Medicago Pasture Soil C:N:P Stoichiometry Mediated by N Fertilization in Northern China
Abstract
1. Introduction
- Previous Research Progress
- Research Entry Point
- Key Issues to be Solved
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Soil Sample Collection and Analysis
2.2.1. Soil Sample Collection
2.2.2. Soil Sample Detection
2.2.3. Above- and Below-Ground Biomass Sampling
2.3. Data Processing and Methods
3. Results Analysis
3.1. Medicago Yield
3.1.1. Above-Ground Biomass
3.1.2. Root Biomass
3.2. Soil pH
3.3. Effects of Nitrogen Application on Soil Nutrients in Grassland Across Different Planting Years
3.3.1. Soil Organic Carbon
3.3.2. Total Nitrogen
3.3.3. Total Phosphorus
3.4. Soil C:N:P Ratios
3.4.1. C:N Ratio
3.4.2. C:P Ratio
3.4.3. N:P Ratio
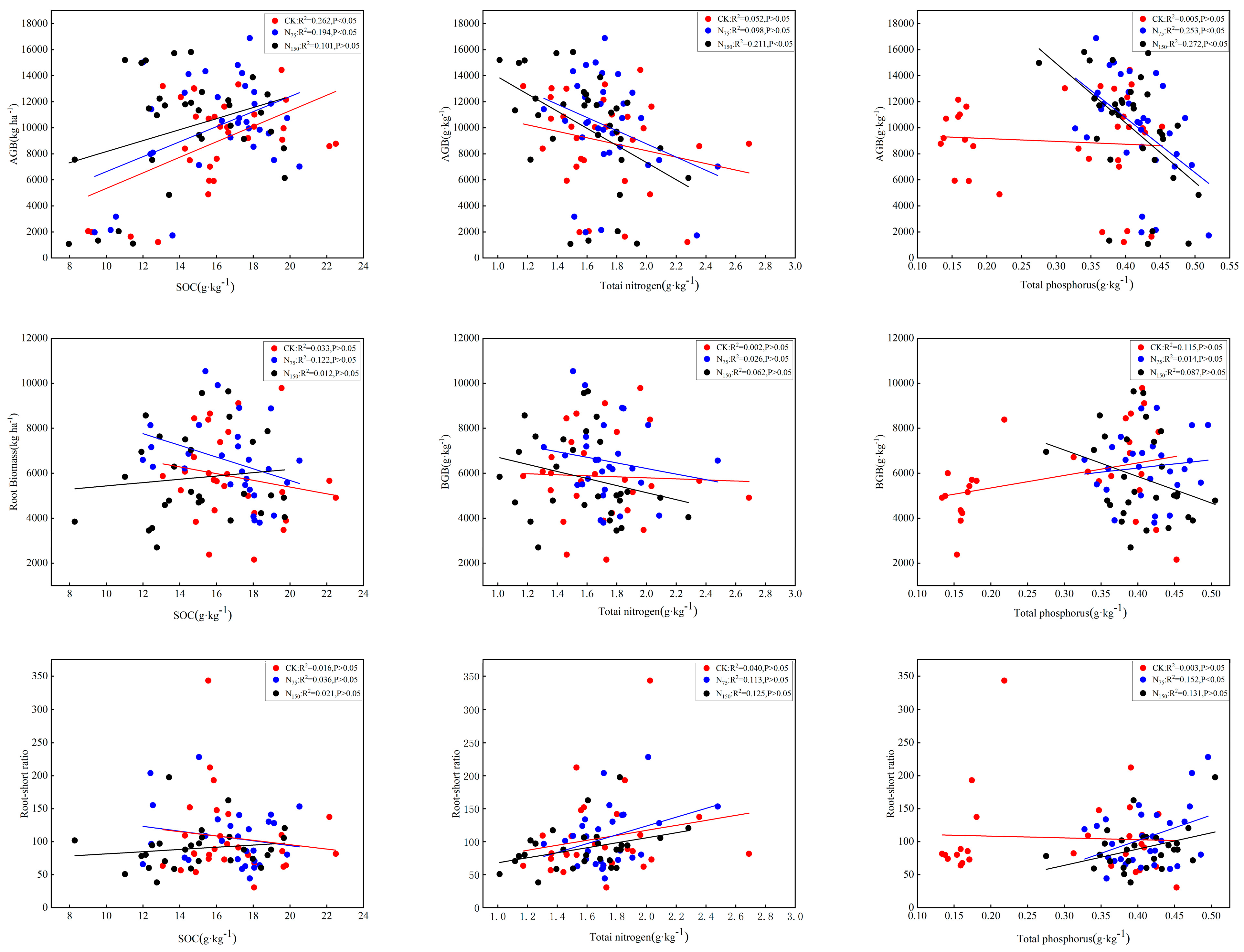
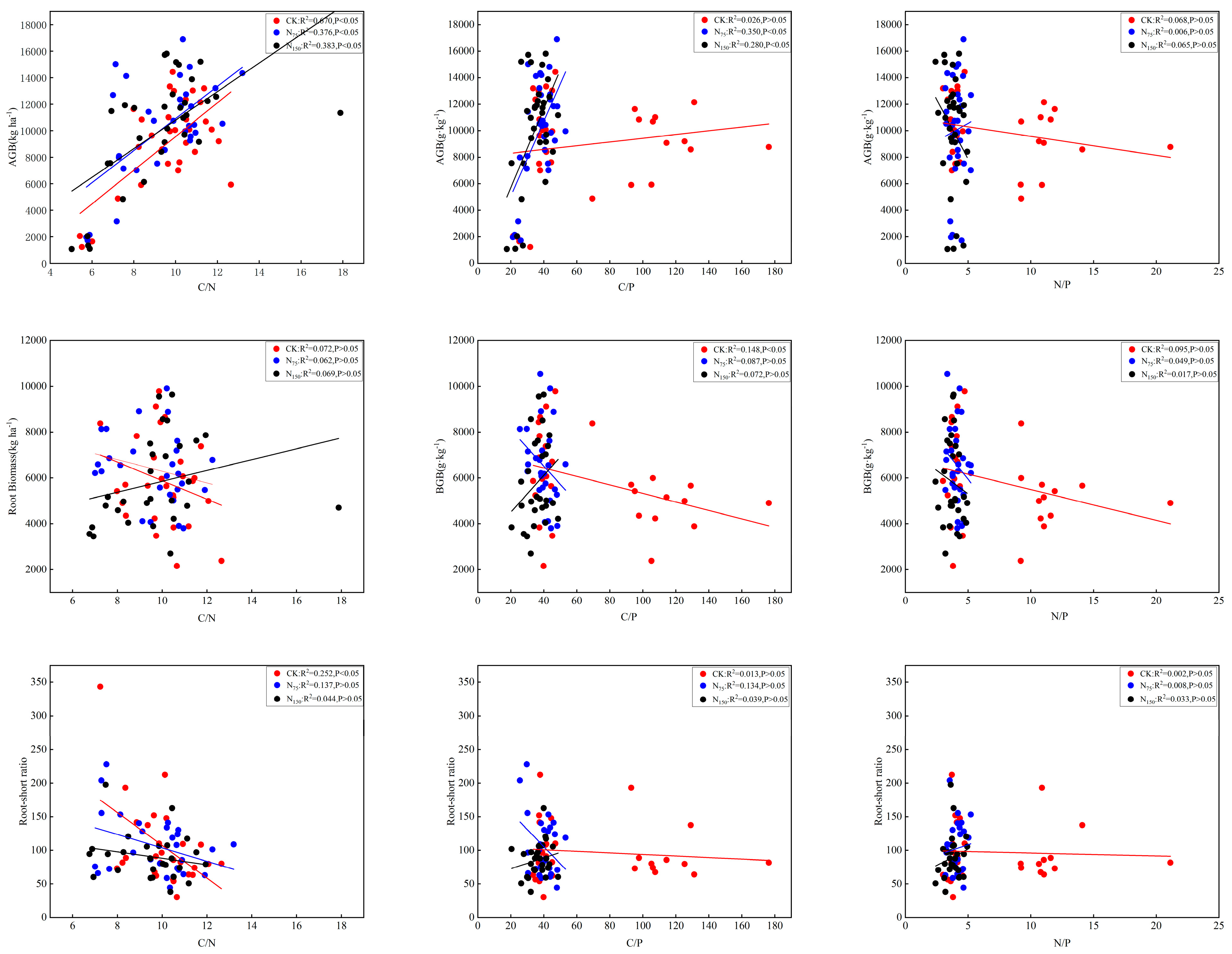
3.5. Relationships Between N Fertilization and Medicago Biomass
3.6. Relationships Between N Fertilization and Soil C:N:P Stoichiometry
4. Discussion
4.1. Effects of Soil C, N, P, and pH on Yield
4.2. Soil C:N, C:P, and N:P Ratios Balance
4.3. Biomass–C:N:P Relationships
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cuo, M.J.; Xu, L.J.; Yuan, B.; Nie, Y.Y.; Wei, J.Q. Pastureland Soil Organic Carbon Storage Regulated by Pasture Species and Age Under Nitrogen and Water Addition in Northern China. Agronomy 2025, 15, 399. [Google Scholar] [CrossRef]
- Nie, Y.Y.; Xu, L.J.; Xin, X.P.; Ye, L.M. Long-term grassland diversity-productivity relationship regulated by management regimes in northern China. Sci. Total Environ. 2024, 949, 175084. [Google Scholar] [CrossRef]
- Bi, Y.X.; Yang, G.W.; Wei, Y.Q.; Wilson, G.W.; Wei, B.; He, Y.J.; Yu, H.Q.; Liu, N.; Zhang, Y.J. Low legume-grass seeding ratio combined with phosphorus fertilization promotes forage yield and soil quality in managed grasslands. Agron. Sustain. Dev. 2024, 44, 36. [Google Scholar] [CrossRef]
- Yang, X.L.; Xiong, J.R.; Du, T.S.; Ju, X.T.; Gan, Y.T.; Li, S.; Xia, L.L.; Shen, Y.J.; Pacenka, S.; Steenhuis, T.S.; et al. Diversifying crop rotation increases food production, reduces net greenhouse gas emissions and improves soil health. Nat. Commun. 2004, 15, 198. [Google Scholar] [CrossRef]
- Xu, L.J.; Nie, Y.Y.; Chen, B.R.; Xin, X.P.; Yang, G.X.; Xu, D.W.; Ye, L.M. Effects of fence enclosure on vegetation community characteristics and productivity of a degraded temperate meadow steppe in northern China. Appl. Sci. 2020, 10, 2952. [Google Scholar] [CrossRef]
- Lu, J.; Tian, H.; Zhang, H.; Xiong, J.; Yang, H.; Liu, Y. Shoot-soil ecological stoichiometry of alfalfa under nitrogen and phosphorus fertilization in the Loess Plateau. Sci. Rep. 2021, 11, 15049. [Google Scholar] [CrossRef]
- Mencel, J.; Mocek-Plociniak, A.; Kryszak, A. Soil Microbial Community and Enzymatic Activity of Grasslands under Different Use Practices: A Review. Agronomy 2022, 12, 1136. [Google Scholar] [CrossRef]
- Wankmuller, F.J.P.; Delval, L.; Lehmann, P.; Baur, M.J.; Cecere, A.; Wolf, S.; Or, D.; Javaux, M.; Carminati, A. Global influence ofsoil texture on ecosystem water limitation. Nature 2024, 635, 631–638. [Google Scholar] [CrossRef]
- Sardans, J.; Penuelas, J. The Role of Plants in the Effects of Global Change on Nutrient Availability and Stoichiometry in the Plant-Soil System. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of soil microbial and enzyme activities are linked to soil C, N and p stoichiometry in afforested ecosystems. For. Ecol. Manag. 2018, 427, 289–295. [Google Scholar] [CrossRef]
