Leaf Traits, Biomass Accumulation and Allocation of Gentiana lawrencei Burkill Along an 800 m Elevation Gradient in Alpine Grasslands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Data Measurement
2.3. Statistical Analysis
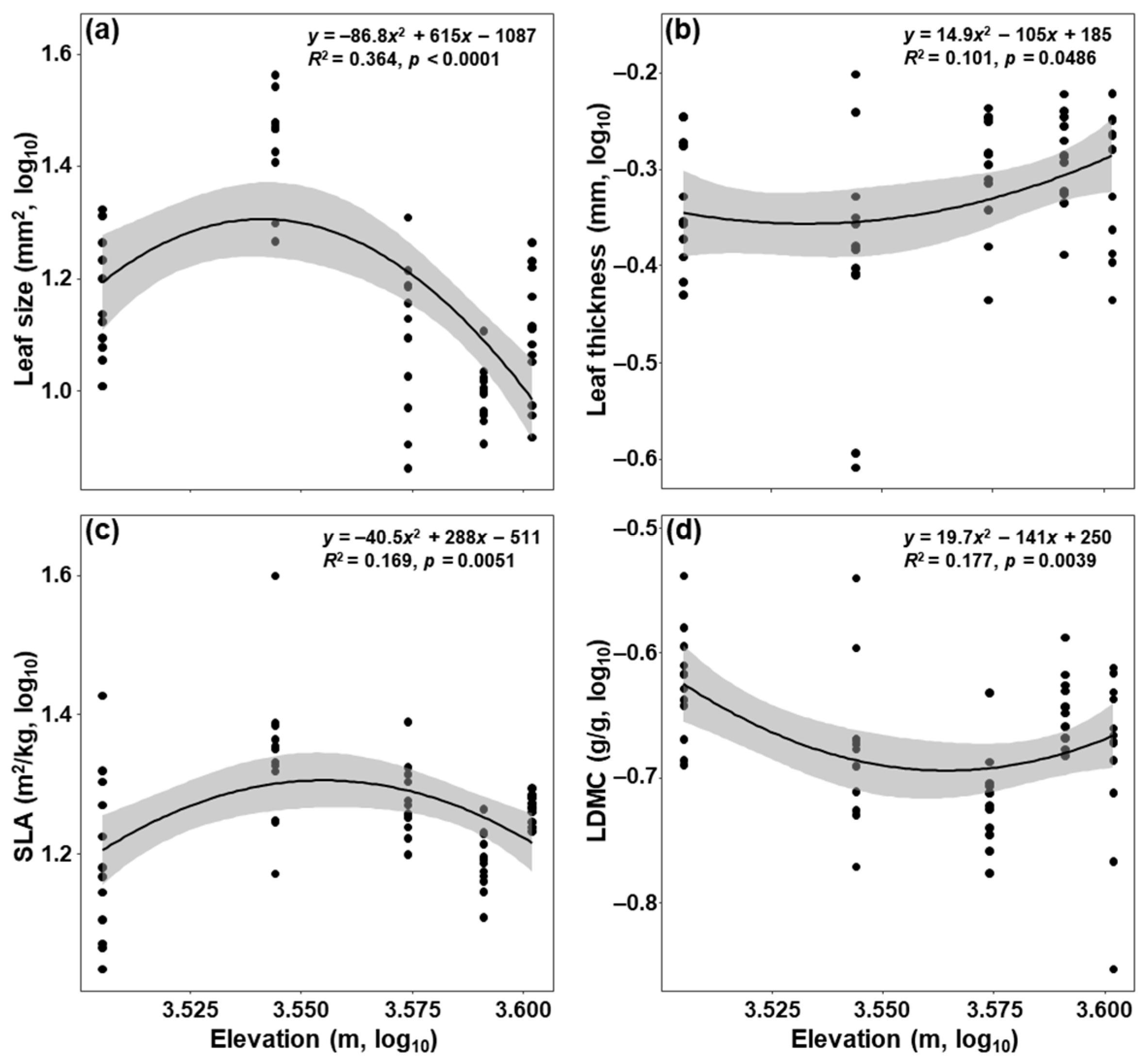
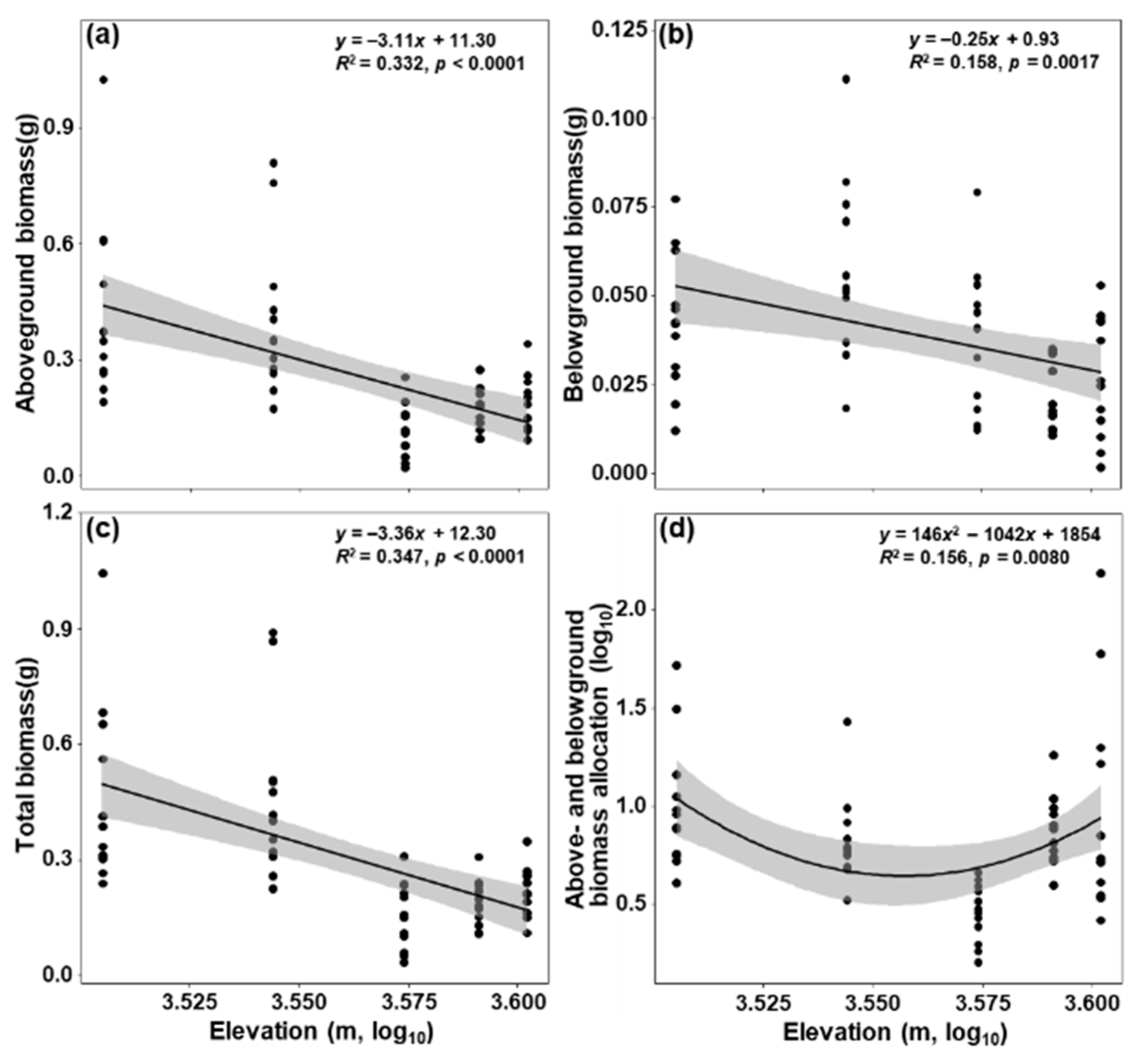
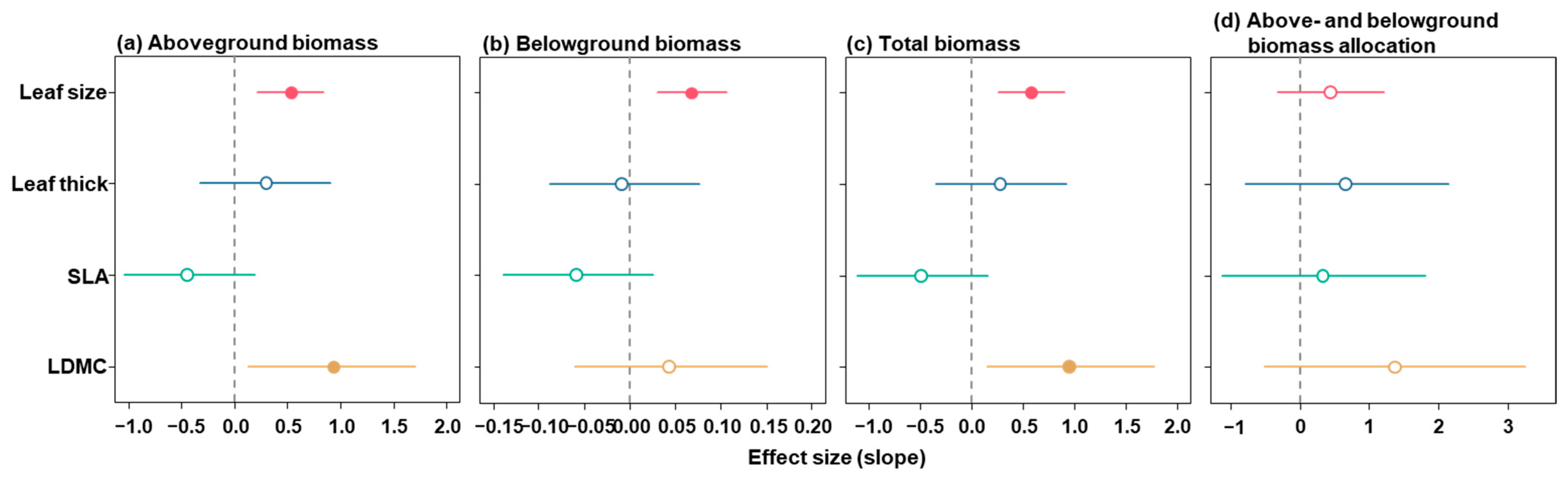
3. Results
4. Discussion
4.1. Influences of Elevation on Leaf Functional Traits
4.2. Impacts of Elevation on Biomass Accumulation and Allocation
4.3. Relationship Between Leaf Functional Traits and Biomass Accumulation and Allocation
4.4. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a.s.l. | Above sea level |
| CV | Coefficient of Variation |
| LDMC | Leaf dry matter content |
| SLA | Specific leaf area |
References
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Malik, A.; Kumar, K.; Kumari, G.; Kumar, N.; Singh, H. Application of Functional Traits in Modelling Productivity and Resilience Under Climate Change. In Plant Functional Traits for Improving Productivity; Springer Nature: Singapore, 2024; pp. 77–96. [Google Scholar]
- Cornelissen, J.H.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Walker, T.W.; Alexander, J.M.; Allard, P.M.; Baines, O.; Baldy, V.; Bardgett, R.D.; Capdevila, P.; Coley, P.D.; David, B.; Defossez, E.; et al. Functional Traits 2.0: The power of the metabolome for ecology. J. Ecol. 2022, 110, 4–20. