Influence of Production Technology Intensity on the Yield and Amino Acid Profile of the Grain Protein of Different Sowing Oat (Avena sativa L.) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.1.1. Site Characteristics, Experimental Design, and Agronomic Practices
2.1.2. Meteorological Conditions
2.2. Methods
2.2.1. Determination of Total Protein Content
2.2.2. Determination of Amino Acids
2.2.3. Determination of the Biological Value of Protein
2.3. Statistical Analysis
3. Results and Discussion
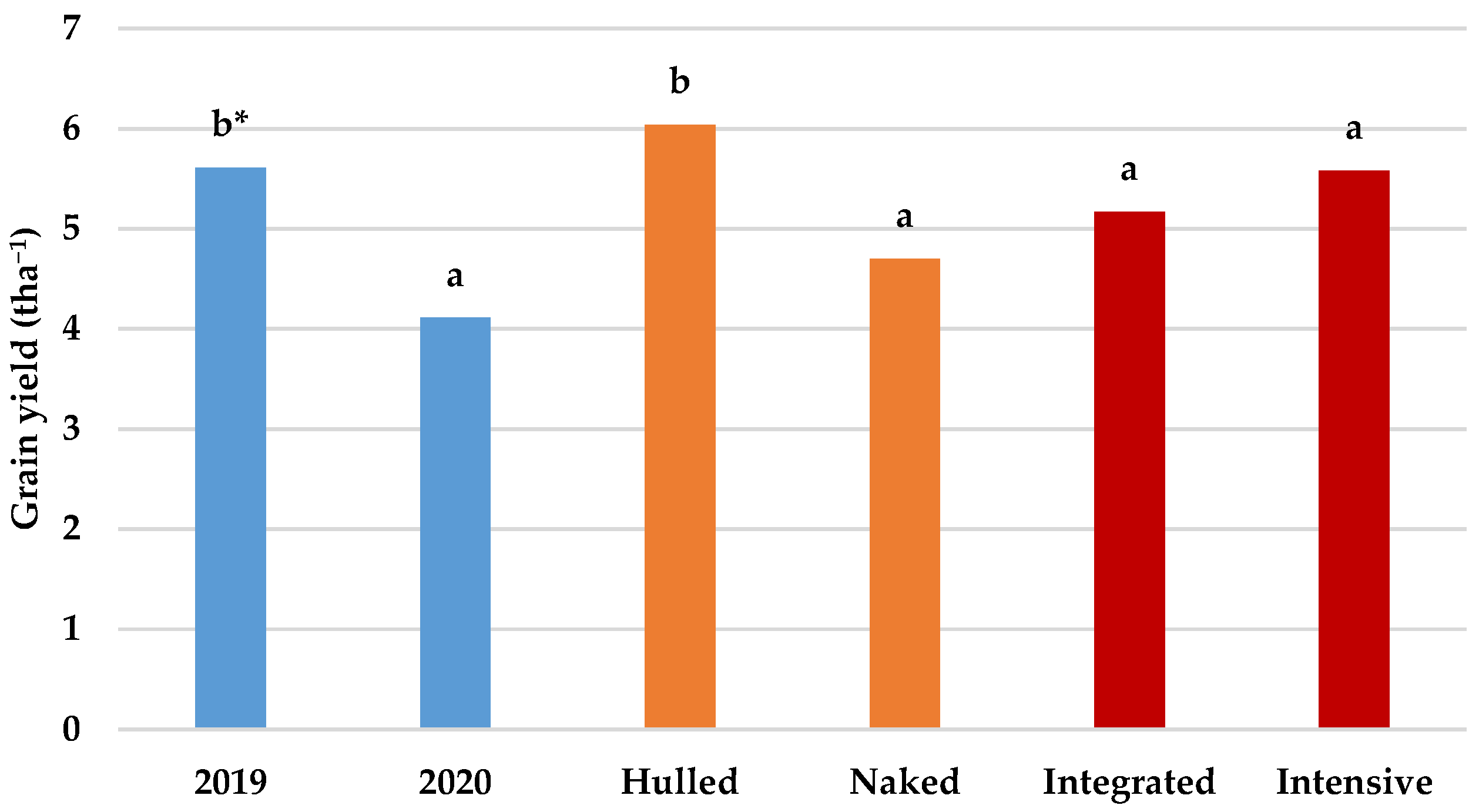
3.1. Grain Yield
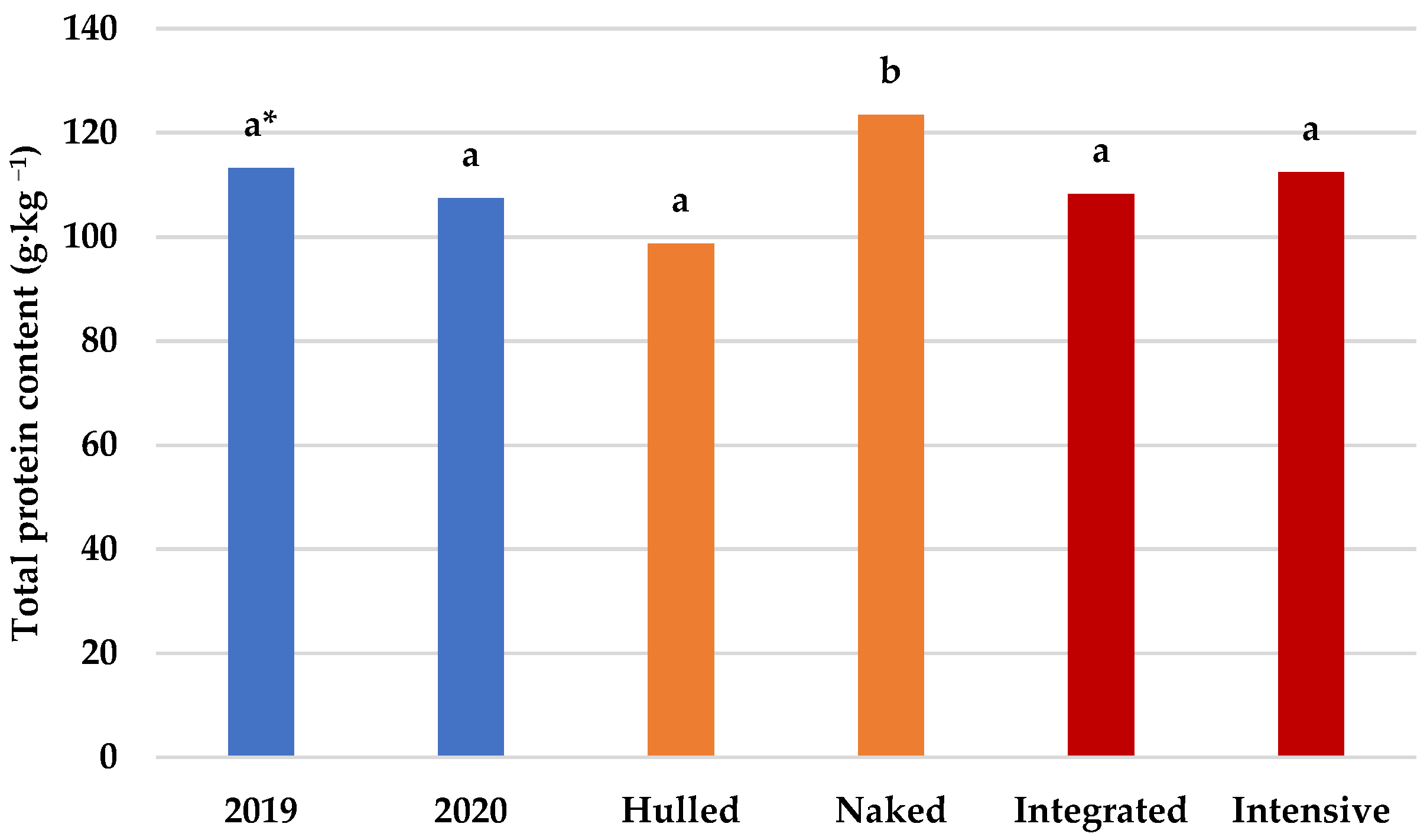
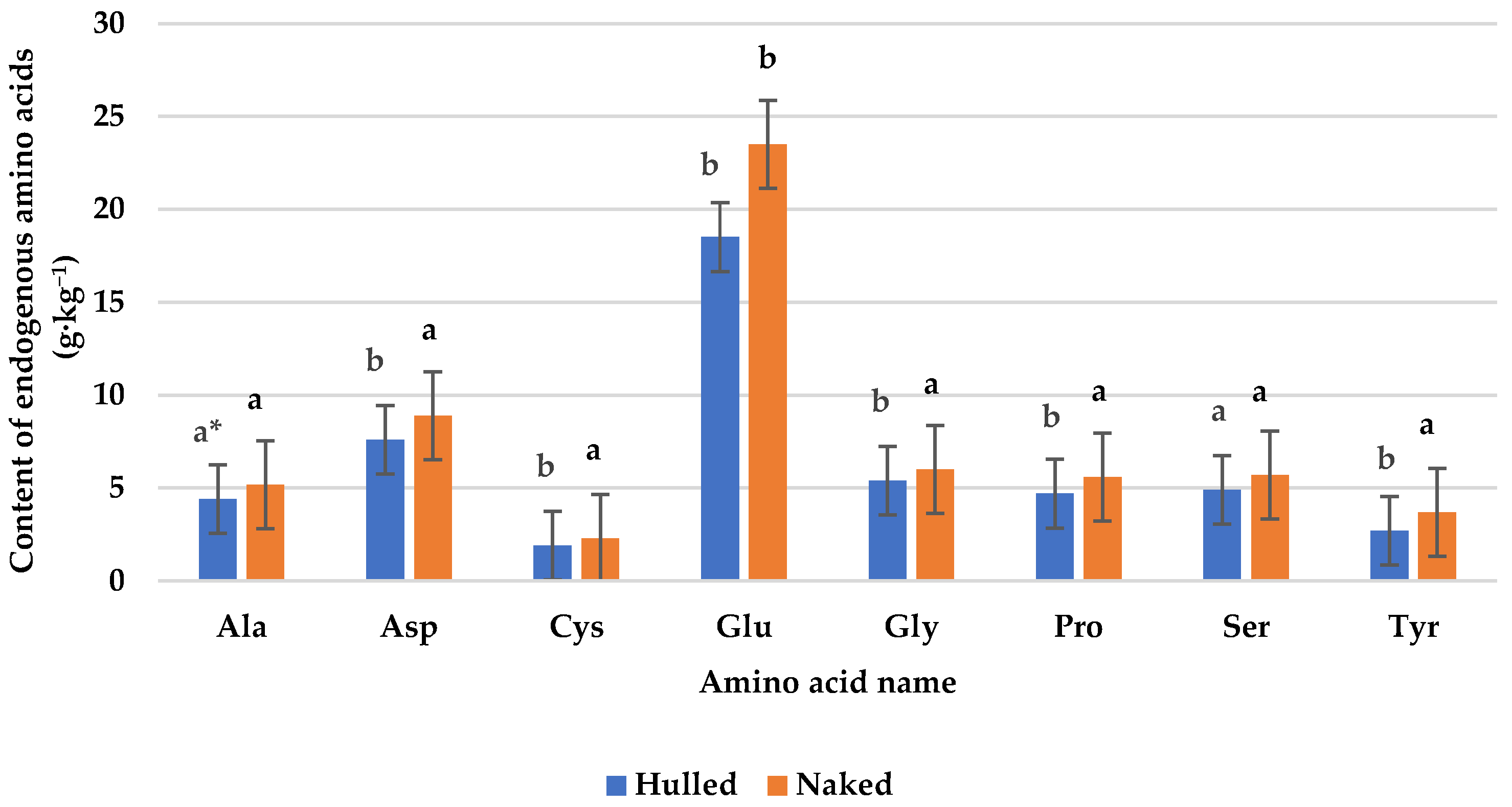
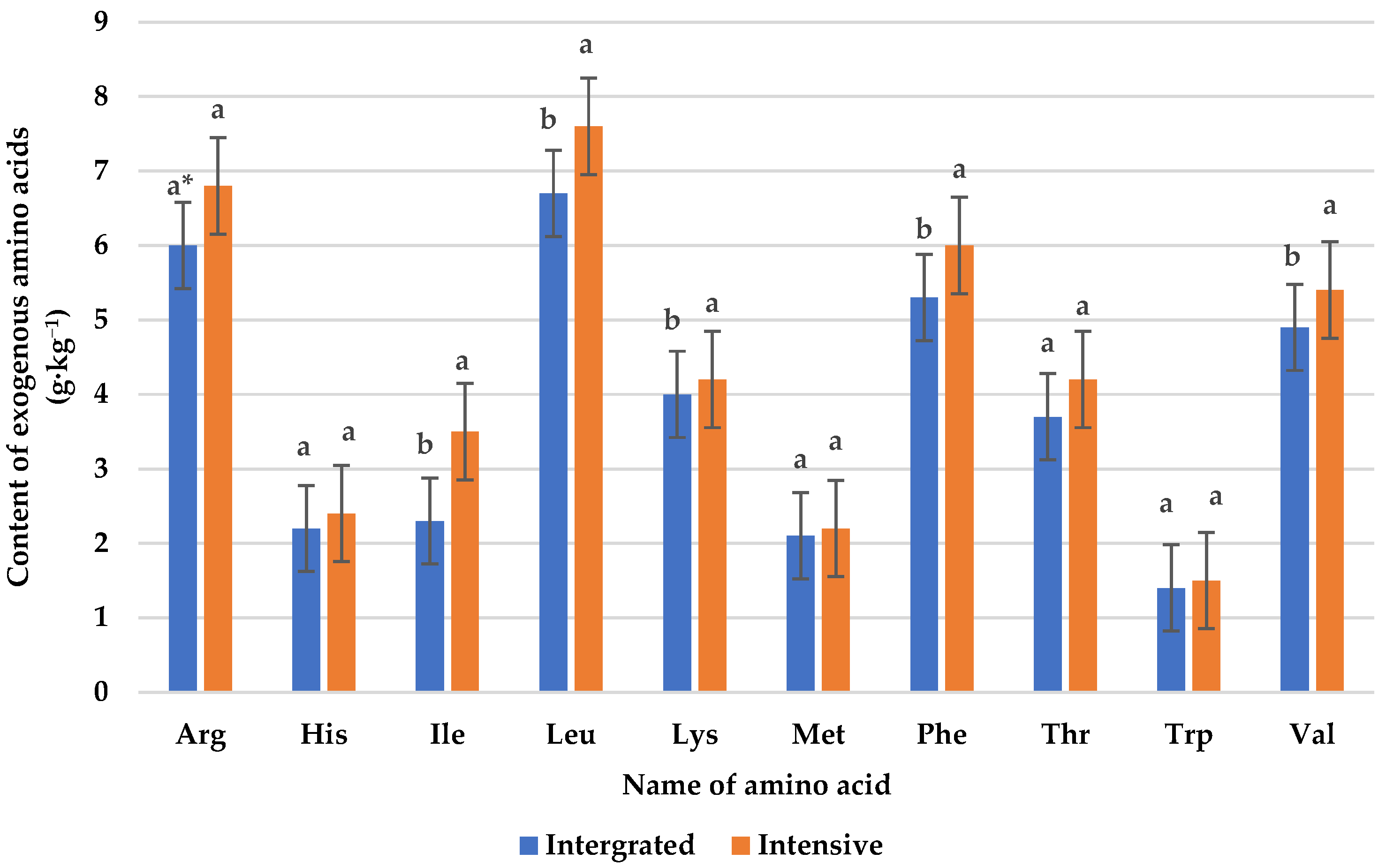
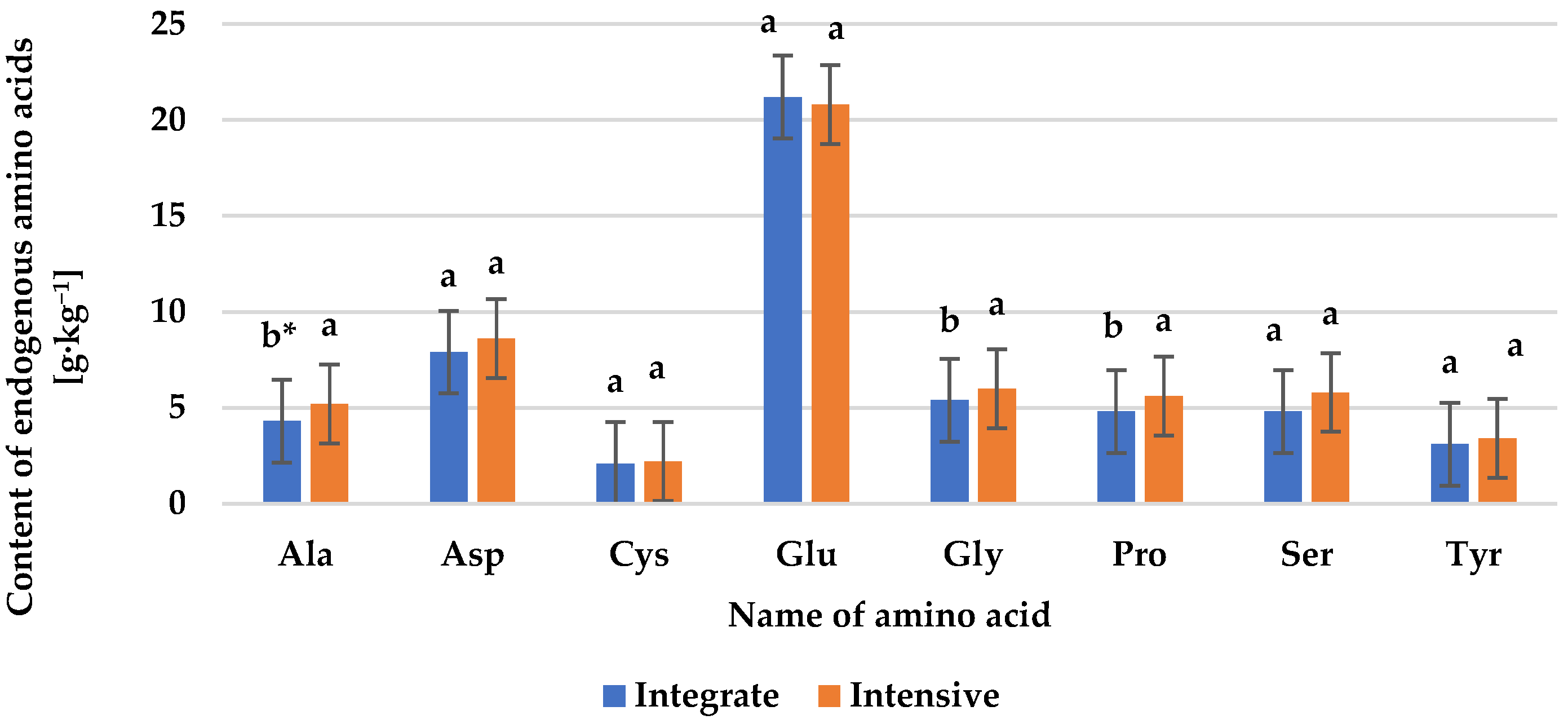
3.2. Protein Content and Amino Acid Composition
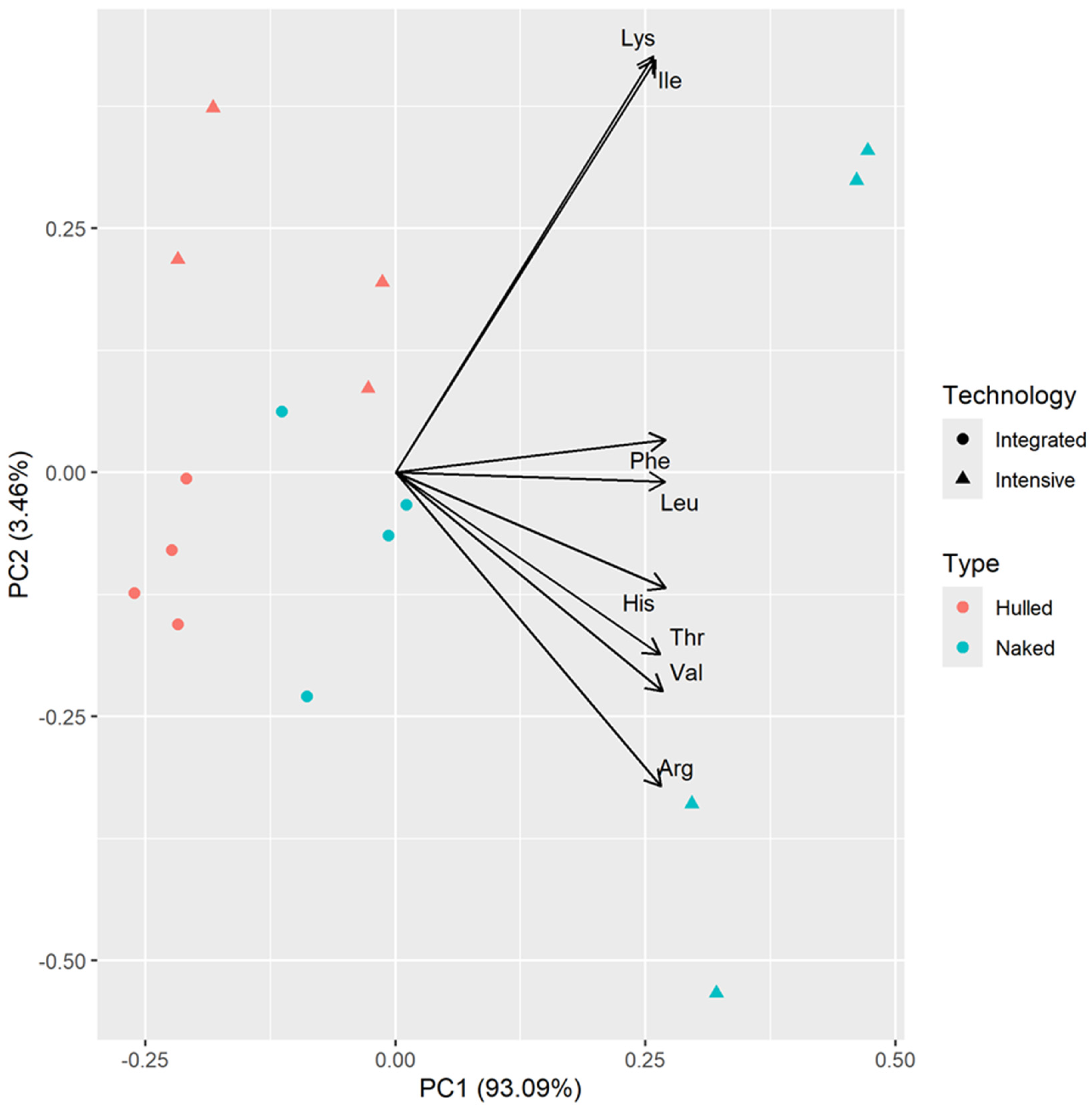