- Huang, Y.P.; Wang, Q.Q.; Zhang, W.J.; Zhu, P.; Xiao, Q.; Wang, C.J.; Wu, L.; Tian, Y.F.; Xu, M.G.; Gunina, A. Stoichiometric imbalance of soil carbon and nutrients drives microbial community structure under long-term fertilization. Appl. Soil Ecol. 2021, 168, 104119. [Google Scholar] [CrossRef]
- Turner, B.L.; Lambers, H.; Condron, L.M.; Cramer, M.D.; Leake, J.R.; Richardson, A.E.; Smith, S.E. Soil microbial biomass and the fate of phosphorus during long-term ecosystem development. Plant Soil 2013, 367, 225–234. [Google Scholar] [CrossRef]
- Xu, L.J.; Li, D.; Wang, D.; Ye, L.M.; Nie, Y.Y.; Fang, H.J.; Xue, W.; Bai, C.L.; Ranst, E.V. Achieving the dual goals of biomass production and soil rehabilitation with sown pasture on marginal cropland: Evidence from a multi-year field experiment in Northeast Inner Mongolia. Front. Plant Sci. 2022, 13, 985864. [Google Scholar] [CrossRef]
- Chen, X.H.; Yan, X.J.; Wang, M.K.; Cai, Y.Y.; Weng, X.F.; Su, D.; Guo, J.X.; Wang, W.Q.; Hou, Y.; Ye, D.L.; et al. Long-term excessive phosphorus fertilization alters soil phosphorus fractions in the acidic soil of pomelo orchards. Soil Tillage Res. 2022, 215, 105214. [Google Scholar] [CrossRef]
- Xu, L.J.; Ye, L.M.; Nie, Y.Y.; Yang, G.X.; Xin, X.P.; Yuan, B.; Yang, X.F. Sown alfalfa pasture decreases grazing intensity while increasing soil carbon: Experimental observations and DNDC model predictions. Front. Plant Sci. 2022, 13, 1019966. [Google Scholar] [CrossRef] [PubMed]
- Poffenbarger, H.J.; Sawyer, J.E.; Barker, D.W.; Olk, D.C.; Six, J.; Castellano, M.J. Legacy effects of long-term nitrogen fertilizer application on the fate of nitrogen fertilizer inputs in continuous maize. Agric. Ecosyst. Environ. 2018, 265, 544–555. [Google Scholar] [CrossRef]
- Zheng, S.M.; Xia, Y.H.; Hu, Y.J.; Chen, X.B.; Rui, Y.C.; Gunina, A.; He, X.Y.; Ge, T.; Wu, J.H.; Su, Y.; et al. Stoichiometry of carbon, nitrogen, and phosphorus in soil: Effects of agricultural land use and climate at a continental scale. Soil Tillage Res. 2021, 209, 104903. [Google Scholar] [CrossRef]
- Ellert, B.H.; Janzen, H.H.; VandenBygaart, A.; Bremer, E. Measuring Change in Soil Organic Carbon Storage. In Soil Sampling and Methods of Analysis; Taylor Francis Group, LLC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Nitrogen Analysis of Soil and Plant Tissues. J. AOAC Int. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Zhang, T.Q. Soil Phosphorus Tests and Transformation Analysis to Quantify Plant Availability: A Review. Soil Fertil. Improv. Integr. Nutr. Manag. A Glob. Perspect. 2012, 1, 19–36. [Google Scholar]
- Schofield, R.K.; Taylor, A.W. The Measurement of Soil pH. Soil Sci. Soc. Am. J. 1955, 19, 164. [Google Scholar] [CrossRef]
- Long, D.S.; McCallum, J.D.; Reardon, C.L.; Engel, R.E. Nitrogen Requirement to Change Protein Concentration of Spring Wheat in Semiarid Pacific Northwest. Agron. J. 2017, 3, 675–683. [Google Scholar] [CrossRef]
- Xu, L.J.; Cheng, S.L.; Fang, H.J.; Xin, X.P.; Xu, X.L.; Tang, H.J. Soil inorganic nitrogen composition and plant functional type determine forage crops nitrogen uptake preference in the temperate cultivated grassland, Inner Mongolia. Soil Sci. Plant Nutr. 2019, 65, 501–510. [Google Scholar] [CrossRef]
