Effects of Low-Temperature Stress During Anthesis Stage on Dry Matter Accumulation and Yield of Winter Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Items and Methods
2.2.1. Accumulation, Transport, and Distribution of Dry Matter
2.2.2. Grain Weight
2.2.3. Fitting of Grain-Filling Process
2.2.4. Determination of Yield Components
2.2.5. Determination of Pollen Viability
2.3. Data Processing
3. Results
3.1. Effects of Low-Temperature Stress During Anthesis Stage on Dry Matter Accumulation, Distribution, and Transport of Winter Wheat
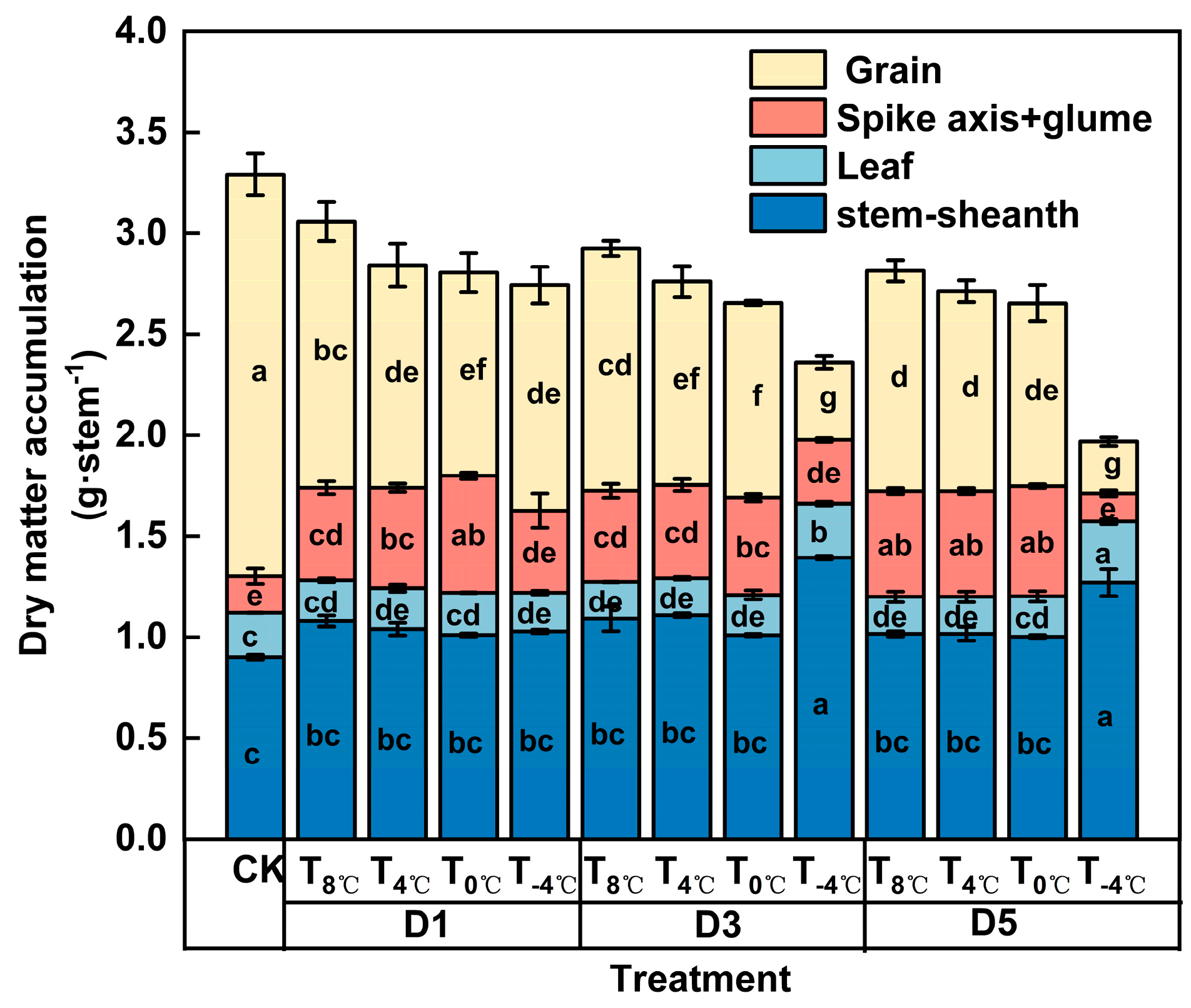
3.1.1. Effect of Low-Temperature Stress on Dry Matter Accumulation at Anthesis Stage
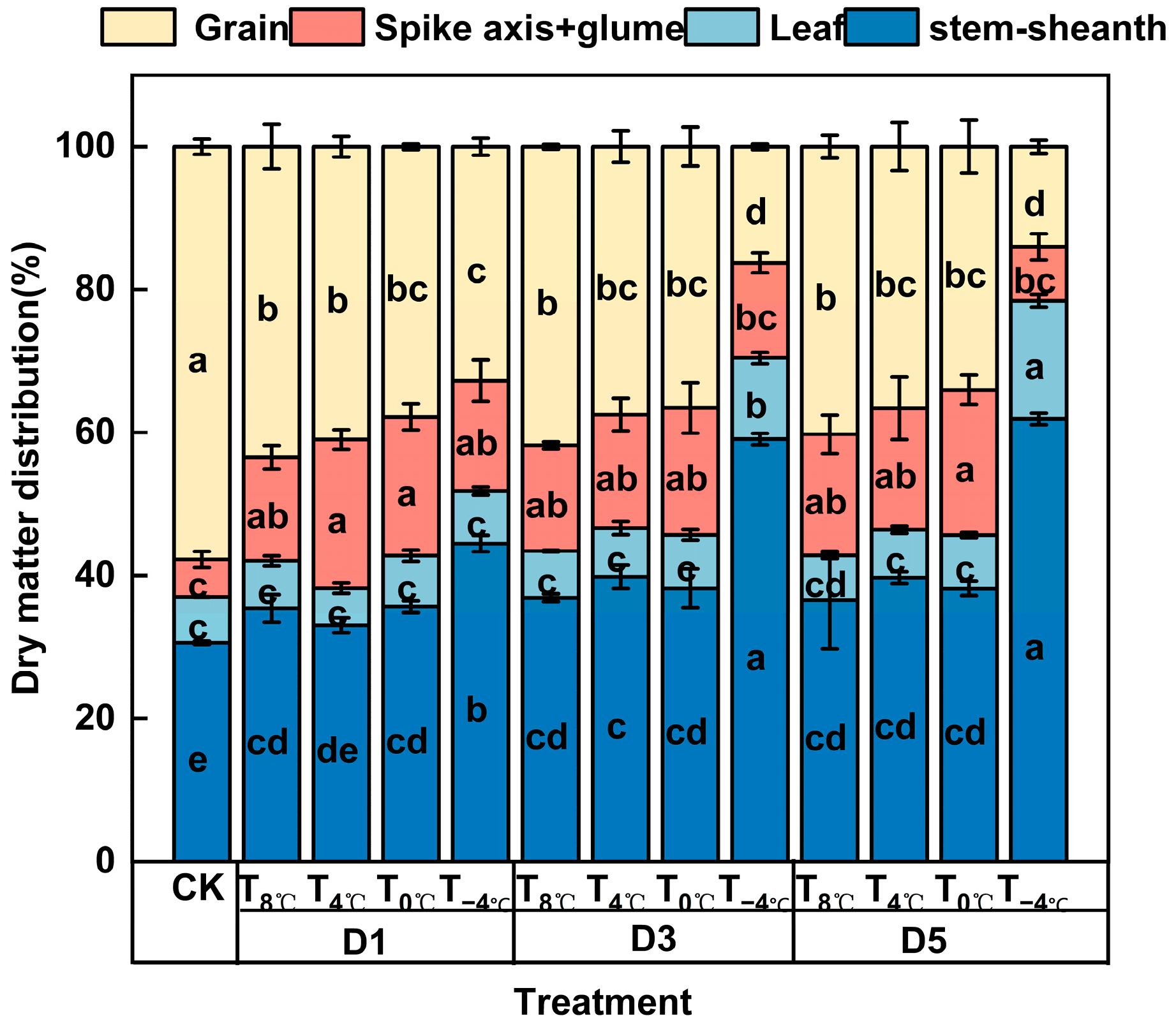
3.1.2. Effect of Low-Temperature Stress on Dry Matter Distribution at Anthesis Stage
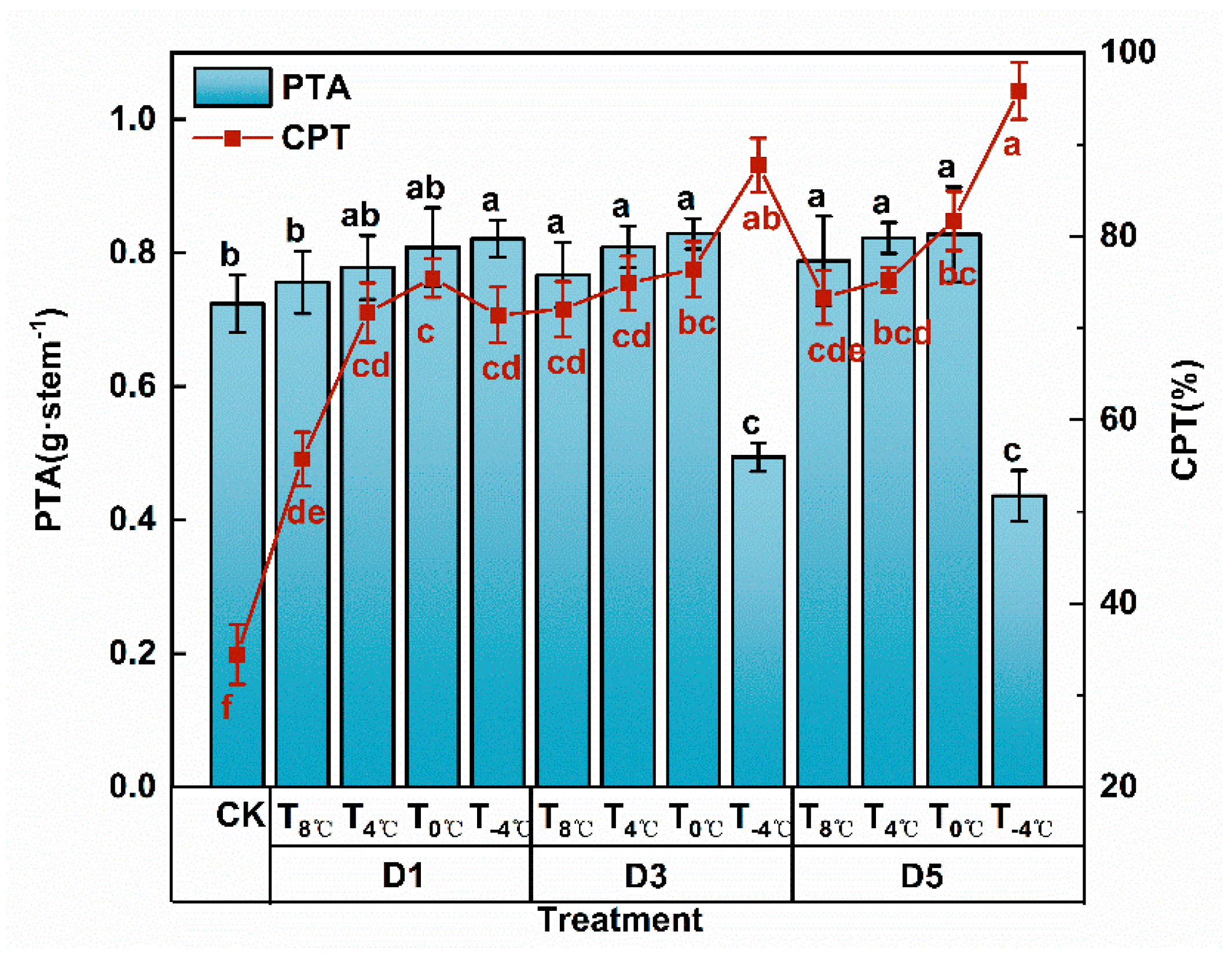
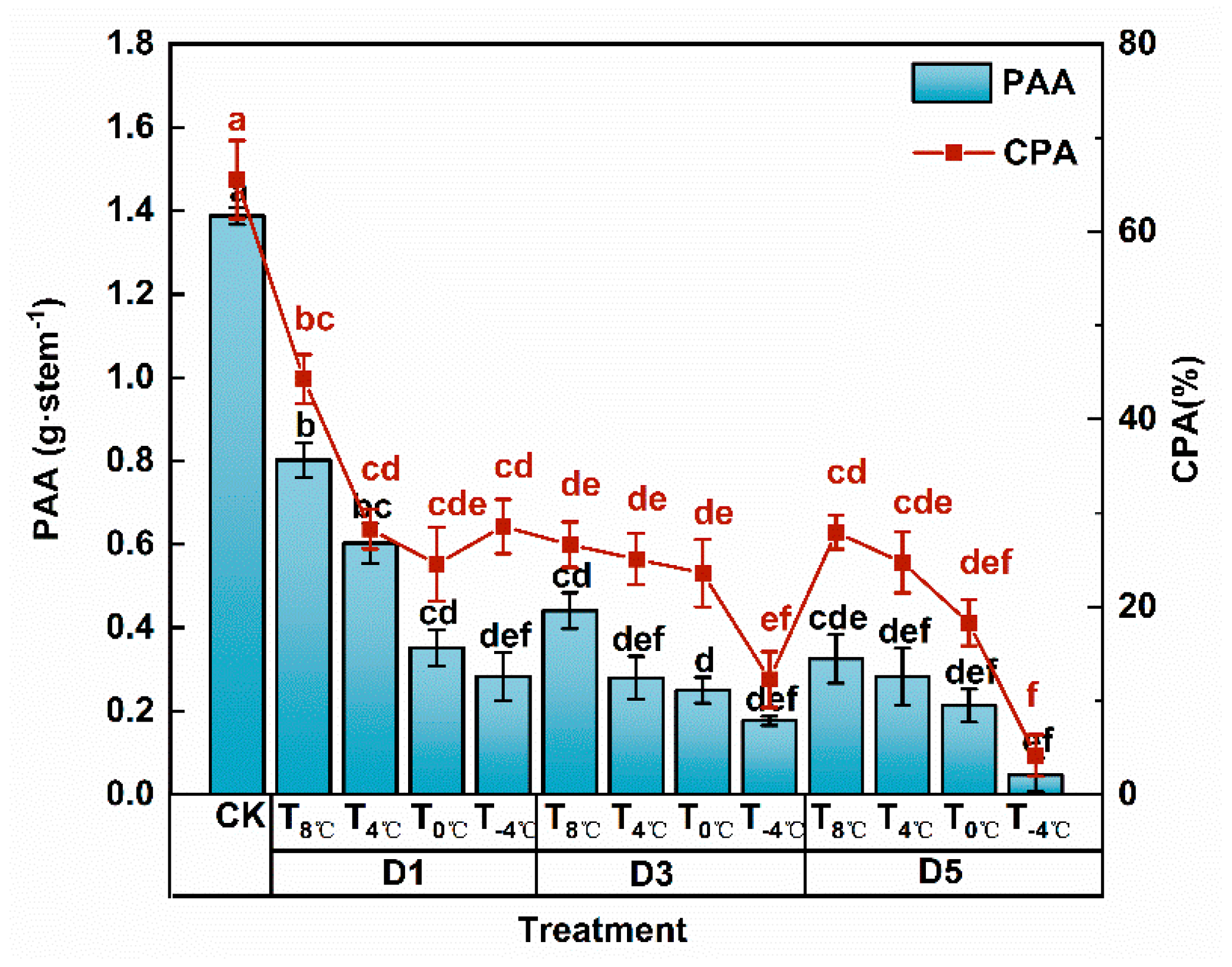
3.1.3. Effect of Low Temperature Stress on Dry Matter Transportation at Anthesis Stage
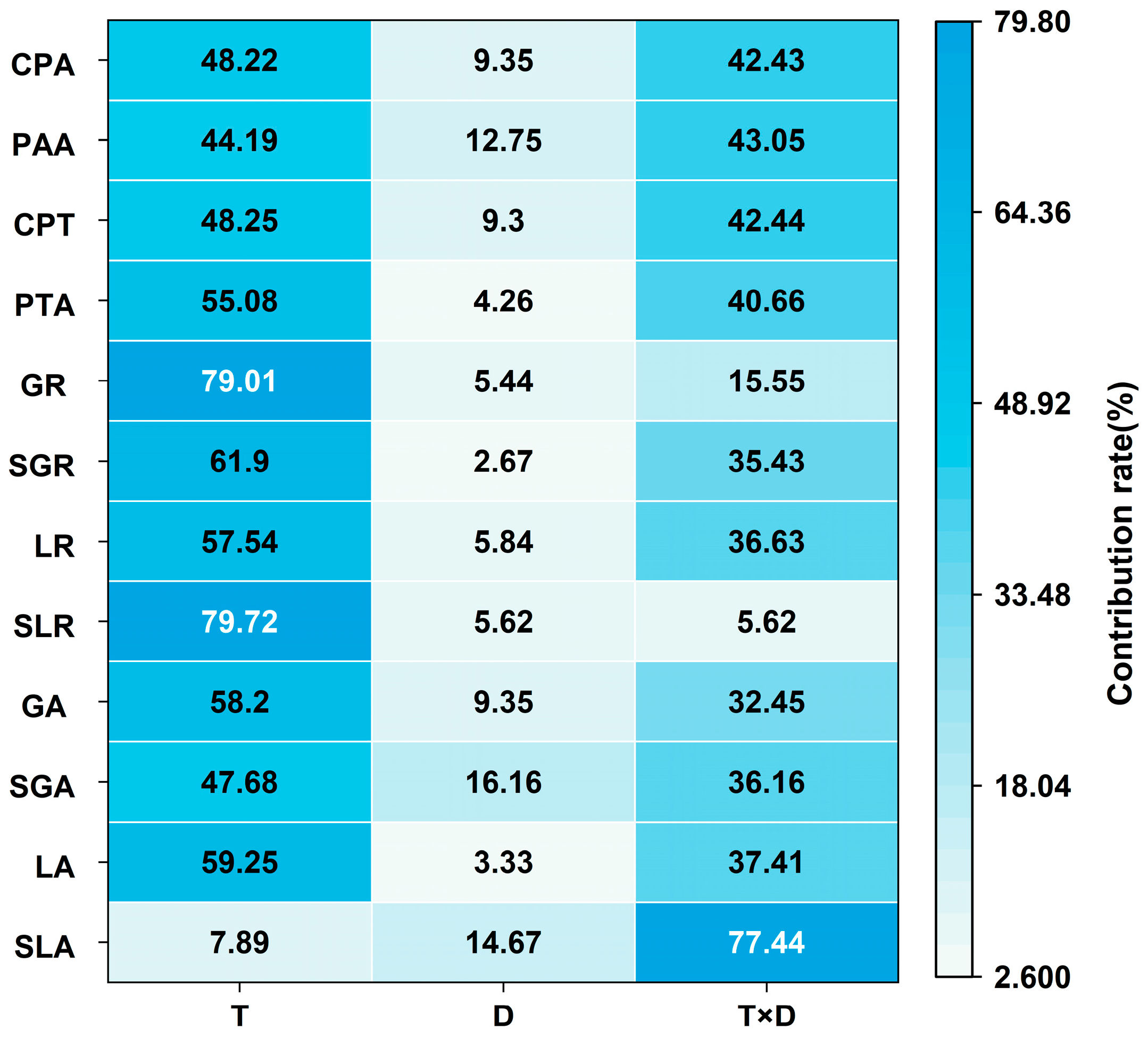
3.1.4. Analysis of Variance of Low Temperature and Duration of Low-Temperature Stress on Dry Matter Accumulation and Distribution at Anthesis Stage
3.2. Effects of Low-Temperature Stress During Anthesis Stage on Grain-Filling Characteristics
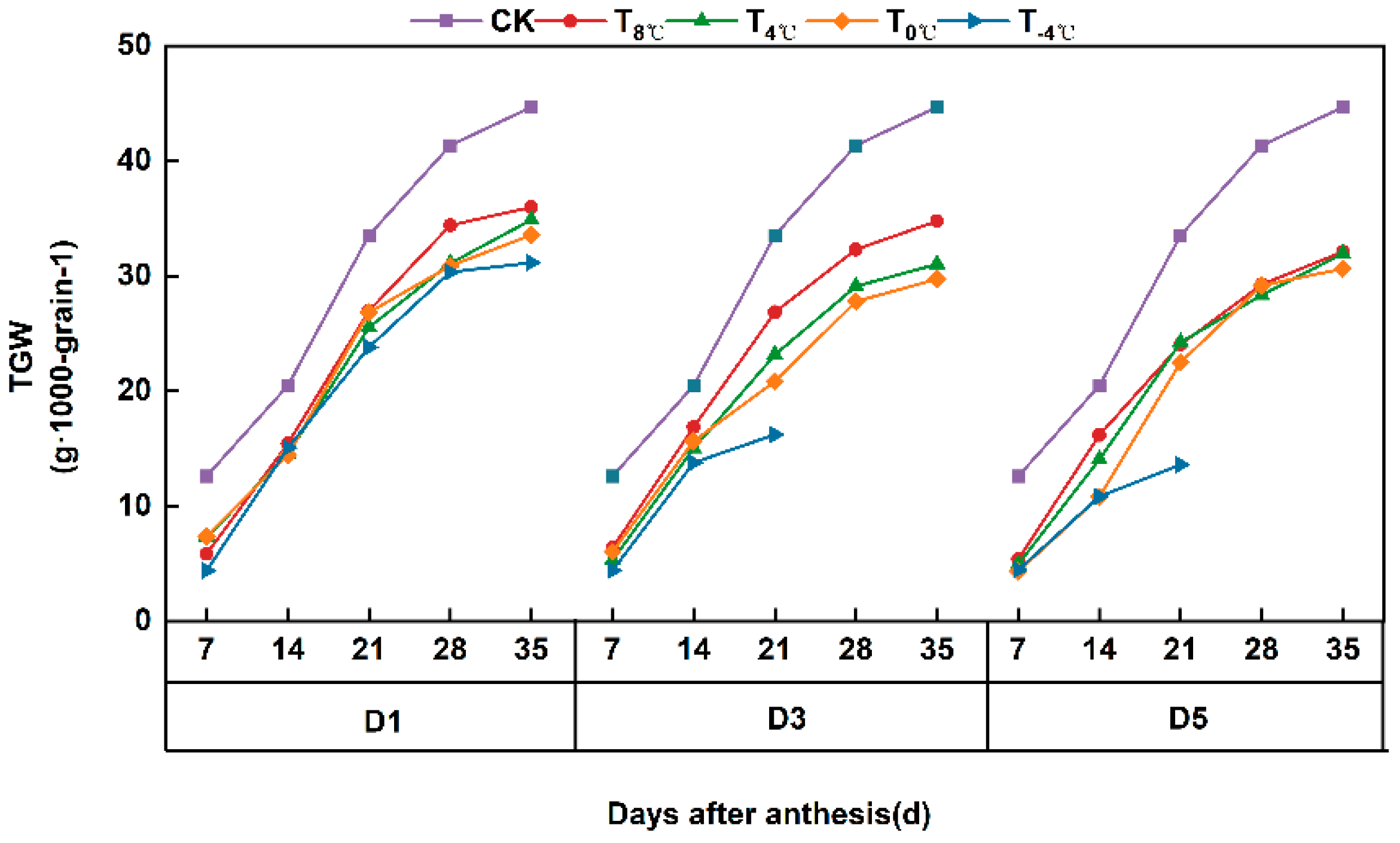
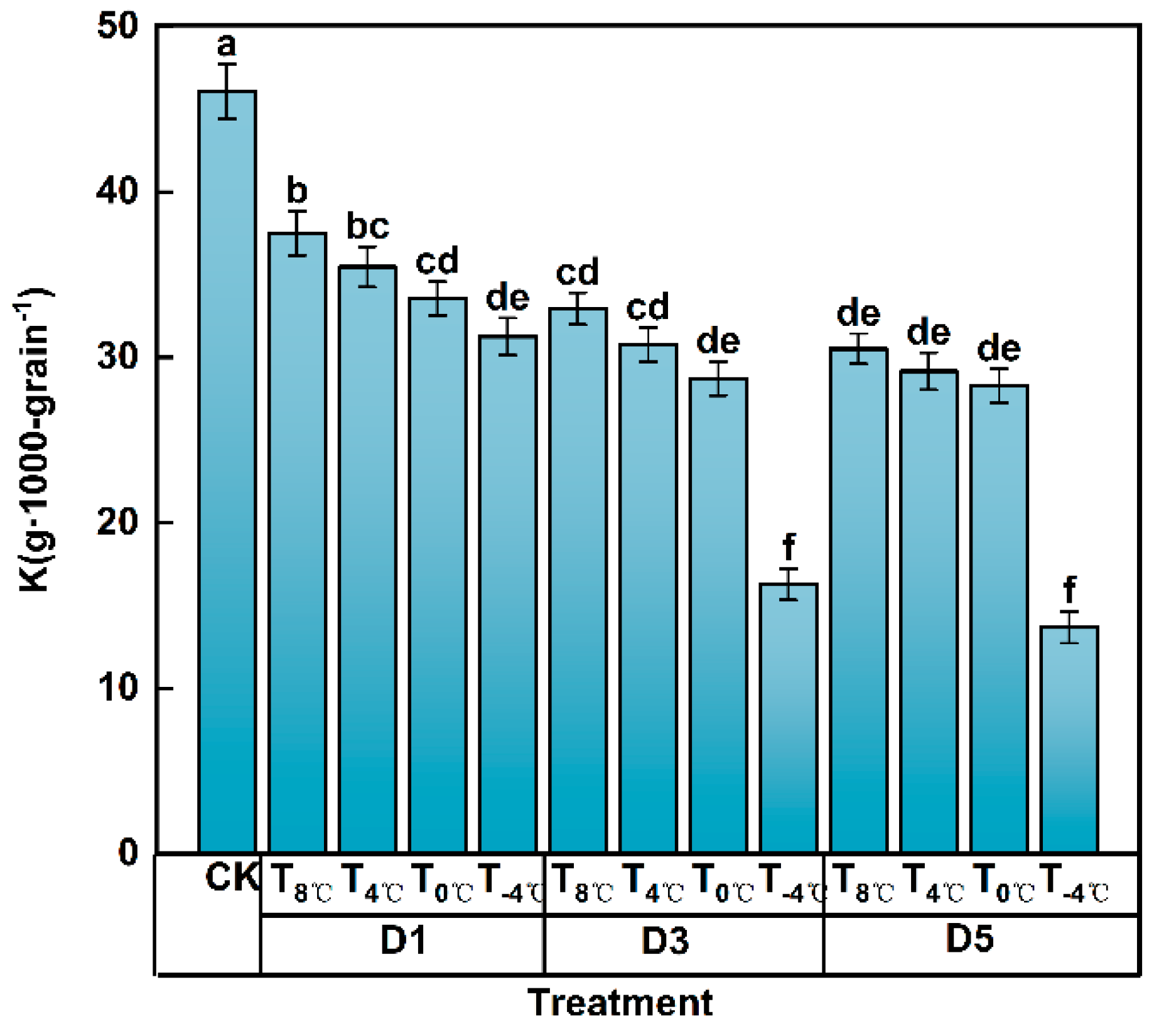
3.2.1. Effect of Low-Temperature Stress on the Variation Process of Thousand Grain Weight of Wheat Grain at Anthesis Stage
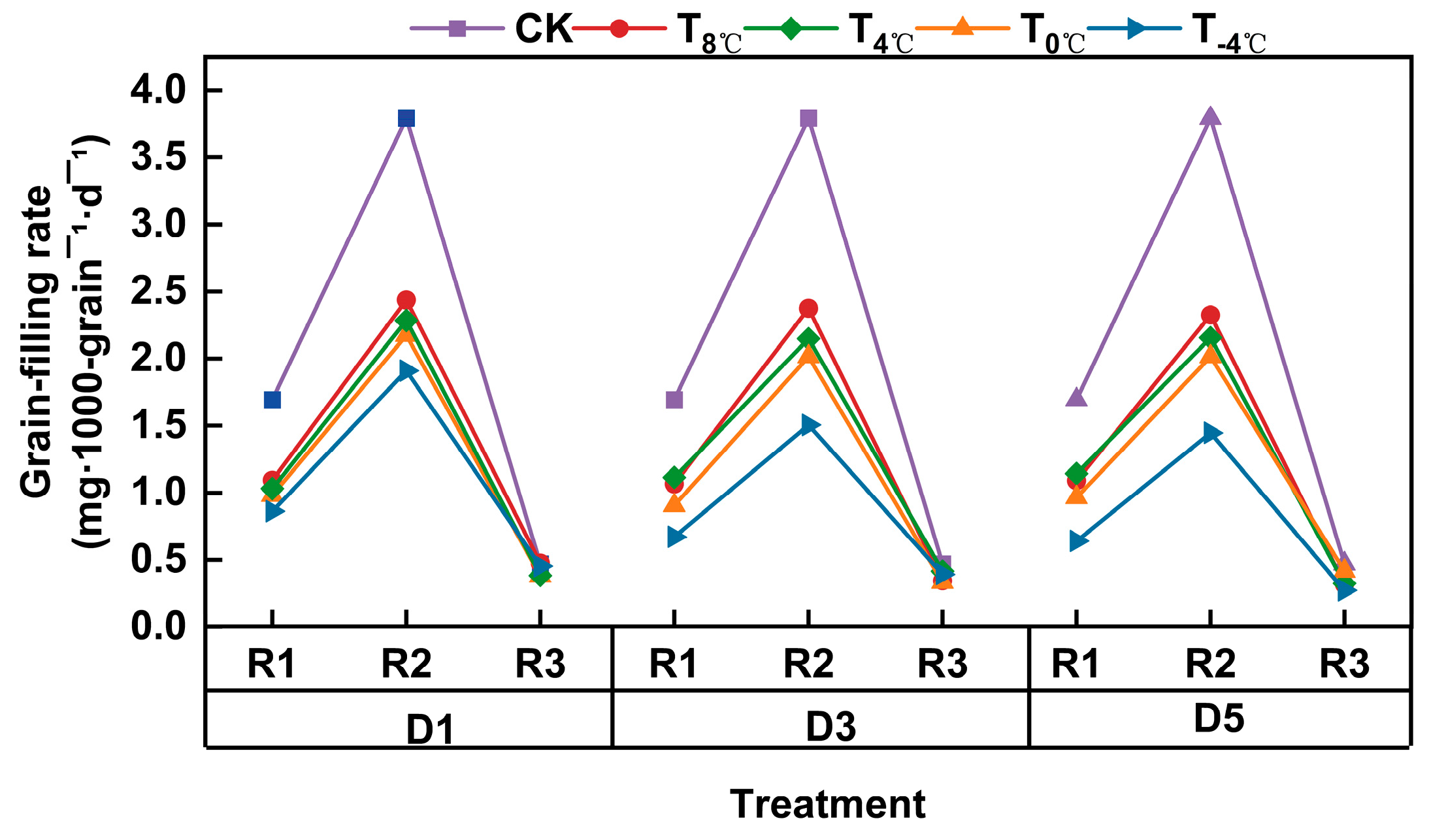
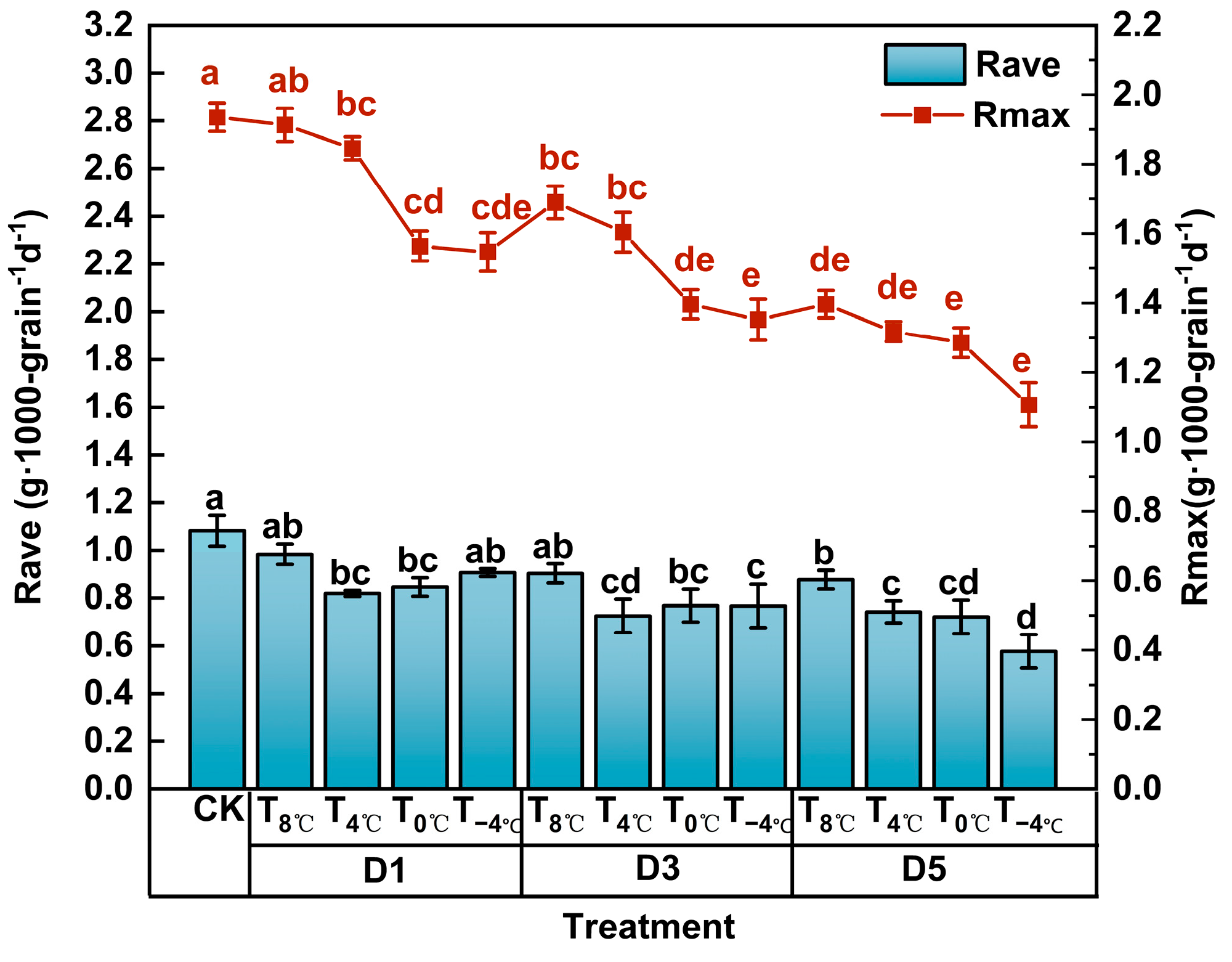
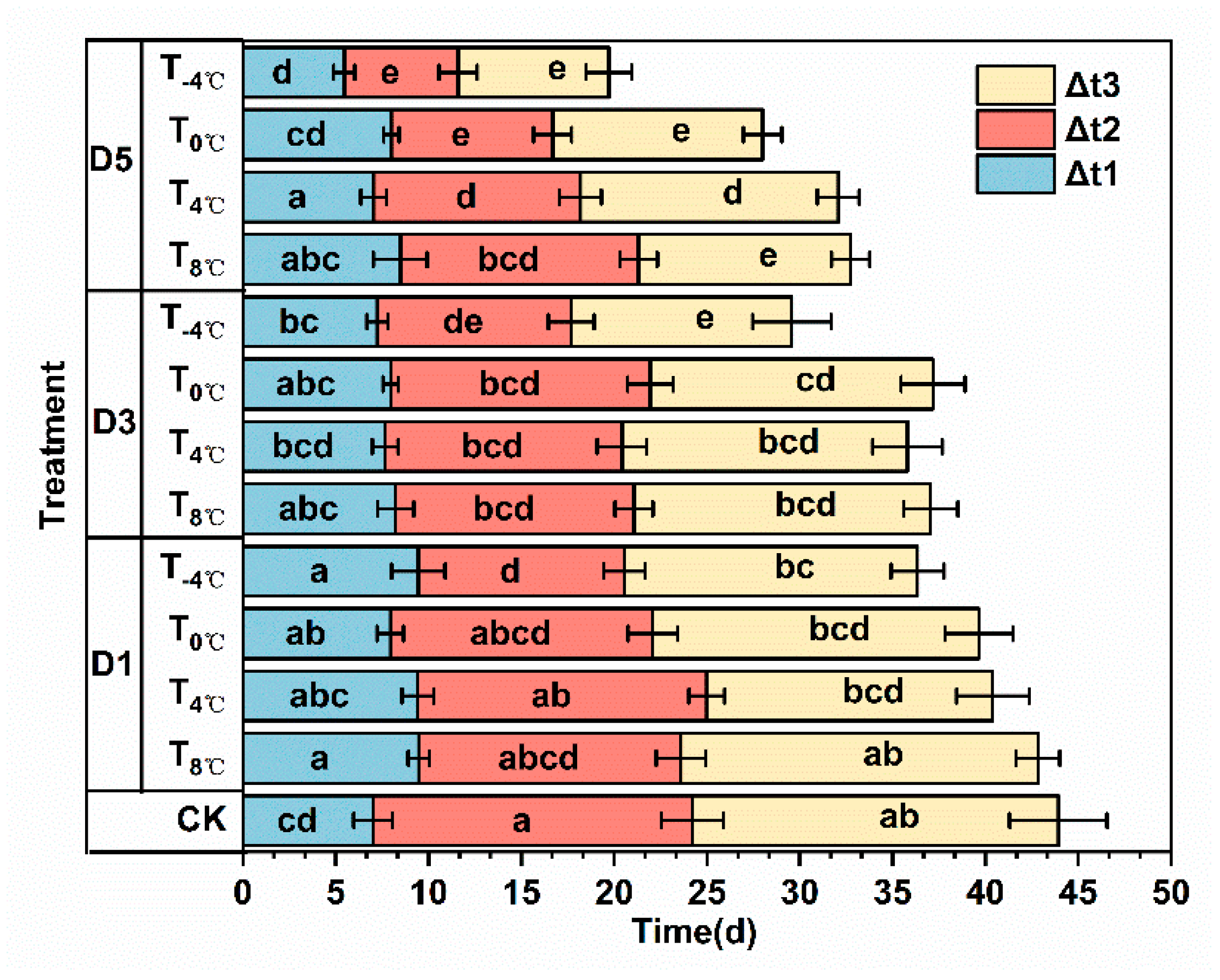
3.2.2. Effect of Low-Temperature Stress on Grain-Filling Parameters at Anthesis Stage
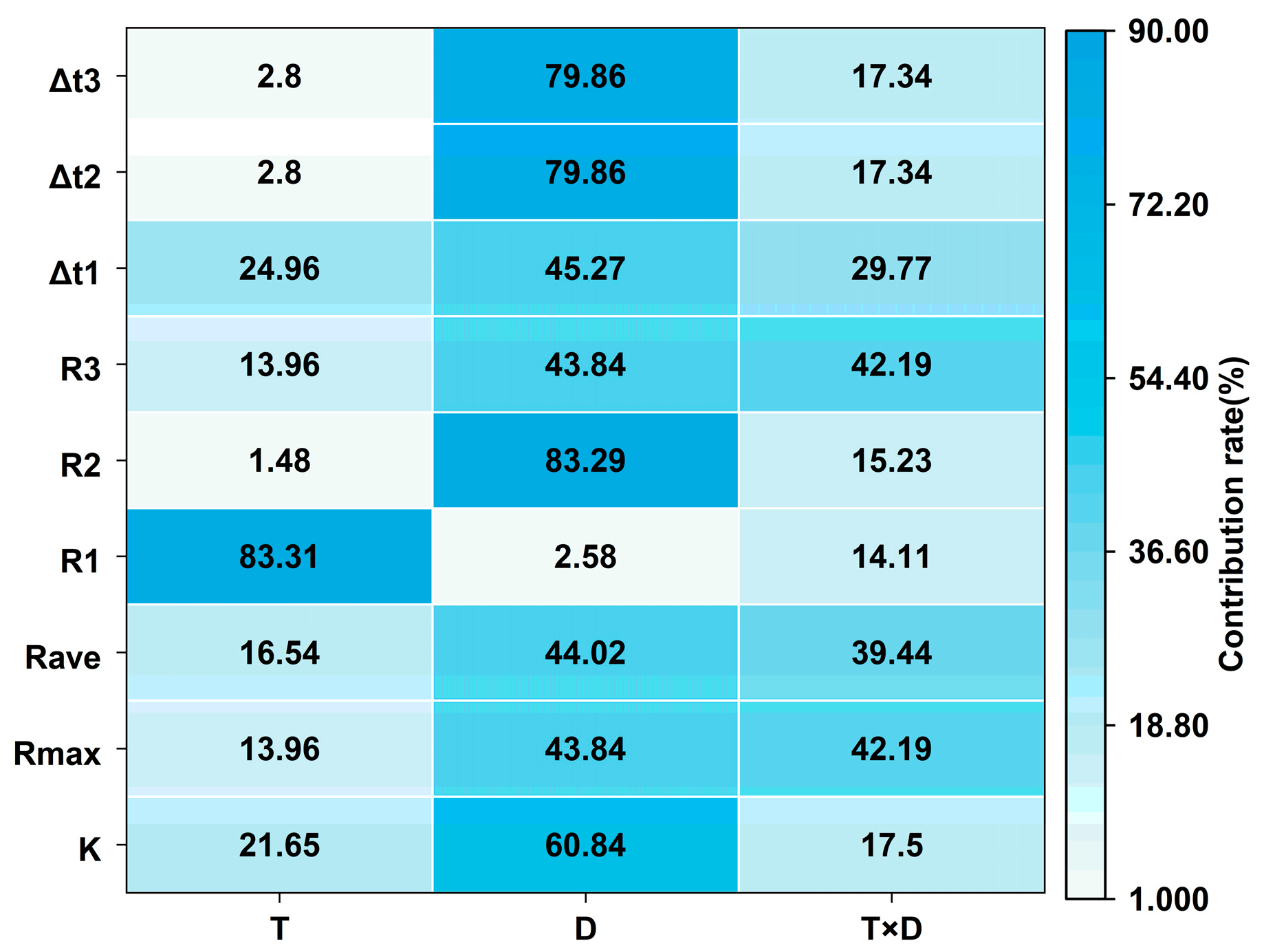
3.2.3. Analysis of Variance of Low Temperature and Duration of Low-Temperature Stress on Grain-Filling Dynamics at Anthesis Stage
3.3. Effect of Low Temperature During Anthesis Stage on Yield
3.3.1. Effect of Low-Temperature Stress on Yield at Anthesis Stage
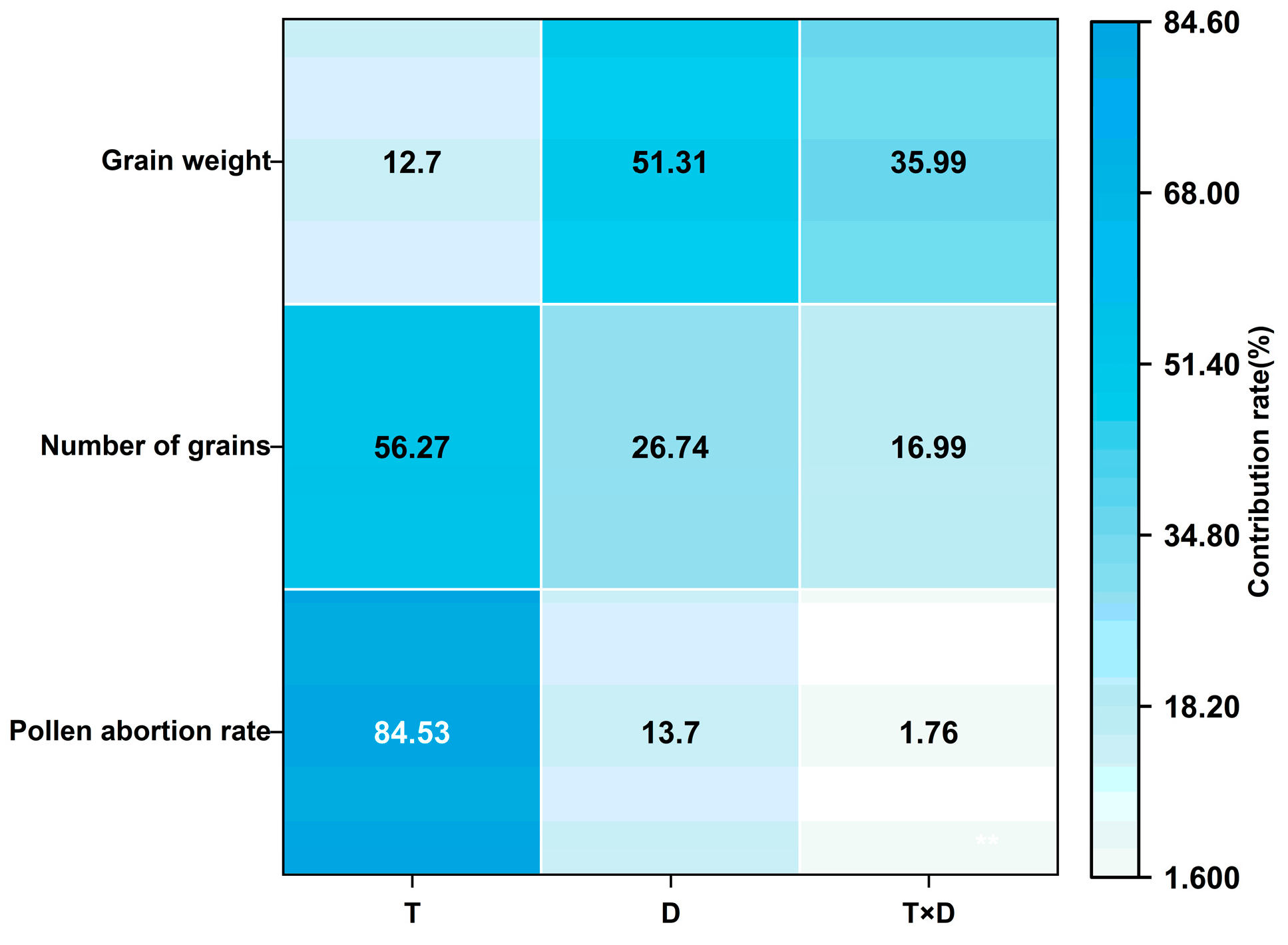
3.3.2. Analysis of Variance of Low Temperature and Duration of Low-Temperature Stress on Wheat Yield at Anthesis Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SA | Stem-sheath dry matter accumulation |
| LA | Leaf dry matter accumulation |
| SGA | Spike axis+glume dry matter accumulation |
| GA | Grain dry matter accumulation |
| SR | Stem-sheath dry matter allocation ratio |
| LR | Leaf dry matter allocation ratio |
| SGR | Spike axis+glume dry matter allocation ratio |
| GR | Grain dry matter allocation ratio |
| PTA | Pre-anthesis translocation amount |
