.
1. Introduction
In the face of the global warming scenario, which increases water scarcity and the spread of pests and diseases and decreases the yield of economically important crops, attention has turned to plants with CAM that have been shown to have adaptive advantages in habitats where water is scarce and high temperatures predominate [1,2,3]. There is now a growing interest in the agricultural potential and growth capacity of these plants in arid or marginal regions [4,5]. The abundance and distribution range of crassulacean acid metabolism (CAM) plants in marginal regions have increased, however the adaptive strategies of water use and the underlying adaptive mechanisms for survival in habitats with scarce water, high temperatures, and high CO2 concentrations are still unknown.
CAM plants optimize water use by fixing CO2 overnight, keeping your stomata open. This adaptation allows them to store CO2 in the form of organic acids, which they then use during the day, when their stomata are closed; a process known as forced CAM [6,7]. The expression of CAM is flexible. While in the C3 pathway, the carbon is directly fixed by the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), and the C4 and CAM plants separate the RuBisCO from the carbon acquisition, either physically in the C4 plants or temporarily in CAM. CO2 binds to the enzyme phosphoenolpyruvate carboxylase (PEPC) and is stored as malic acid in the vacuoles of plant cells, where it is used in sunlight. During severe droughts, gas exchange in CAM plants can even stop completely due to internal CO2 storage [8].
The genus Agave includes 210 species, of which 166 are located in Mexico and 75% of these are endemic to Mexico [9,10]. Agave spp. performs CAM photosynthesis under drought and CO2 enrichment. The first studies in the Agave genus related to gas exchange have been carried out since the 1980s; however, to-date research aimed at knowing the physiological and morphological adaptations related to CO2 uptake only includes a small group of species analyzed [1,11,12,13,14,15]. In A. Americana and A. angustifolia, they have shown that during drought periods, 98% and 85% of CO2 is fixed during the first eight night hours, similar to that reported in plants that perform strong CAM [16,17,18]. Such physiological behavior is stimulated by CO2 enrichment (800 ppm) during the night. At CO2 concentrations of 800 ppm, fixation in A. angustifolia increases (62%) relative to low values of fixed CO2 (200 and 400 ppm) [16]. In Agave salmiana Otto ex Salm-Dyck, the highest CO2 exchange rate occurred between 22:00 and 2:00 h and was higher in plants with abundant soil moisture (irrigated) than in plants with moisture below permanent wilting point [18]. Photosynthetic studies carried out on Agave tequilana during January 2001 to November 2002 indicated that CO2 assimilation occurred during the dry (January to May) and humid months of the year (July to October), and the highest values of daily net CO2 assimilation coincided with cool day/night temperatures and high irradiance [12].
Kohonen et al. [19] analyzed patterns of COS (soil fluxes of carbonyl sulfide) and CO2 fluxes in A. sisalana during November and December 2019 in Kenya. COS and CO2 fluxes followed a similar diurnal pattern, with uptake observed during nighttime, while water (H2O) fluxes showed an opposite cycle with highest evaporation observed during daytime. It was observed that the soil COS fluxes were positive under radiation (i.e., indicating COS emission) and negative (i.e., indicating COS uptake) in the dark, and soil COS emissions increased with increasing soil temperature.
Pimienta-Barrios et al. [20] demonstrated that Agave tequilana grown in different locations in Jalisco, Mexico, can better assimilate CO2 during the winter (578 and 921 mmol/m2/d) in Amatitlan and at the end of the summer (763 mmol/m2/d) and in late winter (572 mmol/m2/d) in Arandas. High temperatures in the summer reduced daily net CO2 uptake at both localities, but in late summer, the nighttime temperatures at Arandas were moderate, which lead to high daily net CO2 uptake. Apparently, this CAM plant species can sequester carbon even during prolonged dry periods, and CO2 assimilation is directly influenced by the temperatures and humidity characteristic of the climates where these plants are grown and adapted.
In contrast, Agave spp. has shown present ample tolerance to different stressful conditions, emphasizing the importance of carrying out studies for the selection of elite individuals with physiological and morphological differences that allow them to adapt even better to abiotic stresses. Agave can tolerate drought and high temperature through control of water loss through leaf surfaces and CO2 exchange [15,16,20]. Added to this, polyploidy has proved an important role in the evolution and speciation of the genus [21,22].
In Agave spp., the occurrence of polyploidy has caused DNA regions to be subjected to genetic events, including recombination, amplification, duplication, transposition, and gene loss, which could have resulted in the high variability and diversity of these genomic regions and favor the formation of new genes that may be fixed in the course of evolution according to the climatic condition and geographical distribution of the species [14,22]. This genus has been shown to inhabit geographical areas where there are high temperatures and water scarcity, which has contributed to the development of morphological (thorns, leaf succulence, and stomatal density) and physiological (CAM) characteristics related to withstanding drought and high temperatures. In addition, in the polyploid species of Agave L., due to the increase in the genome, it is possible that there are more isoforms of protein-coding genes involved in CAM that could be functionally specialized. As a result of these genetic, morphological, and physiological changes, it is possible that the polyploid species of Agave L. adapt in a better way to global warming and drought [10].
Few studies have been carried out to understand the physiological and adaptive differences that the polyploid species of Agave L. present, related to CO2 fixation. Tamayo-Ordoñez et al. [10] described that the sizes of stomata and the suprastomatal cavity increase according to higher levels of ploidy in the accessions analyzed, and the stomatal density is lower as the level of ploidy increases in Agave L. This suggests that polyploid species of Agave spp. could perform CO2 fixation more efficiently compared to their counterparts with lower ploidy levels. In addition, the differences found in the stomatal density could result in a different physiological response to drought among the analyzed accessions, allowing certain accessions of Agave to respond differentially during prolonged periods of drought. In Agave L., adaptations preventing physiological damages due to draught include nocturnal assimilation of CO2, thick cuticles, low stomata density, and succulent leaves. The latter two adaptations enable water stored in the leaf parenchyma to continuously move to the chlorenchyma during dry periods [11], thus conferring the plants the capacity for withstanding up to seven years of drought [23].
According to what has been described in the literature, it seems that Agave spp. can survive drought, maintain their water use efficiency during dry periods, and maintain their productivity [3,24], highlighting the importance of conducting studies in the Agave genus that allow us to understand why CAM plants increase nocturnal CO2 assimilation under higher CO2 concentrations. In the future, it can be proposed for the introduction of new traits to create crop varieties with higher CO2 sequestration and water and light efficiency that could improve productivity in crops of economic interest.
In this research, the goals were to detect physiological and genetic changes related to CO2 uptake in three polyploid species of Agave adapted to two ecoregions of the state of Yucatán, Mexico. The ploidy level of each accession analyzed was corroborated by flow cytometry, and genetic variability was demonstrated by AFLP. Physiological differences in the stomata (related to the stomatal opening and closure) and in the compounds present in the epicuticular waxes were detected, the physiological parameters (photosynthetic rate, transpiration rate, stomatal conductance, and internal CO2 content) were evaluated, and the relative expression of genes related to CO2 fixation (PEPC, PEPCK, and rbcL) indicated the adaptability of Agave against the ecoregions evaluated.
2. Materials and Methods
2.1. Plant Materials
For all the studies reported in this investigation, three representative individuals of each variety analyzed belonging to three species (Agave tequilana Weber, Agave fourcroydes Lem., and Agave angustifolia Haw.) were included in this research (Table S1). The selected Agave accessions included varieties and variants with different ploidy levels (Table S1). All these accessions were directly collected from the field and adapted to two ecoregions: the Regional Roger Orellana-CICY Botanical Garden (RO-CICY) (21°1′39.41 north latitude and −89°38′24.14 east longitude) in the city of Merida and the Germplasm Bank of the Scientific-Technological Park of Yucatán (GB-PCTY) located in the Sierra Papacal, 20 km northwest of Merida (21°07′20″ N, 89°43′41″ W).
The accessions from the GB-PCTY belong to the Agave spp. collection, and their ploidy level was characterized. Herbarium specimens from leaves of each accession were made and deposited in the CICY herbarium (Table S1).
2.2. Determination of Nuclear DNA Content in Agave
The nuclear DNA content of the plants of each ploidy level was estimated by flow cytometry (Table S2). Internal basal leaf tissue aliquots (100–120 mg) were chopped with a razor blade in a Petri dish containing 1.5 mL of 0.1 M citric acid and 0.5% Tween 20, according to Palomino et al. [25]. The sample was filtered through a 50 μm nylon mesh and incubated for 15 min at room temperature. The nuclei in the filtrate were pelleted by centrifugation (90× g for 3 min), suspended in 1 mL of a citric acid/Tween 20 solution, and incubated for 15 min at room temperature. Then, 2 mL of 0.4 M Na2HPO4 was added, and the suspension was supplemented with 125 μL of propidium iodide and RNase to a final concentration of 50 μg/mL−1. Internal standards were selected according to the ploidy level of the studied accessions. Vicia Faba cv. Inovec (2C = 16.19 pg DNA) [26,27] was used to estimate the level of ploidy in diploid Agave accessions, and A. tequilana ‘Azul’, A. fourcroydes ‘Kitam ki’, A. fourcroydes ‘Sac ki’, and A. fourcroydes ‘Yaax ki’—previously characterized by Robert et al. [21]—were used to compare the values of polyploidy accessions collected from the GB-PCTY.
Flow cytometry estimation of the nuclear DNA content was performed in an acoustic focusing flow cytometer (Attune NxT; ThermoFisher, Eugene, OR, USA). Peak means, areas, and coefficients of variation were calculated using Attune NxT 2.1 software. The 2C nuclear DNA content (pg) was then calculated according to [19] by the formula [(mean position of G1 peak of Agave/mean position of G1 peak of Vicia Faba cv. Inovec) × 16.19 (2C value of calibration standard = 16.19 pg DNA)] [26,27,28].
2.3. Variability of Agave L. By AFLP
The AFLP method was performed following Tamayo-Ordoñez et al. [29]. For the selection of the best 4 sets of primers, we initially evaluated 35 combinations of primers (Table S2). AFLP reactions with selective primers were performed with a touchdown PCR program for most primer sets. The amplification conditions were carried out according to Tamayo-Ordoñez et al. [29]. PCR products were electrophoresed in a CEQ 8800 sequencer (Perkin–Elmer Inc., Foster City, CA, USA). The electropherograms obtained were analyzed using software GeneMarker v.1.75 (Perkin-Elmer, Inc., Boston, MA, USA).