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; Wiley: Chichester, UK, 1979. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 3rd ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- IPCC. Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Li, X.; Jiang, L.; Meng, F.; Wang, S.; Niu, H.; Iler, A.M.; Duan, J.; Zhang, Z.; Luo, C.; Cui, S.; et al. Responses of sequential and hierarchical phenological events to warming and cooling in alpine meadows. Nat. Commun. 2016, 7, 12489. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Concepts in alpine plant ecology. Plants 2023, 12, 2666. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Yang, Y.; Zhao, X.; Zhou, H.; Zhou, S.; Yin, X.; Ren, Y.; Dong, H.; Zhang, L.; et al. Influences of elevational gradient on flower size and number of Gentiana lawrencei var. farreri. Ecol. Evol. 2024, 14, e11393. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Zhou, C. Altitude distribution of leaf functional traits of Quercus aquifolioides in southeastern Tibet. J. For. Environ. 2021, 41, 366–372. [Google Scholar]
- Zhou, A.; Ge, B.; Chen, S.; Kang, D.; Wu, J.; Zheng, Y.; Ma, H. Leaf ecological stoichiometry and anatomical structural adaptation mechanisms of Quercus sect. Heterobalanus in southeastern Qinghai–Tibet Plateau. BMC Plant Biol. 2024, 24, 325. [Google Scholar]
