Characteristics of Bacterial Community Structure in Yellow Paddy Soil After Long-Term Chemical Fertilisation, Organic Fertilisation, and Fertilisation Mode Conversion
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling
2.3. Soil Bacteria Assay
2.4. Determination of Soil Chemical Indicators
2.5. Statistical Analysis
3. Results
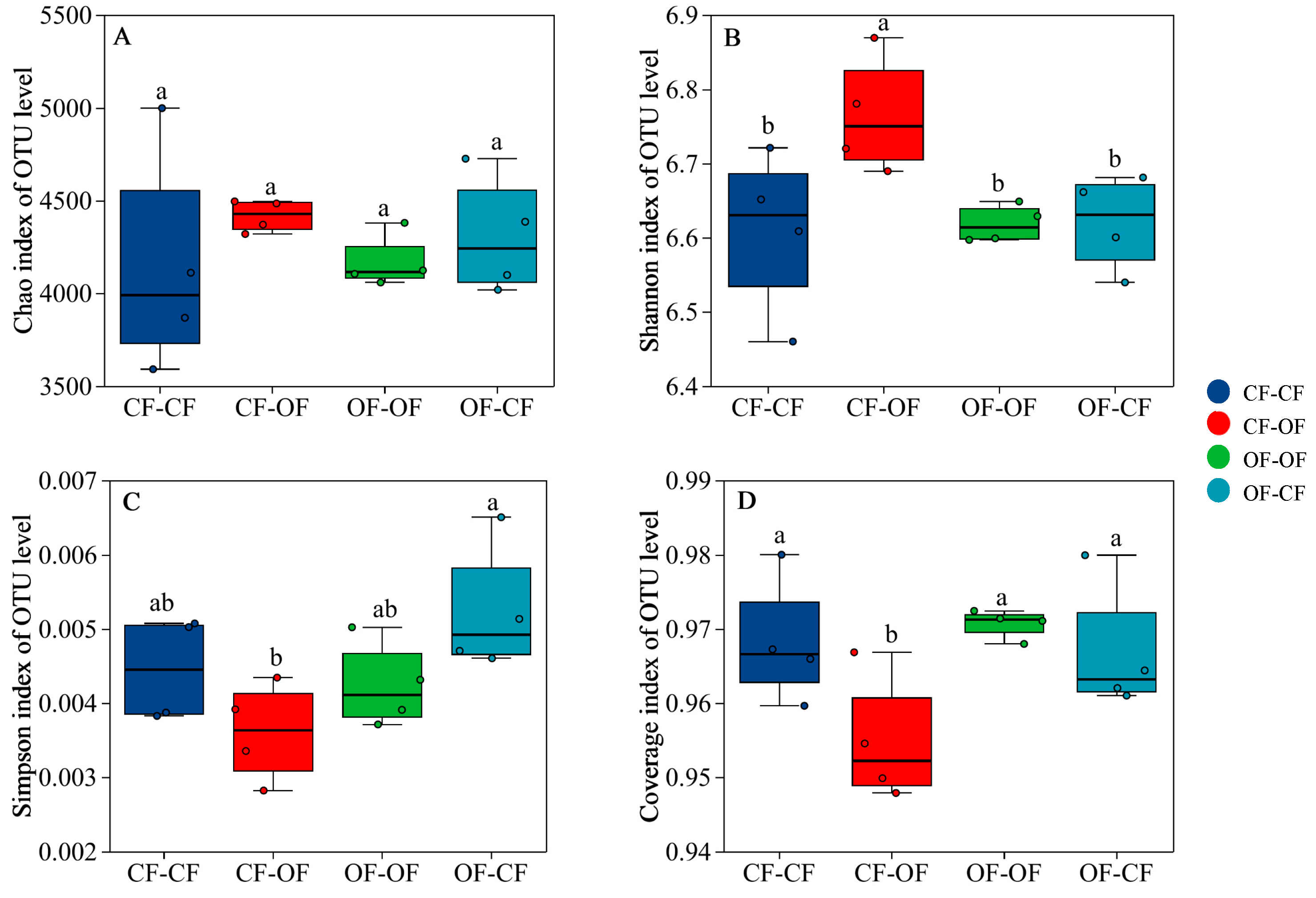
3.1. Effects of Different Fertilisation Treatments on Bacterial Alpha Diversity Indices
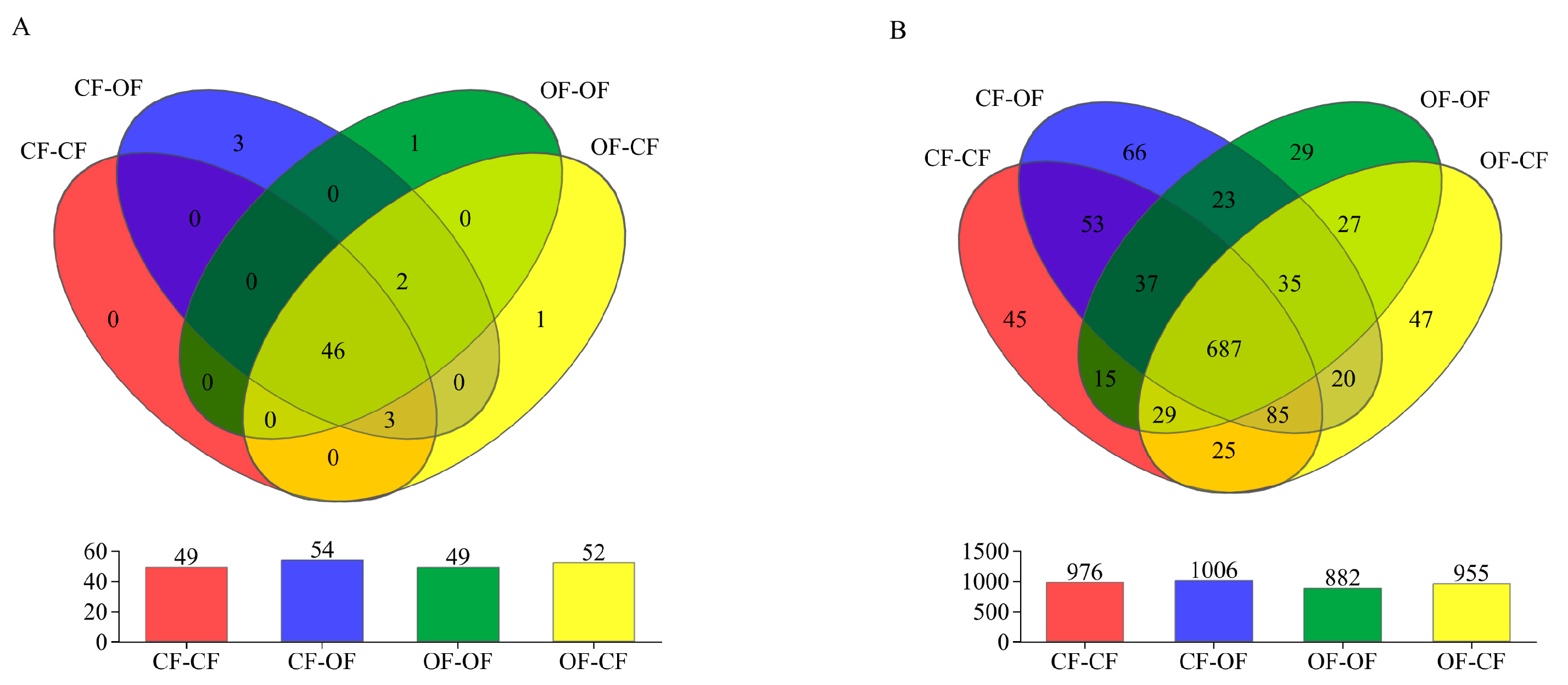
3.2. Effects of Different Fertiliser Treatments on the Number of Bacterial Species
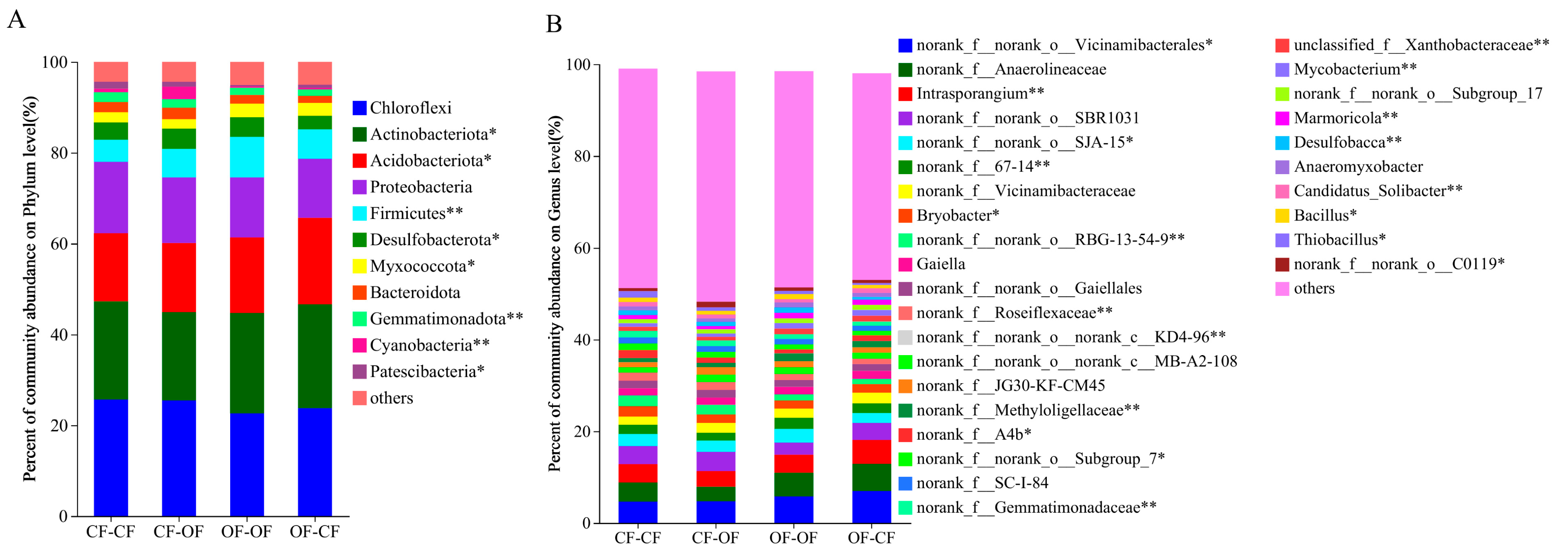
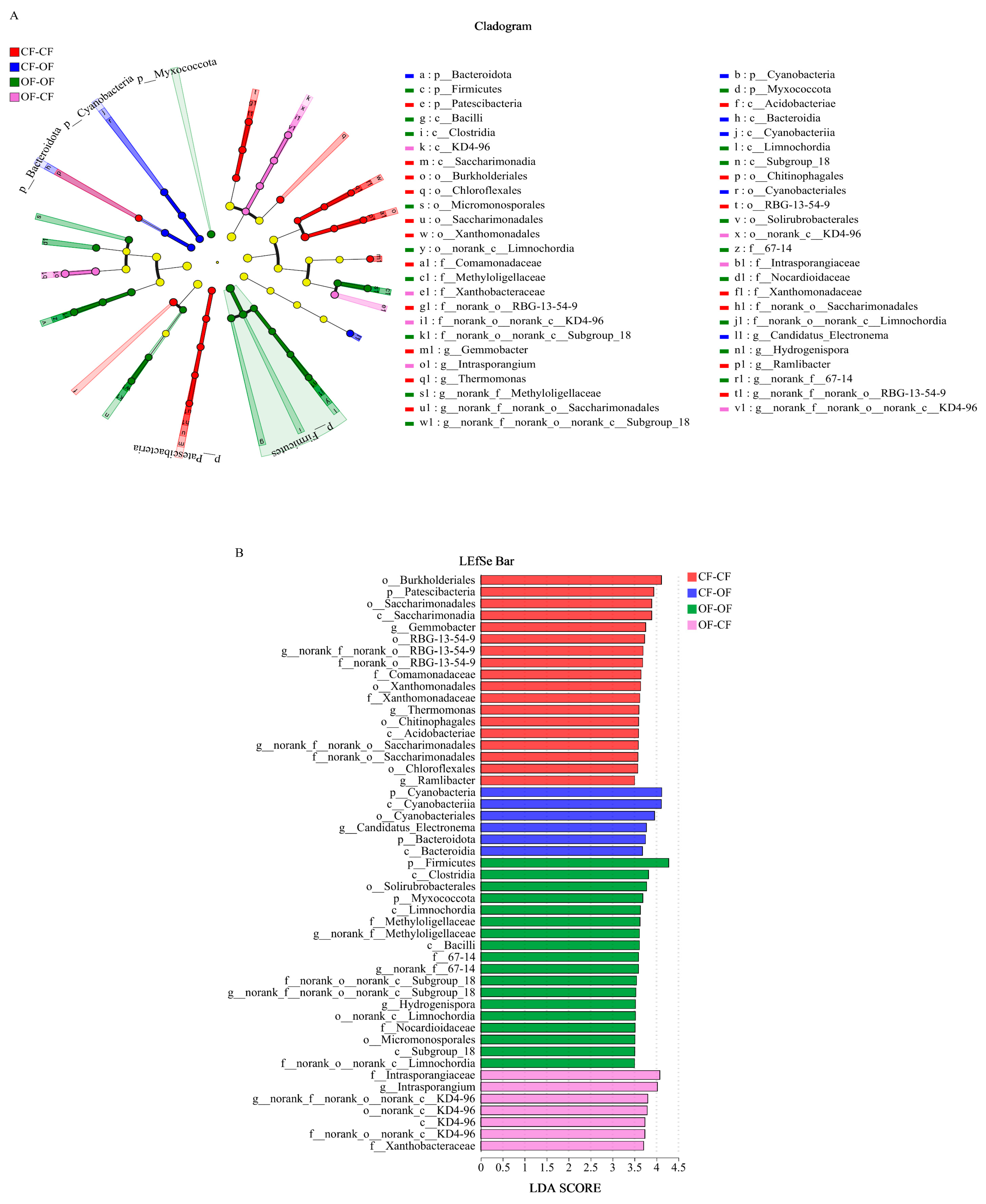
3.3. Effects of Different Fertiliser Applications on Differences in Bacterial Species
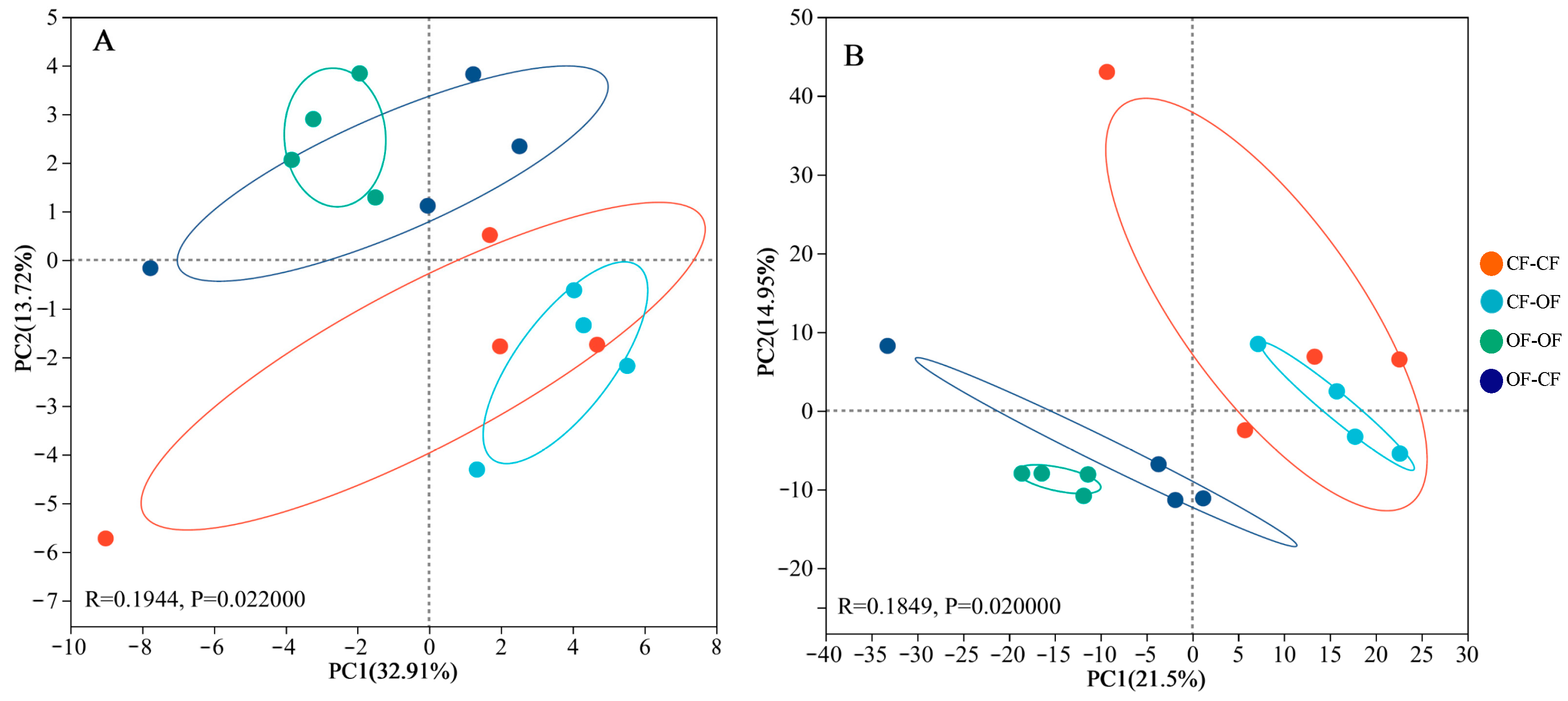
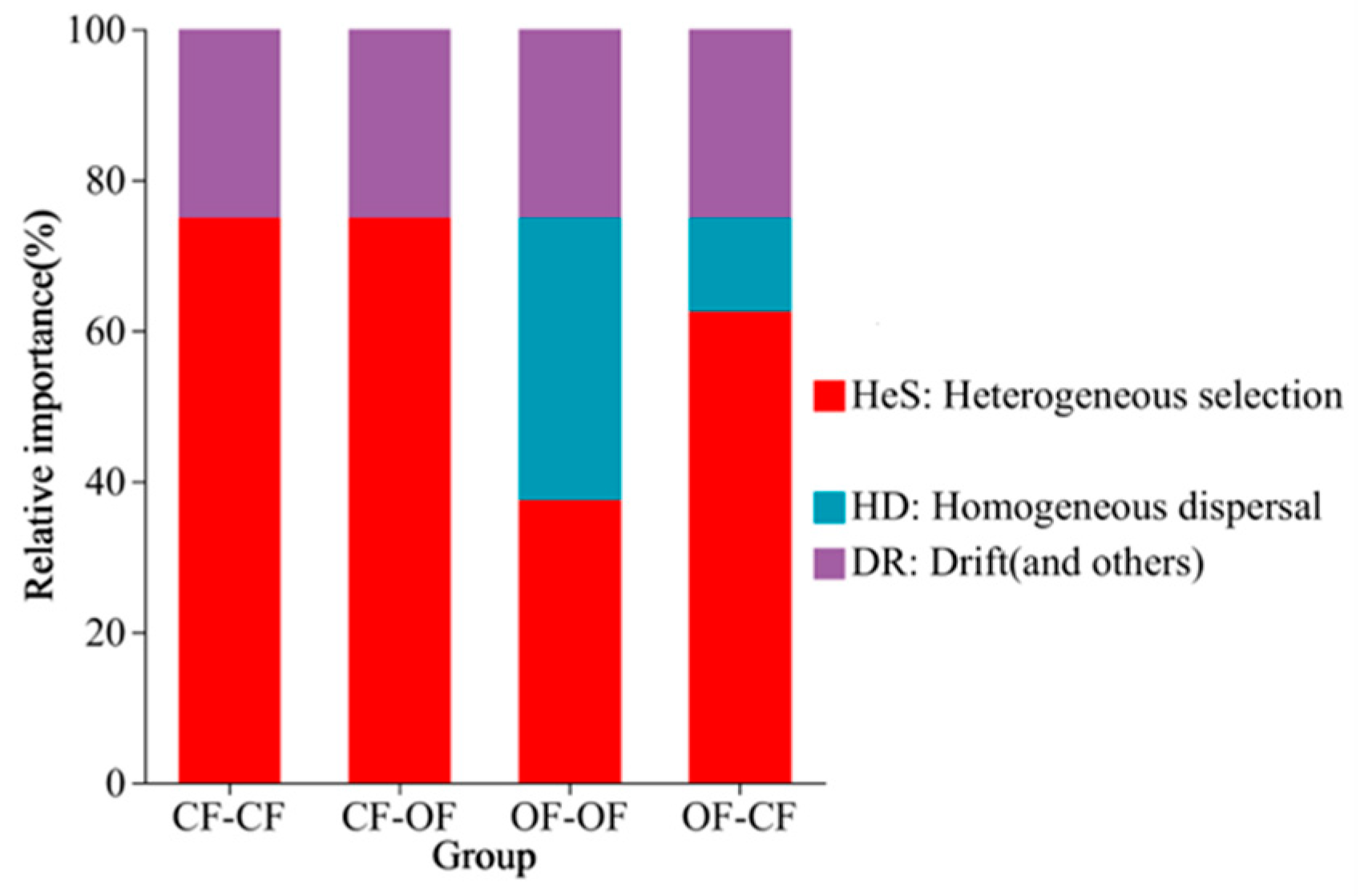
3.4. Effects of Different Fertiliser Applications on Bacterial Assembly Processes
3.5. Environmental Factor Correlation Analysis
4. Discussion
4.1. Fertilisation Pattern Affects Soil Bacterial Alpha Diversity
4.2. Fertilisation Affects the Number of Taxa and Relative Abundance of Soil Bacteria
4.3. Fertilisation Affects Soil Bacterial Beta Diversity and Assembly Processes
4.4. Changes in the Soil Environment Are Important in Influencing the Composition of Soil Bacterial Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long Term Tillage, Cover Crop, and Fertilization Effects on Microbial Community Structure, Activity: Implications for Soil Quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Strecker, T.; Barnard, R.L.; Niklaus, P.A.; Scherer-Lorenzen, M.; Weigelt, A.; Scheu, S.; Eisenhauer, N. Effects of Plant Diversity, Functional Group Composition, and Fertilization on Soil Microbial Properties in Experimental Grassland. PLoS ONE 2015, 10, e0125678. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Zhu, L.; Luan, L.; Chen, Y.; Wang, X.; Zhou, S.; Zou, W.; Han, X.; Duan, Y.; Zhu, B.; Li, Y.; et al. Community Assembly of Organisms Regulates Soil Microbial Functional Potential through Dual Mechanisms. Glob. Change Biol. 2024, 30, e17160. [Google Scholar] [CrossRef]
