Phosphorus Cycling Dominates Microbial Regulation of Synergistic Carbon, Nitrogen, and Phosphorus Gene Dynamics During Robinia pseudoacacia Restoration on the Loess Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. DNA Extraction and Metagenomic Sequencing
2.3. Metagenomic Analysis
2.4. Statistical Analysis
3. Results
3.1. The Changes in Soil Physicochemical Properties and Nutrient Dynamics
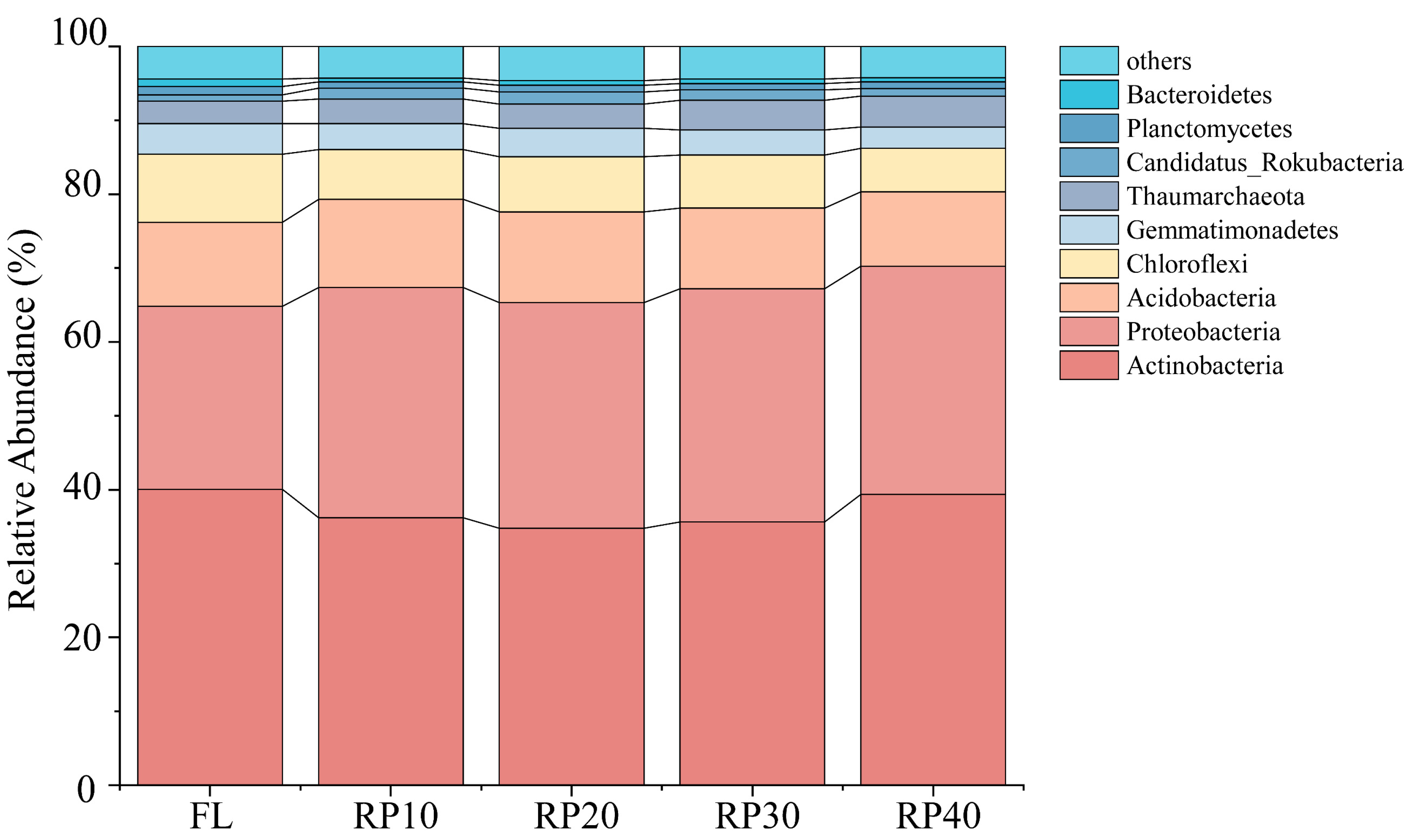
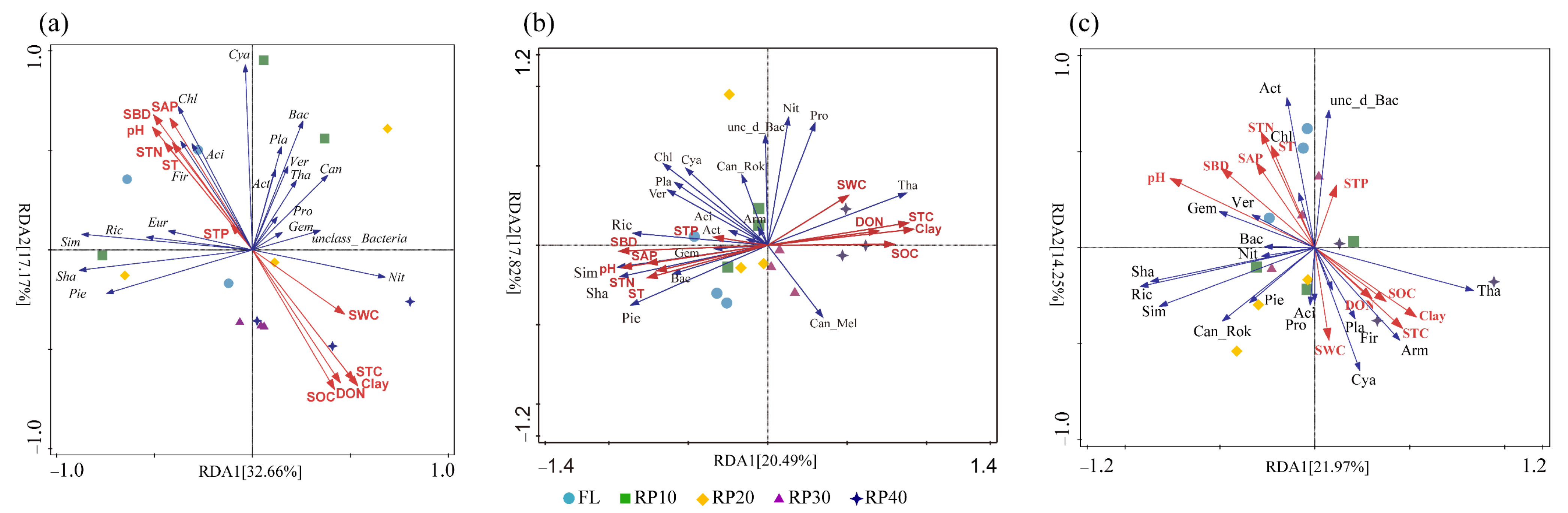
3.2. The Changes in Soil Microbial Composition
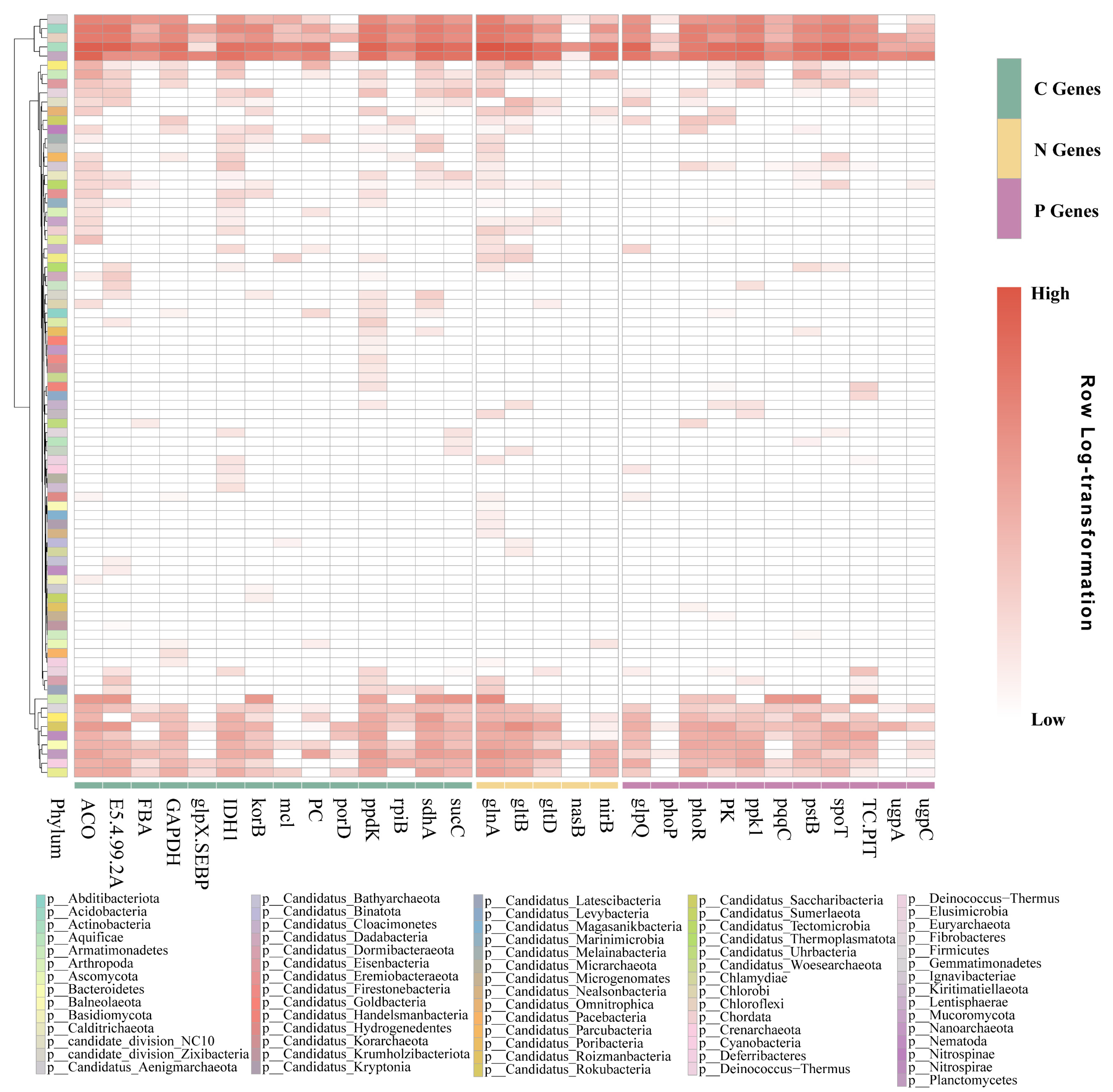
3.3. Co-Occurrence Network Analysis of C, N, and P Cycling Genes
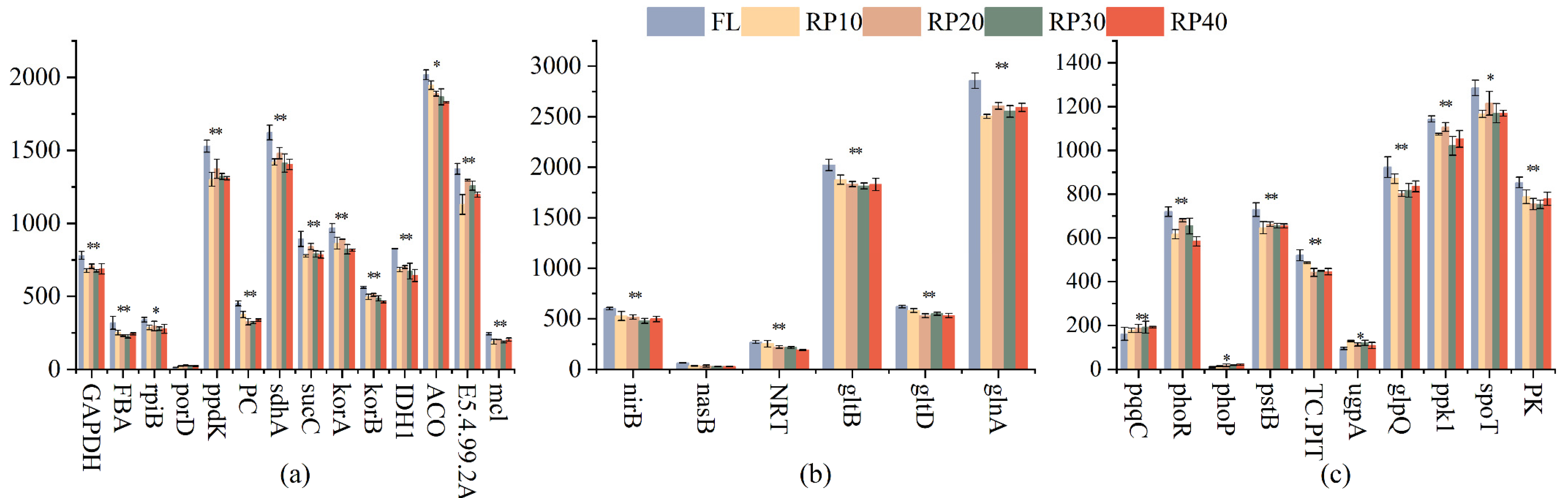
3.4. Changes in C-, N-, and P-Cycling Functional Genes
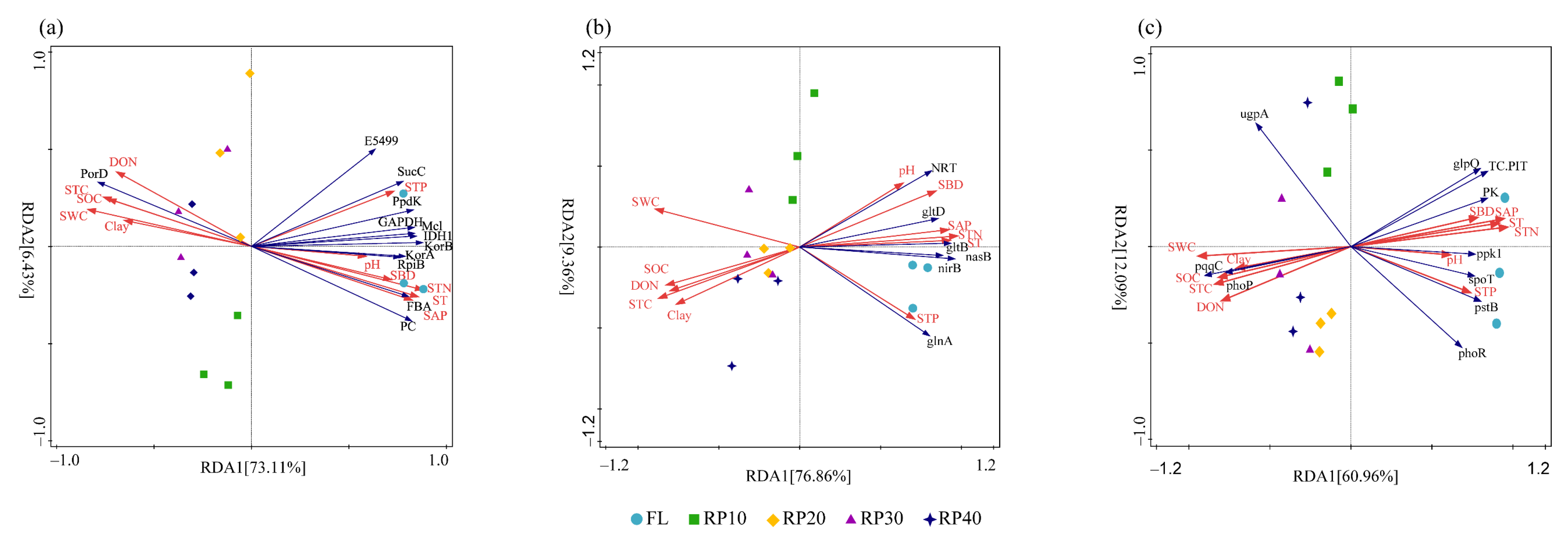
3.5. Driving Factors of Synergistic Changes in C-, N-, and P-Cycling Functional Genes
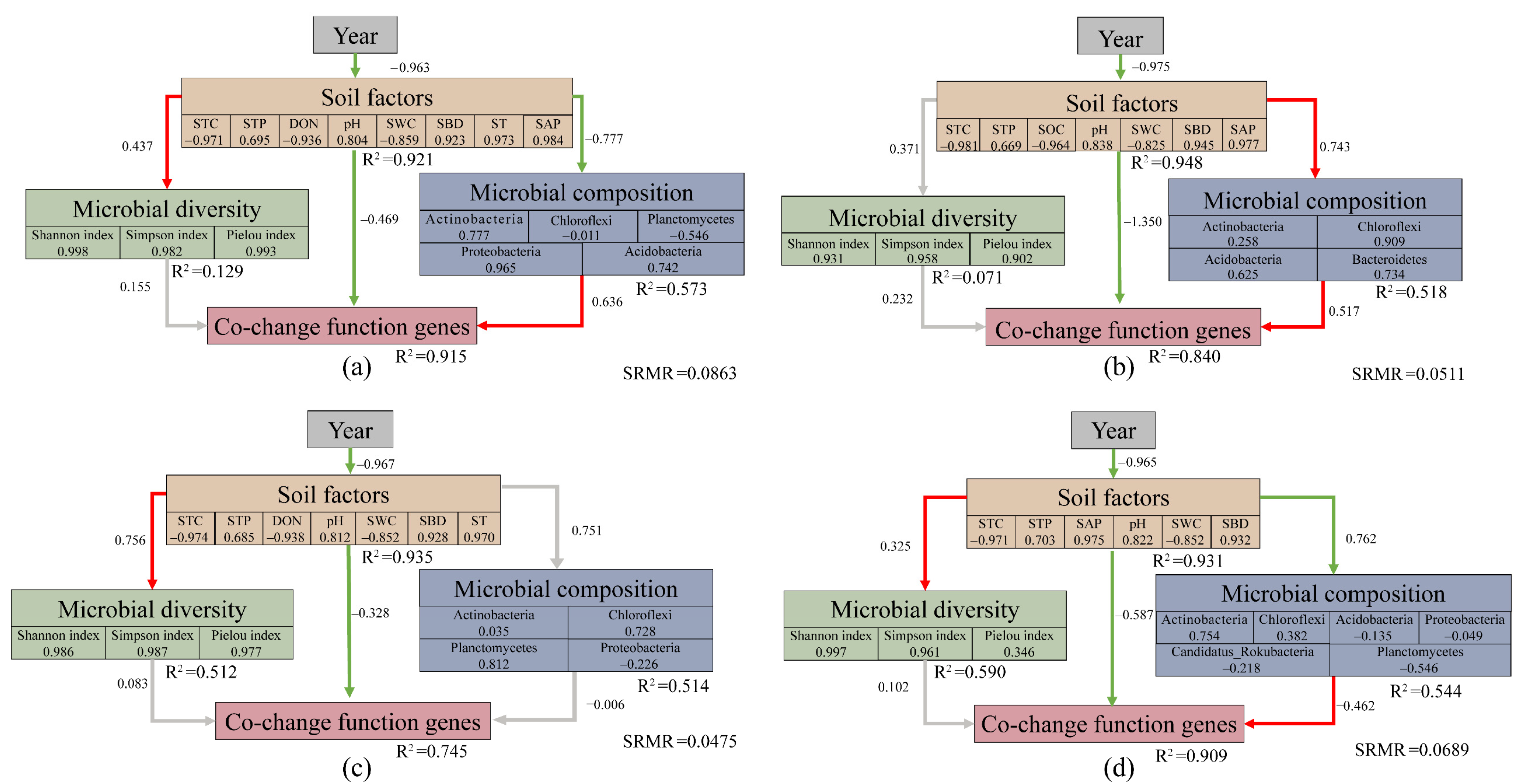
3.6. Structural Equation Modeling (SEM)
4. Discussion
4.1. Soil Physicochemical Properties and Microbial Composition Undergo Significant Changes with the Increasing Restoration Years of R. pseudoacacia
4.2. The Differences and Synergistic Changes in C-, N-, and P-Cycling Functional Genes with the Increasing Restoration Years of R. pseudoacacia
4.3. Drivers of Synergistic Changes in C, N, and P Functional Genes with the Increasing Restoration Years of R. pseudoacacia
4.4. Practical Implications for Ecological Restoration and Soil Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.P.; McCulley, R.L.; Burke, I.C. Carbon fluxes, nitrogen cycling, and soil microbial communities in adjacent urban, native and agricultural ecosystems. Glob. Change Biol. 2005, 11, 575–587. [Google Scholar] [CrossRef]
- Serna-Chavez, H.M.; Fierer, N.; van Bodegom, P.M. Global drivers and patterns of microbial abundance in soil. Glob. Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hu, H.; Anderson, I.C.; Jeffries, T.C.; Zhou, J.; Singh, B.K. Microbial regulation of the soil carbon cycle: Evidence from gene–enzyme relationships. ISME J. 2016, 10, 2593–2604. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Moreno-de las Heras, M. Development of soil physical structure and biological functionality in mining spoils affected by soil erosion in a Mediterranean-Continental environment. Geoderma 2009, 149, 249–256. [Google Scholar] [CrossRef]
