Design, Synthesis, and Herbicidal Activity of Novel 5-Acylbarbituric Acid Derivatives Containing a Pyrimidinedione Moiety
Abstract
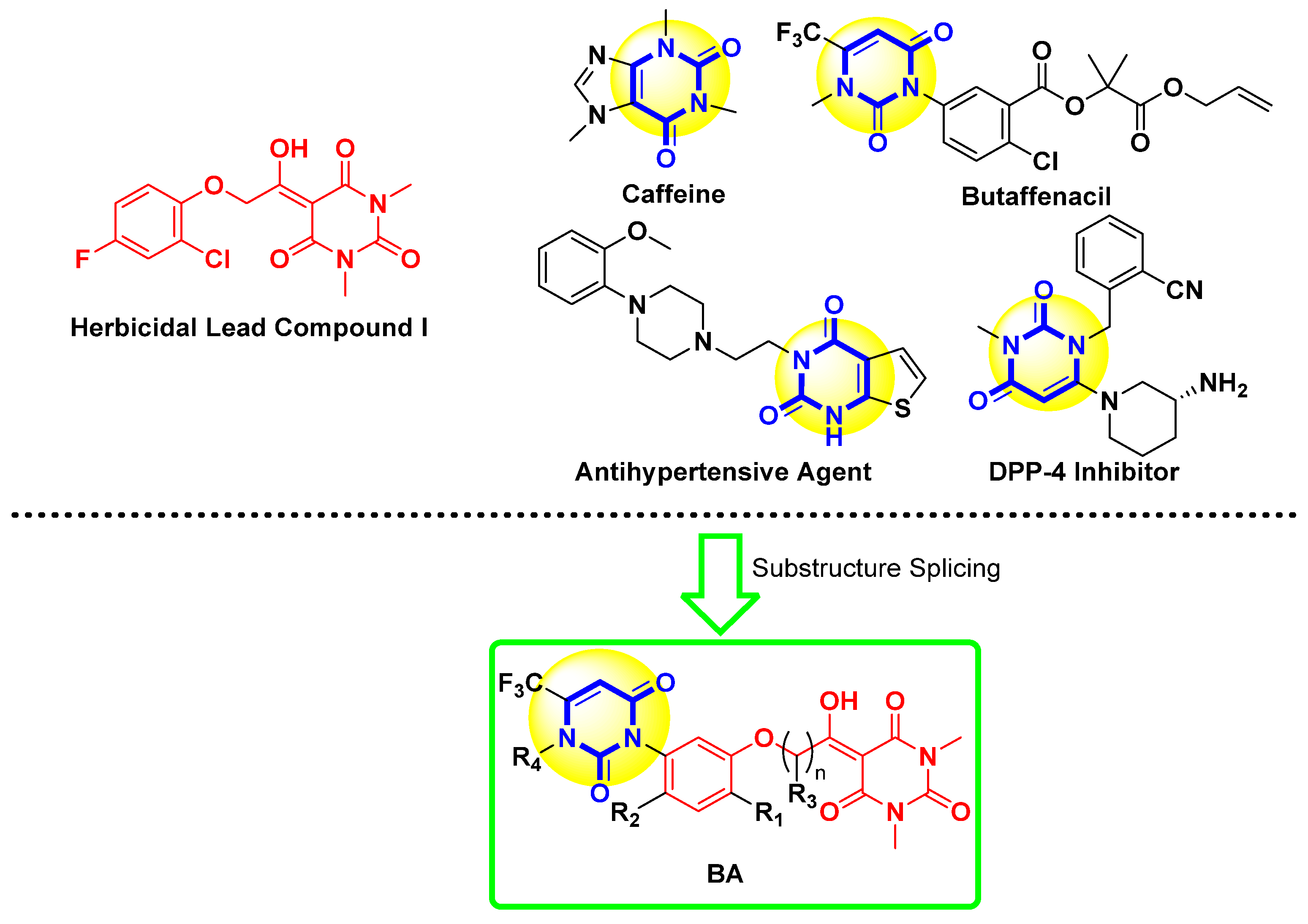
1. Introduction
2. Materials and Methods
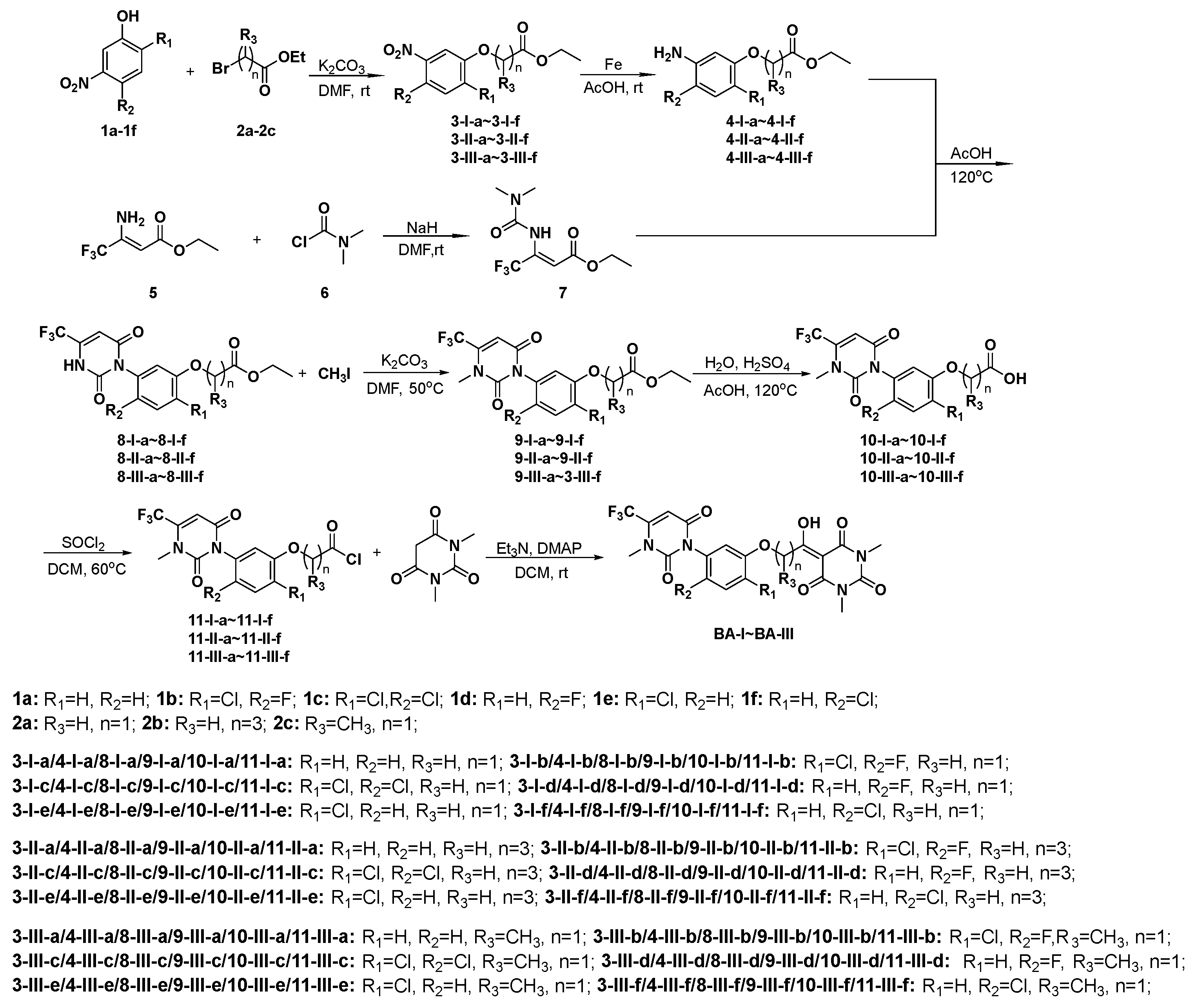
2.1. Chemical Synthesis Procedures
2.1.1. General Method for the Synthesis of Intermediates 3-I-(a-f) to 3-III-(a-f)
2.1.2. General Method for the Synthesis of Intermediates 4-I-(a-f) to 4-III-(a-f)
2.1.3. General Method for the Synthesis of Intermediate 7
2.1.4. General Method for the Synthesis of Intermediates 9-I-(a-f) to 9-III-(a-f)
2.1.5. General Method for the Synthesis of Intermediates 10-I-(a-f) to 10-III-(a-f)
2.1.6. General Method for the Synthesis of Target Compounds BA-I-(1-6) to BA-III-(1-6)
2.2. Evaluation of Herbicidal Activity
2.3. Crops Safety
2.4. Phenotypic Study of Arabidopsis thaliana
2.5. Membrane Permeability Study
2.6. Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
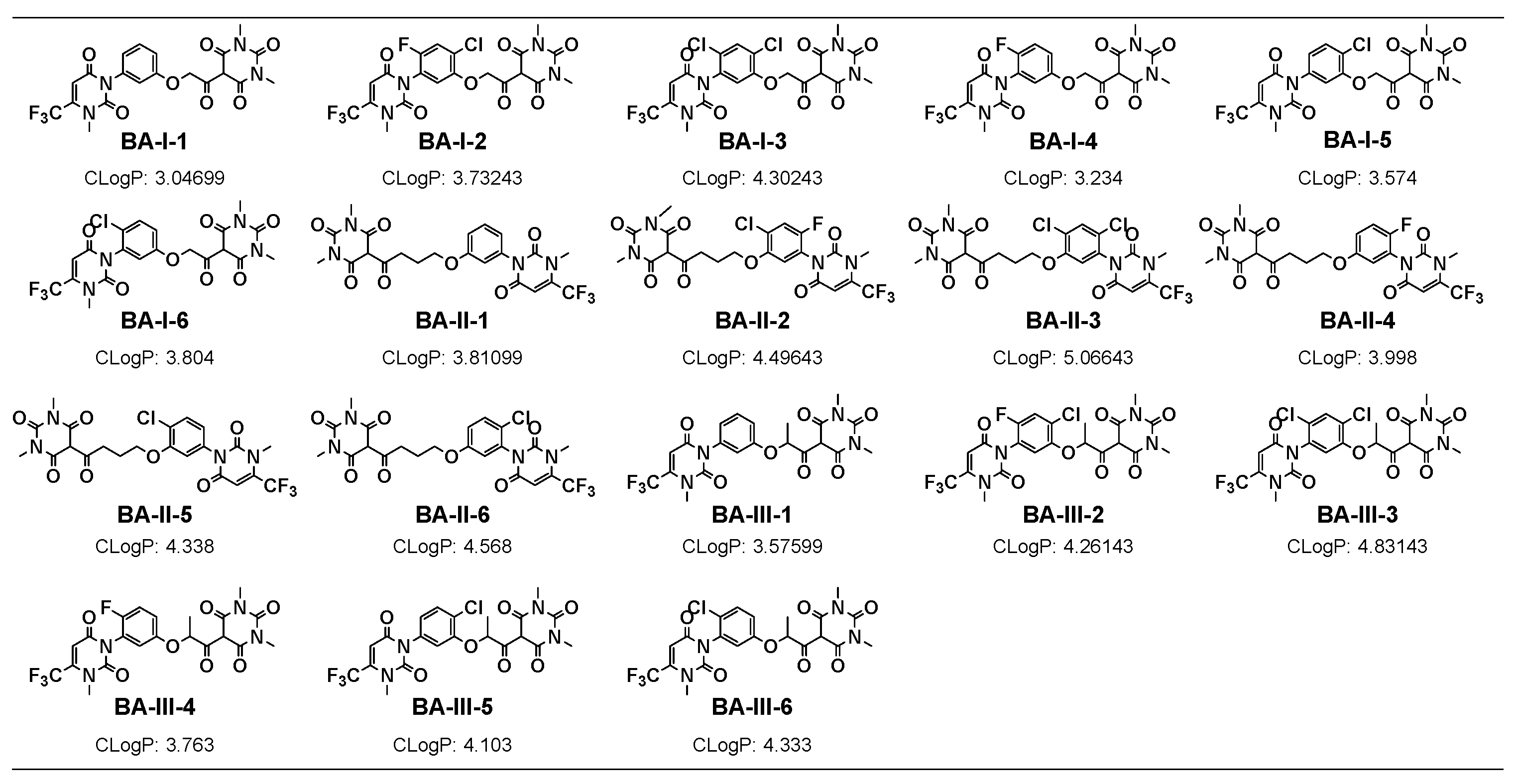
3.1. Chemistry
3.2. Herbicidal Activity and SAR
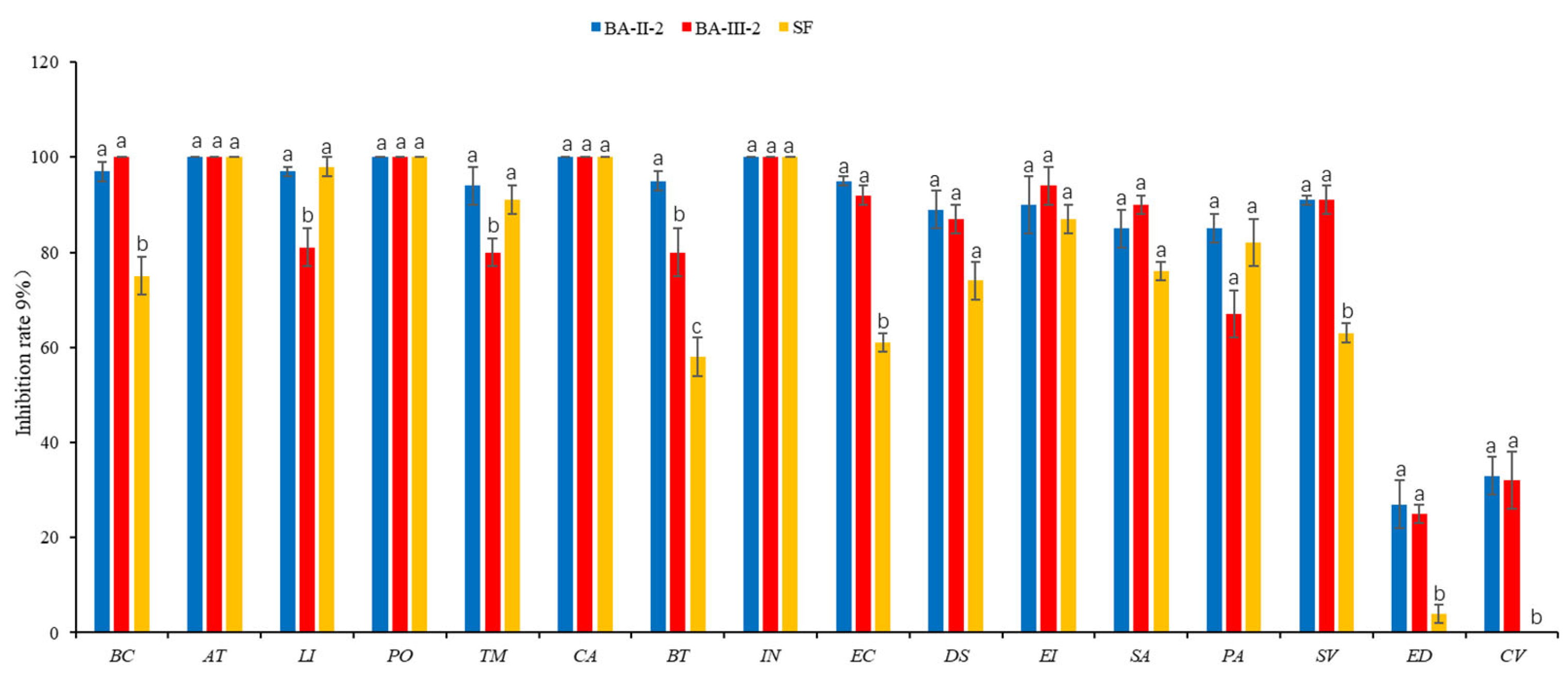
3.3. Herbicidal Spectrum and Crop Safety
3.4. Molecular Mode of Action of the Target Compound
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar]
- Ofosu, R.; Agyemang, E.D.; Márton, A.; Pásztor, G.; Taller, J.; Kazinczi, G. Herbicide resistance: Managing weeds in a changing world. Agronomy 2023, 13, 1595. [Google Scholar] [CrossRef]
- Zhang, P.; Duan, C.B.; Jin, B.; Ali, A.S.; Han, X.Y.; Zhang, H.F.; Zhang, M.Z.; Zhang, W.H.; Gu, Y.C. Recent advances in the natural products-based lead discovery for new agrochemicals. Adv. Agrochem. 2023, 2, 324–339. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products-status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar]
- Ahrens, H.; Lange, G.; Müller, T.; Rosinger, C.; Willms, L.; van Almsick, V. 4-Hydroxyphenylpyruvate dioxygenase inhibitors in combination with safeners: Solutions for modern and sustainable agriculture. Angew. Chem. Int. Ed. 2013, 52, 9388–9398. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef]
- Nadia, A.A.E.; Hajer, H. Mini review on synthesis of pyrimidinthione, pyrimidinedione derivatives and their biological activity. Recent Adv. Petrochem. Sci. 2018, 6, 555676. [Google Scholar]