- Bagavthsingh, G.; Duraisamy, J. Long Term Fertilization on Soil Nutrient Dynamics, Soil Quality and Soil Bacterial Community Structure in an Inceptisol Under Semi-Arid Tropics of Finger Millet (Eleusine Coracana)—Maize (Zea Mays) Cropping Sequence. J. Soil Sci. Plant Nutr. 2024, 24, 3923–3942. [Google Scholar] [CrossRef]
- Fan, P.; Li, J.; Chen, P.; Wei, D.; Zhang, Q.; Jia, Z.; He, C.; Ullah, J.; Wang, Q.; Ruan, Y. Mitigating Soil Degradation in Continuous Cropping Banana Fields through Long-Term Organic Fertilization: Insights from Soil Acidification, Ammonia Oxidation, and Microbial Communities. Ind. Crops Prod. 2024, 213, 118385. [Google Scholar] [CrossRef]
- Dietrich, P.; Buchmann, T.; Cesarz, S.; Eisenhauer, N.; Roscher, C. Fertilization, Soil and Plant Community Characteristics Determine Soil Microbial Activity in Managed Temperate Grasslands. Plant Soil 2017, 419, 189–199. [Google Scholar] [CrossRef]
- Thiombiano, B.A.; Le, Q.B.; Ouédraogo, D. The Role of Responsive Heterogeneity in Sub-Saharan Smallholder Farming Sustainability: Socio-Economic and Biophysical Determinants of Mineral and Organic Fertilizers Used in South Western Burkina Faso. Int. J. Agric. Sustain. 2023, 21, 2219921. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the Yields of Organic and Conventional Agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- Bolo, P.; Mucheru-Muna, M.; Mwirichia, R.K.; Kinyua, M.; Ayaga, G.; Kihara, J. Soil Bacterial Community Is Influenced by Long-term Integrated Soil Fertility Management Practices in a Ferralsol in Western Kenya. J. Sustain. Agric. Environ. 2024, 3, e12090. [Google Scholar] [CrossRef]