All calculations were performed using NTSYS-pc 2.1 (Exeter Software Co., New York, NY, USA) software [30]. Only strong, reproducible, and clearly distinguished bands were used in a binary matrix. In order to find out if the AFLP markers could discriminate between polyploid accessions, the unweighted pair group was analyzed in all the studied accessions with UPGMA method, and genetic distances among accessions were calculated according to [31]. The reliability and robustness of the dendrograms were tested by bootstrap analysis with 1000 replications to assess branch support by using Free Tree software [32]. Analyses of percentages of polymorphism, index of similarity, and marker bands were made with Free Tree software v.1. (https://web.natur.cuni.cz/~flegr/programs/freetree/, accessed on 15 October 2024) [32].
2.4. Determination of Physiological Parameters
Three Agave species (A. tequilana Weber, A. fourcroydes Lem., and A. angustifolia Haw.) were selected for this study, two of which included variants with different ploidy levels. The selected accessions were A. tequilana Weber ‘Azul’ (2n = 2x = 60), A. angustifolia Haw. ‘Marginata’ (2n = 2x = 60), A. angustifolia Haw. ‘Chelem ki’ (2n = 6x = 180), A. fourcroydes Lem. ‘Kitam ki’ (2n = 3x = 90), and A. fourcroydes Lem. ‘Sac ki’ (2n = 5x = 150). The analysis included 3 plants of each Agave accession, with a total of 25 plants, aged 5 to 6 years, and adapted two ecoregions at the RO-CICY at the geographical coordinates 20°58′04″ N, 89°37′18″ W and another was the GB-PCTY at the geographical coordinates 21°07′20″ N, 89°43′41″ W. Both localities were included in the same interval of annual medium temperature around 26 °C. Tall plants from 147 cm to 160 cm were considered, according to Garcia-Castillo et al. [33].
The parameter analysis was carried out directly from the plants that were kept in the field from RO-CICY and GB-PCTY.
RO-CICY is located north of the city at an altitude of approximately 8 m above sea level. It covers an area of 2.5 ha. The land is on a rocky basement of marine limestone from the tertiary, which has given rise to shallow and immature rendzina soils, frequently in transition with lithosols. It is the driest of the subhumid soils, with summer rainfall, canicular, and a low percentage of winter rainfall. This garden houses 21 collections with more than 700 species and a little more than 14,000 individuals of trees, shrubs, and herbs. The following phytogeographic collections are found: Petén, Low Deciduous Forest, Humid Forest, Coastal Dune Scrub, and Endemic Plants of the Yucatán Peninsula.
GB-PCTY has more than 5 hectares of natural forest. The collection includes 96% of native species, and it is estimated to contain 250 species of vascular plants. The vegetation is characteristic of the Low Deciduous Forest with Candelabriform Cacti with calcareous soil and semi-arid climate.
Plants analyzed were under drought (without irrigation) for three months, during the period of March–May 2023, in the Yucatán Peninsula, and subsequently, the parameter analysis was carried out at the end of the drought season [34]. Agave tequilana Weber ‘Azul’ (2n = 2x = 60) was used as a diploid control when comparing results with A. angustifolia Haw. ‘Marginata’ (2n = 2x = 60), and the differences found between these diploid species were related to the adaptive characteristics of the species and not to ploidy level.
Time courses of stomatal conductance (Gs; mmolm−2s−2), photosynthetic rates (Pn; µmol m−2s−1), transpiration rates (Tr; µmol m−2s−1), and internal CO2 content (Ci; µmol mol−1) were monitored using the LI 6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA), as described by Santamaría et al. [35]. Measurements were made during the selected times according to the results obtained from the previously described opening and closing of stomata monitoring test. The evaluated times were 3:00 h, 11:00 h, 16:00 h, and 23:00 h. The selection of these schedules allows us to observe the physiological and genetic changes during CAM night and day phases.
Individual statistical analysis of the behavior of each physiological parameter determined in accessions with different ploidy level was made by F-tests evaluated at <0.05, and the means were compared by Tukey tests (p > 0.05). Relationships between the seven physiological parameters and the ploidy levels of accessions were analyzed using SAS version 9.0 statistical package to perform a principal component analysis (PCA).
2.5. Analysis of Wax Content in Polyploidy Agave
The leaf base was immersed in 1 L of hexane for 40 s. The solvent was evaporated to dryness under reduced pressure to obtain the crude epicuticular wax extract. The chemical identification of epicuticular waxes was performed using Agilent Technologies 6890 (Agilent Technologies, Santa Clara, CA, USA), coupled to a mass selective detector (Agilent 5975B) (Agilent Technologies, Santa Clara, CA, USA). Compounds were separated in an HP-5MS column (5%-phenyl)-methylpolysiloxane, 30 m, 0.32 mm i.d., 0.25 µm film thickness, J&W Scientific. The initial oven temperature started at 70 °C for 5 min, then increased at 10 °C/min until 200 °C for 5 min, and then increased at 20 °C/min until 290 °C and held for 22 min. Injector temperature was set at 250 °C, while interphase was set at 300 °C. The carrier gas (helium) flow was 1.0 mL/min. Analyses of each compound were carried out using MS (identification based on the EI mass spectra in the NIST library) and ST (identification based by comparison on their IE mass spectra and retention time with the corresponding pure standard). The presence of each identified compound was represented as percentages in relation to the total of identified compounds (100%).
2.6. Determination of Stomatal Opening and Closure
In the first instance, in order to know if there are differences in the opening and closing of stomata during the nocturnal period (19:00 h at 7:00 h) of CAM in polyploid species compared to their counterparts with lower levels of ploidy, a determination of stomatal opening and closure was carried out, including two varieties with different ploidy levels representative of the species A. angustifolia Haw. and A. fourcroydes Lem.
Samples of leaves (1 cm3) from the adaxial and abaxial epidermis were collected at 19:00 h, 23:00 h, 3:00 h, and 7:00 h in two ecoregions (RO-CICY and GB-PCTY). The preparation of the samples was made according to Tamayo-Ordoñez et al. [10]. The samples were then mounted on metallic stubs with carbon conductive adhesive tape (Electron Microscopy Science, Jeol Ltd., Tokyo, Japan) and sputter coated with a 150 Å gold layer (Denton Vaccum Desk II, Denton Vacuum Inc., Denton, TX, USA). Length of the guard cells and suprastomatic cavity area, stomatal size, and density were calculated for the abaxial and adaxial epidermis at a magnification of 100X (0.1213 mm2). Counts and measurements were made in ten fields of each leaf for each accession. Sample analysis and image recording were made using a scanning electron microscope (Jeol, JSM-6360LV, Jeol Ltd., Tokyo, Japan). The gathered data were subjected to statistical analysis by Tukey tests evaluated at p > 0.05.
2.7. Determination of Relative Expression of the NADH, rbcL, PEPC, and PEPCK Genes
RNA isolation and cDNA synthesis were conducted according to Tamayo-Ordóñez et al. [14]. The PEPC, PEPCK rbcL, and NADH primers used for relative expression analysis were designed (Table S2) [36], and, as reference genes, the 18S rDNA genes were used according to Tamayo-Ordoñez et al. [22] (Table S3).
Amplifications of the NADH, PEPC, PEPCK, and rbcL genes were carried out as described above and with the same PCR amplification conditions used for the phylogenetic analysis. The melt curve analysis and negative controls for reference and target genes were always included in the experiments to eliminate DNA contamination. The relative expression of each gene was determined by the ∆∆Cq method between the target (NADH, PEPC, PEPCK, and rbcL) and reference (18S rDNA) genes [22], by the following equation: Relative expression = (Eref)Ctref/(Etarget)Ctarget. All analyses included three biological replicates, each with three technical replicates.
3. Results
3.1. Polyploidy in Agave and the Increase in Nuclear DNA
The analysis of DNA content (Table S4) showed linear relationships both between ploidy level and 2C nuclear DNA content (R2 = 0.998; Figure 1A) and between polyploid genome size (Mpb) and 1Cx genome size (pg) (R2 = 1; Figure 1B). In general, the diploid, triploid, pentaploid, and hexaploid accessions had an average 2C nuclear DNA content of 7.53 ± 0.09 pg, 12.00 ± 0.12 pg, 20.28 ± 0.13 pg, and 23.85 ± 0.20 pg, respectively. The accessions presented a size genome of 3865 Mpb, similar to that described for several species of Agave [24,37,38].
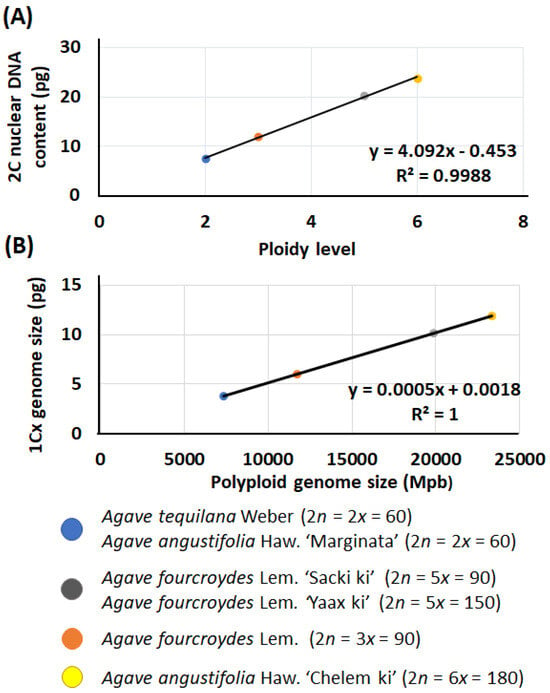
Figure 1.
Estimation of DNA content and genome size in Agave L. (A) Linear relationship between ploidy level and 2C nuclear DNA content. (B) Correlation of polyploidy genome size (Mpb) and 1Cx genome size (pg).
Many authors have described that the increase in genome size in polyploid species is accompanied by genetic changes, resulting in greater variability and genetic diversity in polyploid plants [10,38,39]. The dendrogram obtained from the AFLP marker showed two clusters: cluster I has two subgroups denominated as A and B (Figure 2). Subgroup A showed the clustering of hexaploid and diploid accessions of Agave angustifolia (AAM1-RO, AAM2-RO, AAM3-RO, AAC1-RO, AAC2-RO, AAC3-RO, CHA1-PCTY, CHA5-PCTY, and CHA6-PCTY), and subgroup B included the diploid accessions of A. tequilana (AT1-RO, AT2-RO, AT3-RO, 1444-2-PCTY, 1444-3-PCTY, and 0345b-PCTY; Figure 2A). Cluster II grouped the triploid and pentaploid accessions of A. fourcroydes. According to the global variability, the AFLP marker indicated a closer genetic proximity between A. angustifolia and A. tequilana.
