Sugarcane Responses to Water Deficit Are Modulated by Environmental CO2 Concentration in a Genotype and Scale Dependent-Manner
Abstract
1. Introduction
2. Materials and Methods
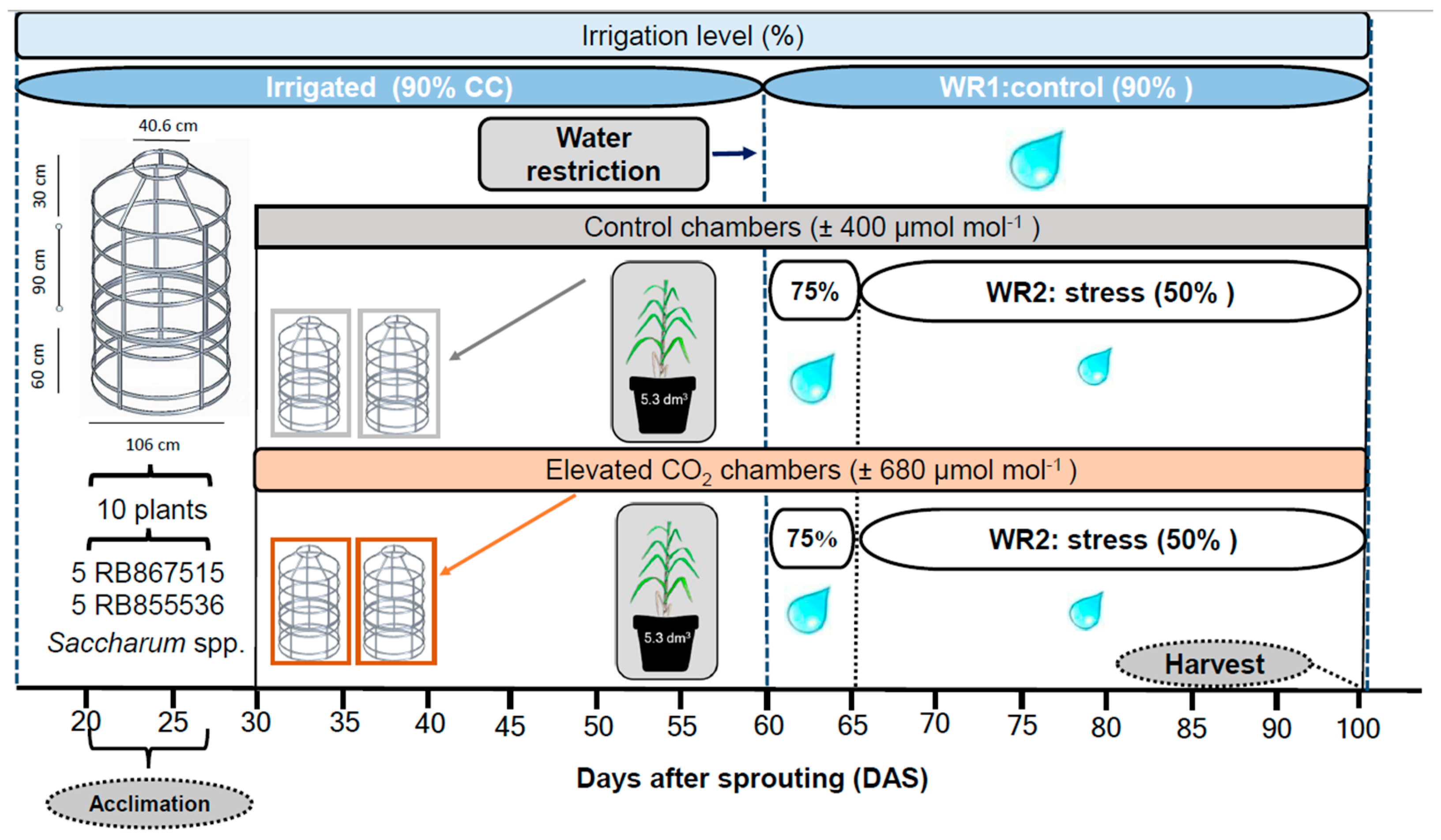
2.1. Plant Material and Experimental Conditions
2.2. Treatment Imposition
2.3. Morphological Measurements
2.4. Physiologic Measurements
2.5. Determination of Carbon and Organic Nitrogen
2.6. Anatomic Measurements
2.7. Experimental Design and Data Analysis
3. Results
Morphological, Physiological, and Anatomic Response to Drought Stress and High CO2
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Guhan, V.; Annadurai, K.; Easwaran, S.; Marimuthu, M.; Balu, D.; Vigneswaran, S.; Navinkumar, C. Assessing the impact of climate change on water requirement and yield of sugarcane over different agro-climatic zones of Tamil Nadu. Sci. Rep. 2024, 14, 8239. [Google Scholar] [CrossRef]
- Da Cruz, T.V.; Machado, R.L. Measuring climate change’s impact on different sugarcane varieties production in the South of Goiás. Sci. Rep. 2023, 13, 11637. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a changing global climate: Scaling up and scaling down in crops. Front. Plant Sci. 2020, 11, 882. [Google Scholar] [CrossRef]
- Walia, S.; Rathore, S.; Kumar, R. Elucidating the Mechanisms, Responses and future prospects of medicinal and aromatic plants to elevated CO2 and elevated temperature. J. Appl. Res. Med. Aromat. Plants 2022, 26, 100365. [Google Scholar] [CrossRef]
- IPCC. A Report of the Intergovernmental Panel on Climate Change. In Climate Change 2023: Synthesis Report; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; p. 36. ISBN 9789291691432. [Google Scholar]
- Vaughan, M.M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.A.; McAuslane, H.J.; Alborn, H.T.; Allen, L.H.; Teal, P.E.A. Interactive effects of elevated [CO2] and drought on the maize phytochemical defense response against Mycotoxigenic Fusarium verticillioides. PLoS ONE 2016, 11, e0159270. [Google Scholar] [CrossRef] [PubMed]
- Balting, D.F.; AghaKouchak, A.; Lohmann, G.; Ionita, M. Northern hemisphere drought risk in a warming climate. NPJ Clim. Atmos. Sci. 2021, 4, 61. [Google Scholar] [CrossRef]
- De Menezes, S.M.; da Silva, G.F.; da Silva, M.M.; de Oliveira Filho, R.A.; Jardim, A.M.d.R.F.; Silva, J.R.I.; Silva, Ê.F.d.F.; Silva, J.V.; dos Santos, M.A.L. Pulse drip irrigation improves yield, physiological responses, and water-use efficiency of sugarcane. Water Conserv. Sci. Eng. 2024, 9, 25. [Google Scholar] [CrossRef]