- Heichel, G.H.; Barnes, D.K.; Vance, C.P. Nitrogen fixation of alfalfa in the seeding year. Crop Sci. 1981, 21, 330–335. [Google Scholar] [CrossRef]
- Page, K.L.; Dang, Y.P.; Dalal, R.C. The Ability of Conservation Agriculture to Conserve Soil Organic Carbon and the Subsequent Impact on Soil Physical, Chemical, and Biological Properties and Yield. Front. Sustain. Food Syst. 2020, 4, 31. [Google Scholar] [CrossRef]
- Galantini, J.; Rosell, R. Long-term fertilization effects on soil organic matter quality and dynamics under different production systems in semiarid Pampean soils. Soil Tillage Res. 2006, 87, 72–79. [Google Scholar] [CrossRef]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Barber, L.D.; Joern, B.C.; Volenec, J.J.; Cunningham, S.M. Supplemental Nitrogen Effects on Alfalfa Regrowth and Nitrogen Mobilization from Roots. Crop Sci. 1996, 36, 1217–1223. [Google Scholar] [CrossRef]
- Ghimire, R.; Norton, J.B.; Pendall, E. Alfalfa-grass biomass, soil organic carbon, and total nitrogen under different management approaches in an irrigated agroecosystem. Plant Soil 2014, 374, 173–184. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Hui, X.L.; Wang, Z.H.; Liu, J.S. Effects of Different Fertilization and Fallowing Practices on Soil Carbon and Nitrogen Mineralization in a Dryland Soil with Low Organic Matter. J. Soil Sci. Plant Nutr. 2019, 19, 108–116. [Google Scholar] [CrossRef]
- Qin, P.Y.; Hu, L.; Liu, Y.D.; Hu, X.; Zhang, X.K.; Rosado, A.S.; Wei, G.H.; Chen, C. Responses of soil microbial communities and nutrient dynamics under continuous alfalfa (Medicago sativa L.) cultivation. Appl. Soil Ecol. 2024, 197, 105356. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of Soil Phosphorus on Legume Production. In Legumes for Soil Health and Sustainable Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 487–510. [Google Scholar]
- Wang, Y.; Huang, Q.; Gao, H.; Zhang, R.; Yang, L.; Guo, Y.; Li, H.; Awasthi, M.K.; Li, G. Long-term cover crops improved soil phosphorus availability in a rain-fed apple orchard. Chemosphere 2021, 275, 130093. [Google Scholar] [CrossRef] [PubMed]
- Ravikiran, K.B.; Santhi, R.; Meena, S.; Sumathi, P. Refinement of Soil Test Crop Response -Integrated Plant Nutrition System based Fertilizer Prescriptions for Pearl Millet Variety Grown Under Inceptisol. Madras Agric. J. 2018, 105, 165–169. [Google Scholar] [CrossRef]
- Guo, K.W.; Xu, Z.S.; Huo, Y.Z.; Sun, Q.; Wang, Y.; Che, Y.H.; Wang, J.C.; Li, W.; Zhang, H.H. Effects of salt concentration, pH, and their interaction on plant growth, nutrient uptake, and photochemistry of alfalfa (Medicago sativa) leaves. Plant Signal. Behav. 2020, 10, 1832373. [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Guolding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.R.; Gao, Y.Z.; Wu, X.; Ma, H.M.; Zheng, C.C.; Wang, X.Y.; Zhang, H.L.; Li, Z.J.; Yang, H.J. The relative contributions of pH, organic anions, and phosphatase to rhizosphere soil phosphorus mobilization and crop phosphorus uptake in maize/alfalfa polyculture. Plant Soil 2019, 447, 117–133. [Google Scholar] [CrossRef]