- Chapin, F.S., III; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology, 2nd ed.; Springer: Cham, Switzerland, 2002. [Google Scholar]
- Tariq, A.; Graciano, C.; Sardans, J.; Zeng, F.; Hughes, A.C.; Ahmed, Z.; Ullah, A.; Ali, S.; Gao, Y.; Peñuelas, J. Plant root mechanisms and their effects on carbon and nutrient accumulation in desert ecosystems under changes in land use and climate. New Phytol. 2024, 242, 916–934. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [PubMed]
- He, J.D.; Xue, J.Y.; Gao, J.; Wang, J.N.; Wu, Y. Adaptations of the floral characteristics and biomass allocation patterns of Gentiana hexaphylla to the altitudinal gradient of the eastern Qinghai-Tibet Plateau. J. Mt. Sci. 2017, 14, 1563–1576. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Sáenz-Ceniceros, A.; Solanki, T.; Robson, T.M.; García-Plazaola, J.I. Alpine forbs rely on different photoprotective strategies during spring snowmelt. Physiol. Plant. 2021, 172, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.; Charlesworth, D. Introduction to Plant Population Biology; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Walther, G.R. Plants in a warmer world. Perspect. Plant Ecol. 2003, 6, 169–185. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Zhang, C.; Willis, C.G.; Klein, J.A.; Ma, Z.; Li, J.; Zhou, H.; Zhao, X. Recovery of plant species diversity during long-term experimental warming of a species-rich alpine meadow community on the Qinghai-Tibet plateau. Biol. Conserv. 2017, 213, 218–224. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Willis, C.G.; Ma, Z. Among-population variation in seed mass for 190 Tibetan plant species: Phylogenetic pattern and ecological correlates. Glob. Ecol. Conserv. 2020, 23, e01163. [Google Scholar] [CrossRef]
- Wu, L.; Ren, Y.; Wan, J.-Z.; Wang, M.; Wang, Z.; Fu, F.; Sun, J.; Fu, Y.; Ma, Z.; Zhang, C. Effects of Precipitation Change and Nitrogen and Phosphorus Additions on Traits and Abundance of Potentilla anserine in an Alpine Meadow. Atmosphere 2022, 13, 1820. [Google Scholar] [CrossRef]
- Royal Botanic Gardens, Kew. Gentiana lawrencei. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:368407-1 (accessed on 15 February 2025).
- World Flora Online. Gentiana lawrencei. WFO Plant List. Available online: https://wfoplantlist.org/taxon/wfo-0000697834-2024-12?page=1 (accessed on 15 February 2025).
- Wu, Z.Y.; Raven, P.H. (Eds.) Gentiana lawrencei. In Flora of China (Vol. 16); Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MI, USA, 2013; Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=210000604 (accessed on 15 February 2025).