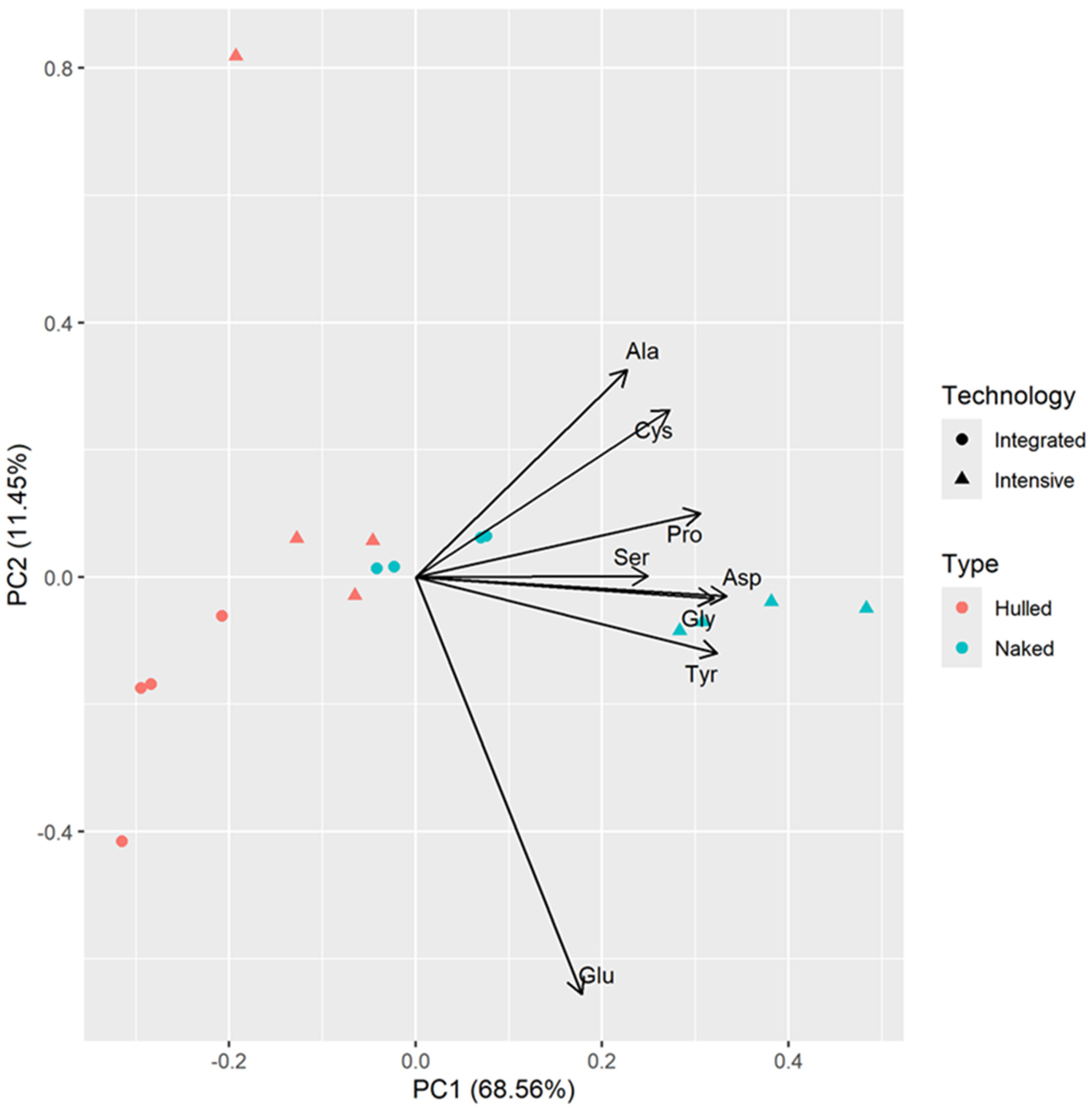
3.3. PCA Analysis
3.4. Biological Value of Oat Protein
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| p.p. | percentage points |
| Arg | Arginine |
| His | Histidine |
| Ile | Isoleusin |
| Leu | Leusine |
| Lys | Lysine |
| Met | Methionine |
| Phe | Phenylalanine |
| Thr | Threonine |
| Trp | Tryptophan |
| Val | Valine |
| Ala | Alanine |
| Asp | Asparagine |
| Cys | Cysteine |
| Glu | Glutamine |
| Gly | Glysine |
| Pro | Proline |
| Ser | Serine |
| Tyr | Tyrosine |
References
- Murphy, J.P.; Hoffman, L.A. The Origin, History, and Production of Oat. In Oat Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1992; pp. 1–28. ISBN 978-0-89118-225-2. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 17 January 2025).