- Van Eerd, L.L.; Congreves, K.A.; Hayes, A.; Verhallen, A.; Hooker, D.C. Long-term tillage and crop rotation effects on soil quality, organic carbon, and total nitrogen. Soil Sci. 2014, 5, 303–315. [Google Scholar]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1997, 2, 87–115. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Lu, M.; Zeng, F.; Lv, S.; Zhang, H.; Zeng, Z.; Peng, W.; Song, T.; Wang, K.; Du, H. Soil C:N:P stoichiometry and its influencing factors in forest ecosystems in southern China. Front. For. Glob. Change 2023, 6, 1142933. [Google Scholar] [CrossRef]
- Adeboye, M.K.A.; Bala, A.; Osunde, A.O.; Uzoma, A.O.; Odofin, A.J.; Lawal, B.A. Assessment of soil quality using soil organic carbon and total nitrogen and microbial properties in tropical agroecosystems. Agric. Sci. 2011, 2, 34–40. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Mcgroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]



| Soil Layers | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | AHN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | PH |
|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 13.62 | 2.20 | 0.52 | 25.76 | 207.04 | 16.25 | 315.96 | 6.77 |
| 10–20 cm | 13.34 | 2.19 | 0.49 | 25.59 | 219.88 | 11.95 | 249.49 | 6.79 |
| 20–40 cm | 10.23 | 1.70 | 0.42 | 24.49 | 161.25 | 8.89 | 192.36 | 7.34 |
| 40–60 cm | 5.08 | 1.02 | 0.35 | 23.59 | 104.78 | 7.68 | 148.77 | 7.94 |
| Process Mode | Above-Ground Biomass (t ha−1) | ||||||
|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | |
| CK | 9.95 ± 1.31 aAB | 10.00 ± 0.86 aAB | 7.22 ± 2.88 aB | 9.32 ± 1.06 bB | 12.97 ± 0.38 aA | 10.72 ± 3.10 aAB | 9.63 ± 1.21 aAB |
| N75 | 10.34 ± 3.12 aA | 10.99 ± 1.35 aA | 9.56 ± 3.17 aA | 12.09 ± 1.56 aA | 12.70 ± 1.86 aA | 10.85 ± 1.29 aA | 11.78 ± 3.17 aA |
| N150 | 9.55 ± 2.41 aBC | 10.94 ± 1.11 aABC | 7.85 ± 2.37 aC | 10.89 ± 1.61 abABC | 14.15 ± 1.52 aA | 13.01 ± 2.61 aAB | 12.26 ± 1.71 aAB |
| Process Mode | Root Biomass (t ha−1) | ||||||
|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | |
| CK | 5.08 ± 0.50 aBC | 5.09 ± 0.63 aBC | 5.09 ± 2.24 abBC | 7.02 ± 0.69 aAB | 7.16 ± 1.64 aAB | 7.80 ± 1.49 aA | 3.78 ± 1.24 aC |
| N75 | 5.93 ± 1.08 aAB | 5.75 ± 0.27 aAB | 7.29 ± 0.86 aA | 7.81 ± 2.09 aA | 7.01 ± 0.57 aA | 7.72 ± 1.76 aA | 4.26 ± 0.59 aB |
| N150 | 4.68 ± 0.42 aA | 5.37 ± 1.50 aA | 3.91 ± 0.53 bA | 6.81 ± 1.91 aA | 6.75 ± 0.71 aA | 7.10 ± 1.72 aA | 6.00 ± 2.60 aA |
| N Level | Soil Layers (cm) | SOC (g kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | ||
| CK | 0–10 | 13.38 ± 1.52 aA II | 23.46 ± 3.43 aA I | 22.99 ± 1.55 aA I | 20.23 ± 2.84 aA I | 20.79 ± 2.34 aA I | 22.36 ± 1.06 aA I | 21.70 ± 1.81 aA I | 22.24 ± 2.59 aA I |
| 10–20 | 13.04 ± 1.37 aA IV | 23.01 ± 2.97 aA I,II | 23.76 ± 1.22 aA I | 19.17 ± 2.05 aA III | 20.45 ± 2.87 aA I,II,III | 19.87 ± 2.45 aA II,III | 20.78 ± 2.32 aA I,II,III | 21.99 ± 2.15 aA I,II,III | |
| 20–40 | 11.14 ± 2.54 aA I | 18.74 ± 3.90 aA I | 16.02 ± 3.01 aB I | 18.43 ± 5.52 aA I | 14.00 ± 1.41 aB I | 11.54 ± 3.71 aB I | 14.41 ± 4.90 aB I | 16.20 ± 2.51 aB I | |
| 40–60 | 4.83 ± 1.46 aB II | 11.75 ± 2.82 aB I | 8.18 ± 2.25 aC I,II | 8.96 ± 4.79 aB I,II | 8.75 ± 2.05 aC I,II | 5.34 ± 1.01 aC II | 7.35 ± 2.40 aC I,II | 8.16 ± 1.01 aC I,II | |
| N75 | 0–10 | 13.41 ± 1.09 aA III | 23.20 ± 2.47 aA I | 23.84 ± 1.87 aA I | 16.80 ± 1.17 abA II | 22.05 ± 2.03 aA I | 22.42 ± 2.34 aA I | 22.72 ± 1.07 aA I | 24.33 ± 0.92 aA I |
| 10–20 | 13.71 ± 1.08 aA III | 20.98 ± 2.52 aAB I | 23.80 ± 1.67 aA I | 16.77 ± 1.56 abA II | 20.99 ± 1.92 aA I | 21.32 ± 2.02 aA I | 21.60 ± 2.14 aA I | 21.92 ± 1.70 aA I | |