- Wang, J.; Xue, C.; Song, Y.; Wang, L.; Huang, Q.; Shen, Q. Wheat and Rice Growth Stages and Fertilization Regimes Alter Soil Bacterial Community Structure, But Not Diversity. Front. Microbiol. 2016, 7, 1207. [Google Scholar] [CrossRef]
- Frąc, M.; Sas-Paszt, L.; Sitarek, M. Changes in the Mineral Content of Soil Following the Application of Different Organic Matter Sources. Agriculture 2023, 13, 1120. [Google Scholar] [CrossRef]
- Scholier, T.; Lavrinienko, A.; Brila, I.; Tukalenko, E.; Hindström, R.; Vasylenko, A.; Cayol, C.; Ecke, F.; Singh, N.J.; Forsman, J.T.; et al. Urban Forest Soils Harbour Distinct and More Diverse Communities of Bacteria and Fungi Compared to Less Disturbed Forest Soils. Mol. Ecol. 2023, 32, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; He, P.; Ai, C.; Zhao, S.; Ding, W.; Song, D.; Zhang, J.; Huang, S.; Abbas, T.; Zhou, W. How Do Soil Bacterial Diversity and Community Composition Respond under Recommended and Conventional Nitrogen Fertilization Regimes? Microorganisms 2020, 8, 1193. [Google Scholar] [CrossRef]
- Knapp, S.; Van Der Heijden, M.G.A. A Global Meta-Analysis of Yield Stability in Organic and Conservation Agriculture. Nat. Commun. 2018, 9, 3632. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, K.A.; Lu, Z.; Shen, Z.; Daba, N.A.; Li, J.; Alam, M.A.; Lisheng, L.; Gilbert, N.; Legesse, T.G.; Huimin, Z. Impacts of Long-Term Chemical Nitrogen Fertilization on Soil Quality, Crop Yield, and Greenhouse Gas Emissions: With Insights into Post-Lime Application Responses. Sci. Total Environ. 2024, 944, 173827. [Google Scholar] [CrossRef]
- Mihelič, R.; Pintarič, S.; Eler, K.; Suhadolc, M. Effects of Transitioning from Conventional to Organic Farming on Soil Organic Carbon and Microbial Community: A Comparison of Long-Term Non-Inversion Minimum Tillage and Conventional Tillage. Biol. Fertil. Soils 2024, 60, 341–355. [Google Scholar] [CrossRef]
- Huertas, V.; Jiménez, A.; Diánez, F.; Chelhaoui, R.; Santos, M. Importance of Dark Septate Endophytes in Agriculture in the Face of Climate Change. JoF 2024, 10, 329. [Google Scholar] [CrossRef]