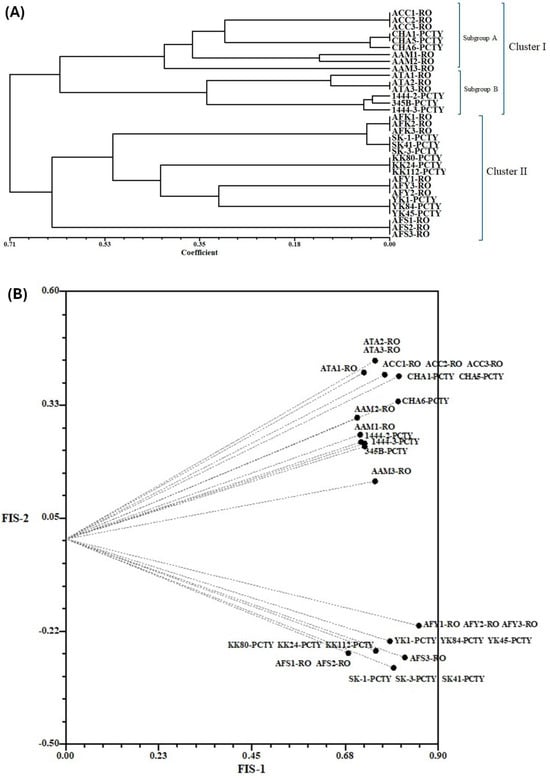
Figure 2.
AFLP analysis in Agave L. accessions. (A) Analysis of genetic variability of Agave L. for the evaluation of the genetic distance, the coefficient NEI72 was used. (B) Genetic relationship of the Agave L. species. For the correlation of the Agave accessions, the CORR coefficient of the statistical program NTSys-PC (version 2.1) was used. Agave angustifolia Haw. ‘Chelem ki’ (ACC), Agave spp. (CHA), Agave angustifolia Haw. ‘Marginata’ (AAM), Agave tequilana Weber. ‘Azul’ (ATA, 1444-2, 1444-3 and 345b), Agave fourcroydes Lem. ‘Kitam ki’ (AFK), Agave fourcroydes Lem. ‘Sacki ki’ (SK, AFS), Agave fourcroydes Lem. ‘Kitam ki’ (KK), and Agave fourcroydes Lem. ‘Yaax ki’ (AFY, YK).
The analysis of similarity indexes and percentages of polymorphism in accessions belonging to the same species (considering ploidy level as an important factor) indicated that Agave accessions containing polyploid varieties had low similarity indexes (0.76) compared to the results from the analysis including only diploid accessions like A. tequilana (2n = 2x = 60) and A. angustifolia (2n = 2x = 60) (0.83). The analysis of A. angustifolia (2n = 2x = 60) and A. angustifolia (2n = 6x = 180) showed percentages of polymorphism of 75 and a similarity index of 0.76, while accessions of A. fourcroydes (2n = 3x = 90) and A. fourcroydes (2n = 5x = 150) showed percentages of polymorphism of 79 and a similarity index of 0.73 (Table S5). Apparently, polyploidy does influence genome size and genetic variability in Agave.
The analysis of correlation of alleles indicated that the accessions of A. tequilana cultivated in the RO-CICY share a greater proportion of alleles, similar in the same proportion as A. angustifolia cultivated in both ecoregions (RO-CICY and GB-PCTY). Likewise, the varieties of A. fourcroydes apparently contain a narrow genetic germplasm, reflected in the conservation of genetic material (Figure 2B).
According to the bimodal karyotype of Agave, this genus is suggested to present allopolyploid origin [40,41]. The use in Agave of ISSR [42,43], AFLP [44], and SSAP [45] molecular markers have indicated variability and genetic diversity between wild and cultivated populations of A. angustifolia, A. tequilana, and A. fourcroydes, among other species. The Agave accessions we analyzed showed high genetic variability. The accessions with the highest level of ploidy—like A. angustifolia ‘Chelem ki’ (2n = 6x = 180), A. fourcroydes ‘Sack ki’ (2n = 5x = 150), and A. fourcroydes ‘Yaax ki’ (2n = 5x = 150)—showed the lowest similarity indexes (<78) in comparison to the other analyzed accessions, suggesting that the combination of genomes is an event that favors the variability and diversity of the species [46,47,48].
3.2. Differences in Stomata and Relative Expression of NADH, PEPC, PEPCK, and rbcL Genes
With the aim of assessing the physiological changes that could be observed in stomata and that could impact the dynamics of CO2 exchange and transpiration, we focused on determining the opening and closing of stomata by scanning electron microscopy in the two Agave species with different levels of ploidy (A. angustifolia and A. fourcroydes) at 19:00 h, 23:00 h, 3:00 h, and 7:00 h. Differences in the opening and closing of stomata on the abaxial and adaxial leaf surfaces were observed (Figure 3 and Table S6).
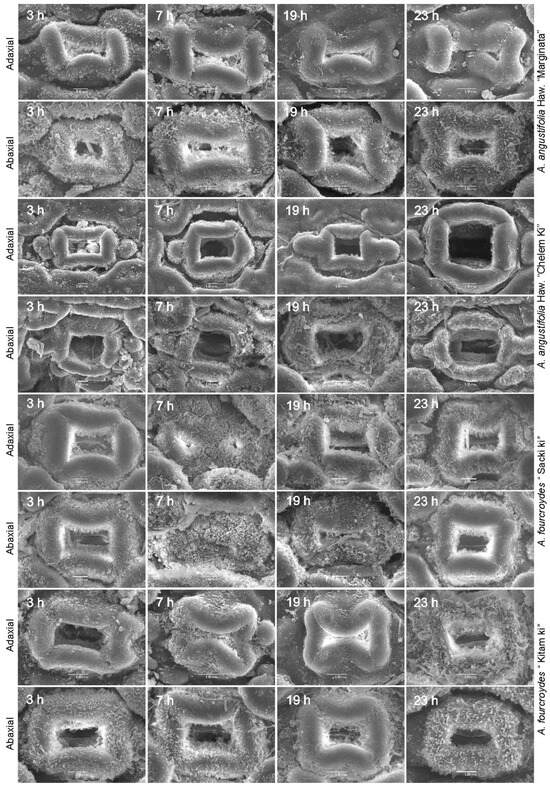
Figure 3.
Scanning electron microscopy of stomata during temporary course in the Agave L. The analysis included A. angustifolia Haw. ‘Marginata’, A. angustifolia Haw. ‘Chelem ki’, A. fourcroydes Lem. ‘Sac ki’, and A. angustifolia Haw. ‘Chelem ki’ for the adaxial and abaxial leaf, during 3:00 h, 7:00 h, 19:00 h, and 23:00 h (bars measure 10 µM).
Comparing different ploidy level accessions from the same species of Agave, those with higher ploidy level (A. angustifolia ‘Chelem ki’ and A. fourcroydes ‘Sac ki’) have a lower stomatal density (32 ± 2 and 52 ± 3), and their stomata are 1.5 to 2 times larger in comparison to their lower-ploidy-level counterparts (A. angustifolia ‘Marginata’ and A. fourcroydes ‘Kitam ki’) (Figure 4 and Table S6). Analyses of guard cell area (μM2) showed larger size in polyploid plants of the species A. angustifolia ‘Chelem ki’ and A. fourcroydes ‘Sac ki’, coinciding with stomata size (Figure S1 and Table S6). Stomatal indexes according to ploidy numbers have also been related to adaptation to stress [17,49,50].
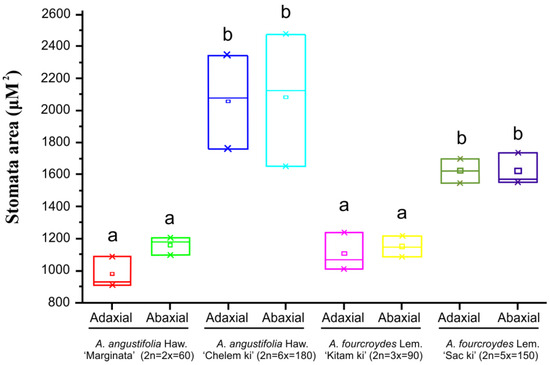
Figure 4.
Stomata area (µM2) of A. fourcroydes Lem. and A. angustifolia Haw. Lowercase letters (a and b) indicate significantly different values (Student’s t-test; p < 0.05). Values are averages of triplicates obtained from analysis of 30 stomata in three leaves of three plants of each accession ± standard error.
Analysis of suprastomatic cavity area (µM2) showed important changes between the polyploid species. A. angustifolia ‘Chelem ki’ showed a greater suprastomatic cavity area (1683 ± 19 µM2) compared to A. angustifolia ‘Marginata’ (317 ± 33 µM2) at 23:00 h (Figure 5 and Table S6). Lower values of suprastomatic cavity area in A. angustifolia ‘Marginata’ were observed at 19:00 h and 23:00 h.
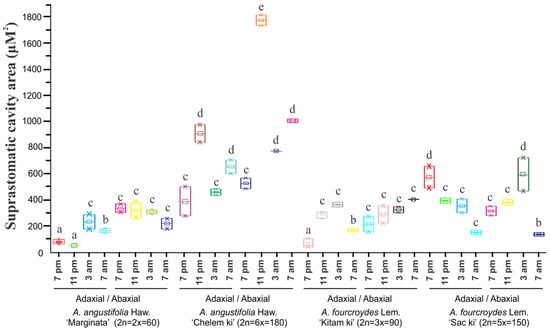
Figure 5.
Suprastomatic cavity area (µM2) of A. fourcroydes Lem. and A. angustifolia Haw. during day and night. Lowercase letters (a, b, c, d, or e) indicate significantly different values (Student’s t-test; p < 0.05). Values are averages of triplicates obtained from three leaves of five plants of each accession ± standard error.
Suprastomatic cavity area in the abaxial section of the leaf did not show significant differences along the temporal course; however, for A. angustifolia ‘Marginata’, the suprastomatic cavity area was higher on the abaxial section of the leaf in comparison to the stomata located on the adaxial part of the leaf (Figure 5).
In the hexaploid species A. angustifolia ‘Chelem ki’, high values of suprastomatic cavity area were observed at 23:00 h in both sections of the leaves (adaxial: 865 ± 37 µM2 and abaxial: 1683 ± 19 µM2; Table S6). The lowest values of suprastomatic cavity on the adaxial and abaxial surfaces were observed at 19:00 h. Similar to what we found in A. angustifolia ‘Marginata’, the suprastomatic opening on the abaxial surface was always greater compared to the same on the adaxial surface.