- Bhargava, S.; Mitra, S. Elevated atmospheric CO2 and the future of crop plants. Plant Breed. 2021, 140, 1–11. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Gonçalves, R.; Alves, R.D.C.; Zingaretti, S.M. Increased [CO2] causes changes in physiological and genetic responses in C4 crops: A brief review. Plants 2020, 9, 1567. [Google Scholar] [CrossRef]
- Clemens, M.E.; Zuniga, A.; Oechel, W. Effects of elevated atmospheric carbon dioxide on the vineyard system of Vitis vinifera: A review. Am. J. Enol. Vitic. 2022, 73, 1–10. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Allen, L.H. Stem juice production of the C4 sugarcane (Saccharum officinarum) is enhanced by growth at double-ambient CO2 and high temperature. J. Plant Physiol. 2009, 166, 1141–1151. [Google Scholar] [CrossRef]
- De Souza, A.P.; Grandis, A.; Leite, D.C.C.; Buckeridge, M.S. Sugarcane as a bioenergy source: History, performance, and perspectives for second-generation bioethanol. Bioenergy Res. 2014, 7, 24–35. [Google Scholar] [CrossRef]
- Voora, V.; Bermúdez, S.; Le, H.; Larrea, C.; Luna, E. Global Market Report: Sugar Cane Prices and Sustainability. International Institute of Sustainable Development. 2023. Available online: https://www.iisd.org/publications/report/2023-global-market-report-sugar-cane (accessed on 1 March 2025).
- Marchiori, P.E.R.; Machado, E.C.; Sales, C.R.G.; Espinoza-Núñez, E.; Magalhães Filho, J.R.; Souza, G.M.; Pires, R.C.M.; Ribeiro, R.V. Physiological plasticity is important for maintaining sugarcane growth under water deficit. Front. Plant Sci. 2017, 8, 2148. [Google Scholar] [CrossRef]
- Grandis, A.; Fortirer, J.S.; Navarro, B.V.; de Oliveira, L.P.; Buckeridge, M.S. Biotechnologies to improve sugarcane productivity in a climate change scenario. Bioenergy Res. 2023, 17, 1–26. [Google Scholar] [CrossRef]
- De Oliveira, H.F.E.; Arriel, F.H.; Soares, F.A.L.; da Silva, E.C.; Mesquita, M.; Silva, T.D.; da Silva, J.L.B.; Sousa, C.M.; da Silva, M.V.; de Carvalho, A.A.; et al. Agronomic performance and technological attributes of sugarcane cultivars under split-irrigation management. Agriengineering 2024, 6, 4337–4352. [Google Scholar] [CrossRef]
- Marin, F.R.; Ribeiro, R.V.; Marchiori, P.E.R. How can crop modeling and plant physiology help to understand the plant responses to climate change? A case study with sugarcane. Theor. Exp. Plant Physiol. 2014, 26, 49–63. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Allen, L.H.; Gesch, R.W. Up-regulation of photosynthesis and sucrose metabolism enzymes in young expanding leaves of sugarcane under elevated growth CO2. Plant Sci. 2006, 171, 123–131. [Google Scholar] [CrossRef]