- Goldenberg-Vilar, A.; Morán-Luis, M.; Vieites, D.R.; Álvarez-Martínez, J.M.; Silió, A.; Mony, C.; Varandas, S.; Monteiro, S.M.; Burgess, D.; Cabecinha, E.; et al. Biogeographical Distribution of River Microbial Communities in Atlantic Catchments. Environ. Microbiol. Rep. 2025, 17, e70065. [Google Scholar] [CrossRef]
- Nicholas, B.; Nicholas, B.; Goodman, M. Abstract 1060 Analyses of Farmland Soil Samples with Differing Tillage Practices: A Comparison of Bacteriological and Fungal Presence, Activity, and Impact on Soil. J. Biol. Chem. 2024, 300, 105813. [Google Scholar] [CrossRef]
- Bao, S. An Exploratory Method for Fractionation of Organic Phosphorus from Grassland Soils; Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Walters, K.E.; Martiny, J.B.H. Alpha-, Beta-, and Gamma-Diversity of Bacteria Varies across Habitats. PLoS ONE 2020, 15, e0233872. [Google Scholar] [CrossRef]
- Custer, G.F.; Van Diepen, L.T.A. Plant Invasion Has Limited Impact on Soil Microbial α-Diversity: A Meta-Analysis. Diversity 2020, 12, 112. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Zhou, Z.; Huang, G.; Wang, X.; Han, Q.; Liu, G. The Application of Mixed Organic and Inorganic Fertilizers Drives Soil Nutrient and Bacterial Community Changes in Teak Plantations. Microorganisms 2022, 10, 958. [Google Scholar] [CrossRef]
- Enagbonma, B.J.; Fadiji, A.E.; Babalola, O.O. Anthropogenic Fertilization Influences a Shift in Barley Rhizosphere Microbial Communities. PeerJ 2024, 12, e17303. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sun, G.; Du, W.; Ai, F.; Yin, Y.; Guo, H. Impacts of Chemical and Organic Fertilizers on the Bacterial Communities, Sulfonamides and Sulfonamide Resistance Genes in Paddy Soil Under Rice-Wheat Rotation. Bull. Iron. Contam. Toxicol. 2023, 110, 20. [Google Scholar] [CrossRef]