- Grossmann, K.; Niggeweg, R.; Christiansen, N.; Looser, R.; Ehrhardt, T. The herbicide saflufenacil (kixorTM) is a new inhibitor of protoporphyrinogen ix oxidase activity. Weed Sci. 2010, 58, 1–9. [Google Scholar] [CrossRef]
- Singh, N.; Shreshtha, A.K.; Thakur, M.S.; Patra, S. Xanthine scaffold: Scope and potential in drug development. Heliyon 2018, 4, e00829. [Google Scholar]
- Zhang, F.; Braun, D.R.; Ananiev, G.E.; Hoffmann, F.M.; Tsai, I.W.; Rajski, S.R.; Bugni, T.S. Biemamides A-E, inhibitors of the TGF-β pathway that block the epithelial to mesenchymal transition. Org. Lett. 2018, 20, 5529–5532. [Google Scholar]
- Dewal, M.B.; Wani, A.S.; Vidaillac, C.; Oupický, D.; Rybak, M.J.; Firestine, S.M. Thieno[2,3-d]pyrimidinedione derivatives as antibacterial agents. Eur. J. Med. Chem. 2012, 51, 145–153. [Google Scholar]
- Lin, X.; Kurz, J.L.; Patel, K.M.; Wun, S.J.; Hussein, W.M.; Lonhienne, T.; West, N.P.; McGeary, R.P.; Schenk, G.; Guddat, L.W. Discovery of a pyrimidine-dione derivative with potent inhibitory activity against Mycobacterium tuberculosis ketol-acid reductoisomerase. Chem. Eur. J. 2021, 27, 3130–3141. [Google Scholar]
- Buckheit, K.W.; Yang, L.; Buckheit, R.W., Jr. Development of dual-acting pyrimidinediones as novel and highly potent topical anti-HIV microbicides. Antimicro. Agents Chemother. 2011, 55, 5243–5254. [Google Scholar]
- El-Sherbeny, M.; El-Ashmawy, M.; El-Subbagh, H.; El-Emam, A.; Badria, F. Synthesis, antimicrobial and antiviral evaluation of certain thienopyrimidine derivatives. Eur. J. Med. Chem. 1995, 30, 445–449. [Google Scholar]
- Mitchell, M.L.; Son, J.C.; Guo, H.Y.; Im, Y.A.; Cho, E.J.; Wang, J.H.; Hayes, J.; Wang, M.; Paul, A.; Lansdon, E.B.; et al. N1-Alkyl pyrimidinediones as non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2010, 20, 1589–1592. [Google Scholar]
- Loksha, Y.M.; Pedersen, E.B.; Loddo, R.; Sanna, G.; Collu, G.; Giliberti, G.; Colla, P.L. Synthesis of novel fluoro analogues of mkc442 as microbicides. J. Med. Chem. 2014, 57, 5169–5178. [Google Scholar]
- Zhang, H.; Peng, X.; Dai, Y.; Shao, J.; Ji, Y.; Sun, Y.; Liu, B.; Cheng, X.; Ai, J.; Duan, W. Discovery of a pyrimidinedione derivative as a potent and orally bioavailable Axl inhibitor. J. Med. Chem. 2021, 64, 3956–3975. [Google Scholar]