- De Souza, A.P.; Gaspar, M.; Da Silva, E.A.; Ulian, E.C.; Waclawovsky, A.J.; Nishiyama, M.Y.; Dos Santos, R.V.; Teixeira, M.M.; Souza, G.M.; Buckeridge, M.S. Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ. 2008, 31, 1116–1127. [Google Scholar] [CrossRef]
- Marchiori, P.E.R.; Machado, E.C.; Ribeiro, R.V. Photosynthetic limitations imposed by self-shading in field-grown sugarcane varieties. Field Crop. Res. 2014, 155, 30–37. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynth. Res. 2003, 77, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C.; Diaz-Ambrona, C.G.H.; Buckeridge, M.S.; Souza, A.; Barbieri, V.; Dourado Neto, D. Sugarcane and climate change: Effects of CO2 on potential growth and development. Acta Hortic. 2008, 802, 331–336. [Google Scholar] [CrossRef]
- Misra, V.; Shrivastava, A.K.; Mall, A.K.; Solomon, S.; Singh, A.K.; Ansari, M.I. Can sugarcane cope with increasing atmospheric CO2 concentration? Aust. J. Crop Sci. 2019, 13, 780–784. [Google Scholar] [CrossRef]
- Walter, L.C.; Rosa, H.T.; Streck, N.A. Mecanismos de aclimatação das plantas à elevada concentração de CO2. Ciência Rural 2015, 45, 1564–1571. [Google Scholar] [CrossRef]
- Sales, C.R.G.; Wang, Y.; Evers, J.B.; Kromdijk, J. Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. J. Exp. Bot. 2021, 72, 5942–5960. [Google Scholar] [CrossRef]
- Brooker, R.; Brown, L.K.; George, T.S.; Pakeman, R.J.; Palmer, S.; Ramsay, L.; Schöb, C.; Schurch, N.; Wilkinson, M.J. Active and adaptive plasticity in a changing climate. Trends Plant Sci. 2022, 27, 717–728. [Google Scholar] [CrossRef]
- Khonghintaisong, J.; Onkaeo, A.; Songsri, P.; Jongrungklang, N. Water use efficiency characteristics and their contributions to yield in diverse sugarcane genotypes with varying drought resistance levels under different field irrigation conditions. Agriculture 2024, 14, 1952. [Google Scholar] [CrossRef]
- Drake, B.; Leadley, P.; Arp, W.; Nassiry, D.; Curtis, P. An open top chamber for field studies of elevated atmospheric CO2 concentration on saltmarsh vegetation. Funct. Ecol. 1989, 3, 363. [Google Scholar] [CrossRef]
- Daros, E.; Oliveira, R.A.d.; Barbosa, G.V.d.S. 45 Anos de Variedades Ridesa Brasil de Cana-de-Açúcar, Editora Graciosa, 1st ed.; 2015; Volume 1, ISBN 978-85-66456-08-0. Available online: https://www.ridesa.com.br/variedades?lightbox=dataItem-ivvjh9l61 (accessed on 20 January 2025).