The behavior of stomata opening in A. fourcroydes showed patterns different to those observed in A. angustifolia. The pentaploid species A. fourcroydes ‘Sack ki’ showed the highest values of suprastomatic cavity area at 3:00 h (576 ± 67 μM2) and 19:00 h (555 ± 43 μM2), both on the abaxial and on the adaxial surfaces. The suprastomatic cavity area was not as large as that reported in the hexaploid species A. angustifolia ‘Chelem ki’ (abaxial: 1683 ± 19 μM2). In addition, in the adaxial leaf section, 75% of the stomata analyzed proved to be closed and the opening of the remaining 25% indicated low values of suprastomatic cavity area (adaxial: 170 ± 4 μM2 and abaxial: 153 ± 4 μM2) (Table S6). Triploid A. fourcroydes ‘Kitam ki’ showed some significant differences over time, indicating that in this species stomata opening is maintained for a longer time, which differs from that observed in A. angustifolia Haw. ‘Chelem ki’, in which immediately after the stomatal opening at 23:00 h, the suprastomatic cavity area decreased drastically (Figure 3 and Figure 5 and Table S6).
Relative expression of the PEPC gene showed significant differences, raising its expression at 3 h and 23 h (Figure 6). The highest values in PEPC expression were identified at 23 h in the accessions cultivated in the RO-CICY (Figure 6A,B), coinciding with the highest values of suprastomatic cavity area. Similar trends were found in the expression of the PEPCK gene, which also indicated higher values at 3 h and 23 h, in plants belonging to both ecoregions (Figure 6C,D). The highest values of rbcL relative expression were observed at 15 h for both plantations (RO-CICY and GB-PCTY; Figure S2). Regarding the NADH gene, there were no significant differences during the evaluated period (Figure S3).
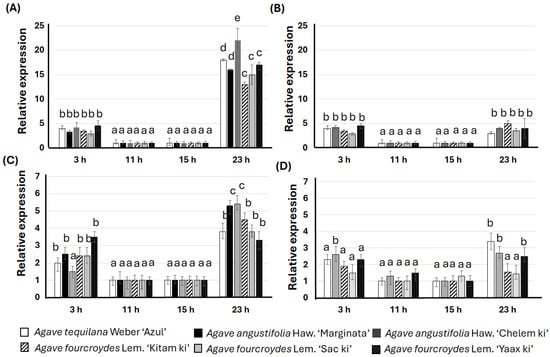
Figure 6.
Expression relative to the phosphoenolpyruvate carboxylase (PEPC) and phosphoenolpyruvate carboxykinase (PEPCK) genes evaluated by RT-qPCR in Agave L. (A) Expression relative to PEPC gene on plants adapted in the Regional Roger Orellana-CICY Botanical Garden (RO-CICY). (B) Expression relative to PEPC gene on plants adapted in the Germplasm Bank of the Scientific-Technological Park of Yucatán (GB-PCTY). (C) Expression relative to PEPCK gene on plants adapted in the RO-CICY. (D) Expression relative to PEPCK gene on plants adapted in the GB-PCTY. Expression analysis was carried out during a temporary course during daytime hours (11:00 h and 15:00 h) and at night (23:00 h and 3:00 h). All values are averages of three replicates ± SE, and lowercase letters (a, b, c, d, or e) indicate significantly different values (Student’s t-test; p < 0.05).
3.3. Differences in Physiological Parameters over Time (Day and Night) and Determination of Epiticular Waxes
In terms of the photosynthetic rate depending on the incidence of photons and CO2 assimilation, our results indicated similar trends in the accessions of A. tequilana Azul, A. fourcroydes (‘Kitam ki’, ‘Sac ki’, and ‘Yaax ki’), and A. angustifolia (‘Marginata’ and ‘Yaax ki’) from the RO-CICY. Most of the varieties evaluated (A. fourcroydes ‘Kitam ki’, A. fourcroydes ‘Sac ki’, A. angustifolia ‘Marginata’, and A. angustifolia ‘Chelem ki’) exhibited negative values of photosynthesis over time, with the exception from 23:00 h, after which a higher photosynthetic rate was detected for all species (Figure S4A). On the contrary, A. tequilana ‘Azul’ and A. fourcroydes ‘Yaax ki’ proved to carry out their photosynthesis throughout the day, with the exception of 15:00 h. Agave species from the GB-PCTY presented photosynthesis at 3:00 h, 11:00 h, 15:00 h, and 23:00 h. The highest photosynthetic rate was reached at 11:00 h (A. tequilana ‘Azul’, A. fourcroydes ‘Sac ki’, and A. fourcroydes Yaax ki’) and at 15:00 h (A. fourcroydes ‘Kitam ki’ and A. angustifolia ‘Chelem ki’; Figure S4A). Apparently, the behavior of CO2 assimilation during daytime in the plantations of Agave in the GB-PCTY suggests a contrasting physiological strategy with respect to Agave species in the RO-CICY, which could be related to the ability to adapt to those ecoregions.
Regarding the transpiration rate in the RO-CICY, it was observed that the polyploid accession of A. fourcroydes ‘Sac ki’ and A. fourcroydes ‘Yaax ki’ presented similar trends. The latter species presented a higher transpiration rate at 11:00 h. However, a substantial increase in this parameter was detected in A. angustifolia ‘Marginata’, A. angustifolia ‘Chelem ki’, A. angustifolia ‘Yaax ki’, and A. fourcroydes ‘Kitam ki’. These trends found in the Agave accessions from these ecoregions reflect the typical physiological characteristics described for CAM plants (Figure S4B). In the Agave taxa cultivated in the GB-PCTY, the transpiration rate was of 1.9 and 2.6 mmol−2s−1 in A. fourcroydes ‘Kitam ki’ and A. fourcroydes ‘Sac ki’, respectively. It should be mentioned that the transpiration value found in the triploid variety A. fourcroydes could be related to its high photosynthetic rate (1.2 µmolm−2s−1; Figure S4A). One of the adaptive strategies described in Agave species that can contribute to diminish the water vapor gradient is the gigas phenomenon [10,22]. As mentioned above, the accessions with higher ploidy level A. angustifolia ‘Chelem ki’ (2n = 6x = 180) and A. fourcroydes ‘Sac ki’ (2n = 5x = 150) have a lower stomatal density and their stomata are larger relative to their lower-ploidy-level counterparts—A. angustifolia ‘Marginata’ (2n = 2x = 60) and A. fourcroydes ‘Kitam ki’ (2n = 3x = 90). These differences in stomatal size and density could be related to the differences found in the transpiration rate.
In general, all the evaluated accessions showed a four-fold increase in internal CO2 during the transition from 23:00 h to 3:00 h, indicating a larger diurnal fixation of internal CO2 (Figure S4C). Agave species cultivated in the RO-CICY indicated variation in internal CO2 content in a range from 200 to 1000 µmol mol−1 during the diel period (3:00 h, 11:00 h, 15:00 h, and 23:00 h). A. tequilana ‘Azul’ and A. angustifolia ‘Chelem ki’ (6x) presented the highest levels of internal CO2 at 3:00 h (12 and 115 µmol mol−1) and 15:00 h (44 and 758 µmol mol−1). The behavior of the assimilation of internal CO2 in Agave species cultivated in the GB-PCTY showed values higher than 300 µmol mol−1 in most of the evaluated times (3:00 h, 15:00 h, and 23:00 h). The highest concentration of intercellular CO2 in Agave species was a function of time, being that A. fourcroydes ‘Sac ki’ showed its highest increase at 3:00 h (483.75 µmol mol−1). Accessions of A. tequilana ‘Azul’ (485.59 µmol mol−1), A. angustifolia ‘Chelem ki’ (711.68 µmol mol−1), and A. fourcroydes ‘Yaax ki’ (719.35 µmol mol−1) showed its highest increase at 15:00 h, and A. fourcroydes ‘Kitam ki’ reached the highest values at 23:00 h (569.66 µmol mol−1; Figure S4). Similarly, in general, the species with lower ploidy levels present accumulation and assimilation of intercellular CO2 during daytime hours; however, in A. angustifolia ‘Chelem ki’ and A. fourcroydes ‘Yaax ki’, the concentration of internal CO2 was different during certain hours (3:00 h and 15:00 h), which was probably associated with the larger stomatal size and lower stomatal density of these varieties.
The A. tequilana, A. angustifolia, and A. fourcroydes accessions cultivated in the RO-CICY with lower ploidy levels presented lower stomatal conductance in function to the intercellular space, reaching a significant increase at 23:00 h (0.061, 0.311, and 0.304 mmolm−2s−1) (Figure S4D) A. fourcroydes ‘Yaax ki’ acquired the highest stomatal conductance at 3:00 h (0.271 mmolm−2s−1) followed by 23:00 h (0.205 mmolm−2s−1) (Figure S4D). A. tequilana ‘Azul’, A. angustifolia, and A. fourcroydes cultivated in the GB-PCTY demonstrated a constant stomatal conductance, reaching the highest value of stomatal movement at 15:00 h in A. fourcroydes ‘Kitam ki’ (0.113 mmolm−2s−1) and A. fourcroydes ‘Sac ki’ (0.087 mmolm−2s−1; Figure S4D). Interestingly, A. fourcroydes ‘Kitam ki’ presented the highest photosynthetic rate (1.233 mmolm−2s−1), transpiration rate (1.902 mmolm−2s−1), and stomatal conductance (0.113 mmolm−2s−1) at 15:00 h, differing from the other accessions analyzed (Figure S4)
In order to know the relationship between photosynthetic rate and stomatal conductance, both parameters were analyzed in parallel. The results indicated a similar pattern in all Agave species from the RO-CICY. It was demonstrated that the photosynthetic rate was according to stomatal conductance, with the exception of A. tequilana (Figure 7A). In contrast, in Agave accessions cultivated in the GB-PCTY, no direct relationship was found between the rate of photosynthesis and stomatal conductance (Figure 7B).
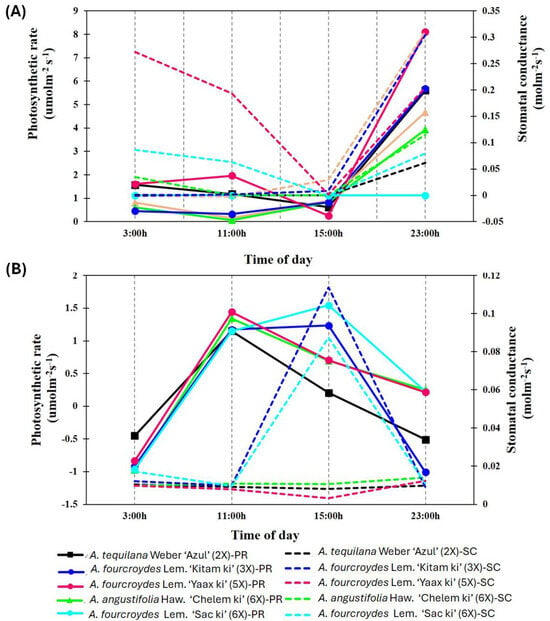
Figure 7.
