The Timing of Sugar Beet Harvesting Significantly Influences Roots Yield and Quality Characteristics
Abstract
:1. Introduction
2. Materials and Methods
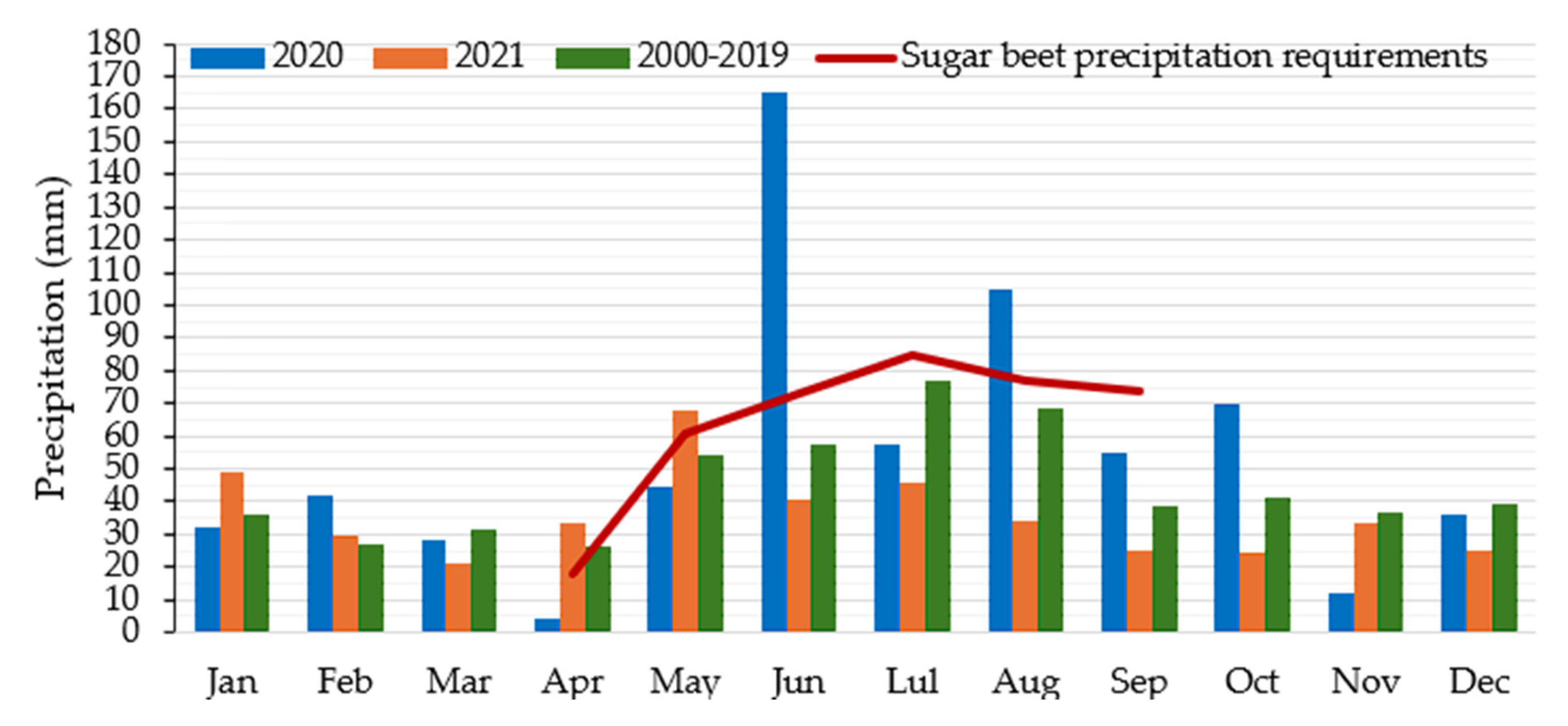
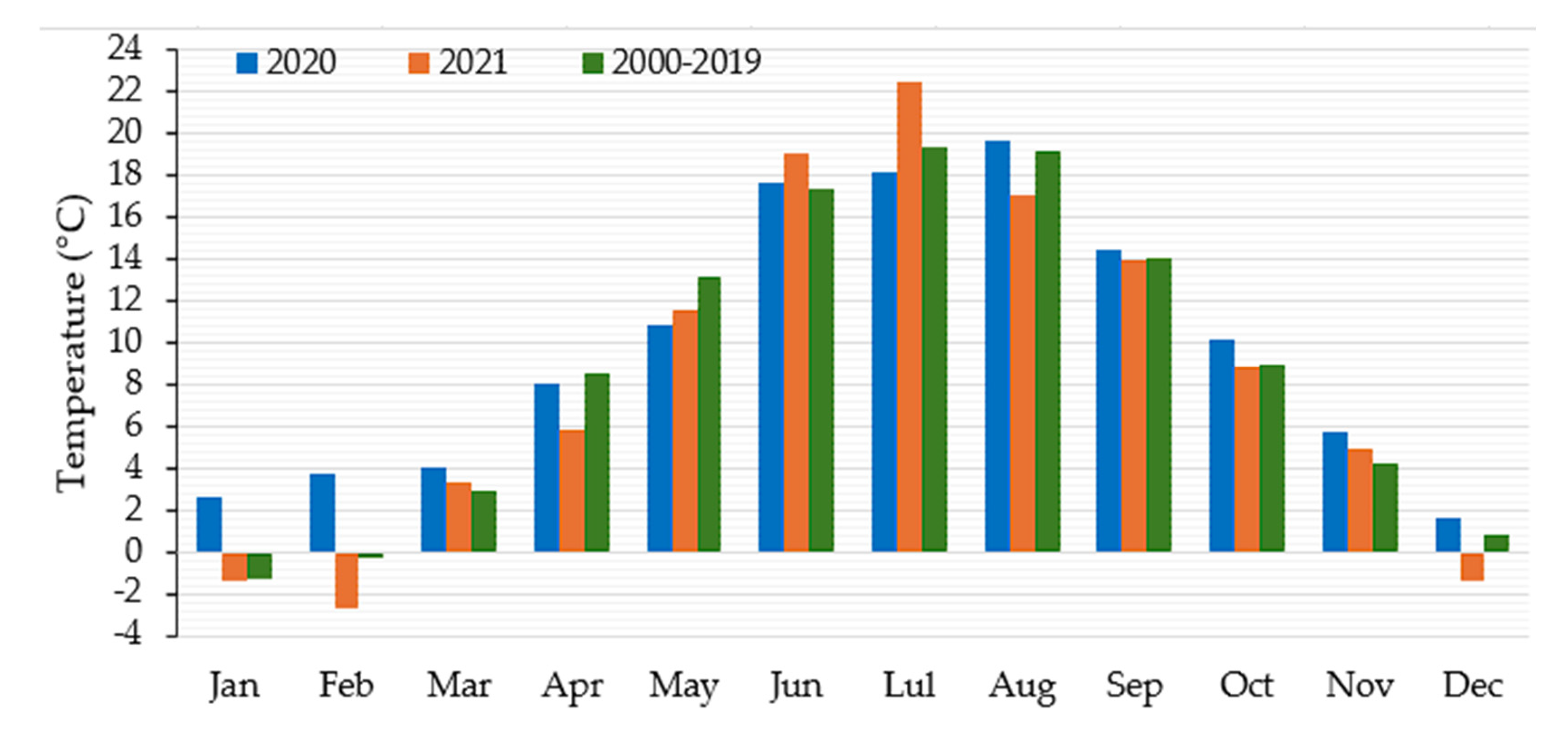
2.1. Experimental Site and Weather Data