- Vital, C.E.; Giordano, A.; de Almeida Soares, E.; Rhys Williams, T.C.; Mesquita, R.O.; Vidigal, P.M.P.; de Santana Lopes, A.; Pacheco, T.G.; Rogalski, M.; de Oliveira Ramos, H.J.; et al. An integrative overview of the molecular and physiological responses of sugarcane under drought conditions. Plant Mol. Biol. 2017, 94, 577–594. [Google Scholar] [CrossRef]
- Van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Fertilization and liming recommendation for the state of São Paulo. In Recomendações de Adubação e Calagem para o Estado de São Paulo; Instituto Agronômico and Fundação IAC: Campinas, Brazil, 1997; p. 262. [Google Scholar]
- Vu, J.C.V.; Allen, L.H. Growth at elevated CO2 delays the adverse effects of drought stress on leaf photosynthesis of the C4 sugarcane. J. Plant Physiol. 2009, 166, 107–116. [Google Scholar] [CrossRef]
- Stokes, C.J.; Inman-Bamber, N.G.; Everingham, Y.L.; Sexton, J. Measuring and modelling CO2 effects on sugarcane. Environ. Model. Softw. 2016, 78, 68–78. [Google Scholar] [CrossRef]
- Singels, A.; Jones, M.; Marin, F.; Ruane, A.; Thorburn, P. Predicting climate change impacts on sugarcane production at sites in Australia, Brazil and South Africa using the canegro model. Sugar Tech 2014, 16, 347–355. [Google Scholar] [CrossRef]
- Hermann, E.R.; Câmara, G.M.S. Um método simples para estimar a área foliar da cana-de-açúcar. Rev. Da Soc. Dos Técnicos Açucareiros e Alcool. Do Bras. 1999, 17, 32–34. [Google Scholar]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413. [Google Scholar] [CrossRef]
- Collatz, G.; Ribas-Carbo, M.; Berry, J. Coupled photosynthesis-stomatal conductance model for leaves of C4 plants. Aust. J. Plant Physiol. 1992, 19, 519–538. [Google Scholar] [CrossRef]
- Johansen, D. Plant Microtechnique; Mcgraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Rodrigues, F.A.; Soares, J.D.R.; Silva, R.A.L.; Penoni, E.D.S.; Pasqual, M.; Pereira, F.J.; de Castro, E.M. Anatomy of vegetative organs and seed histochemistry of Physalis peruviana L. Aust. J. Crop Sci. 2014, 8, 895–900. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Silva, A.A.d.; Rubio, Z.C.C.; Linhares, P.C.A.; Silva, K.R.E.; Pimentel, G.V.; Marchiori, P.E.R. Genotypic variation of sugarcane for salinity tolerance: Morphological and physiological responses. Ciência e Agrotecnologia 2022, 46, e000122. [Google Scholar] [CrossRef]