Photosynthesis rate and stomatal conductance of Agave species. (A) Agave accessions acclimated to the Regional Roger Orellana-CICY Botanical Garden (RO-CICY). (B) Agave accessions acclimated to the Germplasm Bank of the Scientific-Technological Park of Yucatán (GB-PCTY). Physiological response was evaluated in a temporary course during daytime hours (11:00 h and 15:00 h) and at night (23:00 h and 3:00 h). The values of photosynthetic rate (PR) are illustrated with the continuous line, and the trends of the stomatal conductance (SC) are indicated by the semicontinuous line.
The PCA indicated that the first two components (PC1 and PC2) explained 95% of the observed physiological variability (Table S7). Also, the formation of five groups was identified (Figure 8). The first group formed by accessions of A. fourcroydes ‘Yaax ki’ was differentiated from the other Agave accessions cultivated in the Regional Roger Orellana-CICY Botanical Garden (RO-CICY) due to the patterns of its photosynthetic rate at 23:00 h and stomatal conductivity and transpiration rate at 11:00 h and 3:00 h, respectively (Figure S4). In the second group, the accessions of A. tequilana ‘Azul’ cultivated in the RO-CICY present characteristic patterns related to the photosynthetic rate at 3:00 h and internal CO2 content in the morning and at night (11:00 h and 3:00 h) (Figure S4). The third group was formed by A. fourcroydes and A. angustifolia cultivated in the Regional Roger Orellana-CICY Botanical Garden (RO-CICY), demonstrating differences in the physiological parameters associated with the species (Figure 8). For example, A. fourcroydes presented differential patterns in transpiration rate at 23:00 h and photosynthetic rate at 3:00 h and 11:00 h (Figure S4). A. angustifolia showed a physiological response similar to A. tequilana (photosynthetic rate at 3:00 h, intercellular CO2 exchange at 3:00 and 11:00 h (Figure S4). The last two groups included A. tequilana ‘Azul’, A. fourcroydes, and A. angustifolia cultivated in the GB-PCTY, showing the differences in internal CO2 concentration at 23:00 h and stomatal conductance and transpiration rate at 15 h (Figure 8 and Figure S4).
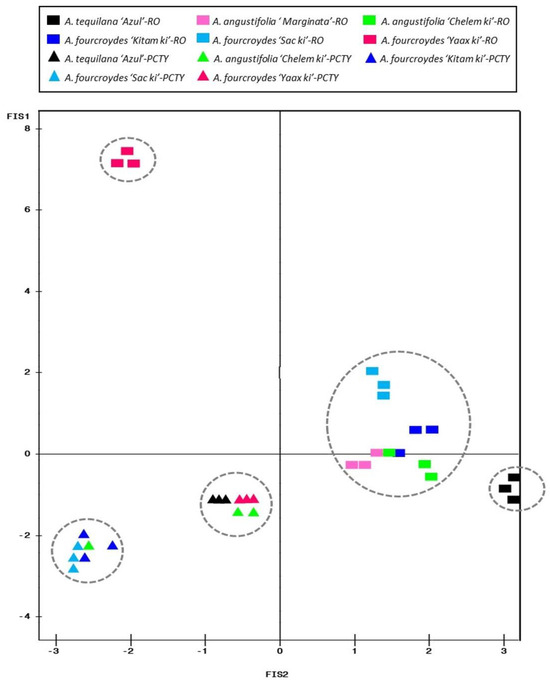
Figure 8.
Principal component analysis (PCA) of physiological parameters in Agave L. In the multivariate analysis, the parameters photosynthesis rate, transpiration rate, stomatal conductance, and internal CO2 content were included. Measurements were made at 15:00 h, 23:00 h, 3:00 h, and 11:00 h located in different ecoregions: Regional Roger Orellana-CICY Botanical Garden (RO-CICY) (symbol box) and Germplasm Bank of the Scientific-Technological Park of Yucatán (GB-PCTY) (triangle symbol). Agave angustifolia Haw. ‘Chelem ki’ (2n = 6x = 180), Agave angustifolia Haw. ‘Marginata’ (2n = 2x = 60), Agave tequilana Weber. ‘Azul’ (2n = 2x = 60), Agave fourcroydes Lem. ‘Kitam ki’ (2n = 3x = 90), Agave fourcroydes Lem. ‘Sacki ki’ (2n = 5x = 150), and Agave fourcroydes Lem. ‘Yaax ki’ (2n = 5x = 150).
In general, the main physiological parameters that allowed discrimination between groups during the transition from early morning daylight hours were photosynthetic rate (3:00 h), stomatal conductance (3:00 h, 11:00 h, and 15:00 h), internal CO2 content (3:00 h and 11:00 h), and transpiration rate (3:00 h, 11:00 h, 15:00 h, and 23:00 h) (Figure 8).
When determining the content of the compounds present in the epicuticular waxes of Agave, all the accessions analyzed showed a variable profile of alkanes, all presented C25 to C34 alkanes. Alkanes mostly represented hentriacontane (C31) and tritriacontane (C33) (Table S8). The highest percentages of both compounds were identified in the polyploid species (A. angustifolia ‘Chelem ki’ and A. fourcroydes ‘Sac ki’). In contrast, diploid species presented C18 to C35 alkanes. In polyploid species, A. angustifolia ‘Chelem ki’ and A. fourcroydes ‘Sac ki’ were characterized by presenting a more closed alkane profile from C26 to C35, when in the diploid species, there is a greater variety of alkanes present.
4. Discussion
Improving the productivity of different agricultural crops, as well as commercial plants, through assisted selection and plant breeding is important in the face of global climate change. Understanding physiology, morphology, and genetic changes in CAM plants will help us better understand the specific changes that enable these plants to inhabit arid and semi-arid lands and tolerate different types of environmental stress. Engineering C3 or C4 plants that viably express the CAM machinery may soon be feasible. CAM engineering is expected to contribute significantly to climate change mitigation by improving plant-derived CO2 capture and storage [7,51,52].
Agaves are mostly constitutive CAM species, with almost entirely CAM-typical gas exchange [53]. However, it remains unknown whether environmental cues alter uptake patterns and photosynthetic modes in CAM species. In this research, physiological changes in stomata of Agave L. accessions were observed. Differences found in stomatal size and density of Agave depending on their ploidy level could be an indicator of differences in water uptake and tolerance to drought, which could be a result of their adaptation to habitats with water deficit and CO2 concentration changes [14,54]. There is evidence that totally compacted cells, or stomata without air spaces, and the development of cuticular waxes diminish the hydric state when the ambient temperature increases. Similarly, research conducted in the Proteaceae family has indicated that the ancient changes in genome size clearly influenced stomatal size, but adaptation to habitat strongly modified the genome–stomatal size relationship [50]. This suggests that an increase in CO2 concentrations could impact stomatal size and frequency; so, it is possible that the polyploid species (A. angustifolia ‘Chelem ki’ and A. fourcroydes ‘Sac ki’) showing larger stomata, lower stomatal density, and higher wax content may be better able to tolerate stress in climates where water is a limiting factor and that this increased tolerance resulted from genetic, physiological, and morphological changes during the polyploidization process and the increased CO2 concentration that are currently experienced [10,16].
In addition, although plants such as Agave can control the loss of water by transpiration—for example, A. americana and A. deserti [55,56]—through their ability to open stomata at night, avoiding desiccation and regulating its temperature through perspiration, there is evidence suggesting that Agave plants with frequent irrigation can alter their metabolism causing the opening of stomata during light hours [57]. In Agave—with the exception of A. fourcroydes ‘Kitam ki’—the opening patterns between 19:00 h and 7:00 h when sunlight is already present can be influenced by the frequent irrigation of plantations, and conditions of culture can result in metabolic and physiological differences in Agave plants.
The increase in the expression of the PEPCK and PEPC genes during the evaluated nocturnal hours (23 h and 3 h) indicates the possible participation of these enzymes in the conversion of oxaloacetate into phosphoenolpyruvate and carbon dioxide. In Agave hybrid N. 11648, RNA sequencing from Agave leaves indicated that the expression of PEPCs and PPCKs peaked at 24 h and 2 h [58], results similar to those found in this investigation. PEPCKs showed stronger transcript abundance at night, and only one PEPCK was expressed at a higher level during the day, increasing in the morning and peaking at 16 h. Differential expression was also demonstrated in three PEPCK genes (PEPCK3, PEPCK5, and PEPCK12), showing a five-fold increase in their expression during the night compared to the day [58].
In the literature, it has been discussed that there is a coordinate regulation of phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase by light and CO2 during C4 photosynthesis in guinea grass [59,60,61]. It is possible that in Agave spp., there is a coregulation of both carboxylases, and future work on transcriptional factors could elucidate this coregulation of both carboxylases. The highest values in rbcL expression coincided at 11:00 h and 15:00 h, times in which PEPC expression was lower. The evolution and diversity of PEPC and presence of isoforms of this enzyme have contributed to the evolution of CAM in plants [62,63,64].
Among the advances in crop improvement by genetic engineering, it was recently reported that overexpression of a CAM-specific A. americana PEPC gene (AaPEPC1) in tobacco overexpressed multiple orthologs of CAM pathway genes, such as genes encoding carbonic anhydrase (CA), malate dehydrogenase (MDH), aluminum-activated malate transporter (ALMT), tonoplast dicarboxylate transporter (tDT), and malic enzyme (ME) [51,52], indicating that these genes present in Agave spp. are potential targets for use in plant improvement of commercial interest.
Conversely, the behavior observed in the Agave species from the RO-CICY suggests that the differences in stomatal size and stomatal density in the abaxial and adaxial leaf surfaces contribute to stomatal movement associated with changes in water potential and temperature of the leaf when the transpiration rate changes. In the accessions from the RO-CICY, similar patterns of photosynthetic rates, transpiration rates, and stomatal conductances are displayed with a main increase at night (23:00 h).