2.2. Experimental Design and Agronomic Operations
2.3. Sample Collection and Laboratory Analyses
2.3.1. Sucrose Determination
2.3.2. Determination of Melassogenic Elements
2.4. Statistical Analysis
3. Results
3.1. Weather Condition
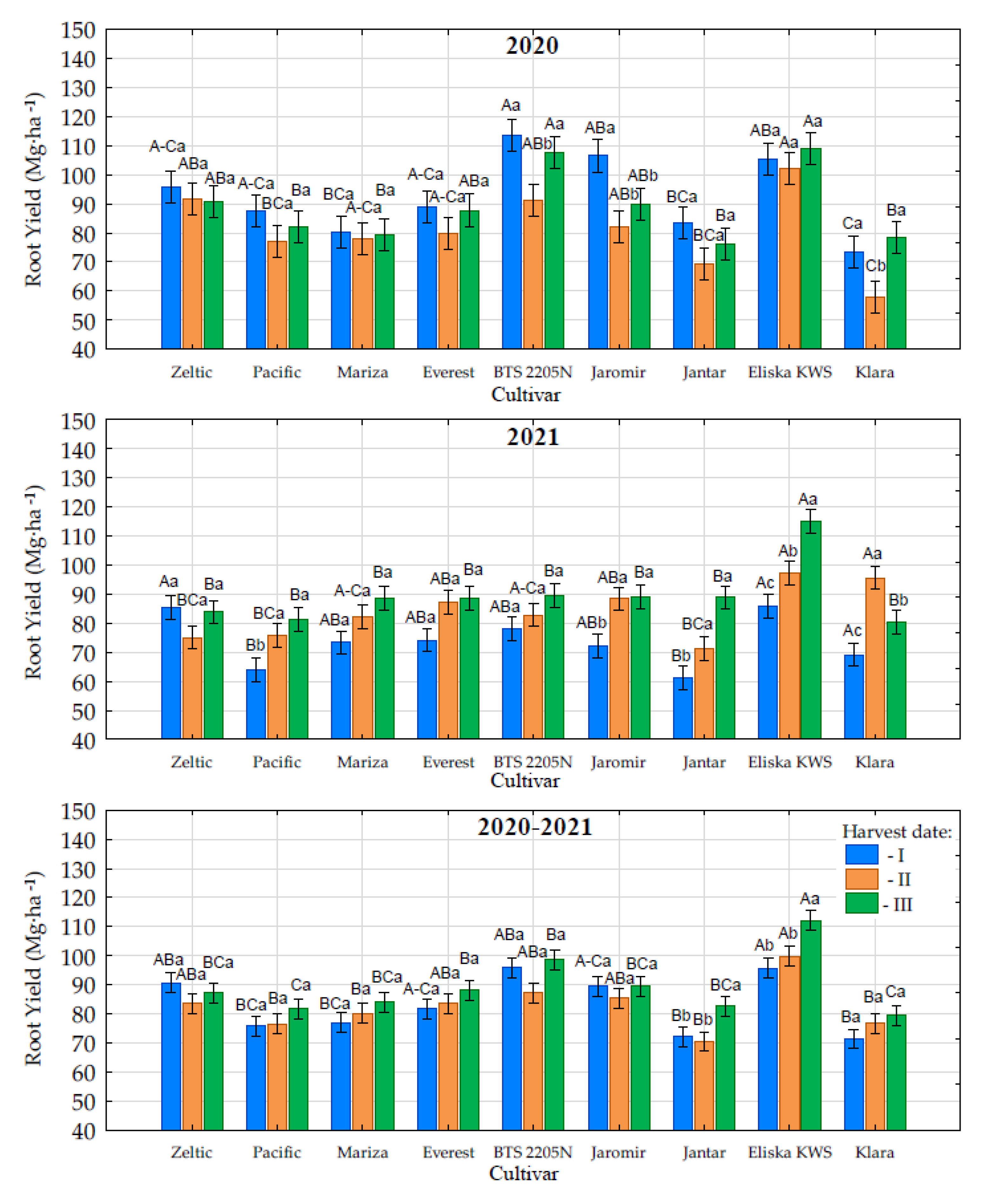
3.2. Root Yield