- Chen, Y.; Chi, J.; Lu, X.; Cai, Y.; Jiang, H.; Zhang, Q.; Zhang, K. Fungal-bacterial composition and network complexity determine soil multifunctionality during ecological restoration. Catena 2023, 230, 107251. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, H.; Yao, Q.; Jiao, F.; Hu, X.; Liu, J.; Jin, J.; Liu, X.; Wang, G. Soil pH and carbon quality index regulate the biogeochemical cycle couplings of carbon, nitrogen and phosphorus in the profiles of Isohumosols. Sci. Total Environ. 2024, 922, 171269. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Liu, J.; Zhang, Z.; Zhang, W.; Qi, Y.; Li, W.; Chen, Y. Changes of soil bacterial community, network structure, and carbon, nitrogen and sulfur functional genes under different land use types. Catena 2023, 231, 107385. [Google Scholar] [CrossRef]
- Liao, J.; Dou, Y.; Yang, X.; An, S. Soil microbial community and their functional genes during grassland restoration. J. Environ. Manag. 2023, 325, 116488. [Google Scholar] [CrossRef]
- Schleuss, P.-M.; Widdig, M.; Heintz-Buschart, A.; Guhr, A.; Martin, S.; Kirkman, K.; Spohn, M. Stoichiometric controls of soil carbon and nitrogen cycling after long-term nitrogen and phosphorus addition in a mesic grassland in South Africa. Soil. Biol. Biochem. 2019, 135, 294–303. [Google Scholar] [CrossRef]
- Niu, L.; Shao, Q.; Ning, J.; Yang, X.; Liu, S.; Liu, G.; Zhang, X.; Huang, H. Evaluation on the degree and potential of ecological restoration in Loess Plateau. J. Nat. Resour. 2023, 38, 779. [Google Scholar] [CrossRef]
- Wen, X.; Zhou, Y.; Liang, X.; Li, J.; Huang, Y.; Li, Q. A novel carbon-nitrogen coupled metabolic pathway promotes the recyclability of nitrogen in composting habitats. Bioresour. Technol. 2023, 381, 129134. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Mao, C.; Qin, S.; Wang, J.; Liu, F.; Blagodatsky, S.; Yang, G.; Zhang, Q.; Zhang, D.; et al. Nitrogen availability regulates topsoil carbon dynamics after permafrost thaw by altering microbial metabolic efficiency. Nat. Commun. 2018, 9, 3951. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Wang, J.; Dijkstra, F.A.; Lei, S.; Zhang, L.; Wang, X.; Liu, G.; Zhang, C. Nitrogen enrichment stimulates rhizosphere multi-element cycling genes via mediating plant biomass and root exudates. Soil. Biol. Biochem. 2024, 190, 109306. [Google Scholar] [CrossRef]
- Richy, E.; Fort, T.; Odriozola, I.; Kohout, P.; Barbi, F.; Martinovic, T.; Tupek, B.; Adamczyk, B.; Lehtonen, A.; Mäkipää, R.; et al. Phosphorus limitation promotes soil carbon storage in a boreal forest exposed to long-term nitrogen fertilization. Glob. Change Biol. 2024, 30, e17516. [Google Scholar] [CrossRef]