- Aknin, K.; Bontemps, A.; Farce, A.; Merlet, E.; Belmont, P.; Helissey, P.; Chavatte, P.; Sari, M.A.; Giorgi-Renault, S.; Desbene-Finck, S. Polycyclic nitrogen heterocycles as potential thymidine phosphorylase inhibitors: Synthesis, biological evaluation, and molecular docking study. J. Enzyme Inhib. Med. Chem. 2022, 37, 252–268. [Google Scholar]
- Günther, J.; Hillig, R.C.; Zimmermann, K.; Kaulfuss, S.; Lemos, C.; Nguyen, D.; Rehwinkel, H.; Habgood, M.; Lechner, C.; Neuhaus, R.; et al. BAY-069, a novel (trifluoromethyl)pyrimidinedione-based bcat1/2 inhibitor and chemical probe. J. Med. Chem. 2022, 65, 14366–14390. [Google Scholar]
- Guile, S.D.; Bantick, J.R.; Cooper, M.E.; Donald, D.K.; Eyssade, C.; Ingall, A.H.; Lewis, R.J.; Martin, B.P.; Mohammed, R.T.; Potter, T.J.; et al. Optimization of monocarboxylate transporter 1 blockers through analysis and modulation of atropisomer interconversion properties. J. Med. Chem. 2007, 50, 254–263. [Google Scholar]
- Jha, V.; Bhadoriya, K.S. Synthesis, pharmacological evaluation and molecular docking studies of pyrimidinedione based DPP-4 inhibitors as antidiabetic agents. J. Mol. Struct. 2018, 1158, 96–105. [Google Scholar]
- Zhang, Z.Y.; Wallace, M.B.; Feng, J.; Stafford, J.A.; Skene, R.J.; Shi, L.H.; Lee, B.; Aertgeerts, K.; Jennings, A.; Xu, R.D.; et al. Design and synthesis of pyrimidinone and pyrimidinedione inhibitors of dipeptidyl peptidase IV. J. Med. Chem. 2011, 54, 510–524. [Google Scholar]
- Li, N.; Wang, L.J.; Jiang, B.; Guo, S.J.; Li, X.Q.; Chen, X.C.; Luo, J.; Li, C.; Wang, Y.; Shi, D.Y. Design, synthesis and biological evaluation of novel pyrimidinedione derivatives as DPP-4 inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 2131–2135. [Google Scholar]
- Park, J.; Ahn, Y.O.; Nam, J.-W.; Hong, M.-K.; Song, N.; Kim, T.; Yu, G.-H.; Sung, S.-K. Biochemical and physiological mode of action of tiafenacil, a new protoporphyrinogen ix oxidase-inhibiting herbicide. Pestic. Biochem. Phys. 2018, 152, 38–44. [Google Scholar]
- Lee, W.H.; Kwon, Y.B.; Lee, K.H.; Choi, J.S.; Seu, Y.B. Synthesis of cyclopropyl ester and amide substituted pyrimidinediones as protoporphyrinogen oxidase-inhibiting herbicides. Bull. Korean Chem. Soc. 2021, 42, 420–428. [Google Scholar]
- Lee, W.H.; Kwon, Y.B.; Kim, J.H.; Lee, K.H.; Maezono, S.M.B.; Choi, J.S.; Seu, Y.B. Design and synthesis of acrylate and acrylamide substituted pyrimidinediones as potential PPO herbicides. Bioorg. Med. Chem. 2021, 31, 115959. [Google Scholar]