- Ho, T.N.; Liu, S.W. A Worldwide Monograph of Gentiana; Science Press: Beijing, China, 2001. [Google Scholar]
- Yang, Y.C.; Ho, T.N.; Lu, S.L.; Huan, R.F.; Wang, Z.X.; Wang, W.Y.; Liu, S.W.; Wu, G.Z.; Zhou, L.H.; Pan, J.T.; et al. Tibetan Medicine Record; Qinghai People’s Publishing House: Xining, China, 1991. [Google Scholar]
- Ma, Z.; Willis, C.G.; Zhang, C.; Zhou, H.; Zhao, X.; Dong, S.; Yao, B.; Huang, X.; Zhao, F.; Yin, G.; et al. Direct and indirect effect of seed size on seedling survival along an experimental light availability gradient. Agric. Ecosyst. Environ. 2019, 281, 64–71. [Google Scholar] [CrossRef]
- Pérez, H.N.; Díaz, S.; Garnier, E.; Lavore, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- de Mendiburu, F.; de Mendiburu, M.F. Package ‘agricolae’. R Package Version 2019, 1, 1143–1149. [Google Scholar]
- Mei, W.; Yu, G.; Greenwell, B. ggtrendline: Add Trendline and Confidence Interval to ‘ggplot’, R Package Version 1.0.3. 2022. Available online: https://CRAN.R-project.org/package=ggtrendline (accessed on 5 December 2024).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘nlme’. Linear and nonlinear mixed effects models. R Package Version 2017, 3, 1–131. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://www.john-fox.ca/Companion/ (accessed on 5 December 2024).
- Fitter, A.H.; Hay, R.K.M. Environmental Physiology of Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Zhang, H.W.; Ma, J.Y.; Sun, W.; Chen, F.H. Altitudinal variation in functional traits of Picea schrenkiana var. tianschanica and their relationship to soil factors in Tianshan Mountains, Northwest China. Acta Ecol. Sin. 2010, 30, 5747–5758. [Google Scholar]
- Losos, J.B. Convergence, adaptation, and constraint. Evolution 2011, 65, 1827–1840. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62.3, 365–392. [Google Scholar] [CrossRef]
- Zhang, X.; Kuang, T.; Dong, W.; Qian, Z.; Zhang, H.; Landis, J.B.; Feng, T.; Li, L.; Sun, Y.; Huang, J.; et al. Genomic convergence underlying high-altitude adaptation in alpine plants. J. Integr. Plant Biol. 2023, 65, 1620–1635. [Google Scholar] [CrossRef]
- McConnaughay, K.D.M.; Coleman, J.S. Biomass allocation in plants: Ontogeny or optimality? A test along three resource gradients. Ecology 1999, 80, 2581–2593. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef]
- Billings, W.D. Arctic and alpine vegetation: Plant adaptations to cold summer climates. In Arctic and Alpine Environments; Ives, J.D., Barry, R.G., Eds.; Methuen: London, UK, 1974; pp. 403–443. [Google Scholar]
- Spehn, E.; Körner, C.; Mountain Biodiversity—A Global Heritage. Mountain Forum Bulletin. 2009. Available online: https://lib2.icimod.org/record/14111 (accessed on 1 December 2024).
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource limitation in plants-an economic analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Chapin, F.S., III. Effects of plant traits on ecosystem and regional processes: A conceptual framework for predicting the consequences of global change. Ann. Bot. 2003, 91, 455–463. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotech. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Morecroft, M.D. Plant Growth and Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Muchow, R.C. Radiation use efficiency. Adv. Agron. 1999, 65, 215–265. [Google Scholar]
- Freschet, G.T.; Kichenin, E.; Wardle, D.A. Explaining within-community variation in plant biomass allocation: A balance between organ biomass and morphology above vs below ground? J. Veg. Sci. 2015, 26, 431–440. [Google Scholar] [CrossRef]