| CPT | Contribution of pre-anthesis translocation to grains |
| PAA | Post-anthesis accumulation amount |
| CPA | Contribution of post-anthesis accumulation to grains |
| TGW | Thousand-grain weight |
| K | Theoretical maximum thousand-grain weight |
| Rave | Average filling rate |
| Rmax | Maximum filling rate |
| R1 | The filling rate in the gradual-increasing stage |
| R2 | The filling rate in the fast-increasing period |
| R3 | The filling rate during the slow growth period |
| Δt1 | The duration of gradual-increasing stage |
| Δt2 | The duration of the fast-increasing period |
| Δt3 | The duration of the slow growth period |
Appendix A
| Treatment | p | |
|---|---|---|
| SA | T | 0.05 |
| D | <0.01 | |
| T × D | <0.01 | |
| LA | T | <0.01 |
| D | 0.04 | |
| T × D | <0.01 | |
| SGA | T | 0.01 |
| D | 0.11 | |
| T × D | 0.14 | |
| GA | T | <0.01 |
| D | <0.01 | |
| T × D | <0.01 | |
| SR | T | <0.01 |
| D | <0.01 | |
| T × D | <0.01 | |
| LR | T | <0.01 |
| D | <0.01 | |
| T × D | <0.01 | |
| SGR | T | 0.07 |
| D | 0.84 | |
| T × D | 0.60 | |
| GR | T | <0.01 |
| D | 0.01 | |
| T × D | <0.01 | |
| PTA | T | <0.01 |
| D | 0.28 | |
| T × D | <0.01 | |
| CPT | T | <0.01 |
| D | 0.13 | |
| T × D | 0.01 | |
| PAA | T | <0.01 |
| D | 0.04 | |
| T × D | <0.01 | |
| CPA | T | <0.01 |
| D | 0.13 | |
| T × D | 0.01 | |
| Treatment | p | |
|---|---|---|
| K | T | <0.01 |
| D | <0.01 | |
| T × D | <0.01 | |
| Rmax | T | 0.10 |
| D | <0.01 | |
| T × D | 0.01 | |
| Rave | T | 0.39 |
| D | 0.04 | |
| T × D | 0.26 | |
| R1 | T | <0.01 |
| D | 0.76 | |
| T × D | 0.79 | |
| R2 | T | <0.01 |
| D | 0.59 | |
| T × D | 0.13 | |
| R3 | T | 0.02 |
| D | <0.01 | |
| T × D | <0.01 | |
| Δt1 | T | 0.04 |
| D | <0.01 | |
| T × D | 0.11 | |
| Δt2 | T | <0.01 |
| D | 0.22 | |
| T × D | 0.02 | |
| Δt3 | T | <0.01 |
| D | 0.21 | |
| T × D | 0.01 | |
| T99 | T | <0.01 |
| D | 0.01 | |
| T × D | <0.01 | |
| Tmax | T | <0.01 |
| D | <0.01 | |
| T × D | 0.11 | |
| Treatment | p | |
|---|---|---|
| Pollen abortion rate | T | <0.01 |
| D | 0.13 | |
| T × D | 0.21 | |
| Number of grains | T | <0.01 |
| D | <0.01 | |
| T × D | 0.24 | |
| Grain weight | T | <0.01 |
| D | <0.01 | |
| T × D | <0.01 | |
References
- Bai, H.; Xiao, D.; Tang, J.; Liu, D.L. Evaluation of wheat yield in North China Plain under extreme climate by coupling crop model with machine learning. Comput. Electron. Agric. 2024, 217, 108651. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Chen, X.; Li, J. Effects of Low-Temperature Stress during the Anther Differentiation Period on Winter Wheat Photosynthetic Performance and Spike-Setting Characteristics. Plants 2022, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, L.; Asseng, S.; Xia, Y.; Tang, L.; Liu, B.; Cao, W.; Zhu, Y. Estimating Spring frost and its impact on yield across winter wheat in China. Agric. For. Meteorol. 2018, 260–261, 154–164. [Google Scholar] [CrossRef]