- Available online: https://globaltopstats.com/how-much-oat-is-produced-globally/ (accessed on 17 January 2025).
- Jia, H.; Zhang, T.; Yin, X.; Shang, M.; Chen, F.; Lei, Y.; Chu, Q. Impact of Climate Change on the Water Requirements of Oat in Northeast and North China. Water 2019, 11, 91. [Google Scholar] [CrossRef]
- Pecio, A.; Bichonski, A. Nitrogen fertilization and fungicide application as elements of oat production. Pol. J. Environ. Stud. 2010, 19, 1297–1305. [Google Scholar]
- Łaba, S.; Cacak-Pietrzak, G.; Łaba, R.; Sułek, A.; Szczepański, K. Food Losses in Consumer Cereal Production in Poland in the Context of Food Security and Environmental Impact. Agriculture 2022, 12, 665. [Google Scholar] [CrossRef]
- Singh, S.; Koli, P.; Ahmed, S.; Kumar, N.; Rana, M.; Singhal, R.; Ren, Y. Exploring the genetic variability in yield, nutritional and digestibility traits in oat grains through ruminant nutrition. Heliyon 2024, 10, 31451. [Google Scholar] [CrossRef]
- Alemayehu, G.F.; Forsido, S.F.; Tola, Y.B.; Amare, E. Nutritional and Phytochemical Composition and Associated Health Benefits of Oat (Avena sativa) Grains and Oat-Based Fermented Food Products. Sci. World J. 2023, 2023, 2730175. [Google Scholar] [CrossRef]
- Sinkovič, L.; Rakszegi, M.; Pipan, B.; Meglič, V. Compositional Traits of Grains and Groats of Barley, Oat and Spelt Grown at Organic and Conventional Fields. Foods 2023, 12, 1054. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Wirkijowska, A.; Gasiński, A.; Średnicka-Tober, D.; Trafiałek, J.; Kazimierczak, R. Oat and Oat Processed Products –Technology, Composition, Nutritional Value, and Health. Appl. Sci. 2023, 13, 11267. [Google Scholar] [CrossRef]
- Ruja, A.; Cozma, A.; Cozma, B.; Horablaga, N.M.; Dinulescu, C.; Alexa, E.; Buzna, C.; Cocan, I.; Berbecea, A.; Dossa, S.; et al. Nutritional, Phytochemical, and Rheological Profiles of Different Oat Varieties and Their Potential in the Flour Industry. Agronomy 2024, 14, 1438. [Google Scholar] [CrossRef]
- Ginter, A.; Zarzecka, K.; Gugała, M.; Mystkowska, I. Nutritional properties of oats and their products. Herbalism 2024, 10, 127–137. [Google Scholar] [CrossRef]
- Biel, W.; Kazimierska, K.; Bashutska, U. Nutritional value of wheat, triticale, barley and oat grains. Acta Sci. Pol. Zootech. 2020, 19, 19–28. [Google Scholar] [CrossRef]
- Boukid, F. Oat proteins as emerging ingredients for food formulation: Where we stand? Eur. Food Res. Technol. 2021, 247, 535–544. [Google Scholar] [CrossRef]
- Kumar, L.; Sehrawat, R.; Kong, Y. Oat proteins: A perspective on functional properties. LWT 2021, 152, 112307. [Google Scholar] [CrossRef]
- Kaur, S.; Seem, K.; Kaur, P.; Rahaman, S.; Kumar, K.; Singh, N.; Singh, B.K. Application of Oats and Their Components in Diverse Functional Food Products. In Oat (Avena sativa); CRC Press: Boca Raton, FL, USA, 2024; pp. 271–331. [Google Scholar]
- Gibiński, M.; Gumul, D.; Korus, J. Prozdrowotne właściwości owsa i produktów owsianych. Żywn Nauka Technol Jakość 2005, 4, 49–60. (In Polish) [Google Scholar]
- Gąsiorowski, H. Oats—Chemistry and Technology; PWRiL: Poznań, Poland, 1995. (In Polish) [Google Scholar]
- Jurga, R. Chemical composition, feeding value and using possibilities of oats and its products przetworów. Przegląd Zbożowo-Młynarski 2011, 5, 28–31. (In Polish) [Google Scholar]
- Kawka, A.; Achremowicz, B. Oats—The plant of the 21st century. Nutritional and industrial use. Nauka Przyr. Technol. 2014, 8, 32–41. (In Polish) [Google Scholar]
- Mel, R.; Malalgoda, M. Oat protein as a novel protein ingredient: Structure, functionality, and factors impacting utilization. Cereal Chem. 2022, 99, 21–36. [Google Scholar] [CrossRef]
- Gil-González, A.B.; Sjögren, L.L.; Bernfur, K.; Olsson, O.; Zambrano, J.A. Genomic identification of expressed globulin storage proteins in oat. Front. Plant Sci. 2024, 15, 1418658. [Google Scholar] [CrossRef]
- Mantai, R.D.; da Silva, J.A.G.; Scremin, O.B.; Carvalho, I.R.; Magano, D.A.; Fachinetto, J.M.; Lautenchleger, F.; da Rosa, J.A.; Peter, C.L.; Berlezi, C.J.D.; et al. Nitrogen levels in oat grains and its relation to productivity. Genet. Mol. Res. 2020, 19, GMR18569. [Google Scholar] [CrossRef]
- Noworolnik, K. Yielding and grain quality of oat depending on soil moisture and nitrogen rate. Żywn Nauka Technol. Jakość 2010, 3, 190–196. (In Polish) [Google Scholar]
- Bartnikowska, E.; Lange, E. The grain of oats—An underestimated source of nutrients and biologically active substances. Part I. General characteristics of oats. Proteins, fats. Biul. IHAR 2020, 215, 209–222. (In Polish) [Google Scholar]
- Barczak, B.; Kozera, W.; Nowak, K.; Majcherczak, E. Effect of fertilization with ammonium nitrate with micronutrients on grain and protein yield of oats of the Komes cultivar. Biul. IHAR 2006, 239, 19–25. (In Polish) [Google Scholar] [CrossRef]
- Strażyński, P.; Tratwal, A.; Sosnowska, D.; Mrówczyński, M. Integrated pest management–status and identification of risks in the context of challenges related to the implementation of the new European Union perspective for 2023–2027. Prog. Plant Prot. 2024, 64, 169–179. [Google Scholar] [CrossRef]
- PN-R-04023:1996; Chemical and Agricultural Analysis of Soil—Determination of the Content of Available Phosphorus in Mineral Soils. Polish Standards Committee: Warsaw, Poland, 1996.
- PN-R-04022:1996/Az1:2002; Chemical and Agricultural Analysis of Soil—Determination of the Content of Available Potassium in Mineral Soils. Polish Standards Committee: Warsaw, Poland, 1996.