- Ding, L.; Lu, Z.; Gao, L.; Guo, S.; Shen, Q. Is nitrogen a key determinant of water transport and photosynthesis in higher plants upon drought stress? Front. Plant Sci. 2018, 9, 1143. [Google Scholar] [CrossRef]
- Lima, W.; Calgaro, M.; Coelho, D.S.; dos Santos, D.B.; de Souza, M.A. Growth of sugar cane varieties under salinity. Rev. Ceres 2016, 63, 265–271. [Google Scholar]
- Taratima, W.; Ritmaha, T.; Jongrungklang, N.; Raso, S.; Maneerattanarungroj, P. Leaf anatomical responses to drought stress condition in hybrid sugarcane leaf (Saccharum officinarum ‘KK3’). Malaysian Appl. Biol. 2019, 48, 181–188. [Google Scholar]
- Haworth, M.; Killi, D.; Materassi, A.; Raschi, A.; Centritto, M. Impaired stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated [CO2]. Front. Plant Sci. 2016, 7, 1568. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Materassi, A.; Raschi, A.; Scutt, C.P.; Centritto, M. The functional significance of the stomatal size to density relationship: Interaction with atmospheric [CO2] and role in plant physiological behaviour. Sci. Total Environ. 2023, 863, 160908. [Google Scholar] [CrossRef]
- Haworth, M.; Killi, D.; Materassi, A.; Raschi, A. Coordination of stomatal physiological behavior and morphology with carbon dioxide determines stomatal control. Am. J. Bot. 2015, 102, 677–688. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate—Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: A review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Drake, P.; Froend, R.; Franks, P. Smaller, Faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.D.; Tschaplinski, T.J.; Norby, R.J. Plant water relations at elevated CO2—Implications for water-limited environments. Plant Cell Environ. 2002, 25, 319–331. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Lau, J.A. Evolutionary context for understanding and manipulating plant responses to past, present and future atmospheric [CO2]. Philos. Trans. R. Soc. B: Biol. Sci. 2012, 367, 613–629. [Google Scholar] [CrossRef]
- Valliere, J. Tradeoffs between growth rate and water-use efficiency in seedlings of native perennials but not invasive annuals. Plant Ecol. 2019, 220, 361–369. [Google Scholar] [CrossRef]