- Watkins-Brandt, K.; Letelier, R.; Spitz, Y.; Church, M.; Böttjer, D.; White, A. Addition of inorganic or organic phosphorus enhances nitrogen and carbon fixation in the oligotrophic North Pacific. Mar. Ecol. Prog. Ser. 2011, 432, 17–29. [Google Scholar] [CrossRef]
- Luo, G.; Xue, C.; Jiang, Q.; Xiao, Y.; Zhang, F.; Guo, S.; Shen, Q.; Ling, N. Soil Carbon, Nitrogen, and Phosphorus Cycling Microbial Populations and Their Resistance to Global Change Depend on Soil C:N:P Stoichiometry. mSystems 2020, 5, e00162-e20. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Li, T.; Yang, J.; Zhao, L.; Yuan, D.; Guo, Z.; Liu, C.; Duan, C. Changes in soil microbe-mediated carbon, nitrogen and phosphorus cycling during spontaneous succession in abandoned PbZn mining areas. Sci. Total Environ. 2024, 920, 171018. [Google Scholar] [CrossRef] [PubMed]
- Séneca, J.; Söllinger, A.; Herbold, C.W.; Pjevac, P.; Prommer, J.; Verbruggen, E.; Sigurdsson, B.D.; Peñuelas, J.; Janssens, I.A.; Urich, T.; et al. Increased microbial expression of organic nitrogen cycling genes in long-term warmed grassland soils. ISME Commun. 2021, 1, 69. [Google Scholar] [CrossRef] [PubMed]
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, abe0725. [Google Scholar]
- Li, H.; Yang, S.; Xu, Z.; Yan, Q.; Li, X.; van Nostrand, J.D.; He, Z.; Yao, F.; Han, X.; Zhou, J.; et al. Responses of soil microbial functional genes to global changes are indirectly influenced by aboveground plant biomass variation. Soil. Biol. Biochem. 2017, 104, 18–29. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Scarlett, K.; Denman, S.; Clark, D.R.; Forster, J.; Vanguelova, E.; Brown, N.; Whitby, C. Relationships between nitrogen cycling microbial community abundance and composition reveal the indirect effect of soil pH on oak decline. ISME J. 2021, 15, 623–635. [Google Scholar] [CrossRef]
- Wen, X.; Zhen, L. Soil erosion control practices in the Chinese Loess Plateau: A systematic review. Environ. Dev. 2020, 34, 100493. [Google Scholar] [CrossRef]
- Li, C.; Fu, B.; Wang, S.; Stringer, L.C.; Wang, Y.; Li, Z.; Liu, Y.; Zhou, W. Drivers and impacts of changes in China’s drylands. Nat. Rev. Earth Environ. 2021, 2, 858–873. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.-J.; Wei, Z.-H.; Fan, M.-C.; Shangguan, Z.-P. Revealing nutrient limitation status of microorganisms in the soil of Robinia pseudoacacia plantation through soil stoichiometry and enzyme metrology. Ying Yong Sheng Tai Xue Bao 2024, 35, 1799–1806. [Google Scholar] [CrossRef]