- Wang, D.W.; Zhang, R.B.; Ismail, I.; Xue, Z.Y.; Liang, L.; Yu, S.Y.; Wen, X.; Xi, Z. Design, herbicidal activity, and QSAR analysis of cycloalka[d]quinazoline-2,4-dione-benzoxazinones as protoporphyrinogen IX oxidase inhibitors. J. Agric. Food Chem. 2019, 67, 9254–9264. [Google Scholar]
- Wang, D.W.; Zhang, H.; Yu, S.Y.; Zhang, R.B.; Liang, L.; Wang, X.; Yang, H.Z.; Xi, Z. Discovery of a potent thieno[2,3-d]pyrimidine-2,4-dione-based protoporphyrinogen IX oxidase inhibitor through an in silico structure-guided optimization approach. J. Agric. Food Chem. 2021, 69, 14115–14125. [Google Scholar]
- Wang, D.W.; Lin, H.Y.; Cao, R.J.; Ming, Z.Z.; Chen, T.; Hao, G.F.; Yang, W.C.; Yang, G.F. Design, synthesis and herbicidal activity of novel quinazoline-2,4-diones as 4-hydroxyphenylpyruvate dioxygenase inhibitors. Pest Manag. Sci. 2015, 71, 1122–1132. [Google Scholar]
- Wang, D.W.; Lin, H.Y.; Cao, R.J.; Yang, S.G.; Chen, Q.; Hao, G.F.; Yang, W.C.; Yang, G.F. Synthesis and herbicidal evaluation of triketone-containing quinazoline-2,4-diones. J. Agric. Food Chem. 2014, 62, 11786–11796. [Google Scholar]
- Lei, K.; Li, P.; Zhou, X.; Wang, S.; Wang, X.; Ji, L.; Liu, R.; Xu, X. Design, synthesis and herbicidal activity of 5-acylbarbituric acid derivatives and study of molecular mode of action. Chin. J. Org. Chem. 2020, 40, 2788–2797. [Google Scholar]
- Lei, K.; Li, P.; Yang, X.F.; Wang, S.B.; Wang, X.K.; Hua, X.W.; Sun, B.; Ji, L.S.; Xu, X.H. Design and synthesis of novel 4-hydroxyl-3-(2-phenoxyacetyl)-pyran-2-one derivatives for use as herbicides and evaluation of their mode of action. J. Agric. Food Chem. 2019, 67, 10489–10497. [Google Scholar]
- Wang, C.D.; Qing, X.S.; Wang, T.; Dai, C.L.; Su, Z.J. Direct synthesis of 6H-chromeno[3,4-b]quinolin-6-ol derivatives from substituted 3-nitro-2H-chromenes and 2-nitrobenzaldehydes mediated by Fe/AcOH system. Synthesis 2018, 50, 1350–1358. [Google Scholar]
- Yang, J.C.; Guan, A.Y.; Wu, Q.; Cui, D.L.; Liu, C.L. Design, synthesis and herbicidal evaluation of novel uracil derivatives containing an isoxazoline moiety. Pest Manag. Sci. 2020, 76, 3395–3402. [Google Scholar]
- Li, N.; Chen, K.; Han, S.B.; Wang, S.M.; He, Y.Q.; Wang, X.K.; Li, P.; Ji, L.S.; Liu, R.; Lei, K. Synthesis, herbicidal activity, and molecular mode of action evaluation of novel aryloxyphenoxypropionate/amide derivatives containing a quinazolinone moiety. J. Agric. Food Chem. 2024, 72, 9445–9456. [Google Scholar]