- Li, P.; Kong, D.; Zhang, H.; Xu, L.; Li, C.; Wu, M.; Jiao, J.; Li, D.; Xu, L.; Li, H.; et al. Different Regulation of Soil Structure and Resource Chemistry under Animal- and Plant-Derived Organic Fertilizers Changed Soil Bacterial Communities. Appl. Soil Ecol. 2021, 165, 104020. [Google Scholar] [CrossRef]
- Shen, C.; He, M.; Zhang, J.; Liu, J.; Wang, Y. Response of Soil Antibiotic Resistance Genes and Bacterial Communities to Fresh Cattle Manure and Organic Fertilizer Application. J. Environ. Manag. 2024, 349, 119453. [Google Scholar] [CrossRef]
- Vincze, É.-B.; Becze, A.; Laslo, É.; Mara, G. Beneficial Soil Microbiomes and Their Potential Role in Plant Growth and Soil Fertility. Agriculture 2024, 14, 152. [Google Scholar] [CrossRef]
- Teste, F.P.; Lambers, H.; Enowashu, E.E.; Laliberté, E.; Marhan, S.; Kandeler, E. Soil Microbial Communities Are Driven by the Declining Availability of Cations and Phosphorus during Ecosystem Retrogression. Soil Biol. Biochem. 2021, 163, 108430. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, R.; Wang, X.; Xu, X.; Ai, C.; He, P.; Liang, G.; Zhou, W.; Zhu, P. Effect of High Soil C/N Ratio and Nitrogen Limitation Caused by the Long-Term Combined Organic-Inorganic Fertilization on the Soil Microbial Community Structure and Its Dominated SOC Decomposition. J. Environ. Manag. 2022, 303, 114155. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut Microbial Metabolites as Multi-Kingdom Intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Chialva, M.; Ghignone, S.; Cozzi, P.; Lazzari, B.; Bonfante, P.; Abbruscato, P.; Lumini, E. Water Management and Phenology Influence the Root-Associated Rice Field Microbiota. FEMS Microbiol. Ecol. 2020, 96, fiaa146. [Google Scholar] [CrossRef]
- Fu, Y.; De Jonge, L.W.; Moldrup, P.; Paradelo, M.; Arthur, E. Improvements in Soil Physical Properties after Long-Term Manure Addition Depend on Soil and Crop Type. Geoderma 2022, 425, 116062. [Google Scholar] [CrossRef]