- Latifmanesh, H.; Deng, A.; Nawaz, M.M.; Li, L.; Chen, Z.; Zheng, Y.; Wang, P.; Song, Z.; Zhang, J.; Zheng, C.; et al. Integrative impacts of rotational tillage on wheat yield and dry matter accumulation under corn-wheat cropping system. Soil Tillage Res. 2018, 184, 100–108. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Z.; Xu, K.; Liu, L.; Zeng, Y.; Ma, S.; Fan, Y. The effect of plant growth regulators on recovery of wheat physiological and yield-related characteristics at booting stage following chilling stress. Acta Physiol. Plant. 2019, 41, 133. [Google Scholar] [CrossRef]
- Gupta, S.M.; Agarwal, A.; Dev, B.; Kumar, K.; Prakash, O.; Arya, M.C.; Nasim, M. Assessment of photosynthetic potential of indoor plants under cold stress. Photosynthetica 2016, 54, 138–142. [Google Scholar] [CrossRef]
- Shahryar, N.; Maali-Amiri, R. Metabolic acclimation of tetraploid and hexaploid wheats by cold stress-induced carbohydrate accumulation. J. Plant Physiol. 2016, 204, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ji, H.; An, J.; Shi, K.; Ma, J.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y. Response of biomass accumulation in wheat to low-temperature stress at jointing and booting stages. Environ. Exp. Bot. 2019, 157, 46–57. [Google Scholar] [CrossRef]
- Obembe, O.S.; Hendricks, N.P.; Tack, J. Decreased wheat production in the USA from climate change driven by yield losses rather than crop abandonment. PLoS ONE 2021, 16, e0252067. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of Low Temperature Stress on Photosynthesis and Allied Traits: A Review. In Physiological Processes in Plants Under Low Temperature Stress; Springer: Singapore, 2022; pp. 199–297. [Google Scholar] [CrossRef]
- Li, X.; Pu, H.; Liu, F.; Zhou, Q.; Cai, J.; Dai, J.; Cao, W.; Jiang, D. Winter Wheat Photosynthesis and Grain Yield Responses to Spring Freeze. Agron. J 2015, 107, 1002–1010. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, A.; Zhou, Q.; Fang, R.; Zhao, Y.; Li, Z.; Zhao, J.; Zhao, M.; Ma, S.; Fan, Y.; et al. Low-temperature at booting reduces starch content and yield of wheat by affecting dry matter transportation and starch synthesis. Front. Plant Sci. 2023, 14, 1207518. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Asseng, S.; Wang, X.; Xia, J.; Zhang, P.; Liu, L.; Tang, L.; Cao, W.; Zhu, Y.; Liu, B. Simulating the effects of low-temperature stress on wheat biomass growth and yield. Agric. For. Meteorol. 2022, 326, 109191. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, R.; Gupta, S.; Gaikwad, K.B.; Jain, N.; Ranjan, R.; Kumar, M. Identification of genotypes and marker validation for grain filling rate and grain filling duration in wheat under conservation agriculture. Indian J. Genet. Plant Breed. 2018, 78, 309–316. [Google Scholar]
- Xie, Y.; Wang, C.; Yang, W.; Feng, M.; Qiao, X.; Song, J. Canopy hyperspectral characteristics and yield estimation of winter wheat (Triticum aestivum) under low temperature injury. Sci. Rep. 2020, 10, 244. [Google Scholar] [CrossRef]
- Ji, H.; Xiao, L.; Xia, Y.; Song, H.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y.; Liu, L. Effects of jointing and booting low temperature stresses on grain yield and yield components in wheat. Agric. For. Meteorol. 2017, 243, 33–42. [Google Scholar] [CrossRef]
- Knott, C.A. Estimated winter wheat yield reduction following spring freeze events in field conditions. Crop Forage Turfgrass Manag. 2020, 6, e20080. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Jin, M.; Yu, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. J. Cereal Sci. 2012, 55, 331–336. [Google Scholar] [CrossRef]
- Si, D. Optimal Temperature Conditions for Wheat Growth and Development. Beijing Agric. 1995, 15. [Google Scholar]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2009, 67, 429–443. [Google Scholar] [CrossRef]
- Zheng, B.; Chapman, S.C.; Christopher, J.T.; Frederiks, T.M.; Chenu, K. Frost trends and their estimated impact on yield in the Australian wheatbelt. J. Exp. Bot. 2015, 66, 3611–3623. [Google Scholar] [CrossRef]
- Barlow, K.M.; Christy, B.P.; O’Leary, G.J.; Riffkin, P.A.; Nuttall, J.G. Simulating the impact of extreme heat and frost events on wheat crop production: A review. Field Crops Res. 2015, 171, 109–119. [Google Scholar] [CrossRef]
- Li, M.X.; Huo, Z.G.; Kong, R.; Jiang, M.Y.; Mi, Q.C. Construction of Spring Low-Temperature Disaster Grade Index for Winter Wheat in the Huang-Huai-Hai Region. J. Appl. Meteorol. Sci. 2024, 35, 45–56. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Ma, Q.; Su, S.; Li, C.; Ding, J.; Zhu, M.; Zhu, X.; Guo, W. Effects of low temperature in spring on fertility of pollen and formation of grain number in wheat. Acta Agron. Sin. 2021, 47, 104–115. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Tian, P.; Ren, D.; Zhang, H.; Ma, G.; Duan, J.; Wang, C.; Feng, W. Mechanism of Irrigation Before Low-Temperature Exposure on Mitigating the Reduction in Yield Loss and Spikelet Abortion at the Jointing Stage of Wheat. Antioxidants 2024, 13, 1451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, N.; Zhang, H.; Huo, Z.; Yang, Z. Effect of Low Temperature on Photosynthetic Characteristics, Senescence Characteristics, and Endogenous Hormones of Winter Wheat “Ji Mai 22” during the Jointing Stage. Agronomy 2023, 13, 2650. [Google Scholar] [CrossRef]
- Zhao, D.; Zeng, J.; Liu, D.; Tian, Y.; Sani, S.R.; Xu, X.; Hao, Y.; He, Z.; Wang, C.; Yang, L.; et al. Identification of genetic loci for powdery mildew resistance in bread wheat. Crop Sci. 2023, 63, 2941–2951. [Google Scholar] [CrossRef]
- Li, M.; Jiang, F.; Huang, L.; Wang, H.; Song, W.; Zhang, X.; Zhang, Y.; Niu, L. Optimization of In Vitro Germination, Viability Tests and Storage of Paeonia ostii Pollen. Plants 2023, 12, 2460. [Google Scholar] [CrossRef]
- Jiang, M.; Xuan, S.; Muneer, M.A.; Sun, B.; Shi, C.; Liu, F.; Wu, R. Response of dry matter partition and yield components to waterlogging and sunlight shortage in different growth stages of wheat. Nat. Hazards 2022, 110, 1133–1152. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Li, J.; Liu, Z.; Zhao, Z.; Zhang, Y.; Zhou, S.; Wang, Z. Improving water use efficiency and grain yield of winter wheat by optimizing irrigations in the North China Plain. Field Crops Res. 2018, 221, 219–227. [Google Scholar] [CrossRef]
- Xu, H.; Hassan, M.A.; Sun, D.; Wu, Z.; Jiang, G.; Liu, B.; Ni, Q.; Yang, W.; Fang, H.; Li, J.; et al. Effects of Low Temperature Stress on Source–Sink Organs in Wheat and Phosphorus Mitigation Strategies. Front. Plant Sci. 2022, 13, 807844. [Google Scholar] [CrossRef]
- Li, J.; Guo, H.; Lou, Q.; Zeng, Y.; Guo, Z.; Xu, P.; Gu, Y.; Gao, S.; Xu, B.; Han, S.; et al. Natural variation of indels in the CTB3 promoter confers cold tolerance in japonica rice. Nat. Commun. 2025, 16, 1613. [Google Scholar] [CrossRef]
- Wu, L.Q.; Cai, Z.H.; Zhang, G.L.; Liu, Y.T.; Zhao, R. Effects of low temperature on physiological characteristics of rice seedlings with different cold tolerance and anatomical structure of root tip. Chin. J. Agrometeorol. 2018, 39, 805–813. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Wang, J.Q.; Zhang, A.M.; Huang, Z.L.; Ma, S.Y.; Fan, Y.H.; Zhang, W.J. Effects of Low Temperature during Panicle Development on Wheat Panicle Development and Yield. Acta Agric. Nucl. Sin. 2022, 36, 2490–2500. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K.; Roitsch, T.; Liu, F. Activities of leaf and spike carbohydrate-metabolic and antioxidant enzymes are linked with yield performance in three spring wheat genotypes grown under well-watered and drought conditions. BMC Plant Biol. 2020, 20, 400. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted Role of Salicylic Acid in Combating Cold Stress in Plants: A Review. J. Plant Growth Regul. 2021, 40, 464–485. [Google Scholar] [CrossRef]
- Fahad, S.; Ihsan, M.Z.; Khaliq, A.; Daur, I.; Saud, S.; Alzamanan, S.; Nasim, W.; Abdullah, M.; Khan, I.A.; Wu, C.; et al. Consequences of high temperature under changing climate optima for rice pollen characteristics-concepts and perspectives. Arch. Agron. Soil Sci. 2018, 64, 1473–1488. [Google Scholar] [CrossRef]
- Jiang, L.N.; Zhang, D.J.; Lin, L.; Shao, Y.; Yu, H.B.; Li, C.X. Effects of Low Temperature on Dry Matter Accumulation and Root Exudates of Wheat Seedlings. J. Triticeae Crops. 2012, 32, 1171–1176. [Google Scholar]
- Ke, Y.Y.; Chen, X.; Ni, Q.Q.; Zhang, L.L.; Liu, L.Z.; Xu, H.; Wei, F.Z.; Li, J.C. Research Progress of the Metabolism of Reactive Oxygen Species and Its Regulation Mechanisms in Wheat under Low Temperature Stress. Barley Cereal Sci. 2021, 38, 1–6+21. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Alleviation of Field Low-Temperature Stress in Winter Wheat by Exogenous Application of Salicylic Acid. J. Plant Growth Regul. 2021, 40, 811–823. [Google Scholar] [CrossRef]
- Yu, M.; Chen, H.; Mao, S.-L.; Dong, K.-M.; Hou, D.-B.; Chen, G.-Y. Contribution of photosynthetic- and yield-related traits towards grain yield in wheat at the individual quantitative trait locus level. Biotechnol. Biotechnol. Equip. 2020, 34, 1188–1197. [Google Scholar] [CrossRef]
- Miao, Y.; Yan, J.; Zhao, D.; Tian, Y.; Yan, J.; Xia, X.; Zhang, Y.; He, Z. Relationship between Grain Filling Parameters and Grain Weight in Leading Wheat Cultivars in the Yellow and Huai Rivers Valley. Acta Agron. Sin. 2018, 44, 260–267. [Google Scholar] [CrossRef]
- Du, S.; Qiao, Y.; Li, W.; Chen, H.; Zhao, Z.; Zhang, X.; Cao, C. Effects of sowing date and rate on grain filling characteristics of winter wheat under chilling injury. Chin. J. Eco-Agric. 2014, 22, 551–559. [Google Scholar]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Nasehzadeh, M.; Ellis, R.H. Wheat seed weight and quality differ temporally in sensitivity to warm or cool conditions during seed development and maturation. Ann. Bot. 2017, 120, 479–493. [Google Scholar] [CrossRef]