- Vlachodimos, K.; Papatheodorou, E.M.; Diamantopoulos, J.; Monokrousos, N. Assessment of Robinia pseudoacacia cultivations as a restoration strategy for reclaimed mine spoil heaps. Environ. Monit. Assess. 2013, 185, 6921–6932. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; Wei, Z.; Zhang, K.; Ma, X.; Shangguan, Z. Broad environmental adaptation of abundant microbial taxa in Robinia pseudoacacia forests during long-term vegetation restoration. Environ. Res. 2024, 242, 117720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.; Gao, D.; Wang, X.; Liu, W.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Ecoenzymatic stoichiometry and nutrient dynamics along a revegetation chronosequence in the soils of abandoned land and Robinia pseudoacacia plantation on the Loess Plateau, China. Soil. Biol. Biochem. 2019, 134, 1–14. [Google Scholar] [CrossRef]
- Ren, C.; Kang, D.; Wu, J.P.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Ren, C.; Wang, T.; Xu, Y.; Deng, J.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. For. Ecol. Manag. 2018, 409, 170–178. [Google Scholar] [CrossRef]
- Li, K.; Han, X.; Ni, R.; Shi, G.; de-Miguel, S.; Li, C.; Shen, W.; Zhang, Y.; Zhang, X. Impact of Robinia pseudoacacia stand conversion on soil properties and bacterial community composition in Mount Tai, China. For. Ecosyst. 2021, 8, 19. [Google Scholar] [CrossRef]
- Tian, H.; Qiao, J.; Zhu, Y.; Jia, X.; Shao, M. Vertical distribution of soil available phosphorus and soil available potassium in the critical zone on the Loess Plateau, China. Sci. Rep. 2021, 11, 3159. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000; pp. 263–270. [Google Scholar]
- Peng, X.; Wang, W. Stoichiometry of Soil Extracellular Enzyme Activity along a Climatic Transect in Temperate Grasslands of Northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Faé, G.S.; Montes, F.; Bazilevskaya, E.; Añó, R.M.; Kemanian, A.R. Making Soil Particle Size Analysis by Laser Diffraction Compatible with Standard Soil Texture Determination Methods. Soil. Sci. Soc. Am. J. 2019, 83, 1244–1252. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Shao, M.-A.; Wang, Y.