| 20–40 | 10.54 ± 2.45 aA III | 15.50 ± 3.29 aB I,II | 16.99 ± 2.44 aB I | 11.72 ± 1.10 abB II,III | 14.85 ± 1.80 aB I,II,III | 14.17 ± 4.02 aB I,II,III | 16.76 ± 0.60 aB I | 16.85 ± 1.59 aB I | |
| 40–60 | 6.08 ± 2.32 aB I | 8.71 ± 3.74 aC I | 9.26 ± 1.79 aC I | 6.71 ± 1.26 aC I | 9.09 ± 2.65 aC I | 6.28 ± 2.84 aC I | 8.49 ± 1.62 aC I | 9.15 ± 1.70 aC I | |
| N150 | 0–10 | 12.93 ± 1.39 aA II | 21.97 ± 2.87 aA I | 20.42 ± 1.79 aA I | 14.41 ± 1.51 bA II | 20.76 ± 2.05 aA I | 20.55 ± 0.92 aA I | 21.83 ± 1.17 aA I | 21.55 ± 1.65 aA I |
| 10–20 | 12.77 ± 1.44 aA II | 21.13 ± 1.83 aAB I | 21.78 ± 1.63 aA I | 14.29 ± 1.57 bA II | 18.83 ± 3.19 aA I | 19.21 ± 2.01 aA I | 19.39 ± 2.55 aA I | 19.69 ± 1.46 aA I | |
| 20–40 | 9.52 ± 1.41 aB I | 15.44 ± 468 aB I | 15.26 ± 6.41 aAB I | 9.37 ± 1.83 bB I | 12.68 ± 2.52 aB I | 11.35 ± 5.33 aB I | 11.35 ± 3.40 aB I | 11.56 ± 5.14 aB I | |
| 40–60 | 4.44 ± 1.28 aC II | 8.67 ± 2.60 aC I,II | 12.09 ± 3.88 aB I | 8.45 ± 3.69 aB I,II | 8.40 ± 2.65 aB I,II | 5.88 ± 4.70 aB I,II | 5.80 ± 1.57 aC I,II | 7.25 ± 3.01 aB I,II | |
| N Level | Soil Layers (cm) | TN (g kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | ||
| CK | 0–10 | 2.18 ± 0.23 aA I | 2.56 ± 0.31 aA I | 2.16 ± 0.23 aA I | 2.12 ± 0.14 aA I | 2.17 ± 0.15 aA I | 2.13 ± 0.10 aA I | 2.09 ± 0.24 aA I | 2.15 ± 0.21 aA I |
| 10–20 | 2.19 ± 0.18 aA I,II | 2.60 ± 0.34 aA I | 2.11 ± 0.19 aA II | 2.09 ± 0.19 aA II | 2.09 ± 0.15 aA II | 1.92 ± 0.28 aA II | 2.00 ± 0.26 aA II | 2.20 ± 0.24 aA I,II | |
| 20–40 | 1.86 ± 0.34 aA I,II | 2.24 ± 0.38 aAB I | 1.50 ± 0.29 aB II,III | 1.71 ± 0.33 aA I,II,III | 1.47 ± 0.12 aB II,III | 1.11 ± 0.39 aB III | 1.28 ± 0.48 aB II,III | 1.55 ± 0.24 aB II,III | |
| 40–60 | 1.05 ± 0.43 aB II | 1.56 ± 0.38 aB I | 0.80 ± 0.23 aC II,III | 1.14 ± 0.25 aB I,II | 0.80 ± 0.14 aC II,III | 0.55 ± 0.15 aC III | 0.78 ± 0.26 aB II,III | 0.82 ± 0.20 aC II,III | |
| N75 | 0–10 | 2.00 ± 0.56 aAB I | 2.52 ± 0.38 aA I | 2.25 ± 0.21 aA I | 2.28 ± 0.20 aA I | 2.23 ± 0.16 aA I | 2.17 ± 0.12 aA I | 2.17 ± 0.12 aA I | 2.28 ± 0.08 aA I |
| 10–20 | 2.21 ± 0.23 aA I | 2.36 ± 0.29 aA I | 2.19 ± 0.19 aA I | 2.27 ± 0.17 aA I | 2.11 ± 0.22 aA I | 2.04 ± 0.13 aA I | 2.09 ± 0.18 aA I | 2.15 ± 0.15 aA I | |
| 20–40 | 1.72 ± 0.33 aAB I,II | 1.96 ± 0.34 aAB I | 1.56 ± 0.28 aB I,II | 1.70 ± 0.07 aB I,II | 1.42 ± 0.21 aB I,II | 1.31 ± 0.38 aB II | 1.58 ± 0.07 aB I,II | 1.62 ± 0.12 aB I,II | |
| 40–60 | 1.20 ± 0.44 aB I,II | 1.44 ± 0.27 aB I | 0.88 ± 0.22 aC II,III | 0.89 ± 0.17 aC II,III | 0.75 ± 0.29 aC II,III | 0.69 ± 0.22 aC III | 0.84 ± 0.18 aC II,III | 0.90 ± 0.11 aC II,III | |
| N150 | 0–10 | 2.17 ± 0.21 aA I | 2.27 ± 0.14 aA I | 2.03 ± 0.29 aA I | 2.06 ± 0.18 aA I | 2.12 ± 0.20 aA I | 2.07 ± 0.12 aA I | 0.29 ± 0.16 aA I | 2.15 ± 0.12 aA I |
| 10–20 | 2.12 ± 0.21 aA I | 2.36 ± 0.27 aA I | 2.00 ± 0.23 aA I | 2.08 ± 0.13 aA I | 2.03 ± 0.19 aA I | 1.87 ± 0.20 aA I | 1.88 ± 0.24 aA I | 1.98 ± 0.23 aA I | |
| 20–40 | 1.63 ± 0.20 aB I,II | 1.89 ± 0.52 aAB I | 1.41 ± 0.57 aAB I,II | 1.33 ± 0.36 aB I,II | 1.34 ± 0.31 aB I,II | 0.96 ± 0.40 aB II | 1.06 ± 0.32 aB I,II | 1.07 ± 0.46 aB I,II | |
| 40–60 | 0.92 ± 0.14 aC I,II | 1.31 ± 0.32 aB I | 0.83 ± 0.27 aB I,II | 1.21 ± 0.52 aB I,II | 0.84 ± 0.21 aC I,II | 0.63 ± 0.42 aB II | 0.59 ± 0.14 aC II | 0.69 ± 0.26 aB I,II | |
| N Level | Soil Layers (cm) | TP (g kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | ||