- Bay, S.K.; Dong, X.; Bradley, J.A.; Leung, P.M.; Grinter, R.; Jirapanjawat, T.; Arndt, S.K.; Cook, P.L.M.; LaRowe, D.E.; Nauer, P.A.; et al. Trace Gas Oxidizers Are Widespread and Active Members of Soil Microbial Communities. Nat. Microbiol. 2021, 6, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Ning, D.; He, Z.; Zhang, P.; Spencer, S.J.; Gao, S.; Shi, W.; Wu, L.; Zhang, Y.; Yang, Y.; et al. Small and Mighty: Adaptation of Superphylum Patescibacteria to Groundwater Environment Drives Their Genome Simplicity. Microbiome 2020, 8, 51. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Zhang, L.; Zeng, L.; Liu, Y.; Wang, X.; He, P.; Li, S.; Liang, G.; Zhou, W.; et al. The Stronger Impact of Inorganic Nitrogen Fertilization on Soil Bacterial Community than Organic Fertilization in Short-Term Condition. Geoderma 2021, 382, 114752. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Zhao, Z.-Y.; Xiong, X.-B.; Wang, N.; Zhou, R.; Zhang, Z.-M.; Ding, F.; Hao, M.; Wang, S.; Ma, Y.; et al. Microplastics Affect Soil Bacterial Community Assembly More by Their Shapes Rather than the Concentrations. Water Res. 2023, 245, 120581. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, Z.; Sun, S.; Zhang, Q.; Sun, S.; Hang, X.; Ravanbakhsh, M.; Wei, Z.; Li, R.; Wang, S.; et al. Investigating Protistan Predators and Bacteria within Soil Microbiomes in Agricultural Ecosystems under Organic and Chemical Fertilizer Applications. Biol. Fertil. Soils 2024, 60, 1009–1024. [Google Scholar] [CrossRef]
- Tang, S.; Ma, Q.; Marsden, K.A.; Chadwick, D.R.; Luo, Y.; Kuzyakov, Y.; Wu, L.; Jones, D.L. Microbial Community Succession in Soil Is Mainly Driven by Carbon and Nitrogen Contents Rather than Phosphorus and Sulphur Contents. Soil Biol. Biochem. 2023, 180, 109019. [Google Scholar] [CrossRef]
- Li, R.; Ren, C.; Wu, L.; Zhang, X.; Mao, X.; Fan, Z.; Cui, W.; Zhang, W.; Wei, G.; Shu, D. Fertilizing-Induced Alterations of Microbial Functional Profiles in Soil Nitrogen Cycling Closely Associate with Crop Yield. Environ. Res. 2023, 231, 116194. [Google Scholar] [CrossRef]