-Q. Spatial patterns of soil total nitrogen and soil total phosphorus across the entire Loess Plateau region of China. Geoderma 2013, 197–198, 67–78. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil. Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Baldrian, P.; Trögl, J.; Frouz, J.; Šnajdr, J.; Valášková, V.; Merhautová, V.; Cajthaml, T.; Herinková, J. Enzyme activities and microbial biomass in topsoil layer during spontaneous succession in spoil heaps after brown coal mining. Soil. Biol. Biochem. 2008, 40, 2107–2115. [Google Scholar] [CrossRef]
- Buchfink, B.; Reuter, K.; Drost, H.-G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Feng, M.; Li, T.; Zeng, C.; He, B.; Zhang, D. Changes in soil water repellency and soil erosion resistance as affected by land uses in karst environments. J. Environ. Manag. 2024, 368, 122102. [Google Scholar] [CrossRef]
- Mehnaz, K.R.; Keitel, C.; Dijkstra, F.A. Phosphorus availability and plants alter soil nitrogen retention and loss. Sci. Total Environ. 2019, 671, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Yu, X.; Chen, M.; Liang, Y. Vegetation recovery reshapes the composition and enhances the network connectivity of phoD-harboring microorganisms to promote P availability in a karst ecosystem. Sci. Total Environ. 2024, 918, 170561. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-L.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, J. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Goodfellow, M.; Williams, S.T. Ecology of actinomycetes. Annu. Rev. Microbiol. 1983, 37, 189–216. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Wang, Q. Roots have greater effects on the accumulation of soil microbial residue carbon in microaggregate fractions than leaf litter in a subtropical forest. Geoderma 2024, 442, 116803. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Sierra, B.E.G.; et al. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef]
- Javed, Z.; Tripathi, G.D.; Mishra, M.; Dashora, K. Actinomycetes—The microbial machinery for the organic-cycling, plant growth, and sustainable soil health. Biocatal. Agric. Biotechnol. 2021, 31, 101893. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Kielak, A.M.; Castellane, T.C.L.; Campanharo, J.C.; Colnago, L.A.; Costa, O.Y.A.; Corradi Da Silva, M.L.; Van Veen, J.A.; Lemos, E.G.M.; Kuramae, E.E. Characterization of novel Acidobacteria exopolysaccharides with potential industrial and ecological applications. Sci. Rep. 2017, 7, 41193. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil Acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, Y.; Ye, S.; Liu, S.; Stirling, E.; Gilbert, J.A.; Faust, K.; Knight, R.; Jansson, J.K.; Cardona, C.; et al. Earth microbial co-occurrence network reveals interconnection pattern across microbiomes. Microbiome 2020, 8, 82. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu Linwei Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu Liyou Yang, Y.; Tiedje, J.M.; Zhou, J. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Du, T.; Hu, Q.; Mao, W.; Yang, Z.; Chen, H.; Sun, L.; Zhai, M. Metagenomics insights into the functional profiles of soil carbon, nitrogen, and phosphorus cycles in a walnut orchard under various regimes of long-term fertilisation. Eur. J. Agron. 2023, 148, 126887. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Vyas, P.; Gulati, A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009, 9, 174. [Google Scholar] [CrossRef]
- Langhans, C.; Beusen, A.H.W.; Mogollón, J.M.; Bouwman, A.F. Phosphorus for Sustainable Development Goal target of doubling smallholder productivity. Nat. Sustain. 2022, 5, 57–63. [Google Scholar] [CrossRef]
- He, X.; Abs, E.; Allison, S.D.; Tao, F.; Huang, Y.; Manzoni, S.; Abramoff, R.; Bruni, E.; Bowring, S.P.K.; Chakrawal, A.; et al. Emerging multiscale insights on microbial carbon use efficiency in the land carbon cycle. Nat. Commun. 2024, 15, 8010. [Google Scholar] [CrossRef]
- Dai, S.; Wang, B.; Ye, R.; Zhang, D.; Xie, Z.; Yu, N.; Cai, C.; Huang, C.; Zhao, J.; Zhang, F.; et al. Structural Evolution of Bacterial Polyphosphate Degradation Enzyme for Phosphorus Cycling. Adv. Sci. 2024, 11, 2309602. [Google Scholar] [CrossRef]
- Yang, L.; Du, L.; Li, W.; Wang, R.; Guo, S. Divergent responses of phoD- and pqqC-harbouring bacterial communities across soil aggregates to long fertilization practices. Soil. Tillage Res. 2023, 228, 105634. [Google Scholar] [CrossRef]
- Lidbury, I.D.E.A.; Scanlan, D.J.; Murphy, A.R.J.; Christie-Oleza, J.A.; Aguilo-Ferretjans, M.M.; Hitchcock, A.; Daniell, T.J. A widely distributed phosphate-insensitive phosphatase presents a route for rapid organophosphorus remineralization in the biosphere. Proc. Natl. Acad. Sci. USA 2022, 119, e2118122119. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, S. The microbial phosphorus cycle in aquatic ecosystems. Nat. Rev. Microbiol. 2024, 1–17. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, H.; Huang, Y.; Wu, S.; Tian, J.; Niu, Y.; Zou, C.; Jia, C.; Jin, M.; Huang, J.; et al. Glutamine synthetase gene glnA plays a vital role in curdlan biosynthesis of Agrobacterium sp. CGMCC 2020, 11546. Int. J. Biol. Macromol. 2020, 165, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, W.; Hou, L.; Zakem, E.J.; Wan, X.; Zhao, Z.; Liu, L.; Hunt, K.A.; Jiao, N.; Kao, S.-J.; et al. Nitrifier Adaptation to Low Energy Flux Controls Inventory of Reduced Nitrogen in the Dark Ocean. Proc. Natl. Acad. Sci. USA 2020, 117, 4823–4830. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, B.; Liu, J.; Wang, X.; Han, F.; Zhou, J. Effects of Different Ages of Robinia pseudoacacia Plantations on Soil Physiochemical Properties and Microbial Communities. Sustainability 2020, 12, 9161. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, X.; Wu, X.; Zheng, F.; Li, S.; Zhuang, Y.; Man, X.; Degré, A. Microbial Regulation of Aggregate Stability and Carbon Sequestration under Long-Term Conservation Tillage and Nitrogen Application. Sustain. Prod. Consum. 2024, 44, 74–86. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, J.; Ye, C.; Li, S.; Duan, S.; Wang, Z.; Huang, H.; Liu, Y.; Deng, W.; Mei, X.; et al. Foliar Pathogen Infection Manipulates Soil Health through Root Exudate-Modified Rhizosphere Microbiome. Microbiol. Spectr. 2022, 10, e0241822. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, Z.; Li, Y.; Wang, G.; Liu, X.; Tang, C.; Lian, T.; Adams, J.; Liu, J.; Liu, J.; et al. Soil microbial metabolism on carbon and nitrogen transformation links the crop-residue contribution to soil organic carbon. NPJ Biofilms Microbiomes 2022, 8, 14. [Google Scholar] [CrossRef]
- Gill, A.L.; Grinder, R.M.; See, C.R.; Chapin, F.S.; DeLancey, L.C.; Fisk, M.C.; Groffman, P.M.; Harms, T.; Hobbie, S.E.; Knoepp, J.D.; et al. Soil carbon availability decouples net nitrogen mineralization and net nitrification across United States Long Term Ecological Research sites. Biogeochemistry 2023, 162, 13–24. [Google Scholar] [CrossRef]
- Mosley, O.E.; Gios, E.; Close, M.; Weaver, L.; Daughney, C.; Handley, K.M. Nitrogen cycling and microbial cooperation in the terrestrial subsurface. ISME J. 2022, 16, 2561–2573. [Google Scholar] [CrossRef]

| Soil Properties | Sample Plot | ||||
|---|---|---|---|---|---|
| FL | RP10 | RP20 | RP30 | RP40 | |
| STC (g kg−1) | 5.83 ± 0.24e | 10.37 ± 0.29d | 14.80 ± 0.052c | 19.39 ± 0.54b | 22.99 ± 0.34a |