- Houot, V.; Etienne, P.; Petitot, A.; Barbier, S.; Blein, J.; Suty, L. Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J. Exp. Bot. 2001, 52, 1721–1730. [Google Scholar]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comp. Chem. 2010, 31, 455–461. [Google Scholar]
- Tanchuk, V.Y.; Tanin, V.O.; Vovk, I.; Poda, G. A new improved hybrid scoring function for molecular docking and scoring based on autodock and autodock vina. Chem. Biol. Drug Des. 2016, 87, 618–625. [Google Scholar]
- Di, M.E.; Toti, D.; Polticelli, F. Dockingapp: A user friendly interface for facilitated docking simulations with autodock vina. J. Comput. Aid. Mol. Des. 2017, 31, 213–218. [Google Scholar]
- Chaudhari, R.; Li, Z. Pymine: A pymol plugin to integrate and visualize data for drug discovery. BMC Res. Not. 2015, 8, 517. [Google Scholar]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar]
- Tang, Q.Y.; Lin, Y.X. Maximum entropy-minimum residual model: An optimum solution to comprehensive evaluation and multiple attribute decision making. Entropy 2025, 27, 203. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current contributions of organofluorine compounds to the agrochemical industry. iScience 2020, 23, 101467. [Google Scholar]
- Ojima, I. Exploration of fluorine chemistry at the multidisciplinary interface of chemistry and biology. J. Org. Chem. 2013, 78, 6358–6383. [Google Scholar]
- Rao, W.J.; Li, M.L.; You, X.L.; Wei, Z.H.; Zhang, M.X.; Wang, L.Y.; Cai, H. The role of fluorine-substituted positions on the phase rransition in organic–inorganic hybrid perovskite compounds. Inorg. Chem. 2021, 60, 14706–14712. [Google Scholar]
- Jeschke, P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag. Sci. 2010, 66, 10–27. [Google Scholar]
- Yan, Z.Z.; Yang, Z.H.; Deng, X.L.; Lin, D.; Wu, M.F.; Li, J.M.; Chen, A.Y.; Ye, J.; Hu, A.X.; Liao, H.D. Novel aryloxyphenoxypropionate derivates containing benzofuran moiety: Design, synthesis, herbicidal activity, docking study and theoretical calculation. Pestic. Biochem. Physiol. 2019, 154, 78–87. [Google Scholar]
- Zuo, Y.; Wu, Q.; Su, S.W.; Niu, C.W.; Xi, Z.; Yang, G.F. Synthesis, herbicidal activity, and QSAR of novel N-benzothiazolylpyrimidine-2,4-diones as protoporphyrinogen oxidase inhibitors. J. Agric. Food Chem. 2016, 64, 552–562. [Google Scholar] [PubMed]