- Smith, N.G.; Keenan, T.F. Mechanisms underlying leaf photosynthetic acclimation to warming and elevated CO2 as inferred from least-cost optimality theory. Glob. Chang. Biol. 2020, 26, 5202–5216. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Araus, J.L.; van Heerden, P.D.R.; Foyer, C.H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 2011, 62, 3135–3153. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Silva, M.; Jifon, J.L.; da Silva, J.; dos Santos, C.M.; Sharma, V. Relationships between physiological traits and productivity of sugarcane in response to water deficit. J. Agric. Sci. 2014, 152, 104–118. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbó, M.; Bota, J.; Galmés, J.; Henkle, M.; Martínez-Cañellas, S.; Medrano, H. Decreased rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2006, 172, 73–82. [Google Scholar] [CrossRef]
- Varone, L.; Ribas-Carbo, M.; Cardona, C.; Gallé, A.; Medrano, H.; Gratani, L.; Flexas, J. Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Bot. 2012, 75, 235–247. [Google Scholar] [CrossRef]
- Perlikowski, D.; Czyżniejewski, M.; Marczak, Ł.; Augustyniak, A.; Kosmala, A. Water deficit affects primary metabolism differently in two lolium multiflorum/festuca arundinacea introgression forms with a distinct capacity for photosynthesis and membrane regeneration. Front. Plant Sci. 2016, 7, 1063. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Yu, T.; Liu, X.; Li, Y.; Peng, S.; Huang, J. Heterogeneity of photosynthesis within leaves is associated with alteration of leaf structural features and leaf N content per leaf area in rice. Funct. Plant Biol. 2015, 42, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Ghosh, S. Regulation of glutamine synthetase isoforms in two differentially drought-tolerant rice (Oryza sativa L.) Cultivars under water deficit conditions. Plant Cell Rep. 2013, 32, 183–193. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar] [CrossRef]
- Singh, N.; Singh, D.; Singh, A. Biological seed priming mitigates the effects of water stress in sunflower seedlings. Physiol. Mol. Biol. Plants 2015, 21, 207–214. [Google Scholar] [CrossRef] [PubMed]
| pH (KCl) | K | P | Na | Ca | Mg | Al | H+ + Al | Zn | Fe | Mn | Cu | B | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg dm−3 | cmol dm−3 | mg dm−3 | |||||||||||
| 6.0 | 51.8 | 20.6 | 11.2 | 2.3 | 0.28 | 0.06 | 2.01 | 3.4 | 32.4 | 21.9 | 6.9 | 0.2 | 22.9 |
| Variety | Variable | 400 μmol·mol−1 | 680 μmol·mol−1 | F (p < 0.05) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Drought | Control | Drought | Drought | (CO2) | Drought × (CO2) | ||||||
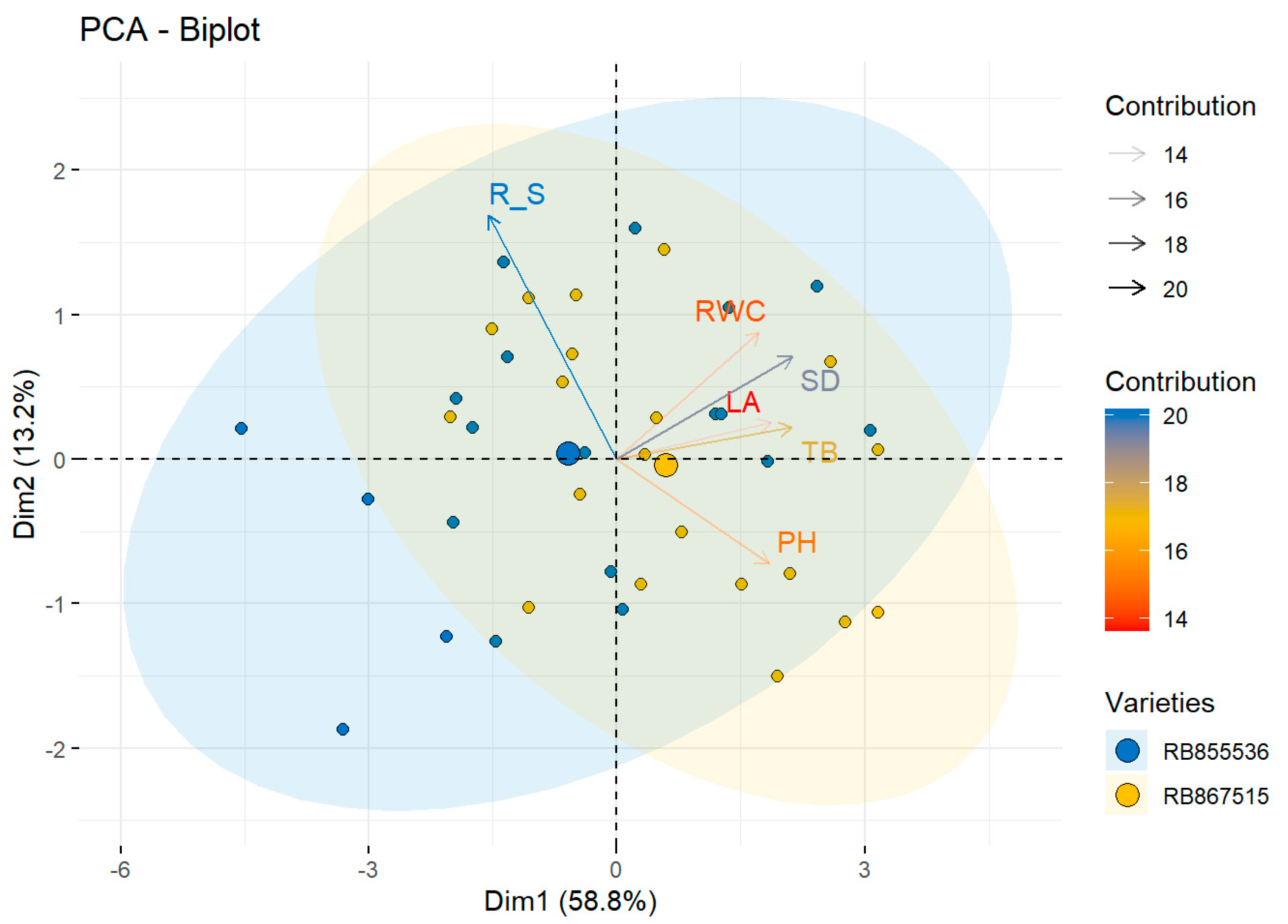
| RB867515 | TB | 26.2 ± 1.2 | a | 19.7 ± 1.1 | b | 26.0 ± 1.7 | a | 22.3 ± 1.8 | ab | * | NS | * |
| PH | 149.0 ± 3.1 | b | 146.6 ± 3.4 | b | 171.8 ± 2.8 | a | 143.2 ± 1.2 | b | * | * | * | |