| CK | 0–10 | 0.48 ± 0.04 aA I | 0.20 ± 0.03 bA III | 0.17 ± 0.01 bA III | 0.21 ± 0.04 bA III | 0.46 ± 0.03 aA I,II | 0.46 ± 0.02 aA I,II | 0.42 ± 0.04 aA II | 0.43 ± 0.02 aA II |
| 10–20 | 0.44 ± 0.03 aAB I,II | 0.18 ± 0.05 bA III | 0.17 ± 0.02 bA III | 0.18 ± 0.03 bA III | 0.46 ± 0.01 aA I | 0.41 ± 0.02 aAB II | 0.40 ± 0.03 aA II | 0.44 ± 0.05 aA I,II | |
| 20–40 | 0.39 ± 0.03 aB I | 0.15 ± 0.01 bAB II | 0.15 ± 0.02 bA II | 0.15 ± 0.02 bA II | 0.39 ± 0.02 aB I | 0.33 ± 0.04 aC I | 0.33 ± 0.09 aAB I | 0.38 ± 0.03 aA I | |
| 40–60 | 0.30 ± 0.03 bC II | 0.12 ± 0.01 bB III | 0.12 ± 0.01 bB III | 0.16 ± 0.03 bA III | 0.31 ± 0.03 aC I,II | 0.35 ± 0.05 aBC I,II | 0.29 ± 0.03 aB II | 0.37 ± 0.06 aA I | |
| N75 | 0–10 | 0.50 ± 0.03 aA I,II | 0.49 ± 0.04 aA I,II | 0.55 ± 0.07 aA I | 0.47 ± 0.05 aA I,II | 0.49 ± 0.04 aA I,II | 0.50 ± 0.03 aA I,II | 0.43 ± 0.04 aA II | 0.45 ± 0.03 aA II |
| 10–20 | 0.50 ± 0.04 aA I,II | 0.45 ± 0.04 aA II,III,IV | 0.53 ± 0.04 aA I | 0.50 ± 0.06 aA I,II | 0.48 ± 0.03 aAB I,II,III | 0.43 ± 0.01 aAB III,IV | 0.40 ± 0.03 aA IV | 0.41 ± 0.04 aAB IV | |
| 20–40 | 0.42 ± 0.05 aAB I,II | 0.39 ± 0.07 aAB I,II | 0.42 ± 0.03 aB I | 0.42 ± 0.03 aAB I | 0.41 ± 0.02 aB I,II | 0.39 ± 0.07 aB I,II | 0.33 ± 0.04 aB II | 0.37 ± 0.02 aAB I,II | |
| 40–60 | 0.39 ± 0.05 aB I | 0.34 ± 0.06 aB I | 0.32 ± 0.06 aC I | 0.35 ± 0.06 aB I | 0.29 ± 0.07 aC I | 0.29 ± 0.06 aC I | 0.27 ± 0.03 aB I | 0.34 ± 0.08 aB I | |
| N150 | 0–10 | 0.53 ± 0.02 aA I,II | 0.49 ± 0.04 aA I,II,III | 0.54 ± 0.04 aA I | 0.47 ± 0.03 aA II,III | 0.48 ± 0.05 aA I,II,III | 0.49 ± 0.02 aA I,II,III | 0.45 ± 0.03 aA III | 0.44 ± 0.04 aA III |
| 10–20 | 0.43 ± 0.11 aAB I | 0.46 ± 0.03 aAB I | 0.49 ± 0.06 aAB I | 0.48 ± 0.04 aA I | 0.48 ± 0.03 aA I | 0.41 ± 0.02 aB I | 0.39 ± 0.05 aA I | 0.41 ± 0.05 aAB I | |
| 20–40 | 0.43 ± 0.05 aAB I | 0.39 ± 0.07 aBC I,II | 0.38 ± 0.07 aBC I,II | 0.40 ± 0.08 aA I,II | 0.38 ± 0.05 aAB I,II | 0.39 ± 0.04 aBC I,II | 0.28 ± 0.06 aB II | 0.35 ± 0.01 aBC I,II | |
| 40–60 | 0.34 ± 0.02 abB I,II | 0.31 ± 0.04 aC I,II | 0.33 ± 0.03 aC I,II | 0.39 ± 0.08 aA I | 0.34 ± 0.07 aB I,II | 0.33 ± 0.05 aC I,II | 0.25 ± 0.04 aB II | 0.33 ± 0.05 aC II | |
| N Level | Soil Layers (cm) | C:N Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | ||
| CK | 0–10 | 6.13 ± 0.30 aA III | 9.21 ± 1.07 aA II | 10.72 ± 0.78 aA I | 9.59 ± 1.55 aA I,II | 9.54 ± 0.49 aA I,II | 10.50 ± 0.40 aA I | 10.41 ± 0.46 aAB I,II | 10.35 ± 0.55 aA I,II |
| 10–20 | 5.95 ± 0.27 aA V | 8.05 ± 0.27 aAB IV | 11.32 ± 0.66 aA I | 9.21 ± 0.97 aA III,IV | 9.79 ± 1.13 aA II,III,IV | 10.41 ± 0.40 aA I,II | 10.42 ± 0.47 aAB I,II | 10.03 ± 0.56 aA II,III | |
| 20–40 | 5.93 ± 0.27 aA III | 8.32 ± 0.48 aAB II,III | 10.68 ± 0.72 aA I,II | 11.05 ± 3.59 aA I | 9.51 ± 0.66 aA I,II | 10.69 ± 1.16 aA I,II | 11.36 ± 0.70 aA I | 10.49 ± 0.46 aA I,II | |
| 40–60 | 4.74 ± 0.61 aB II | 7.58 ± 0.49 aB I,II | 10.96 ± 3.27 aA I,II | 9.58 ± 8.15 aA I,II | 11.37 ± 3.47 aA I | 9.91 ± 1.20 aA I,II | 9.54 ± 0.45 aB I,II | 10.23 ± 1.17 aA I,II | |
| N75 | 0–10 | 7.28 ± 2.16 aA II | 9.35 ± 1.31 aA I | 10.63 ± 0.28 aA I | 7.40 ± 0.36 bA I | 9.87 ± 0.35 aA I | 10.31 ± 0.58 aA I | 10.48 ± 0.18 aA I | 10.66 ± 0.08 aA I |
| 10–20 | 6.22 ± 0.33 aA V | 8.89 ± 0.07 aA III | 10.88 ± 0.50 aA I | 7.37 ± 0.16 bA IV | 10.02 ± 0.85 aA II | 10.42 ± 0.31 aA I,II | 10.35 ± 0.50 aA I,II | 10.20 ± 0.13 aA II | |