In general, the physiological parameters we analyzed in this study allowed to discriminate different groups, among which, the Agave accessions from RO-CICY showed contrasting tendencies that were expressed in three groups (Figure 8). A. fourcroydes ‘Kitam ki’, A. fourcroydes ‘Sac ki’, A. fourcroydes ‘Yaax ki’, and A. angustifolia ‘Chelem ki’ formed a group according to the physiological parameters, and in these species, it was also possible to observe some differential adaptations related to the stomata and the profile of alkanes present in waxes, as compared to diploid species (Table S7). The differences observed in stomata could influence the way they respond to CO2 assimilation and how they perform their photosynthetic metabolism according to the variables of the ecoregions evaluated. The diploid species of Agave, adapted to the RO-CICY, showed unique differences that did not allow them to group with other species of Agave L. Apparently, the ecoregions in which different varieties of Agave have acclimated influence their physiological response, while polyploidy may be playing a crucial role in the genetic diversity and functional specialization of genes, leading to physiological adaptations of Agave species. Moreover, the differences in gene expression we found correlated with the highest values of suprastomatic cavity area, photosynthesis rate, and internal CO2 content, suggesting that the physiological adaptations of each species of Agave are accompanied by transcriptional regulation of the PEPC, PEPCK, and rbcL genes involved in CAM [8,15].
The leaf succulence in Agave is a factor that allows sustaining the opening of stomata and keeping the photosynthetic tissue active during the dry period [12]. Although Agave spp. were not irrigated for three months, the succulence of these plants played an important role in allowing the Agave accessions to photosynthesize and assimilate CO2, without problem. In Agave, it has been described that CO2 exchange is affected by drought and CO2 enrichment [16,17]. A. americana, A. tequilana, A. asperrima Jacobi, A. cupreata Trel. et. A. Berger, A. durangensis Gentry, and A. salmiana Otto ex Salm-Dyck have been shown to affect their growth rate, biomass distribution, leaf thickness, and proline content at different water potentials (Ψ). In species not adapted to dry regions, biomass production was inhibited (−3.5 MPa), but in species adapted to dry regions, such as A. tequilana, A. durangensis, A. lechuguilla Torr., and A. salmiana, the number of leaves and plant coverage were maintained [65].
It is important to note that solar radiation in both ecoregions was different: in the RO-CICY derived from the abundance of cultivated species such as palms and trees, the Agave accessions were adapted to shade. In the GB-PCTY, Agave accessions are adapted to the open field. These differences in solar radiation could lead to changes in soil moisture and, therefore, explain the differences found when evaluating the physiological parameters in Agave adapted to the two ecoregions. Winter et al. [16] reported that A. angustifolia did not switch to a C3 pattern at night when water supply was limited, unlike A. deserti [66]. Two weeks under drought stress manifested as a continuous decrease in CO2 fixation by light. Light harvesting was accompanied by an increase in the rate of dark CO2 fixation. In this research, the species with lower ploidy levels present accumulation and assimilation of intercellular CO2 during daytime hours; on the contrary, in A. angustifolia ‘Chelem ki’ and A. fourcroydes ‘Yaax ki’, the concentration of internal CO2 was different during certain hours (3:00 h and 15:00 h). Pimienta-Barrios et al. [12] reported that the assimilation of CO2 by young plants of A. tequilana during the day and night occurs through the combination of the photosynthetic pathways C3 (day) and CAM (night) and are an example of photosynthetic plasticity that is reflected in the optimization of water use, the carbon balance in the plant, and the use of light in favorable temperature and humidity conditions during the summer. The use of the C3 photosynthetic pathway allows some CAM plants to maximize carbon gain when environmental conditions are favorable, particularly when water availability increases [7,11].
The contribution of nocturnal CO2 fixation to carbon gain in CAM tissues is variably responsive to environmental stimuli, which include night temperature, day length, light intensity, atmospheric CO2 concentration [62], and humidity [1]. For example, increases in both light and dark CO2 fixation following exposure to high concentrations of atmospheric CO2 have been reported for A. deserti [67] and Agave salmiana [68]. Agave tequilana F.A.C. Weber was shown to fix CO2 during the day and night in humid periods [20], Agave deserti Engelm. switches to a mainly diurnal uptake under continuous irrigation [66], and Agave angustifolia Haw. shows limited photosynthetic plasticity, with about 25% of total uptake occurring during the day under well-watered conditions [16]. In Agave fourcryodes Lem., diurnal uptake during drought was reduced compared to nocturnal [68,69]. In Agave sisalana Perrine, a study monitoring CO2 showed that high productivity periods of A. sisalana occurred during the initial wet period with a mean CO2 uptake of −1.1 µmol m−2s−1; high productivity was related to significant daytime and nighttime carbon uptake. Furthermore, it was shown that with decreasing soil moisture, the mean diurnal net CO2 exchange became an important source of carbon from +1.0 to +4.0 µmol m−2s−1 [3]. This research indicates that differential patterns in internal CO2 content in Agave accessions could reflect the flexibility of CAM, as reported in Ananas comosus [70], Mesembryanthemum crystallinum [71], and Opuntia elatior [72].
In their research, Heyduk et al. [73] stated that despite the difficulties to resolve the phylogeny of the subfamily Agavoideae, ancestral state reconstruction showed three independent origins of CAM in the group. These origins are associated with a shift in climate space toward warmer, drier habitats. The large genera of Agave and Yucca have a center of diversity in the southwestern deserts of North America; however, a number of species have distributions outside of the iconic desert range. These derived species may suggest that ancestrally the Agavoideae were composed of non-desert inhabitants and that established lineages migrated to more arid regions after early radiation within the group. A movement into arid regions would require that those desert regions to be in place already and that the species that moved there had an ability to grow in arguably some of the toughest conditions on the planet. In addition, morphological data (3D venation and large cells) indicated that the last common ancestor of Yucca and Agave was C3 with CAM-like leaf anatomy. Sage [74] suggested that leaf and cell succulence may arise first in a C3 ancestor, followed by evolution of PEPC function to recapture respired CO2, eventually leading to circadian control and full-fledged CAM function [75,76]. According to Pimienta-Barrios et al. [12] and what was observed in this research, it is possible that in some Agave spp., the assimilation of CO2 occurs through the combination of the photosynthetic pathways C3 (day) and CAM (night), demonstrating the flexibility of Agave spp.
Although each Agave spp. analyzed showed specific differences, the polyploid species are the ones that demonstrated greater differences in stomata, gene regulation, determination of waxes, and physiological parameters. It seems that polyploidy is also playing an important role in the adaptation of Agave to climates where water scarcity is a limitation. It is then possible that polyploidy is an event that has favored the formation of new gene variants, which could be functionally specialized to acquire better Agave responses to biotic stress, such as drought and high CO2 concentrations (Figure 9). Future studies of stomata, regulation of genes associated with CAM, and physiological data in polyploid Agave species under water stress and thermal shock and under controlled environmental conditions will allow us to better understand how Agave spp. adapt to abiotic stress.
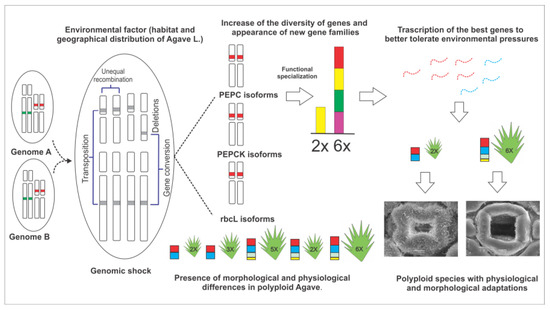
Figure 9.
Genetic and physiological changes in Agave L. for its adaptation to stressful habitats. Agave has a bimodal karyotype and the occurrence of polyploidy (hybridization and genome duplication) in the genus could cause genes related to CAM (PEPC, rbcL, and PEPCK) to have undergone different genetic changes (deletions, inversions, translocations, and insertions), varying the number of copies and contributing to originating isoforms of these genes, which will be fixed according to the pressure of the different abiotic stresses of the species’ habitats, and it is possible to specialize functionally, enhancing carbon fixation. Additionally, during the evolution of the genus, abiotic stresses and speciation within the genus could cause certain Agave species to change geographical location and differ in their habitats, which could be accompanied by morphological and physiological adaptations that together with the genetic background of each species allow them to better adapt to habitats where water is scarce.
5. Conclusions
Currently, engineering of the CAM pathway in non-CAM crops has been proposed as an approach to improve WUE while maintaining high productivity. Understanding the key mechanistic elements of CAM photosynthesis is essential to guide bioengineering and breeding improvements targeting productivity in crops of economic importance. This research showed that polyploid Agave species showed specific differences such as variation in the timing of stomatal opening and closing, differences in the regulation of PEPC, PEPCK, and rbcL genes, and higher wax content and flexibility in physiological parameters. These characteristics position these species as ideal models to study and understand CAM mechanisms and in the future to use bioengineering to improve economically important crops.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15040817/s1: Figure S1: Guard cells area (µM2) of A. fourcroydes Lem. and A. angustifolia Haw. during temporary course; Figure S2: Relative expression of the rbcL gene evaluated by RT-qPCR in Agave L.; Figure S3: Expression relative of the NADH gene evaluated by RT-qPCR in Agave L.; Figure S4: Determination of physiological parameters in Agave accessions; Table S1: Information about the 33 accessions of Agave spp. collected in Mexico used in this research; Table S2: Selection of the best combination of primers for AFLP studies from Agave L; Table S3: Primers used in research; Table S4: Nuclear DNA amount and genome size in Agave spp.; Table S5: Polymorphism and index of genetic variation in Agave L.; Table S6: Features of stomata in Agave L.; Table S7: Eigenvalues of the most descriptive physiological characteristics based on principal component analysis in Agave L.; Table S8: Compounds identified in the epicuticular waxes of Agave L.