- Cao, X.; Liu, J.; Zhang, L.; Mao, W.; Li, M.; Wang, H.; Sun, W. Response of Soil Microbial Ecological Functions and Biological Characteristics to Organic Fertilizer Combined with Biochar in Dry Direct-Seeded Paddy Fields. Sci. Total Environ. 2024, 948, 174844. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Alori, E.T.; Osemwegie, O.O.; Ibaba, A.L.; Daramola, F.Y.; Olaniyan, F.T.; Lewu, F.B.; Babalola, O.O. The Importance of Soil Microorganisms in Regulating Soil Health. Commun. Soil Sci. Plant Anal. 2024, 55, 2636–2650. [Google Scholar] [CrossRef]
- Esposito, A.; Del Duca, S.; Vitali, F.; Bigiotti, G.; Mocali, S.; Semenzato, G.; Papini, A.; Santini, G.; Mucci, N.; Padula, A.; et al. The Great Gobi A Strictly Protected Area: Characterization of Soil Bacterial Communities from Four Oases. Microorganisms 2024, 12, 320. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial Community Structure after Long-Term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef]
- Geng, H.T.; Wang, X.D.; Ye, Z.Q.; Zhou, W.J. Effect of Combined Application of Fungal Residue and Chemical Fertilizer on Soil Microbial Community Composition and Diversity in Paddy Soil. Huanjing Kexue 2023, 44, 2338–2347. [Google Scholar] [CrossRef]
- Wu, C.; Yin, Y.; Yang, X.; Feng, L.; Tang, H.; Tao, J. A Markov-based Model for Predicting the Development Trend of Soil Microbial Communities in Saline-alkali Land in Wudi County. Concurr. Comput. 2019, 31, e4754. [Google Scholar] [CrossRef]
- Tan, X.; Kan, L.; Su, Z.; Liu, X.; Zhang, L. The Composition and Diversity of Soil Bacterial and Fungal Communities Along an Urban-To-Rural Gradient in South China. Forests 2019, 10, 797. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil Aggregate Size Mediates the Impacts of Cropping Regimes on Soil Carbon and Microbial Communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]