| SD | 11.7 ± 0.3 | a | 10.6 ± 0.3 | a | 11.7 ± 0.7 | a | 11.6 ± 0.3 | a | NS | NS | NS | |
| LA | 43.9 ± 0.02 | b | 14.8 ± 0.01 | c | 53.1 ± 0.02 | a | 18.7 ± 0.01 | c | * | * | * | |
| R/S | 0.38 ± 0.3 | b | 0.53 ± 0.04 | a | 0.44 ± 0.05 | ab | 0.50 ± 0.02 | ab | * | NS | * | |
| RB855536 | TB | 23.6 ± 1.8 | a | 15.4 ± 1.8 | b | 23.7 ± 1.5 | a | 17.2 ± 2.7 | b | * | NS | * |
| PH | 142.2 ± 5.5 | a | 134.2 ± 5.3 | a | 144.8 ± 4.1 | a | 134.4 ± 1.6 | a | * | NS | NS | |
| SD | 12.2 ± 0.6 | a | 9.6 ± 0.6 | b | 12 ± 0.3 | a | 9.9 ± 0.4 | b | * | NS | * | |
| LA | 43.7 ± 0.03 | a | 13.2 ± 0.01 | b | 42.9 ± 0.02 | a | 17.3 ± 0.01 | b | * | NS | * | |
| R/S | 0.43 ± 0.04 | a | 0.48 ± 0.05 | a | 0.48 ± 0.02 | a | 0.51 ± 0.02 | a | NS | NS | NS | |
| Variety | Variable | 400 μmol·mol−1 | 680 μmol·mol−1 | F (p < 0.05) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Drought | Control | Drought | Drought | (CO2) | Drought × (CO2) | ||||||
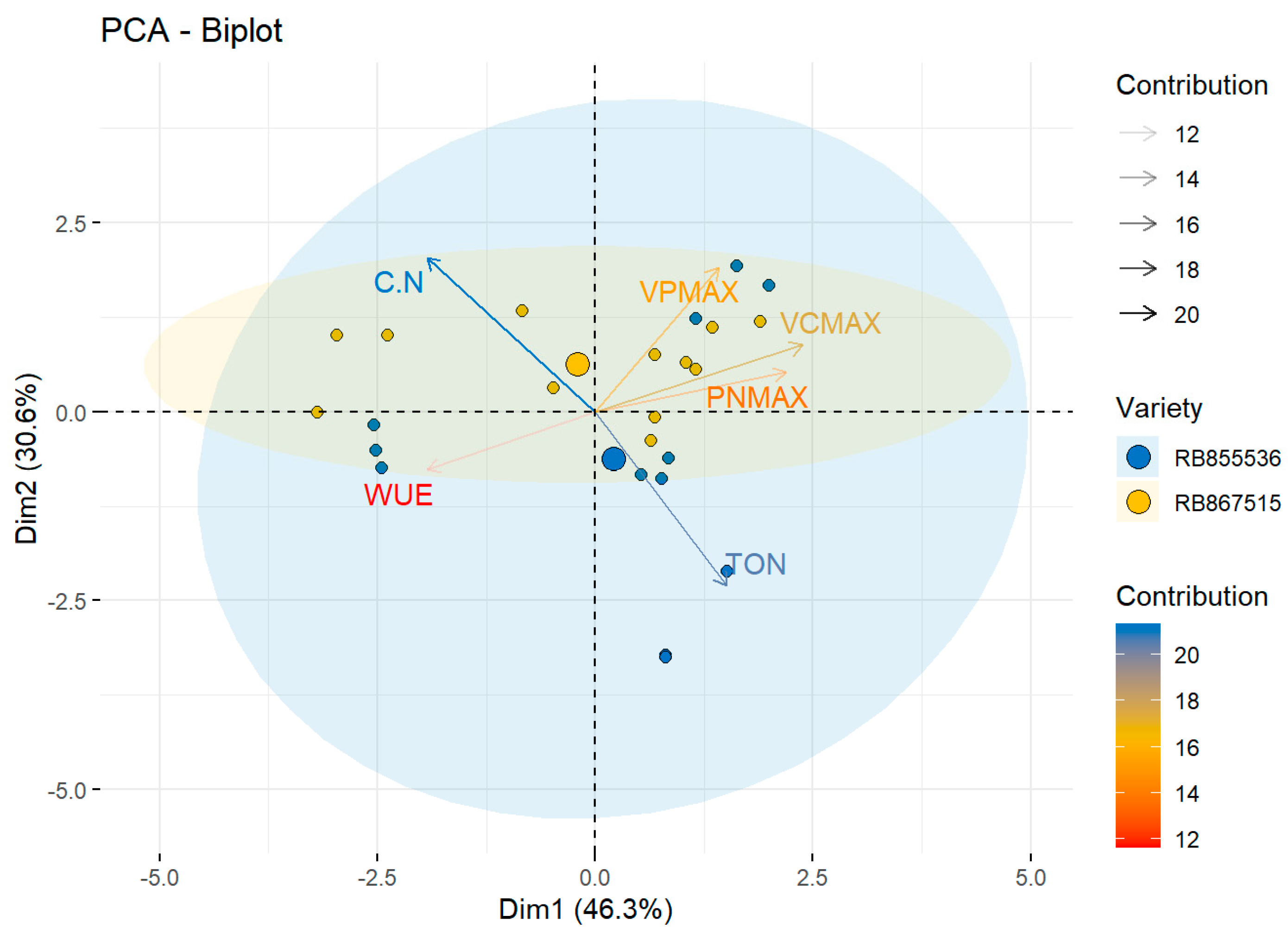
| RB867515 | RWC | 85.5 ± 2.4 | a | 78.7 ± 1.9 | b | 82.5 ± 1.7 | ab | 84.4 ± 1.4 | ab | NS | NS | * |
| WUE | 6.9 ± 0.6 | b | 7.6 ± 0.7 | b | 11.2 ± 0.6 | a | 12.9 ± 0.3 | a | NS | * | * | |
| PNmax | 15.3 ± 0.2 | a | 10.6 ± 0.5 | b | 14.6 ± 0.6 | a | 6.4 ± 1.1 | c | * | * | ||
| Vcmax | 23.3 ± 1.1 | a | 12.0 ± 1.4 | b | 21.1 ± 1.1 | a | 8.5 ± 0.7 | b | * | * | * | |
| Vpmax | 0.3 ± 0.03 | a | 0.28 ± 0.005 | a | 0.24 ± 0.02 | a | 0.18 ± 0.03 | a | NS | NS | NS | |
| TON | 0.56 ± 0.01 | ab | 0.57 ± 0.05 | a | 0.58 ± 0.01 | a | 0.44 ± 0.05 | b | * | NS | * | |
| C/N | 69.8 ± 1.8 | b | 72.3 ± 7.1 | b | 69.8 ± 1.9 | b | 91.4 ± 1.4 | a | * | * | * | |
| RB855536 | RWC | 81.1 ± 2.7 | ab | 72.1 ± 2.9 | b | 86.4 ± 2.7 | a | 77.3 ± 3.3 | ab | * | NS | * |
| WUE | 6.3 ± 0.5 | b | 9.2 ± 0.4 | b | 12.8 ± 0.4 | b | 23.1 ± 3.1 | a | * | * | * | |
| PNmax | 16.7 ± 0.4 | a | 11.1 ± 0.8 | b | 16.5 ± 0.5 | a | 11.1 ± 1.4 | b | * | NS | * | |
| Vcmax | 21.6 ± 0.4 | a | 13.5 ± 0.7 | b | 20.2 ± 0.6 | a | 9.9 ± 0.1 | c | * | * | * | |
| Vpmax | 0.32 ± 0.02 | a | 0.17 ± 0.03 | b | 0.14 ± 0.05 | b | 0.15 ± 0.05 | b | * | * | * | |
| TON | 0.54 ± 0.08 | c | 0.88 ± 0.02 | a | 0.62 ± 0.08 | b | 0.50 ± 0.08 | c | * | * | * | |
| C/N | 73.5 ± 1.1 | b | 45.6 ± 0.9 | d | 63.5 ± 0.8 | c | 80.0 ± 1.6 | a | * | * | * | |
| Variety | Variable | 400 μmol·mol−1 | 680 μmol·mol−1 | F (p < 0.05) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Drought | Control | Drought | Drought | (CO2) | Drought X (CO2) | ||||||
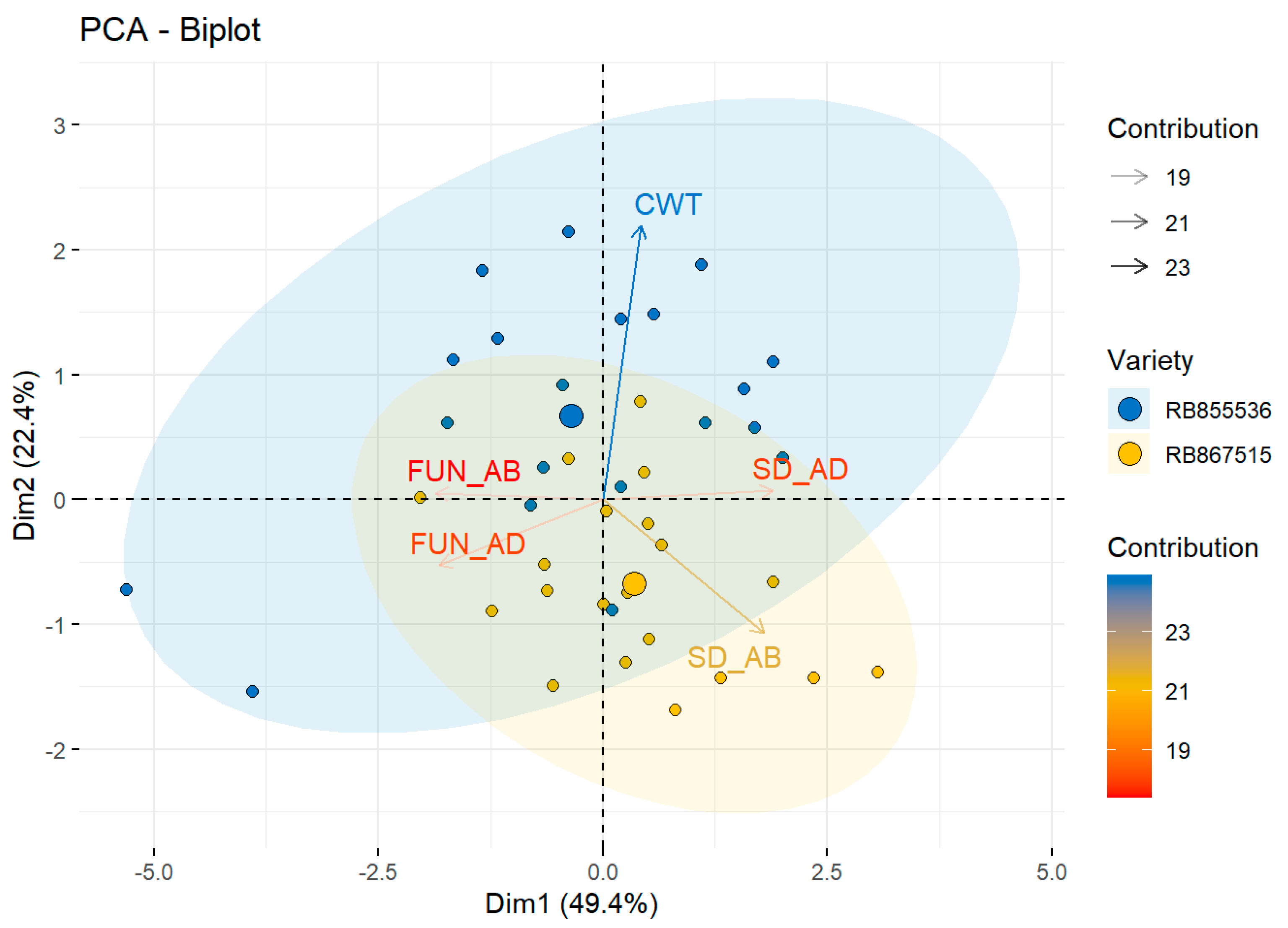