Author Contributions
Conceptualization, B.A.A.-G., L.F.S.-T., E.S.-L., C.L.-O., Y.d.J.T.-O. and M.C.T.-O.; methodology, L.C.R.-Z., F.B.-P. and V.H.R.-G.; validation, L.C.R.-Z., F.B.-P. and V.H.R.-G.; data curation, F.A.T.-O., S.D.-D., J.A.R.-d.l.G. and A.V.C.-Q.; formal analysis, L.C.R.-Z., F.B.-P. and V.H.R.-G.; software, E.S.-L., C.L.-O., E.A.-C., J.A.R.-d.l.G. and G.d.J.S.-S.; supervision, E.A.-C., J.A.R.-d.l.G. and G.d.J.S.-S.; visualization E.A.-C., J.A.R.-d.l.G. and G.d.J.S.-S.; investigation, A.V.C.-Q., F.A.T.-O. and S.D.-D.; resources, E.S.-L., C.L.-O., A.V.C.-Q., F.A.T.-O. and S.D.-D. writing—original draft preparation, Y.d.J.T.-O., B.A.A.-G., M.C.T.-O. and L.F.S.-T.; writing—review and editing, Y.d.J.T.-O., B.A.A.-G., M.C.T.-O. and L.F.S.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “Consejo Nacional de Humanidades, Ciencias y Tecnologías” (CONACYT) with the Science Projects, CB-50268 and CB-155356.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to express their gratitude to the staff in charge of the Germplasm Bank of the Scientific-Technological Park of Yucatán (GB-PCTY) and Regional Roger Orellana-CICY Botanical Garden (RG-CICY) for the facilities granted for the collection of plant material. Also, we thank Dolezel Jaroslav for the donation of standards for flow cytometry. Thanks are also given to Miriam Monforte González for her contribution and orientation to the sequencing services, Maria J. Garcia Castillo by RNA extractions, Fabiola Escalante Erosa by the determination of waxes, and Miguel Keb-Llanes and María Teresa Patricia Pulido Salas for their support in determining physiological parameters.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flores, J.; Briones, O.; Andrade, J.L. Physiological ecology of Mexican CAM plants: History, progress, and opportunities. Bot. Sci. 2022, 100, 290–324. [Google Scholar] [CrossRef]
- Burgos, A.; Miranda, E.; Vilaprinyo, E.; Meza-Canales, I.D.; Alves, R. CAM models: Lessons and implications for CAM evolution. Front. Plant Sci. 2022, 13, 893095. [Google Scholar] [CrossRef]
- Skogberg, M.; Kohonen, K.M.; Lohila, A.; Merbold, L.; Räsänen, M.; Vuorinne, I.; Pellikka, P.; Vesala, T.; Kübert, A. Ecosystem-scale crassulacean acid metabolism (CAM) gas exchange of a sisal (Agave sisalana) plantation. Agric. Ecosyst. Environ. 2025, 381, 109435. [Google Scholar] [CrossRef]
- Carvajal, M.A.; Quiroz, M.; Alaniz, A.J.; Vergara, P.M.; Valenzuela-Aguayo, F.; Hidalgo-Corrotea, C. The global land-water-climate nexus of drought-tolerant succulent plants for bioenergy in abandoned croplands and arid marginal lands. J. Environ. Manag. 2025, 379, 124747. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Z.; Tu, C.; Lv, Z.; Liu, S.; Fan, Y. Response of an obligate CAM plant to competition and increased watering intervals. Physiol. Plant. 2025, 177, e70093. [Google Scholar] [CrossRef]
- Cenciareli, L.C.; Justi, M.S.; Ferreira-Silva, S.L.; de Almeida, L.F.R.; Sershen; Lima Neto, M.C. Physiological and biochemical changes associated with the induction of facultative CAM in Pereskia aculeata under drought stress and recovery. Plant Physiol. Biochem. 2025, 222, 109681. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Mohanta, Y.K.; Kaushik, P.; Kumar, J. Physiology, genomics, and evolutionary aspects of desert plants. J. Adv. Res. 2024, 58, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Cushman, J.C. Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiol. 2001, 127, 1439–1448. [Google Scholar] [CrossRef]
- García-Mendoza, A.J.; Franco Martínez, I.S.; Sandoval Gutiérrez, D. Cuatro especies nuevas de Agave (Asparagaceae, Agavoideae) del sur de México. Acta Bot. Mex. 2019, 126. [Google Scholar] [CrossRef]
- Tamayo-Ordóñez, M.C.; Rodriguez-Zapata, L.C.; Narváez-Zapata, J.A.; Tamayo-Ordóñez, Y.; Ayil-Gutiérrez, B.; Barredo-Pool, F.; Sánchez-Teyer, L. Morphological features of different polyploids for adaptation and molecular characterization of CC-NBS-LRR and LEA gene families in Agave L. J. Plant Physiol. 2016, 195, 80–94. [Google Scholar] [CrossRef]
- Pimienta-Barrios, E.; Zañudo-Hernández, J.; Nobel, P.S.; García-Galindo, J. Respuesta fisiológica a factores ambientales del agave azul (Agave tequilana Weber). Sci. Cuba. 2005, 7, 5–97. [Google Scholar]
- Pimienta-Barrios, E.; Zañudo-Hernández, J.; García-Galindo, J. Fotosíntesis estacional en plantas jóvenes de Agave tequilana. Agrociencia. 2006, 40, 69–709. [Google Scholar]
- Campos, H.; Trejo, C.; Peña-Valdivia, C.B.; García-Nava, R.; Conde-Martínez, F.V.; Cruz-Ortega, M.d.R. Photosynthetic acclimation to drought stress in Agave salmiana Otto ex Salm-Dyck seedlings is largely dependent on thermal dissipation and enhanced electron flux to photosystem I. Photosynth. Res. 2014, 122, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Ordóñez, Y.J.; Narváez-Zapata, J.A.; Tamayo-Ordóñez, M.C.; Sánchez-Teyer, L.F. Retroelements and DNA Methylation Could Contribute to Diversity of 5S rDNA in Agave L. J. Mol. Evol. 2018, 86, 404–423. [Google Scholar] [CrossRef]
- Holtum, J.A.; Winter, K. Limited photosynthetic plasticity in the leaf-succulent CAM plant Agave angustifolia grown at different temperatures. Funct. Plant Biol. 2014, 41, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Garcia, M.; Holtum, J.A. Nocturnal versus diurnal CO2 uptake: How flexible is Agave angustifolia? J. Exp. Bot. 2014, 65, 3695–3703. [Google Scholar] [CrossRef]
- Males, J.; Griffiths, H. Stomatal biology of CAM plants. Plant Physiol. 2017, 174, 550–560. [Google Scholar] [CrossRef]
- Cruz-Vasconcelos, S.T.; Ruiz-Posadas, L.d.M.; Garcia-Moya, E.; Sandoval-Villa, M.; Cruz-Huerta, N. Growth and CO2 exchange rate of maguey pulquero (Agave salmiana) obtained by seed. Agrociencia 2020, 54, 911–926. [Google Scholar] [CrossRef]
- Kohonen, K.M.; Skogberg, M.; Kübert, A.; Räsänen, M.; Merbold, L.; Buchmann, N.; Mammarella, I.; Pellikka, P.; Vesala, T. COS, CO2 and H2O eddy covariance flux measurements over Agave sisalana. In Proceedings of the EGU General Assembly 2024, Vienna, Austria, 14–19 April 2024. [Google Scholar] [CrossRef]
- Pimienta-Barrios, E.; Robles-Murguia, C.; Nobel, P.S. Net CO2 Uptake for Agave tequilana in a warm and a temperate environment. Biotropica 2001, 33, 312–318. [Google Scholar] [CrossRef]
- Robert, M.L.; Lim, K.Y.; Hanson, L.; Sanchez-Teyer, F.; Bennett, M.D.; Leitch, A.R.; Leitch, I.J. Wild and agronomically important Agave species (Asparagaceae) show proportional increases in chromosome number, genome size, and genetic markers with increasing ploidy. Bot. J. Linn. Soc. 2008, 158, 215–222. [Google Scholar] [CrossRef]
- Tamayo-Ordóñez, Y.J.; Narvaez-Zapata, J.A.; Sánchez-Teyer, L.F. Comparative characterization of ribosomal DNA regions in different Agave accessions with economical importance. Plant Mol. Biol. Rep. 2015, 33, 2014–2029. [Google Scholar] [CrossRef]
- Stewart, J.R. Agave as a model CAM crop system for a warming and drying world. Front. Plant Sci. 2015, 6, 684. [Google Scholar] [CrossRef]
- Davis, S.C.; Abatzoglou, J.T.; LeBauer, D.S. Expanded potential growing region and yield increase for Agave americana with future climate. Agronomy 2021, 11, 2109. [Google Scholar] [CrossRef]
- Palomino, G.; Martínez, J.; Cepeda-Cornejo, V.; Pimienta-Barrios, E. Nuclear genome size and cytotype analysis in Agave cupreata Trel. & Berger (Agavaceae). Caryologia 2012, 65, 281–294. [Google Scholar] [CrossRef][Green Version]
- Doležel, J. Application of flow cytometry for the study of plant genomes. J. Appl. Genet. 1997, 3, 285–302. [Google Scholar]
- Doležel, J.; Greilhuber, J.; Lucretti, S.; Meister, A.; Lysák, M.A.; Nardi, L.; Obermayer, R. Plant genome size estimation by flow cytometry: Inter-laboratory comparison. Ann. Bot. 1998, 82, 17–26. [Google Scholar] [CrossRef]
- Doležel, J.; Bartoš, J.A.N. Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 2005, 95, 99–110. [Google Scholar] [CrossRef]
- Tamayo-Ordoñez, M.; Huijara-Vasconselos, J.; Quiroz-Moreno, A.; Ortíz-García, M.; Sánchez-Teyer, L.F. Plant Tissue Culture and Molecular Markers. In Plant Cell Culture Protocols; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 877, pp. 343–356. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc. Numerical Taxonomy and Multivariate Analysis System, version 2.1; Exeter Software: Setauket, NY, USA, 2000. [Google Scholar]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
- Pavlíek, A. Free Tree-freeware program for construction of phylogenetic trees on the basis of distance data and bootstrap/jackknife analysis of the tree robustness. Application in the RAPD analysis of genus Frenkelia. Folia Biol. 1999, 45, 97–99. [Google Scholar]
- García-Castillo, M.J.; Rodríguez-Zapata, L.C.; Sanchez-Teyer, L.F. Differential expression of CesA genes and the relationship with fiber content in henequen. Trop. Subtrop. Agroecosyst. 2022, 25, 3. [Google Scholar] [CrossRef]
- Estrada-Medina, H.; Cobos-Gasca, V.; Acosta-Rodríguez, J.L.; Fierro, S.P.; Castilla-Martínez, M.; Castillo-Carrillo, C.; Franco-Brito, S.; López-Castillo, D.; López-Díaz, M.; Luna-Flores, W.; et al. La sequía de la península de Yucatán. Tecnol. Cienc. Agua. 2016, 7, 151–165. [Google Scholar]