- Zhao, K.; Tao, Y.; Liu, M.; Yang, D.; Zhu, M.; Ding, J.; Zhu, X.; Guo, W.; Zhou, G.; Li, C. Does temporary heat stress or low temperature stress similarly affect yield, starch, and protein of winter wheat grain during grain filling? J. Cereal Sci. 2022, 103, 103408. [Google Scholar] [CrossRef]
- Wang, R.; Yan, C.; Zhan, X.; Sun, G.; Qian, Z.; Qi, X.; Mou, Q.; Xiao, S. Effect of low temperature in spring on yield and photosynthetic characteristics of wheat. Acta Agron. Sin. 2018, 44, 288–296. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, Y.; Yuan, S.; Wang, P.; Duan, W.; Bai, J.; Sun, H.; Wang, N.; Zhang, F.; Zhang, L.; et al. Functional Analysis of Wheat TaPaO1 Gene Conferring Pollen Sterility Under Low Temperature. J. Plant Biol. 2018, 61, 25–32. [Google Scholar] [CrossRef]
- Aya, K.; Ueguchi-Tanaka, M.; Kondo, M.; Hamada, K.; Yano, K.; Nishimura, M.; Matsuoka, M. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 2009, 21, 1453–1472. [Google Scholar] [CrossRef]
- Sakata, T.; Oda, S.; Tsunaga, Y.; Shomura, H.; Kawagishi-Kobayashi, M.; Aya, K.; Saeki, K.; Endo, T.; Nagano, K.; Kojima, M.; et al. Reduction of Gibberellin by Low Temperature Disrupts Pollen Development in Rice. Plant Physiol. 2014, 164, 2011–2019. Available online: https://pubmed.ncbi.nlm.nih.gov/24569847/ (accessed on 18 March 2025). [CrossRef] [PubMed]
- Zhang, W.; Zhao, Y.; Li, L.; Xu, X.; Yang, L.; Luo, Z.; Wang, B.; Ma, S.; Fan, Y.; Huang, Z. The Effects of Short-Term Exposure to Low Temperatures During the Booting Stage on Starch Synthesis and Yields in Wheat Grain. Front. Plant Sci. 2021, 12, 684784. [Google Scholar] [CrossRef]
- Scheres, B.; van der Putten, W.H. The plant perceptron connects environment to development. Nature 2017, 543, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, G.; Zhang, Y.; Song, Q.; Chen, Z.; Wang, J.; Guo, J.; Niu, N.; Wang, J.; Ma, S. Comparative studies of mitochondrial proteomics reveal an intimate protein network of male sterility in wheat (Triticum aestivum L.). J. Exp. Bot. 2015, 66, 6191–6203. [Google Scholar] [CrossRef]
- Cai, D.; Shoukat, M.R.; Zheng, Y.; Tan, H.; Sun, M.; Yan, H. Improving Wheat Grain Yield and Nitrogen Use Efficiency by Optimizing the Fertigation Frequency Using Center Pivot Irrigation System. Water 2023, 15, 1932. [Google Scholar] [CrossRef]
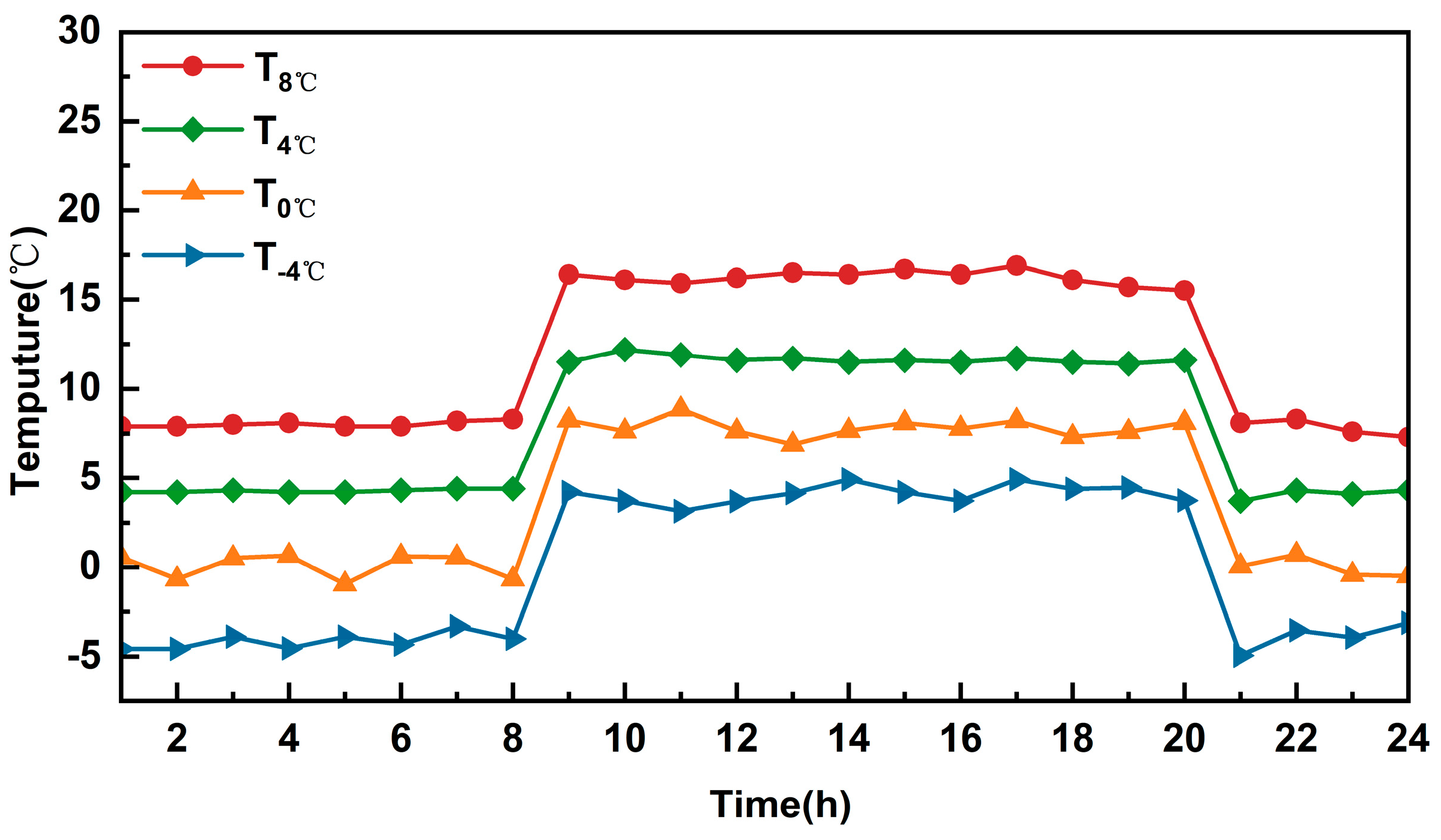
| Temperature (℃) (Daytime 8:00–20:00/Night-time 20:00–8:00) | Duration of Days (d) |
|---|---|
| CK (22/12) | --- |
| T8 °C (16/8) | D1(1) |
| T4 °C (12/4) | D1(1) |
| T0 °C (8/0) | D1(1) |
| T−4 °C (4/−4) | D1(1) |
| T8 °C (16/8) | D3(3) |
| T4 °C (12/4) | D3(3) |