- AACC Method 46-11.02; Crude Protein–Improved Kjeldahl Method, Copper Catalyst Modyfication. AACC: St. Paul, MN, USA, 2010; p. 39.
- PN-ISO 24333:2010; Cereal Grains and Cereal Products—Sampling. ISO: Geneva, Switzerland, 2010.
- Sułek, A.; Cacak-Pietrzak, G.; Różewicz, M.M.; Nieróbca, A.; Grabiński, J.; Studnicki, M.; Sujka, K.; Dziki, D. Effect protein technology intensity on the grain yield, proteincontent and amino acid profile in common and durum wheat grain. Plants 2023, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Lv, Y.; Li, T.; Tang, J.; Yang, X.; Zhou, H. Effects of drought stress during critical periods on the photosynthetic characteristics and production performance of Naked oat (Avena nuda L.). Sci. Rep. 2022, 12, 11199. [Google Scholar] [CrossRef]
- Krajewski, W.T.; Szempliński, W.; Bielski, S. Yielding of naked and hulled varieties of spring barley in variable nitrogen fertilization. Ann. UMCS Sect. E 2013, 68, 18–29. (In Polish) [Google Scholar]
- Hackett, R. A comparison of husked and naked oats under Irish conditions. Ir. J. Agric. Food Res. 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Batalova, G.A.; Shevchenko, S.N.; Tulyakova, M.V.; Rusakova, I.I.; Zheleznikova, V.A.; Lisitsyn, E.M. Breeding of naked oats having high-quality grain. Russ. Agric. Sci. 2016, 42, 407–410. [Google Scholar] [CrossRef]
- Zobnina, I.V.; Korelina, V.A.; Batakova, O.B. Assessment of stability and plasticity of hulless spring oat varieties by yield and 1000 seed weight in the Northern Russia. RUDN J. Agron. Anim. Ind. 2024, 19, 76–89. [Google Scholar] [CrossRef]
- Güngör, H.; Çakır, M.F.; Dumlupınar, Z. Determination of grain yield and agricultural traits of some oat cultivars at different locations. Black Sea J Agric. 2023, 6, 350–355. [Google Scholar] [CrossRef]
- Ju, Z.; Liu, K.; Zhao, G.; Ma, X.; Jia, Z. Nitrogen Fertilizer and Sowing Density Affect Flag Leaf Photosynthetic Characteristics, Grain Yield, and Yield Components of Oat in a Semiarid Region of Northwest China. Agronomy 2022, 12, 2108. [Google Scholar] [CrossRef]
- Mantai, R.D.; da Silva, J.A.; Carbonera, R.; Carvalho, I.R.; Lautenchleger, F.; Pereira, L.M. Technical and agronomic efficiency of nitrogen use on the yield and quality of oat grains. Rev. Bras. Eng. Agric. E Ambient. 2021, 25, 529–537. [Google Scholar] [CrossRef]
- Świderska Ostapiak, M.; Stankowski, S. The effect of nitrogen sulphur fertilization on yield and yield components of hulied and naked oats. Zesz. Probl. Post. Nauk Roln. 2002, 484, 711–717. (In Polish) [Google Scholar]
- Bibi, H.; Hameed, S.; Iqbal, M.; Al-Barty, A.; Darwish, H.; Khan, A.; Anwar, S.; Mian, I.A.; Ali, M.; Zia, A.; et al. Evaluation of exotic oat (Avena sativa L.) varieties for forage and grain yield in response to different levels of nitrogen and phosphorous. Peer J. 2021, 9, e12112. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.W.; Martinez-Martin, P.; Xu, Y.; Cowan, A.; Pont, S.; Griffiths, I.; Sungurtas, J.; Clarke, S.; Goodacre, R.; Marshall, A.; et al. Assessing the impact of nitrogen supplementation in oats across multiple growth locations and years with targeted phenotyping and high-resolution metabolite profiling approaches. Food Chem. 2021, 355, 129585. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Liang, G.; Jia, Z.; Ju, Z.; Ma, X.; Zhou, Q. Comprehensive Evaluation of Low Nitrogen Tolerance in Oat (Avena sativa L.) Seedlings. Agronomy 2023, 13, 604. [Google Scholar] [CrossRef]
- Pan, J.; Ju, Z.; Ma, X.; Duan, L.; Jia, Z. Genome-wide characterization of TCP family and their potential roles in abiotic stress resistance of oat (Avena sativa L.). Front. Plant Sci. 2024, 15, 1382790. [Google Scholar] [CrossRef]
- Bednarek, W.; Tkaczyk, P.; Dresler, S.; Jawor, E. Relationship between oat yield and some soil properties and nitrogen fertilization. Acta Agroph. 2013, 20, 29–38. (In Polish) [Google Scholar]
- Da Silva, J.A.G.; Neto, C.J.G.; Fernandes, S.B.V.; Mantai, R.D.; Scremin, O.B.; Pretto, R. Nitrogen efficiency in oat on grain yield with stability. Rev. Bras. Eng. Agric. E Ambient. 2016, 20, 1095–1100. [Google Scholar] [CrossRef]
- Sponchiado, J.C.; Souza, C.A.; Sangoi, L.; Coelho, C.M.M.; Stefen, D.L.V. Late nitrogen topdressing increases nutritional and industrial quality of white oat (‘Avena sativa’) grain. Aust. J. Crop Sci. 2020, 14, 1355–1361. [Google Scholar] [CrossRef]
- Said, M.S.; Hameed, R.M.; Majeed, H.A. Effect of nitrogen fertilizer and seeding rates on growth and grain yield in oats (Avena sativa L.). Plant Arch. 2019, 19, 1554–1558. [Google Scholar]
- Tomple, B.M.; Hawan, J.I. Enhacing seed productivity and feed value of oats (Avena sativa L.) with different seeding rate and nitrogen fertilizing levels in gyeongbuk area. J. Agric. Sci. 2018, 52, 61–72. [Google Scholar]
- Joshi, R.V.; Patel, B.J.; Patel, K.M. Effect of nitrogen levels and time of application on growth, yield, quality, nitrogen, phosphorus content and uptake for seed production of oat (Avena sativa L.). Forage Res. 2015, 41, 104–108. [Google Scholar]
- Błażewicz, J.; Kawa-Rygielska, J.; Leszczyńska, D.; Grabiński, J.; Gasiński, A. Influence of Variety and Nitrogen Fertilization on the Technological Parameters of Special Malts Prepared from Naked and Hulled Oat Varieties. Agronomy 2021, 11, 2566. [Google Scholar] [CrossRef]
- Khan, T.A.; Nadeem, F.; Chen, L.; Wang, X.; Zeng, Z.; Hu, Y. Enhancing naked oat (Avena nuda L.) productivity with minimal indirect nitrogen loss and maximum nitrogen use efficiency through integrated use of different nitrogen sources. PLoS ONE 2019, 14, e0213808. [Google Scholar] [CrossRef]