- Zhao, L.X.; Jiang, M.J.; Hu, J.J.; Zou, Y.L.; Cheng, Y.; Ren, T.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, and herbicidal activity of novel diphenyl ether derivatives containing fast degrading tetrahydrophthalimide. J. Agric. Food Chem. 2020, 68, 3729–3741. [Google Scholar]
- Qin, X.; Sun, L.; Wen, X.; Yang, X.; Tan, Y.; Jin, H.; Cao, Q.; Zhou, W.; Xi, Z.; Shen, Y. Structural insight into unique properties of protoporphyrinogen oxidase from Bacillus subtilis. J. Struct. Biol. 2010, 170, 76–82. [Google Scholar]
- Wang, B.; Wen, X.; Xi, Z. Molecular simulations bring new insights into protoporphyrinogen IX oxidase/protoporphyrinogen IX interaction modes. Mol. Inf. 2016, 35, 476–482. [Google Scholar]

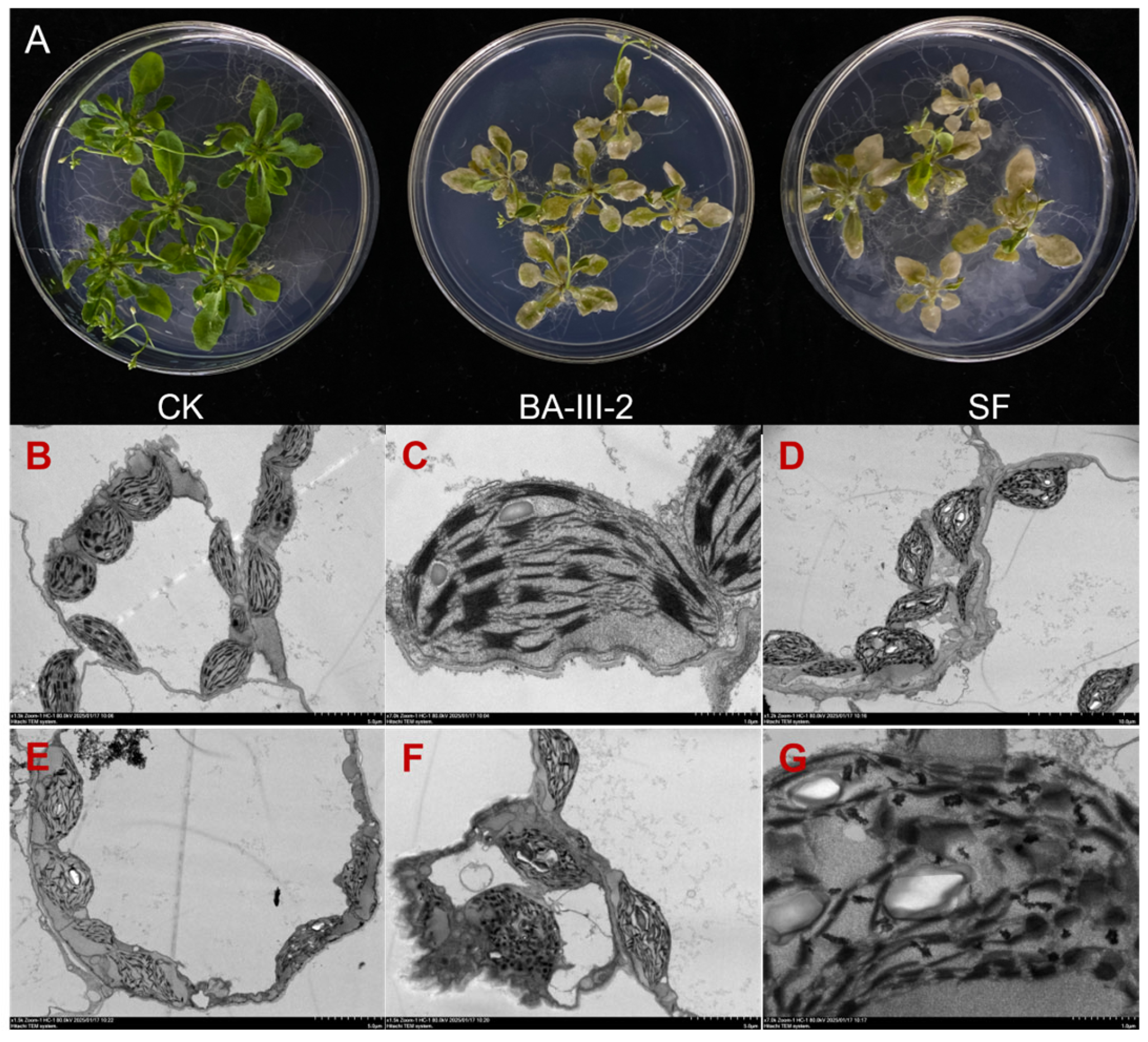
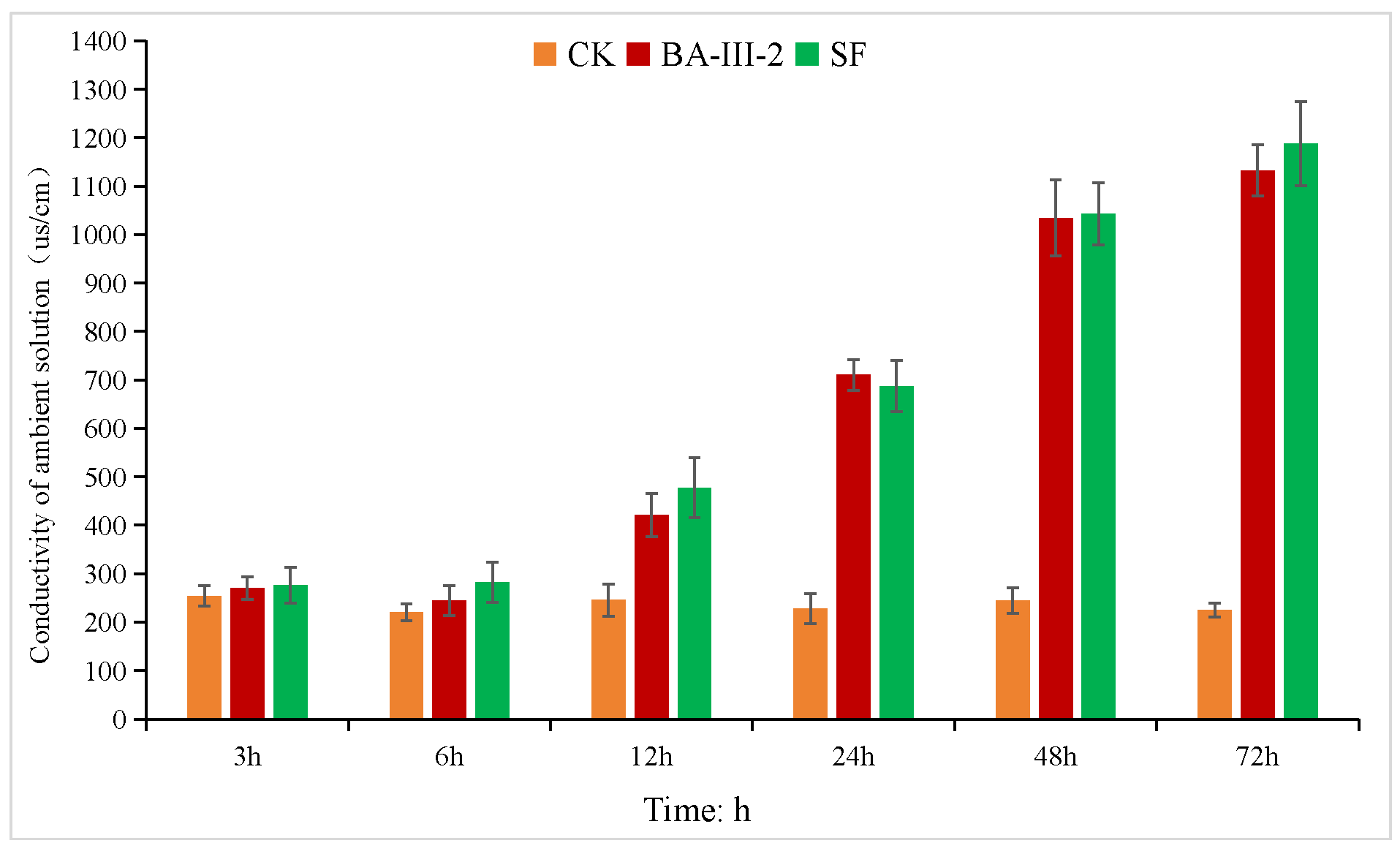
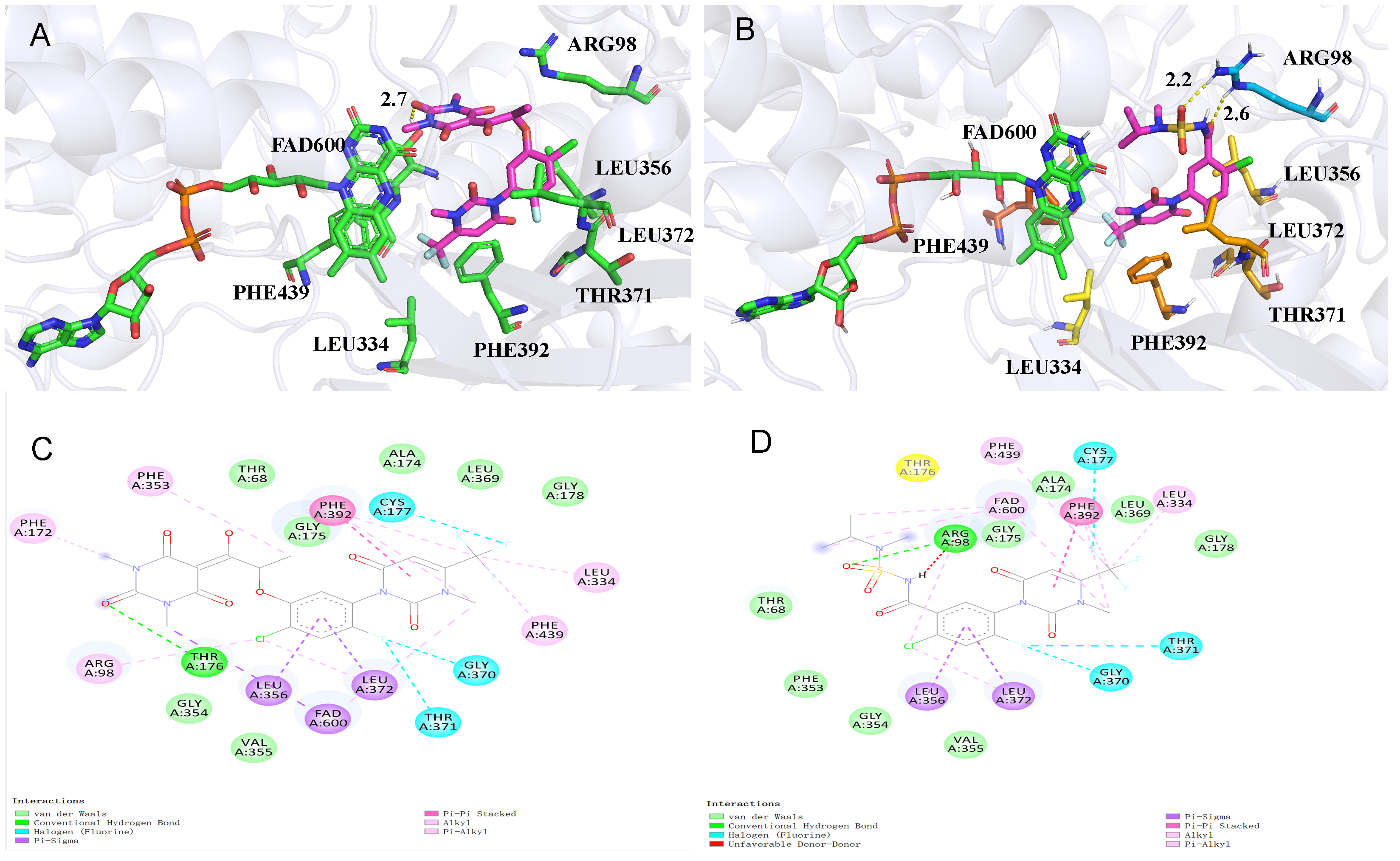
| Comp. | B. campestris | A. tricolor | E. crusgalli | D. sanguinalis | Sum Inhibition 2 |
|---|---|---|---|---|---|
| BA-I-1 | 4 ± 2 Ii | 16 ± 5 Fef | 0 Gh | 7 ± 2 Ij | 27 |
| BA-I-2 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| BA-I-3 | 33 ± 3 Gg | 79 ± 5 Dc | 52 ± 2 Dd | 61 ± 2 De | 225 |
| BA-I-4 | 33 ± 7 Gg | 66 ± 6 Ed | 30 ± 7 Ef | 21 ± 2 Gh | 150 |
| BA-I-5 | 91 ± 2 ABCb | 96 ± 4 ABab | 97 ± 3 Aa | 96 ± 1 Aab | 380 |
| BA-I-6 | 0 Ii | 18 ± 3 Fe | 0 Gh | 7 ± 2 Ij | 24 |
| BA-II-1 | 39 ± 3 Gf | 90 ± 3 BCb | 46 ± 7 De | 60 ± 6 De | 236 |
| BA-II-2 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| BA-II-3 | 83 ± 2 DEd | 100 Aa | 74 ± 1 Bb | 82 ± 3 Bc | 338 |
| BA-II-4 | 90 ± 3 CDbc | 100 Aa | 99 ± 1 Aa | 96 ± 3 Aab | 384 |
| BA-II-5 | 99 ± 2 ABa | 100 Aa | 100 Aa | 100 Aa | 399 |
| BA-II-6 | 0 Ii | 66 ± 5 Ed | 1 ± 0 Gh | 13 ± 3 Hi | 80 |
| BA-III-1 | 61 ± 2 Fe | 81 ± 3 Dc | 22 ± 5 Fg | 33 ± 2 Fg | 196 |
| BA-III-2 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| BA-III-3 | 84 ± 5 CDEcd | 90 ± 1 BCb | 98 ± 3 Aa | 100 Aa | 373 |
| BA-III-4 | 78 ± 6 Ed | 84 ± 4 CDc | 60 ± 3 Cc | 67 ± 3 Cd | 290 |
| BA-III-5 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| BA-III-6 | 2 ± 6 Ii | 12 ± 5 Ff | 51 ± 1 Dde | 45 ± 5 Ef | 110 |
| I | 22 ± 7 Hh | 64 ± 7 Ed | 0 Gh | 5 ± 1 Ij | 91 |
| SF | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| Comp. | Dosage (g ha−1) | BC | AR | EC | DS | Sum Inhibition 2 |
|---|---|---|---|---|---|---|
| BA-I-2 | 75 | 100 Aa | 97 ± 2 Aa | 98 ± 2 Aa | 96 ± 4 ABab | 391 |