| T0 °C (8/0) | D3(3) |
| T−4 °C (4/−4) | D3(3) |
| T8 °C (16/8) | D5(5) |
| T4 °C (12/4) | D5(5) |
| T0 °C (8/0) | D5(5) |
| T−4 °C (4/−4) | D5(5) |
| Duration of Days (d) | Temperature (°C) | Equation | R2 |
|---|---|---|---|
| D1 | CK | 0.98 | |
| T8 °C | ) | 0.99 | |
| T4 °C | 0.98 | ||
| T0 °C | 0.97 | ||
| T−4 °C | 0.99 | ||
| D3 | T8 °C | 0.99 | |
| T4 °C | 0.97 | ||
| T0 °C | 0.97 | ||
| T−4 °C | 0.99 | ||
| D5 | T8 °C | 0.99 | |
| T4 °C | 0.96 | ||
| T0 °C | 0.94 | ||
| T−4 °C | 0.98 |
| Duration of Days (d) | Temperature (°C) | Pollen Abortion Rate (%) | Number of Grains (pcs) | Grain Weight (g·spike−1) |
|---|---|---|---|---|
| D1 | CK | 6.60 ± 0.52 g | 47.67 ± 2.87 a | 2.20 ± 0.16 a |
| T8 °C | 9.57 ± 1.13 g | 38.80 ± 2.80 b | 1.32 ± 0.27 bc | |
| T4 °C | 14.04 ± 4.31 ef | 35.47 ± 3.13 bc | 1.10 ± 0.06 cd | |
| T0 °C | 18.59 ± 1.91 cd | 31.27 ± 3.60 cd | 1.03 ± 0.02 cd | |
| T−4 °C | 22.93 ± 2.70 ab | 29.20 ± 3.42 de | 0.90 ± 0.11 f | |
| D3 | T8 °C | 11.96 ± 0.50 f | 39.40 ± 3.26 b | 1.02 ± 0.0 cd |
| T4 °C | 15.19 ± 1.86 ef | 35.60 ± 2.42 bc | 1.01 ± 0.08 de | |
| T0 °C | 19.23 ± 1.53 bc | 31.87 ± 1.15 cd | 0.97 ± 0.01 ef | |
| T−4 °C | 22.01 ± 3.43 ab | 25.00 ± 1.15 e | 0.59 ± 0.10 g | |
| D5 | T8 °C | 13.03 ± 1.29 f | 38.60 ± 1.6 b | 0.96 ± 0.07 cd |
| T4 °C | 16.86 ± 2.01 de | 34.27 ± 3.60 bc | 0.87 ± 0.16 de | |
| T0 °C | 20.08 ± 0.40 ab | 30.07 ± 3.47 cd | 0.74 ± 0.13 ef | |
| T−4 °C | 23.26 ± 1.26 a | 22.87 ± 4.05 e | 0.38 ± 0.01 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Chen, Q.; Agathokleous, E.; Zhang, J.; Yang, Z.; Takin, M. Effects of Low-Temperature Stress During Anthesis Stage on Dry Matter Accumulation and Yield of Winter Wheat. Agronomy 2025, 15, 761. https://doi.org/10.3390/agronomy15040761
Jiang X, Chen Q, Agathokleous E, Zhang J, Yang Z, Takin M. Effects of Low-Temperature Stress During Anthesis Stage on Dry Matter Accumulation and Yield of Winter Wheat. Agronomy. 2025; 15(4):761. https://doi.org/10.3390/agronomy15040761
Chicago/Turabian StyleJiang, Xiaodong, Qiuhui Chen, Evgenios Agathokleous, Jianqu Zhang, Zaiqiang Yang, and M’Ponkrou Takin. 2025. "Effects of Low-Temperature Stress During Anthesis Stage on Dry Matter Accumulation and Yield of Winter Wheat" Agronomy 15, no. 4: 761. https://doi.org/10.3390/agronomy15040761
APA StyleJiang, X., Chen, Q., Agathokleous, E., Zhang, J., Yang, Z., & Takin, M. (2025). Effects of Low-Temperature Stress During Anthesis Stage on Dry Matter Accumulation and Yield of Winter Wheat. Agronomy, 15(4), 761. https://doi.org/10.3390/agronomy15040761