| STN (g kg−1) | 0.28 ± 0.0037e | 0.50 ± 0.015d | 0.69 ± 0.024c | 0.92 ± 0.0060b | 1.06 ± 0.016a |
| STP (g kg−1) | 0.59 ± 0.0039a | 0.55 ± 0.0055c | 0.57 ± 0.0068b | 0.55 ± 0.0065bc | 0.57 ± 0.0051b |
| DON (mg kg−1) | 11.13 ± 0.57e | 18.38 ± 0.85d | 34.21 ± 2.018c | 56.87 ± 1.89a | 46.86 ± 1.21b |
| SAP (mg kg−1) | 4.46 ± 0.11a | 3.48 ± 0.089b | 2.87 ± 0.10c | 2.12 ± 0.033d | 2.27 ± 0.036d |
| SOC (mg kg−1) | 49.85 ± 3.17e | 124.57 ± 3.65d | 180.94 ± 1.18c | 359.02 ± 5.37a | 323.60 ± 5.63b |
| SWC (%) | 9.78 ± 0.16c | 13.54 ± 0.061b | 14.27 ± 0.35ab | 15.20 ± 0.11a | 13.76 ± 0.56b |
| pH | 8.41 ± 0.0058a | 8.30 ± 0.012ab | 8.25 ± 0.012b | 8.10 ± 0.015c | 7.72 ± 0.094d |
| SBD (g cm−3) | 1.29 ± 0.0095a | 1.24 ± 0.0032b | 1.20 ± 0.0060c | 1.15 ± 0.0096d | 1.09 ± 0.0149e |
| ST (%) | 18.72 ± 0.171a | 16.22 ± 0.10b | 15.18 ± 0.18c | 13.66 ± 0.17e | 14.35 ± 0.15d |
| Clay (%) | 18.30 ± 0.25d | 19.10 ± 0.18cd | 19.92 ± 0.17bc | 20.43 ± 0.18b | 22.20 ± 0.44a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, N.; Wang, Y.; Wu, H.; Hao, H.; Sailike, A.; Yu, Z.; Li, S.; Shi, R.; Hao, W.; Zhang, W. Phosphorus Cycling Dominates Microbial Regulation of Synergistic Carbon, Nitrogen, and Phosphorus Gene Dynamics During Robinia pseudoacacia Restoration on the Loess Plateau. Agronomy 2025, 15, 797. https://doi.org/10.3390/agronomy15040797
Peng N, Wang Y, Wu H, Hao H, Sailike A, Yu Z, Li S, Shi R, Hao W, Zhang W. Phosphorus Cycling Dominates Microbial Regulation of Synergistic Carbon, Nitrogen, and Phosphorus Gene Dynamics During Robinia pseudoacacia Restoration on the Loess Plateau. Agronomy. 2025; 15(4):797. https://doi.org/10.3390/agronomy15040797
Chicago/Turabian StylePeng, Ning, Yan Wang, Huifeng Wu, Hongjian Hao, Ahejiang Sailike, Zhouchang Yu, Shicai Li, Runhao Shi, Wenfang Hao, and Wei Zhang. 2025. "Phosphorus Cycling Dominates Microbial Regulation of Synergistic Carbon, Nitrogen, and Phosphorus Gene Dynamics During Robinia pseudoacacia Restoration on the Loess Plateau" Agronomy 15, no. 4: 797. https://doi.org/10.3390/agronomy15040797
APA StylePeng, N., Wang, Y., Wu, H., Hao, H., Sailike, A., Yu, Z., Li, S., Shi, R., Hao, W., & Zhang, W. (2025). Phosphorus Cycling Dominates Microbial Regulation of Synergistic Carbon, Nitrogen, and Phosphorus Gene Dynamics During Robinia pseudoacacia Restoration on the Loess Plateau. Agronomy, 15(4), 797. https://doi.org/10.3390/agronomy15040797