| 37.5 | 99 ± 1 Aa | 98 ± 4 Aa | 93 ± 3 ABb | 97 ± 3 Aab | 386 | |
| 18.8 | 83 ± 3 Dd | 96 ± 4ABab | 84 ± 3 BCb | 84 ± 3 ABCbc | 346 | |
| 9.4 | 83 ± 5 BCbc | 95 ± 4 Aa | 45 ± 1 Cd | 61 ± 4 Ccd | 283 | |
| BA-I-3 | 75 | 37 ± 5 Ee | 68 ± 6 Dd | 53 ± 5 Ff | 62 ± 7 De | 220 |
| 37.5 | 34 ± 3 Fg | 55 ± 1 Ee | 18 ± 1 Gh | 38 ± 5 Def | 145 | |
| 18.8 | 28 ± 6 Ff | 51 ± 3 DEe | 5 ± 2 Gfg | 14 ± 2 Hh | 99 | |
| 9.4 | 10 ± 1 Ee | 39 ± 4 Ee | 0 Fg | 0 Gh | 49 | |
| BA-I-4 | 75 | 13 ± 2 Ff | 41 ± 3 Ee | 18 ± 4 Hh | 11 ± 2 Fg | 83 |
| 37.5 | 11 ± 3 Gh | 21 ± 3 Ff | 6 ± 5 Hi | 7 ± 6 Eg | 45 | |
| 18.8 | 5 ± 3 Hh | 13 ± 4 Ff | 0 Gg | 0 Ii | 18 | |
| 9.4 | 0 Ff | 3 ± 2 Hh | 0 Fg | 0 Gh | 3 | |
| BA-I-5 | 75 | 97 ± 4 Aa | 83 ± 5 BCb | 85 ± 5 Cc | 51 ± 6 Ef | 316 |
| 37.5 | 97 ± 3 Aa | 84 ± 3 Bb | 63 ± 2 De | 44 ± 8 De | 289 | |
| 18.8 | 91 ± 4 BCDbc | 78 ± 6 Cc | 28 ± 7 Fe | 46 ± 5 Ff | 243 | |
| 9.4 | 88 ± 4 Bb | 76 ± 4 CDc | 32 ± 5 De | 35 ± 6 De | 230 | |
| BA-II-2 | 75 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| 37.5 | 100 Aa | 100 Aa | 100 Aa | 98 ± 2 Aa | 398 | |
| 18.8 | 97 ± 1 ABCab | 100 Aa | 95 ± 1 Aa | 91 ± 2 Aa | 383 | |
| 9.4 | 96 ± 1 Aa | 100 Aa | 89 ± 2 Aa | 88 ± 3 Aa | 372 | |
| BA-II-3 | 75 | 87 ± 4 Bb | 100 Aa | 90 ± 2 BCb | 91 ± 3 Bb | 368 |
| 37.5 | 61 ± 3 Dd | 100 Aa | 81 ± 4 Cc | 88 ± 3 Ab | 330 | |
| 18.8 | 59 ± 8 Ee | 92 ± 3 ABb | 60 ± 6 Dc | 74 ± 7 DEde | 286 | |
| 9.4 | 48 ± 2 Dd | 84 ± 4 BCb | 44 ± 4 Cd | 57 ± 6 Ccd | 233 | |
| BA-II-4 | 75 | 75 ± 4 Cc | 87 ± 1 Bb | 67 ± 5 Ee | 74 ± 3 Cd | 302 |
| 37.5 | 53 ± 5 Ee | 81 ± 5 Bcb | 41 ± 9 Ef | 56 ± 7 Cd | 231 | |
| 18.8 | 34 ± 6 Ff | 57 ± 6 Dd | 23 ± 7 Fe | 26 ± 1 Gg | 140 | |
| 9.4 | 7 ± 4 Ee | 25 ± 2 Ff | 2 ± 1 Fg | 7 ± 1 Fg | 41 | |
| BA-II-5 | 75 | 95 ± 2 Aa | 100 Aa | 96 ± 1 ABa | 92 ± 3 Bb | 382 |
| 37.5 | 88 ± 3 Bb | 99 ± 3 Aa | 91 ± 3 Bb | 96 ± 4 Aab | 373 | |
| 18.8 | 91 ± 2 BCDbc | 100 Aa | 77 ± 4 Cb | 80 ± 5 BCDcd | 348 | |
| 9.4 | 85 ± 3 BCb | 97 ± 5 Aa | 50 ± 2 Ccd | 63 ± 6 Cc | 295 | |
| BA-III-2 | 75 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| 37.5 | 100 Aa | 100 Aa | 100 Aa | 96 ± 6 Aab | 396 | |
| 18.8 | 100 Aa | 100 Aa | 92 ± 1 Aba | 87 ± 4 ABab | 380 | |
| 9.4 | 100 Aa | 97 ± 6 Aa | 86 ± 6 ABa | 77 ± 5 Bb | 360 | |
| BA-III-3 | 75 | 82 ± 5 BCb | 77 ± 4 Cc | 76 ± 3 Dd | 79 ± 4 Cc | 314 |