| Trial Soil | pH | OM (g∙kg−1) | TN (g∙kg−1) | AP (mg∙kg−1) | AK (mg∙kg−1) |
|---|---|---|---|---|---|
| CF | 6.74 | 48.17 | 1.90 | 15.43 | 264.33 |
| OF | 6.42 | 57.24 | 2.76 | 16.83 | 335.00 |
| Treatment Code | Organic Fertiliser (g∙pot−1) | Total Amount (g∙pot−1) | ||
|---|---|---|---|---|
| N | P2O5 | K2O | ||
| CF-CF | 0.00 | 1.60 | 0.80 | 0.80 |
| CF-OF | 592.59 | 1.60 | 0.77 | 3.56 |
| OF-OF | 592.59 | 1.60 | 0.77 | 3.56 |
| OF-CF | 0.00 | 1.60 | 0.80 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Huang, X.; Zhu, H.; Liu, Y.; Zhang, Y.; Zhang, S.; Xiong, H.; Yang, H.; Li, Y. Characteristics of Bacterial Community Structure in Yellow Paddy Soil After Long-Term Chemical Fertilisation, Organic Fertilisation, and Fertilisation Mode Conversion. Agronomy 2025, 15, 749. https://doi.org/10.3390/agronomy15030749
Yang Y, Huang X, Zhu H, Liu Y, Zhang Y, Zhang S, Xiong H, Yang H, Li Y. Characteristics of Bacterial Community Structure in Yellow Paddy Soil After Long-Term Chemical Fertilisation, Organic Fertilisation, and Fertilisation Mode Conversion. Agronomy. 2025; 15(3):749. https://doi.org/10.3390/agronomy15030749
Chicago/Turabian StyleYang, Yehua, Xingcheng Huang, Huaqing Zhu, Yanling Liu, Yarong Zhang, Song Zhang, Han Xiong, Huan Yang, and Yu Li. 2025. "Characteristics of Bacterial Community Structure in Yellow Paddy Soil After Long-Term Chemical Fertilisation, Organic Fertilisation, and Fertilisation Mode Conversion" Agronomy 15, no. 3: 749. https://doi.org/10.3390/agronomy15030749
APA StyleYang, Y., Huang, X., Zhu, H., Liu, Y., Zhang, Y., Zhang, S., Xiong, H., Yang, H., & Li, Y. (2025). Characteristics of Bacterial Community Structure in Yellow Paddy Soil After Long-Term Chemical Fertilisation, Organic Fertilisation, and Fertilisation Mode Conversion. Agronomy, 15(3), 749. https://doi.org/10.3390/agronomy15030749