- Gąsiorowska, B.; Cybulska, A.; Makarewicz, A. The influence of sowing density on content of selected plant nutrients in oats grain. Biul. IHAR 2011, 28, 16–24. (In Polish) [Google Scholar]
- Yan, W.; Frégeau-Reid, J.; Pageau, D.; Martin, R. Genotype-by-environment interaction and trait associations in two genetic populations of oat. Crop Sci. 2016, 56, 1136–1145. [Google Scholar] [CrossRef]
- Howarth, C.J.; Martinez-Martin, P.M.J.; Cowan, A.A.; Griffiths, I.M.; Sanderson, R.; Lister, S.J.; Langdon, T.; Clarke, S.; Fradgley, N.; Marshall, A.H. Genotype and Environment Affect the Grain Quality and Yield of Winter Oats (Avena sativa L.). Foods 2021, 10, 2356. [Google Scholar] [CrossRef]
- Biel, W.; Bobko, K.; Maciorowski, R. Chemical composition and nutritive value of husked and naked oats grain. J. Cereal Sci. 2009, 49, 413–418. [Google Scholar] [CrossRef]
- Brand, T.S.; Cruywagen, C.W.; Brandt, D.A.; Viljoen, M.; Burger, W.W. Variation in the chemical composition, physical characteristics and Energy values of cereal grains producted in the Western Cape area of South Africa. S. Afr. J. Anim. Sci. 2003, 33, 117–126. [Google Scholar]
- Sunilkumar, B.A.; Leonova, S.; Öste, R.; Olsson, O. Identification and characterization of high protein oat lines from a mutagenized oat population. J. Cereal Sci. 2017, 75, 100–107. [Google Scholar] [CrossRef]
- Podolska, G.; Nita, Z.; Mikos, M. Effect of sowing rates and nitrogen fertilization on grain yield and chemical composition of naked short-shoot oat (STH 5630). Fragm. Agron. 2009, 26, 100–107. [Google Scholar]
- Sterna, V.; Zute, S.; Brunava, L. Oat grain composition and its nutrition benefice. Agric. Sci. Procedia 2016, 8, 252–256. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Ahmad, A.; Sohail, A.; Asad, M.J. Nutritional and functional characterization of different oat (Avena sativa L.) cultivars. Int. J. Food Prop. 2020, 23, 1373–1385. [Google Scholar] [CrossRef]
- Hospodarenko, H.; Karpenko, V.; Liubych, V.; Novikov, V. Characterization of amino acid content of grain of new wheat varieties and lines. Agric. Sci. Pract. 2018, 5, 12–18. [Google Scholar] [CrossRef]
- Johansson, E.; Henriksson, T.; Prieto-Linde, M.L.; Andersson, S.; Ashraf, R.; Rahmatov, M. Diverse wheat-alien introgression lines as a basis for durable resistance and quality characteristics in bread wheat. Front. Plant Sci. 2020, 11, 1067. [Google Scholar]
- Besaliev, I.N.; Panfilov, A.L.; Karavaytsev, Y.A.; Reger, N.S.; Kholodilina, T.N. Content of prolin and exogenous amino acids in spring wheat grain in dry conditions. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012116. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Y.; Shi, Z.; Rentsch, D.; Ward, J.L.; Hassall, K.; Sparks, C.A.; Huttly, A.K.; Buchner, P.; Powers, S.; et al. Wheat amino acid transporters highly expressed in grain cells regulate amino acid accumulation in grain. PLoS ONE 2021, 16, e0246763. [Google Scholar] [CrossRef]
- Jaśkiewicz, B.; Szczepanek, M. Amino acids content in triticale grain depending on meteorological, agrotechnical and genetic factors. Agric. Sci. Crop Sci. Anim. Sci. Res. Rural Dev. 2008, 2, 28–34. [Google Scholar] [CrossRef]
- Biel, W. Chemical Composition and Nutritious Value of Protein from Various Oat Forms, with a Particular Consideration Goven to Short-Shoot Forms with the DW6 Dwarfness Gen Introduced; West Pomeranian University of Technology: Szczecin, Poland, 2013; p. 71. ISBN 978-83-7663-159-2. (In Polish) [Google Scholar]
- Shewry, P.R.; Piironen, V.; Lampi, A.M.; Nystrom, L.; Li, L.; Rakszegi, M.; Ward, J.L. Phytochemical and fiber components in oat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9777–9784. [Google Scholar] [CrossRef]
- Witkowicz, R.; Pisulewska, E.; Kidacka, A.; Mickowska, B. Composition of amino quality of proteins in grains of oats with yellow and brown glume (Avena sativa). Żywn Nauka Technol. Jakość 2016, 23, 125–140. (In Polish) [Google Scholar] [CrossRef]
- Zorovski, P.; Georgieva, T. Exogenous amino acid contents of winter and spring oat cultivars (Avena sativa L.) grown in Central South Bulgaria. In Proceedings of the AGRISAFE Final Conference, Climate Change: Challenges and Opportunities in Agriculture, Budapest, Hungary, 21–23 March 2011; Veisz, O., Ed.; Budapest Agricultural Research Institute of the Hungarian Academy of Sciences: Budapest, Hungary, 2011; pp. 481–484. [Google Scholar]
- Ralcewicz, M.; Knapowski, T. The effect of some agrotechnical factors on grain yield and amino acid composition of protein of oat. Biul. IHAR 2006, 239, 193–204. (In Polish) [Google Scholar] [CrossRef]
- Vilmane, L.; Zute, S.; Straumīte, E.; Galoburda, R. Protein, Amino Acid and Gluten Content in Oat (Avena Sativa L.) Grown in Latvia. Proc. LAS. Sect. B. Nat. Exact Appl. Sci. 2015, 69, 170–177. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, D.; Wang, C.; Zhao, H.; Zhu, Y.; Guo, T. Responses of amino acid composition to nitrogen application in high-and low—Protein wheat cultivars at two planting environments. Crop Sci. 2016, 56, 1277–1287. [Google Scholar] [CrossRef]
- Nowak, K.; Spychaj-Fabisiak, E.; Barczak, B.; Knapowski, T.; Kozera, W. Preliminary study of the amino acid composition of spelt grain fertilised with nitrogen and micronutrients. Inż. Ap. Chem. 2015, 54, 265–266. (In Polish) [Google Scholar]