| 20–40 | 6.09 ± 0.25 aA III | 7.85 ± 0.41 aAB II | 10.95 ± 0.44 aA I | 6.89 ± 0.46 aA I,II | 10.57 ± 1.28 aA I | 10.90 ± 1.27 aA I | 10.58 ± 0.32 aA I | 10.43 ± 0.65 aA I | |
| 40–60 | 5.02 ± 0.14 aA IV | 5.81 ± 1.80 aB III,IV | 10.95 ± 2.05 aA I,II | 7.56 ± 0.48 aA II,III,IV | 14.44 ± 6.52 aA I | 8.60 ± 1.97 aA II,III,IV | 10.22 ± 0.47 aA I,II,III | 10.25 ± 1.72 aA I,II,III | |
| N150 | 0–10 | 5.96 ± 0.31 aA II | 9.78 ± 1.71 aA I | 10.17 ± 0.86 aA I | 6.98 ± 0.25 bA II | 9.78 ± 0.23 aA I | 9.94 ± 0.58 aAB I | 10.47 ± 0.33 aA I | 10.02 ± 0.26 aA I |
| 10–20 | 6.00 ± 0.09 aA VI | 9.00 ± 0.53 aA IV | 10.95 ± 0.69 aA I | 6.87 ± 0.44 bA V | 9.20 ± 0.78 aA III,IV | 10.28 ± 0.41 aAB I,II | 10.30 ± 0.53 aA I,II | 10.03 ± 0.65 aA II,III | |
| 20–40 | 5.82 ± 0.18 aAB IV | 8.12 ± 0.67 aAB III | 10.66 ± 0.80 aA I,II | 7.22 ± 0.75 aA III | 9.60 ± 0.79 aA II | 11.83 ± 1.33 aA I | 10.80 ± 1.00 aA I,II | 10.75 ± 0.17 aA I,II | |
| 40–60 | 4.75 ± 0.98 aB II | 6.51 ± 0.67 aB II | 18.07 ± 12.81 aA I | 6.98 ± 0.71 aA II | 10.56 ± 4.43 aA I,II | 8.88 ± 1.57 aB I,II | 9.73 ± 1.28 aA I,II | 10.35 ± 1.02 aA I,II | |
| N Level | Soil Layers (cm) | C:P Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | ||
| CK | 0–10 | 27.88 ± 1.65 aA IV | 120.18 ± 24.62 aA I,II | 137.66 ± 10.91 aAB I | 98.67 ± 27.85 aA II | 45.61 ± 2.91 aA III,IV | 48.21 ± 2.60 aA III,IV | 51.42 ± 5.05 aA III | 51.52 ± 4.89 aA III |
| 10–20 | 29.68 ± 4.56 aA II | 151.32 ± 77.69 aA I | 139.26 ± 8.24 aA I | 110.90 ± 25.30 aA I | 44.61 ± 5.86 aA II | 48.10 ± 6.82 aA II | 52.50 ± 5.19 aA II | 50.07 ± 4.80 aAB II | |
| 20–40 | 28.75 ± 5.85 aA II | 125.67 ± 23.43 aA I | 104.99 ± 23.58 aB I | 124.03 ± 44.83 aA I | 36.38 ± 3.09 aAB II | 34.57 ± 9.54 aB II | 42.81 ± 7.06 aA II | 42.55 ± 3.42 aB II | |
| 40–60 | 16.20 ± 3.94 aB III | 101.11 ± 34.41 aA I | 71.39 ± 19.39 aC I | 65.29 ± 50.43 aA I,II | 28.31 ± 7.40 aB II,III | 15.91 ± 4.62 aC III | 24.67 ± 5.70 aB III | 22.84 ± 5.24 aC III | |
| N75 | 0–10 | 26.69 ± 0.93 abA IV | 47.68 ± 4.99 bA I,II | 43.63 ± 3.68 bA II | 35.70 ± 3.08 bA III | 44.82 ± 4.64 aA II | 45.54 ± 6.33 aAB II | 52.97 ± 4.69 aA I | 53.96 ± 3.26 aA I |
| 10–20 | 27.62 ± 2.83 aA IV | 46.56 ± 1.77 bA II | 45.06 ± 3.13 bA II | 33.60 ± 3.17 bA III | 44.19 ± 3.86 aA II | 49.06 ± 4.78 aA I,II | 54.25 ± 6.13 aA I | 53.34 ± 2.22 aAB I | |
| 20–40 | 24.65 ± 2.60 aA IV | 39.71 ± 5.03 bA II,III | 39.83 ± 3.71 bA II,III | 27.64 ± 2.07 bB IV | 36.36 ± 4.15 aAB III | 36.30 ± 5.98 aB III | 51.79 ± 5.84 aA I | 45.61 ± 5.43 aB I,II | |
| 40–60 | 15.23 ± 3.56 aB IV | 24.94 ± 8.02 bB I,II,III,IV | 29.45 ± 2.94 bB I,II,III | 19.03 ± 2.02 aC III,IV | 32.18 ± 7.68 aB I | 20.55 ± 7.36 aC II,III,IV | 30.76 ± 2.61 aB I,II | 28.33 ± 6.91 aC I,II,III | |
| N150 | 0–10 | 24.19 ± 1.96 bAB V | 45.11 ± 5.53 bA I,II | 37.89 ± 2.02 bA III | 30.83 ± 3.80 bA IV | 43.57 ± 3.14 aA II | 41.95 ± 2.50 aAB II,III | 49.05 ± 2.82 aA I | 48.73 ± 0.88 aAB I |
| 10–20 | 32.21 ± 11.47 aA III,IV | 45.67 ± 1.37 bA I,II | 44.90 ± 3.79 bA I,II | 29.68 ± 0.76 bA IV | 39.32 ± 4.25 aAB II,III | 46.77 ± 4.32 aA I,II | 49.52 ± 2.98 aA I | 49.20 ± 7.33 aA I | |
| 20–40 | 22.09 ± 3.14 aAB I | 38.82 ± 5.28 bA I | 37.70 ± 11.62 bA I | 23.84 ± 3.96 bAB I | 33.24 ± 2.04 aAB I | 27.87 ± 10.62 aBC I | 39.54 ± 5.81 aB I | 33.05 ± 14.20 aBC I | |
| 40–60 | 13.00 ± 3.51 aB II | 27.31 ± 6.82 bB I,II | 37.72 ± 15.20 bA I | 20.54 ± 6.83 aB I,II | 27.33 ± 11.58 aB I,II | 17.52 ± 12.37 aC I,II | 23.47 ± 6.30 aC I,II | 21.30 ± 7.54 aC I,II | |