- Guo, H.; Weiner, J.; Mazer, S.J.; Zhao, Z.; Du, G.; Li, B. Reproductive allometry in Pedicularis species changes with elevation. J. Ecol. 2012, 100, 452–458. [Google Scholar] [CrossRef]
- Qi, W.; Bu, H.; Cornelissen, J.H.; Zhang, C.; Guo, S.; Wang, J.; Zhou, X.; Li, W.; Du, G. Untangling interacting mechanisms of seed mass variation with elevation: Insights from the comparison of inter-specific and intra-specific studies on eastern Tibetan angiosperm species. Plant Ecol. 2015, 216, 283–292. [Google Scholar] [CrossRef]
- Kiełtyk, P. Patterns of floral allocation along an elevation gradient: Variation in Senecio subalpinus growing in the Tatra Mountains. Alp. Bot. 2021, 131, 117–124. [Google Scholar] [CrossRef]
- Rixen, C.; Wipf, S.; Rumpf, S.B.; Giejsztowt, J.; Millen, J.; Morgan, J.W.; Nicotra, A.B.; Venn, S.; Zong, S.; Dickinson, K.J.; et al. Intraspecific trait variation in alpine plants relates to their elevational distribution. J. Ecol. 2022, 110, 860–875. [Google Scholar] [CrossRef]
- Institute of Soil Science. The Soil Atlas of China; Cartographic Publishing House: Beijing, China, 1986. [Google Scholar]
| Elevation | 3200 m | 3500 m | 3750 m | 3900 m | 4000 m | |
|---|---|---|---|---|---|---|
| Leaf size (mm2) | Mean | 14.670 b | 28.365 a | 12.945 bc | 9.887 c | 12.858 bc |
| Standard Deviation | 3.693 | 5.032 | 3.614 | 1.165 | 3.132 | |
| Coefficient of Variation | 0.252 | 0.177 | 0.279 | 0.118 | 0.244 | |
| Leaf thickness (mm) | Mean | 0.465 ab | 0.422 b | 0.503 ab | 0.516 a | 0.496 ab |
| Standard Deviation | 0.066 | 0.104 | 0.062 | 0.052 | 0.073 | |
| Coefficient of Variation | 0.142 | 0.246 | 0.124 | 0.101 | 0.147 | |
| SLA (m2/kg) | Mean | 16.147 b | 22.538 a | 18.961 ab | 15.498 b | 18.519 ab |
| Standard Deviation | 4.530 | 5.903 | 2.254 | 1.438 | 0.801 | |
| Coefficient of Variation | 0.281 | 0.262 | 0.119 | 0.093 | 0.043 | |
| LDMC (mg/mg) | Mean | 0.238 a | 0.212 ab | 0.192 b | 0.226 a | 0.210 ab |
| Standard Deviation | 0.023 | 0.030 | 0.016 | 0.014 | 0.029 | |
| Coefficient of Variation | 0.098 | 0.143 | 0.084 | 0.062 | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, L.; Wang, Z.; Shuai, L.; Zhang, X.; Huang, Y.; Wang, Y.; Ma, Z.; Zhang, C. Leaf Traits, Biomass Accumulation and Allocation of Gentiana lawrencei Burkill Along an 800 m Elevation Gradient in Alpine Grasslands. Agronomy 2025, 15, 723. https://doi.org/10.3390/agronomy15030723
Yang Y, Zhang L, Wang Z, Shuai L, Zhang X, Huang Y, Wang Y, Ma Z, Zhang C. Leaf Traits, Biomass Accumulation and Allocation of Gentiana lawrencei Burkill Along an 800 m Elevation Gradient in Alpine Grasslands. Agronomy. 2025; 15(3):723. https://doi.org/10.3390/agronomy15030723
Chicago/Turabian StyleYang, Yuan, Longxin Zhang, Zuoyi Wang, Linlin Shuai, Xiaoying Zhang, Yufang Huang, Ying Wang, Zhen Ma, and Chunhui Zhang. 2025. "Leaf Traits, Biomass Accumulation and Allocation of Gentiana lawrencei Burkill Along an 800 m Elevation Gradient in Alpine Grasslands" Agronomy 15, no. 3: 723. https://doi.org/10.3390/agronomy15030723
APA StyleYang, Y., Zhang, L., Wang, Z., Shuai, L., Zhang, X., Huang, Y., Wang, Y., Ma, Z., & Zhang, C. (2025). Leaf Traits, Biomass Accumulation and Allocation of Gentiana lawrencei Burkill Along an 800 m Elevation Gradient in Alpine Grasslands. Agronomy, 15(3), 723. https://doi.org/10.3390/agronomy15030723