| RB867515 | SD_AD | 115.4 ± 1.6 | a | 109.1 ± 4.2 | a | 86.9 ± 4.1 | b | 120.9 ± 5.4 | a | * | NS | * |
| FUN_AD | 3.6 ± 0.08 | a | 3.7 ± 0.1 | a | 3.6 ± 0.09 | a | 3.5 ± 0.2 | a | NS | NS | NS | |
| SD_AB | 55.4 ± 0.9 | b | 62.2 ± 3.1 | ab | 50.1 ± 3.72 | c | 71.3 ± 4.6 | a | * | NS | * | |
| FUN_AB | 3.4 ± 0.1 | a | 3.5 ± 0.08 | a | 3.3 ± 0.2 | a | 3.2 ± 0.1 | a | NS | NS | NS | |
| CWT | 2.6 ± 0.1 | a | 2.2 ± 0.05 | b | 2.5 ± 0.09 | ab | 2.5 ± 0.06 | ab | * | NS | * | |
| RB855536 | SD_AD | 137.4 ± 2.4 | a | 98.4 ± 3.6 | b | 99.4 ± 5.2 | b | 84.7 ± 7.2 | b | * | * | * |
| FUN_AD | 3.4 ± 0.08 | a | 3.5 ± 0.2 | a | 3.5 ± 0.07 | a | 3.8 ± 0.4 | a | NS | NS | NS | |
| SD_AB | 61.9 ± 3.9 | a | 53.5 ± 2.9 | ab | 41.7 ± 1.4 | b | 43.6 ± 3.1 | b | NS | * | * | |
| FUN_AB | 3.4 ± 0.1 | a | 3.5 ± 0.2 | a | 3.6 ± 0.2 | a | 3.9 ± 0.3 | a | NS | NS | NS | |
| CWT | 3.2 ± 0.08 | a | 3.2 ± 0.1 | a | 3.0 ± 0.1 | a | 2.3 ± 0.09 | b | NS | * | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardenal-Rubio, Z.C.; Pinzón-Sandoval, E.H.; Linhares, P.C.A.; da Silva, A.A.; de Souza, C.R.; Assefa, M.K.; Barbosa, J.P.R.A.D.; Marchiori, P.E.R. Sugarcane Responses to Water Deficit Are Modulated by Environmental CO2 Concentration in a Genotype and Scale Dependent-Manner. Agronomy 2025, 15, 726. https://doi.org/10.3390/agronomy15030726
Cardenal-Rubio ZC, Pinzón-Sandoval EH, Linhares PCA, da Silva AA, de Souza CR, Assefa MK, Barbosa JPRAD, Marchiori PER. Sugarcane Responses to Water Deficit Are Modulated by Environmental CO2 Concentration in a Genotype and Scale Dependent-Manner. Agronomy. 2025; 15(3):726. https://doi.org/10.3390/agronomy15030726
Chicago/Turabian StyleCardenal-Rubio, Zulma Catherine, Elberth Hernando Pinzón-Sandoval, Paulo Cássio Alves Linhares, Antonia Almeida da Silva, Claudia Rita de Souza, Mewael Kiros Assefa, João Paulo Rodrigues Alves Delfino Barbosa, and Paulo Eduardo Ribeiro Marchiori. 2025. "Sugarcane Responses to Water Deficit Are Modulated by Environmental CO2 Concentration in a Genotype and Scale Dependent-Manner" Agronomy 15, no. 3: 726. https://doi.org/10.3390/agronomy15030726
APA StyleCardenal-Rubio, Z. C., Pinzón-Sandoval, E. H., Linhares, P. C. A., da Silva, A. A., de Souza, C. R., Assefa, M. K., Barbosa, J. P. R. A. D., & Marchiori, P. E. R. (2025). Sugarcane Responses to Water Deficit Are Modulated by Environmental CO2 Concentration in a Genotype and Scale Dependent-Manner. Agronomy, 15(3), 726. https://doi.org/10.3390/agronomy15030726