- Santamaría, J.M.; Herrera, J.L.; Robert, M.L. Stomatal physiology of a micropropagated CAM plant; Agave tequilana (Weber). Plant Growth Regul. 1995, 16, 211–214. [Google Scholar] [CrossRef]
- Tamayo-Ordoñez, M.; Rodríguez-Zapata, L.C.; Sánchez-Teyer, L.F. Construction and characterization of a partial binary bacterial artificial chromosome (BIBAC) of Agave tequilana var. azul (2X) and its application for gene identification. Afr. J. Biotechnol. 2012, 11, 15950–15958. [Google Scholar]
- Moreno-Salazar, S.F.; Esqueda, M.; Martínez, J.; Palomino, G. Tamaño del genoma y cariotipo en Agave angustifolia y A. rhodacantha de Sonora, México. Rev. Fitotec. Mex. 2007, 30, 13–23. [Google Scholar]
- Chen, Z.J. Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010, 15, 57–71. [Google Scholar] [CrossRef]
- Moghe, G.D.; Shiu, S.H. The causes and molecular consequences of polyploidy in flowering plants. Ann. N. Y. Acad. Sci. 2014, 1320, 16–34. [Google Scholar] [CrossRef] [PubMed]
- McKain, M.R.; Wickett, N.; Zhang, Y.; Ayyampalayam, S.; McCombie, W.R.; Chase, M.W.; Pires, J.C.; de Pamphilis, C.W.; Leebens-Mack, J. Phylogenomic analysis of transcriptome data elucidates co-occurrence of a paleopolyploid event and the origin of bimodal karyotypes in Agavoideae (Asparagaceae). Am. J. Bot. 2012, 99, 397–406. [Google Scholar] [CrossRef]
- Palomino, G.; Martínez, J.; Méndez, I.; Cepeda-Cornejo, V.; Barba-González, R.; Rodríguez-Garay, B. Nuclear genome size and cytotype analysis in Agave parviflora Torr. subsp. flexiflora Gentry (Asparagales, Asparagaceae). Caryologia 2015, 68, 159–168. [Google Scholar] [CrossRef]
- Vargas-Ponce, O.; Zizumbo-Villarreal, D.; Martínez-Castillo, J.; Coello-Coello, J.; Colunga-Garcíamarín, P. Diversity and structure of landraces of Agave grown for spirits under traditional agriculture: A comparison with wild populations of A. angustifolia (Agavaceae) and commercial plantations of A. tequilana. Am. J. Bot. 2009, 96, 448–457. [Google Scholar] [CrossRef]
- Aguirre-Dugua, X.; Eguiarte, L.E. Genetic diversity, conservation and sustainable use of wild Agave cupreata and Agave potatorum extracted for mezcal production in Mexico. J. Arid. Environ. 2013, 90, 36–44. [Google Scholar] [CrossRef]
- Sánchez-Teyer, F.; Moreno-Salazar, S.; Esqueda, M.; Barraza, A.; Robert, M.L. Genetic variability of wild Agave angustifolia populations based on AFLP: A basic study for conservation. J. Arid. Environ. 2009, 73, 611–616. [Google Scholar] [CrossRef]
- Bousious, A.; Saldana-Oyarzabal, I.; Valenzuela-Zapata, A.G.; Wood, C.; Pearce, S.R. Isolation and characterization of Ty1-copia retrotransposon sequences in the blue agave (Agave tequilana Weber var. azul) and their development as SSAP markers for phylogenetic analysis. Plant Sci. 2007, 172, 291–298. [Google Scholar] [CrossRef]
- Good-Avila, S.V.; Souza, V.; Gaut, B.S.; Eguiarte, L.E. Timing and rate of speciation in Agave (Agavaceae). Proc. Natl. Acad. Sci. USA 2006, 103, 9124–9129. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Blancas, J.; Lira, R. Mexican ethnobotany: Interactions of people and plants in Mesoamerica. In Ethnobotany of Mexico: Interactions of People and Plants in Mesoamerica; Springer: New York, NY, USA, 2016; pp. 1–19. [Google Scholar] [CrossRef]
- Colunga-GarcíaMarín, P.; Zizumbo-Villarreal, D. Domestication of plants in Maya lowlands. Econ. Bot. 2004, 58, S101–S110. [Google Scholar] [CrossRef]
- Balao, F.; Herrera, J.; Talavera, S. Phenotypic consequences of polyploidy and genome size at the microevolutionary scale: A multivariate morphological approach. New Phytol. 2011, 192, 256–265. [Google Scholar] [CrossRef]
- Jordan, G.J.; Carpenter, R.J.; Koutoulis, A.; Price, A.; Brodribb, T.J. Environmental adaptation in stomatal size independent of the effects of genome size. New Phytol. 2015, 205, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Yuan, G.; Weston, D.J.; Tuskan, G.A. Engineering crassulacean acid metabolism in C3 and C4 plants. Cold Spring Harb. Perspect. Biol. 2024, 16, a041674. [Google Scholar] [CrossRef]
- Sage, R.F.; Gilman, I.S.; Smith, J.A.C.; Silvera, K.; Edwards, E.J. Atmospheric CO2 decline and the timing of CAM plant evolution. Ann. Bot. 2023, 132, 753–770. [Google Scholar] [CrossRef]
- Winter, K. Ecophysiology of constitutive and facultative CAM photosynthesis. J. Exp. Bot. 2019, 70, 6495–6508. [Google Scholar] [CrossRef]
- Driever, S.M.; Kromdijk, J. Will C3 crops enhanced with the C4 CO2-concentrating mechanism live up to their full potential (yield)? J. Exp. Bot. 2013, 64, 3925–3935. [Google Scholar] [CrossRef]
- Ehrler, W.L. Daytime stomatal closure in Agave americana as related to enhanced water-use efficiency. In Physiological Systems in Semiarid Environments; Hoff, C.C., Riedesel, M.L., Eds.; University of New Mexico Press: Albuquerque, NM, USA, 1969; pp. 239–247. [Google Scholar]
- Nobel, P.S.; Hartsock, T.L. Resistance analysis of nocturnal carbon dioxide uptake by a Crassulacean acid metabolism succulent, Agave deserti. Plant Physiol. 1978, 61, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Geydan, T.D.; Melgarejo, L. Metabolismo ácido de las crasuláceas. Acta Biol. Colom. 2005, 10, 3–16. [Google Scholar]
- Yang, Z.; Yang, Q.; Liu, Q.; Li, X.; Wang, L.; Zhang, Y.; Ke, Z.; Lu, Z.; Shen, H.; Li, J.; et al. A chromosome-level genome assembly of Agave hybrid NO.11648 provides insights into the CAM photosynthesis. Hortic. Res. 2024, 11, uhad269. [Google Scholar] [CrossRef]
- Bailey, K.J.; Gray, J.E.; Walker, R.P.; Leegood, R.C. Coordinate regulation of phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase by light and CO2 during C4 photosynthesis. Plant Physiol. 2007, 144, 479–486. [Google Scholar] [CrossRef]
- O’Leary, B.; Park, J.; Plaxton, W.C. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): Recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem. J. 2011, 436, 15–34. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, L.S.; Zhang, G.Q.; Zheng, B.-Q.; Liu, Z.-J.; Wang, Y. Evolutionary history of PEPC genes in green plants: Implications for the evolution of CAM in orchids. Mol. Phylogenetics Evol. 2016, 94, 559–564. [Google Scholar] [CrossRef]
- Borland, A.M.; Barrera-Zambrano, V.A.; Ceusters, J.; Shorrock, K. The photosynthetic plasticity of crassulacean acid metabolism: An evolutionary innovation for sustainable productivity in a changing world. New Phytol. 2011, 191, 619–633. [Google Scholar] [CrossRef]
- Heckmann, D. C4 photosynthesis evolution: The conditional Mt. Fuji. Curr. Opin. Plant Biol. 2016, 31, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Valegård, K.; Hasse, D.; Andersson, I.; Gunn, L.H. Structure of Rubisco from Arabidopsis thaliana in complex with 2-carboxyarabinitol-1, 5-bisphosphate. Acta Crystallogr. D Struct. Biol. 2018, 74, 1–9. [Google Scholar] [CrossRef]
- Ramírez-Tobías, H.M.; Peña-Valdivia, C.B.; Aguirre, J.R. Respuestas bioquímico-fisiológicas de especies de Agave a la restricción de humedad. Bot. Sci. 2014, 92, 131–139. [Google Scholar]
- Hartsock, T.L.; Nobel, P.S. Watering converts a CAM plant to daytime CO2 uptake. Nature 1976, 262, 574–576. [Google Scholar] [CrossRef]
- Graham, E.A.; Nobel, P.S. Long-term effects of a doubled atmospheric CO2 concentration on the CAM species Agave deserti. J. Exp. Bot. 1996, 47, 61–69. [Google Scholar] [CrossRef]
- Nobel, P.S. Responses of some North American CAM plants to freezing temperatures and doubled CO2 concentrations: Implications of global climate change for extending cultivation. J. Arid. Environ. 1996, 34, 187–196. [Google Scholar] [CrossRef]
- Nobel, P.S.; Berry, W.L. Element responses of agaves. Am. J. Bot. 1985, 72, 686–694. [Google Scholar] [CrossRef]
- Zhu, J.; Goldstein, G.; Bartholomew, D.P. Gas exchange and carbon isotope composition of Ananas comosus in response to elevated CO2 concentration and temperature. Plant Cell Environ. 1999, 22, 999–1007. [Google Scholar] [CrossRef]
- Winter, K.; von Willert, D.J. NaCl-induzierter Crassulaceensäurestoffwechsel bei Mesembryanthemum crystallinum. Z. Pflanzenphysiol. 1972, 67, 166–170. [Google Scholar] [CrossRef]
- Winter, K.; Garcia, M.; Holtum, J.A. Drought-stress-induced up-regulation of CAM in seedlings of a tropical cactus, Opuntia elatior, operating predominantly in the C3 mode. J. Exp. Bot. 2011, 62, 4037–4042. [Google Scholar] [CrossRef]
- Heyduk, K.; McKain, M.R.; Lalani, F.; Leebens-Mack, J. Evolution of a CAM anatomy predates the origins of Crassulacean acid metabolism in the Agavoideae (Asparagaceae). Mol. Phylogenetics Evol. 2016, 105, 102–113. [Google Scholar] [CrossRef]
- Sage, R.F. Are crassulacean acid metabolism and C4 photosynthesis incompatible? Funct. Plant Biol. 2002, 29, 775–785. [Google Scholar] [CrossRef]
- Sage, R.F. The evolution of C4 photosynthesis. New Phytol. 2004, 161, 341–370. [Google Scholar] [CrossRef]
- Sage, R.F.; Sage, T.L.; Kocacinar, F. Photorespiration and the evolution of C4 photosynthesis. Annu. Rev. Plant Biol. 2012, 63, 19–47. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).