| 37.5 | 78 ± 5 Cc | 76 ± 6 Cc | 75 ± 1 Cd | 74 ± 2 Bc | 303 | |
| 18.8 | 58 ± 6 Ee | 73 ± 2 Cc | 48 ± 4 Ed | 46 ± 3 Ff | 224 | |
| 9.4 | 45 ± 4 Dd | 67 ± 6 Dd | 16 ± 4 Ef | 22 ± 5 Ef | 150 | |
| BA-III-4 | 75 | 55 ± 5 Dd | 71 ± 1 Dd | 41 ± 4 Gg | 53 ± 5 Ef | 221 |
| 37.5 | 40 ± 3 Ff | 63 ± 7 Dd | 33 ± 5 Fg | 35 ± 5 Df | 171 | |
| 18.8 | 17 ± 3 Gg | 45 ± 5 Ee | 9 ± 2 Gf | 11 ± 2 Hh | 82 | |
| 9.4 | 8 ± 3 Ee | 12 ± 4 Gg | 0 Fg | 3 ± 2 FGgh | 23 | |
| BA-III-5 | 75 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| 37.5 | 100 Aa | 98 ± 4 Aa | 96 ± 2 ABab | 95 ± 6 Aab | 388 | |
| 18.8 | 99 ± 1 ABa | 91 ± 2 Bb | 66 ± 2 Dc | 68 ± 6 Ee | 325 | |
| 9.4 | 84 ± 3 BCbc | 86 ± 5 Bb | 54 ± 2 Cc | 55 ± 4 Cd | 278 | |
| SF | 75 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 |
| 37.5 | 100 Aa | 100 Aa | 100 Aa | 100 Aa | 400 | |
| 18.8 | 89 ± 3 CDcd | 100 Aa | 84 ± 3 BCb | 75 ± 2 CDEd | 348 | |
| 9.4 | 78 ± 4 Cc | 97 ± 4 Aa | 77 ± 5 Bb | 63 ± 1 Cc | 314 |
| Comp. | Injury (%) | |||
|---|---|---|---|---|
| T. aestivum | Z. mays | G. hirsutum | G. max | |
| BA-II-2 | 36 ± 5 Aa | 49 ± 7 Aa | 52 ± 2 Bb | 100 Aa |
| BA-III-2 | 5 ± 1 Bb | 27 ± 4 ABb | 42 ± 2 Cc | 100 Aa |
| SF | 7 ± 3 Bb | 15 ± 2 Bb | 100 Aa | 62 ± 4 Bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Wang, S.; Fu, S.; Gao, W.; Kim, J.; Park, P.; Liu, R.; Lei, K. Design, Synthesis, and Herbicidal Activity of Novel 5-Acylbarbituric Acid Derivatives Containing a Pyrimidinedione Moiety. Agronomy 2025, 15, 777. https://doi.org/10.3390/agronomy15040777
Chen K, Wang S, Fu S, Gao W, Kim J, Park P, Liu R, Lei K. Design, Synthesis, and Herbicidal Activity of Novel 5-Acylbarbituric Acid Derivatives Containing a Pyrimidinedione Moiety. Agronomy. 2025; 15(4):777. https://doi.org/10.3390/agronomy15040777
Chicago/Turabian StyleChen, Ke, Shumin Wang, Shuyue Fu, Wei Gao, Junehyun Kim, Phumbum Park, Rui Liu, and Kang Lei. 2025. "Design, Synthesis, and Herbicidal Activity of Novel 5-Acylbarbituric Acid Derivatives Containing a Pyrimidinedione Moiety" Agronomy 15, no. 4: 777. https://doi.org/10.3390/agronomy15040777
APA StyleChen, K., Wang, S., Fu, S., Gao, W., Kim, J., Park, P., Liu, R., & Lei, K. (2025). Design, Synthesis, and Herbicidal Activity of Novel 5-Acylbarbituric Acid Derivatives Containing a Pyrimidinedione Moiety. Agronomy, 15(4), 777. https://doi.org/10.3390/agronomy15040777