| N Level | Soil Layers (cm) | N:P Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | ||
| CK | 0–10 | 4.56 ± 0.36 aA III | 13.25 ± 3.11 aA I | 12.86 ± 0.85 aA I | 10.12 ± 1.19 aA II | 4.78 ± 0.27 aA III | 4.59 ± 0.15 aA III | 4.94 ± 0.49 aA III | 4.98 ± 0.45 aA III |
| 10–20 | 4.98 ± 0.72 aA II | 17.14 ± 8.90 aA I | 12.31 ± 0.39 aA I | 11.91 ± 1.40 aA I | 4.55 ± 0.25 aA II | 4.63 ± 0.74 aA II | 5.04 ± 0.42 aA II | 4.98 ± 0.25 aA II | |
| 20–40 | 4.81 ± 0.73 aA III | 15.04 ± 2.30 aA I | 9.77 ± 1.75 aB II | 11.23 ± 1.98 aA II | 3.82 ± 0.11 aB II | 3.32 ± 1.04 aA II | 3.77 ± 0.61 bB II | 4.06 ± 0.32 aB II | |
| 40–60 | 3.52 ± 1.23 aA III | 13.32 ± 4.24 aA I | 6.79 ± 1.37 aC II | 7.10 ± 0.95 aB II | 2.53 ± 0.16 aC III | 1.65 ± 0.56 aB III | 2.60 ± 0.63 aC III | 2.32 ± 0.76 aC III | |
| N75 | 0–10 | 3.96 ± 0.98 aAB II | 5.18 ± 0.79 bA I | 4.11 ± 0.44 bA I,II | 4.82 ± 0.33 bA I,II | 4.53 ± 0.35 aA I,II | 4.40 ± 0.36 aA I,II | 5.05 ± 0.37 aA I,II | 5.06 ± 0.29 aA I,II |
| 10–20 | 4.44 ± 0.33 aA II | 5.24 ± 0.23 bA I | 4.14 ± 0.16 bA II | 4.56 ± 0.47 bAB II | 4.42 ± 0.27 aA II | 4.70 ± 0.34 aA I,II | 5.24 ± 0.49 aA I | 5.23 ± 0.22 aA I | |
| 20–40 | 4.04 ± 0.33 aAB II,III | 5.04 ± 0.46 bA I | 3.65 ± 0.45 bA II,III | 4.01 ± 0.17 bB II,III | 3.50 ± 0.63 aAB III | 3.37 ± 0.66 aB III | 4.88 ± 0.43 aA I | 4.36 ± 0.35 aB I,II | |
| 40–60 | 3.03 ± 0.65 aB II | 4.26 ± 0.27 bA I | 2.75 ± 0.37 bB II | 2.53 ± 0.36 bC II | 2.49 ± 0.71 aB II | 2.29 ± 0.44 aC II | 3.02 ± 0.33 aB II | 2.81 ± 0.62 aC II | |
| N150 | 0–10 | 4.06 ± 0.26 aAB III,IV | 4.66 ± 0.33 bA I,II | 3.75 ± 0.32 bA IV | 4.41 ± 0.51 bA I,II,III | 4.45 ± 0.28 aA I,II,III | 4.22 ± 0.15 aA II,III,IV | 4.68 ± 0.24 aA I,II | 4.87 ± 0.22 aA I |
| 10–20 | 5.36 ± 1.88 aA I | 5.10 ± 0.36 bA I | 4.10 ± 0.33 bA I | 4.33 ± 0.17 bA I | 4.27 ± 0.21 aA I | 4.57 ± 0.51 aA I | 4.82 ± 0.38 aA I | 4.91 ± 0.68 aA I | |
| 20–40 | 3.79 ± 0.45 aAB I,II | 4.78 ± 0.53 bA I | 3.49 ± 0.95 bAB I,II | 3.38 ± 0.85 bA I,II | 3.49 ± 0.41 aB I,II | 2.37 ± 0.82 aB II | 3.68 ± 0.53 bB I,II | 3.05 ± 1.27 aB II | |
| 40–60 | 2.70 ± 0.40 aB I,II | 4.14 ± 0.66 bA I | 2.45 ± 0.64 bB II | 2.96 ± 1.05 bA I,II | 2.54 ± 0.57 aC II | 1.91 ± 1.11 aB II | 2.43 ± 0.68 aC II | 2.05 ± 0.66 aB II | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Xu, L.; Wei, J.; Cuo, M.; Zhang, H.; Nie, Y.; Guo, M.; Li, J.; Liu, X. Medicago Pasture Soil C:N:P Stoichiometry Mediated by N Fertilization in Northern China. Agronomy 2025, 15, 724. https://doi.org/10.3390/agronomy15030724
Yuan B, Xu L, Wei J, Cuo M, Zhang H, Nie Y, Guo M, Li J, Liu X. Medicago Pasture Soil C:N:P Stoichiometry Mediated by N Fertilization in Northern China. Agronomy. 2025; 15(3):724. https://doi.org/10.3390/agronomy15030724
Chicago/Turabian StyleYuan, Bo, Lijun Xu, Jiaqiang Wei, Meji Cuo, Hongzhi Zhang, Yingying Nie, Mingying Guo, Jinxia Li, and Xinwei Liu. 2025. "Medicago Pasture Soil C:N:P Stoichiometry Mediated by N Fertilization in Northern China" Agronomy 15, no. 3: 724. https://doi.org/10.3390/agronomy15030724
APA StyleYuan, B., Xu, L., Wei, J., Cuo, M., Zhang, H., Nie, Y., Guo, M., Li, J., & Liu, X. (2025). Medicago Pasture Soil C:N:P Stoichiometry Mediated by N Fertilization in Northern China. Agronomy, 15(3), 724. https://doi.org/10.3390/agronomy15030724