- Biel, W.; Hury, G.; Maciorowski, R.; Kotlarz, A.; Jaśkowska, I. Effect of nitrogen fertilization on chemical composition of spelt wheat (Triticum aestivum ssp. spelta L.) two varieties. Acta Sci. Pol. Zootech. 2010, 9, 5–14. (In Polish) [Google Scholar]
- Pátek, M. Branched-chain amino acids. In Amino Acid Biosynthesis—Pathways, Regulation and Metabolic Engineering; Springer: Berlin/Heidelberg, 2007; pp. 129–162. [Google Scholar] [CrossRef]
- Gaudichon, C. Evolution and significance of amino acid scores for protein quality. Front. Nutr. 2024, 11, 1437853. [Google Scholar] [CrossRef]
- Bai, Y.; Khoddami, A.; Messina, V.; Zhang, Z.; Tan, D.K.Y. Response of Wheat Genotypes Stressed by High Temperature in Terms of Yield and Protein Composition Across Diverse Environments in Australia. Agriculture 2025, 15, 514. [Google Scholar] [CrossRef]
- Zyukin, D.A.; Glushkov, I.A. Comparative assessment of fertilizer systems and intensification levels in crop production. In Proceedings of the BIO Web of Conferences, EDP Sciences. International Scientific and Practical Conference “Agriculture and Food Security: Technology, Innovation, Markets, Human Resources” (FIES 2024), Kazan, Russia, 28–29 November 2025; Volume 161, p. 00002. [Google Scholar] [CrossRef]


| Technology | Fertilisation (kg∙ha−1) | Sowing Protection | |||||
|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | Herbicides | Fungicides | Insecticides | Retardants | |
| Integrated | 90 | 70 | 100 | Mustang 306 SE (florasulan) 0.8 l∙ha−1 | Seguris 215 S.C. (isopyrazole, epoxiconazole) 1.0 l∙ha−1 | Fury 100 EW (zeta-cypermethrin) 0.1 l∙ha−1 | - |
| Intensive | 120 | 80 | 105 | Mustang 306 SE (florasulan) 0.6 l∙ha−1 | Seguris 215 S.C. (isopyrazole, epoxiconazole) 1.0 l∙ha−1 Amistar 250 SC (azoxystrobin) 0.6 l∙ha−1 Artea 330 EC (propiconazole + cyproconazole) 0.5 l∙ha−1 | Fury 100 EW (zeta-cypermethrin) 0.1 l∙ha−1 | Modus 250 EW (trinexapac ethyl) 0.4 l∙ha−1 |
| Month | Temperature (°C) | Precipitation (mm) | ||||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 1981–2018 | 2019 | 2020 | 1981–2018 | |
| March | 5.5 | 4.5 | 2.3 | 23 | 25 | 33 |
| April | 9.6 | 8.5 | 8.3 | 35 | 12 | 36 |
| May | 12.9 | 11.1 | 13.9 | 86 | 113 | 56 |
| June | 21.7 | 18.4 | 16.6 | 39 | 189 | 62 |
| July | 18.6 | 18.6 | 18.9 | 34 | 50 | 78 |
| Factors | Amino Acids Content (g∙kg−1) | ||
|---|---|---|---|
| Total Content | Endogenous | Exogenous | |
| Growing Season | |||
| 2019 | 100.14 a * | 57.67 a | 42.48 a |
| 2020 | 94.24 a | 53.495 a | 40.75 a |
| Grain Form | |||
| Hulled | 88.53 a | 50.17 a | 38.36 a |
| Naked | 105.86 b | 60.99 b | 44.87 b |
| Production Technology | |||
| Integrated | 92.86 a | 53.44 a | 39.42 a |
| Intensive | 101.53 a | 57.72 a | 43.81 b |
| Exogenous Amino Acid | Amino Acid Content Perg | Amino Acid Score CS [%] | AASDF [%] | Biological Value [%] | |
|---|---|---|---|---|---|
| Reference Protein | Studied Specimen | ||||
| Hulled | |||||
| Isoleucine Leucine Lysine Methionine + Cysteine Phenylalanine + Tyrosine Threonine Tryptphan Valine | 4.0 7.0 5.5 3.5 6.0 4.0 1.0 5.0 | 3.22 6.74 3.94 3.74 7.95 3.64 1.11 4.76 | 80.5 96.3 72.0 106.8 132.5 91.0 111.0 95.2 | 19 3 28 0 0 9 0 4 | 81 97 72 107 133 91 111 96 |
| Naked | |||||
| Isoleucine Leucine Lysine Methionine + Cysteine Phenylalanine + Tyrosine Threonine Tryptphan Valine | 4.0 7.0 5.5 3.5 6.0 4.0 1.0 5.0 | 3.52 7.52 4.24 4.83 9.80 4.26 1.72 5.51 | 88.0 107.4 77.1 138.0 163.3 106.6 172.0 110.2 | 12 0 23 0 0 0 0 0 | 88 108 77 138 164 107 172 111 |
| Exogenous Amino Acid | Amino Acid Content Perg | Amino Acid Score CS [%] | AASDF [%] | Biological Value [%] | |
|---|---|---|---|---|---|
| Reference Protein | Studied Specimen | ||||
| Integrated technology | |||||
| Isoleucine Leucine Lysine Methionine + Cysteine Phenylalanine + Tyrosine Threonine Tryptphan Valine | 4.0 7.0 5.5 3.5 6.0 4.0 1.0 5.0 | 3.21 6.70 3.95 4.14 8.32 3.73 1.38 4.86 | 80.2 95.7 71.8 118.3 138.7 93.3 138.0 97.2 | 20 4 18 0 0 7 0 3 | 80 96 72 118 139 93 138 97 |
| Intensive technology | |||||
| Isoleucine Leucine Lysine Methionine + Cysteine Phenylalanine + Tyrosine Threonine Tryptphan Valine | 4.0 7.0 5.5 3.5 6.0 4.0 1.0 5.0 | 3.53 7.56 4.23 4.25 9.45 4.17 1.45 5.41 | 88.3 108.0 76.9 121.4 157.5 104.3 145.0 82.0 | 12 0 23 0 0 0 0 18 | 88 108 77 121 156 104 145 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułek, A.; Cacak-Pietrzak, G.; Różewicz, M.; Nieróbca, A.; Studnicki, M.; Podolska, G. Influence of Production Technology Intensity on the Yield and Amino Acid Profile of the Grain Protein of Different Sowing Oat (Avena sativa L.) Cultivars. Agronomy 2025, 15, 803. https://doi.org/10.3390/agronomy15040803
Sułek A, Cacak-Pietrzak G, Różewicz M, Nieróbca A, Studnicki M, Podolska G. Influence of Production Technology Intensity on the Yield and Amino Acid Profile of the Grain Protein of Different Sowing Oat (Avena sativa L.) Cultivars. Agronomy. 2025; 15(4):803. https://doi.org/10.3390/agronomy15040803
Chicago/Turabian StyleSułek, Alicja, Grażyna Cacak-Pietrzak, Marcin Różewicz, Anna Nieróbca, Marcin Studnicki, and Grażyna Podolska. 2025. "Influence of Production Technology Intensity on the Yield and Amino Acid Profile of the Grain Protein of Different Sowing Oat (Avena sativa L.) Cultivars" Agronomy 15, no. 4: 803. https://doi.org/10.3390/agronomy15040803
APA StyleSułek, A., Cacak-Pietrzak, G., Różewicz, M., Nieróbca, A., Studnicki, M., & Podolska, G. (2025). Influence of Production Technology Intensity on the Yield and Amino Acid Profile of the Grain Protein of Different Sowing Oat (Avena sativa L.) Cultivars. Agronomy, 15(4), 803. https://doi.org/10.3390/agronomy15040803