3.3. Sucrose Content
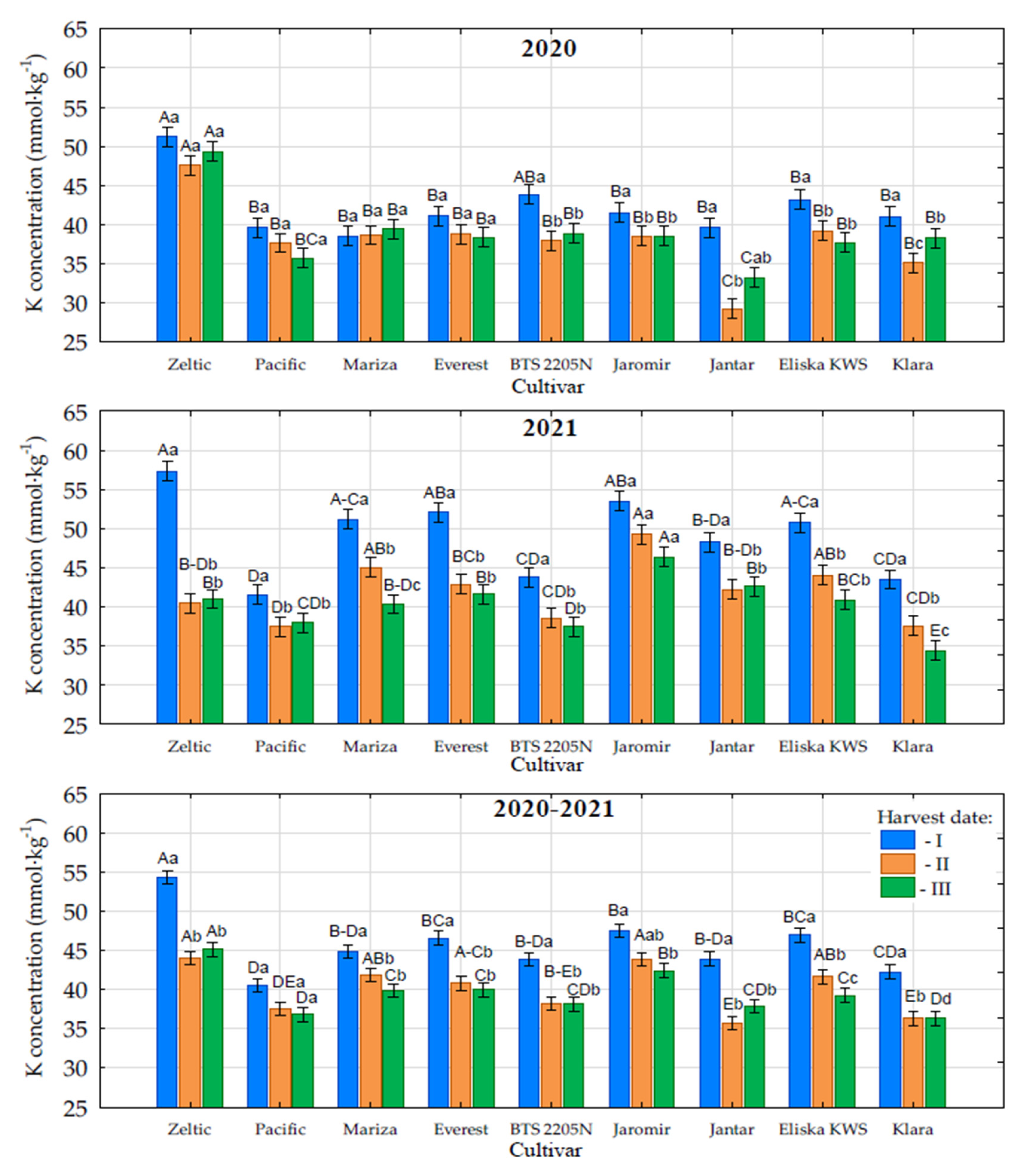
3.4. Potassium Content
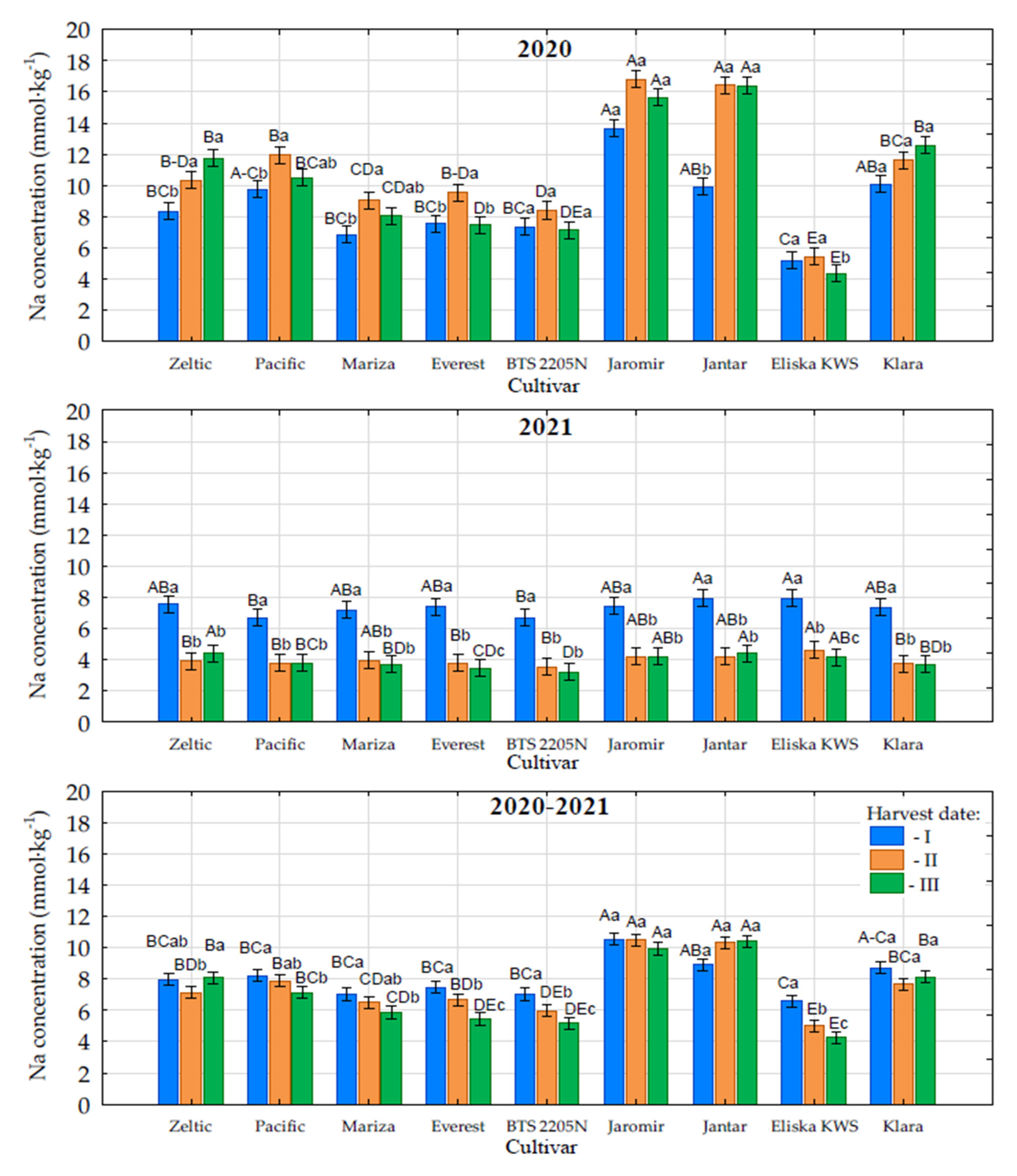
3.5. Sodium Content
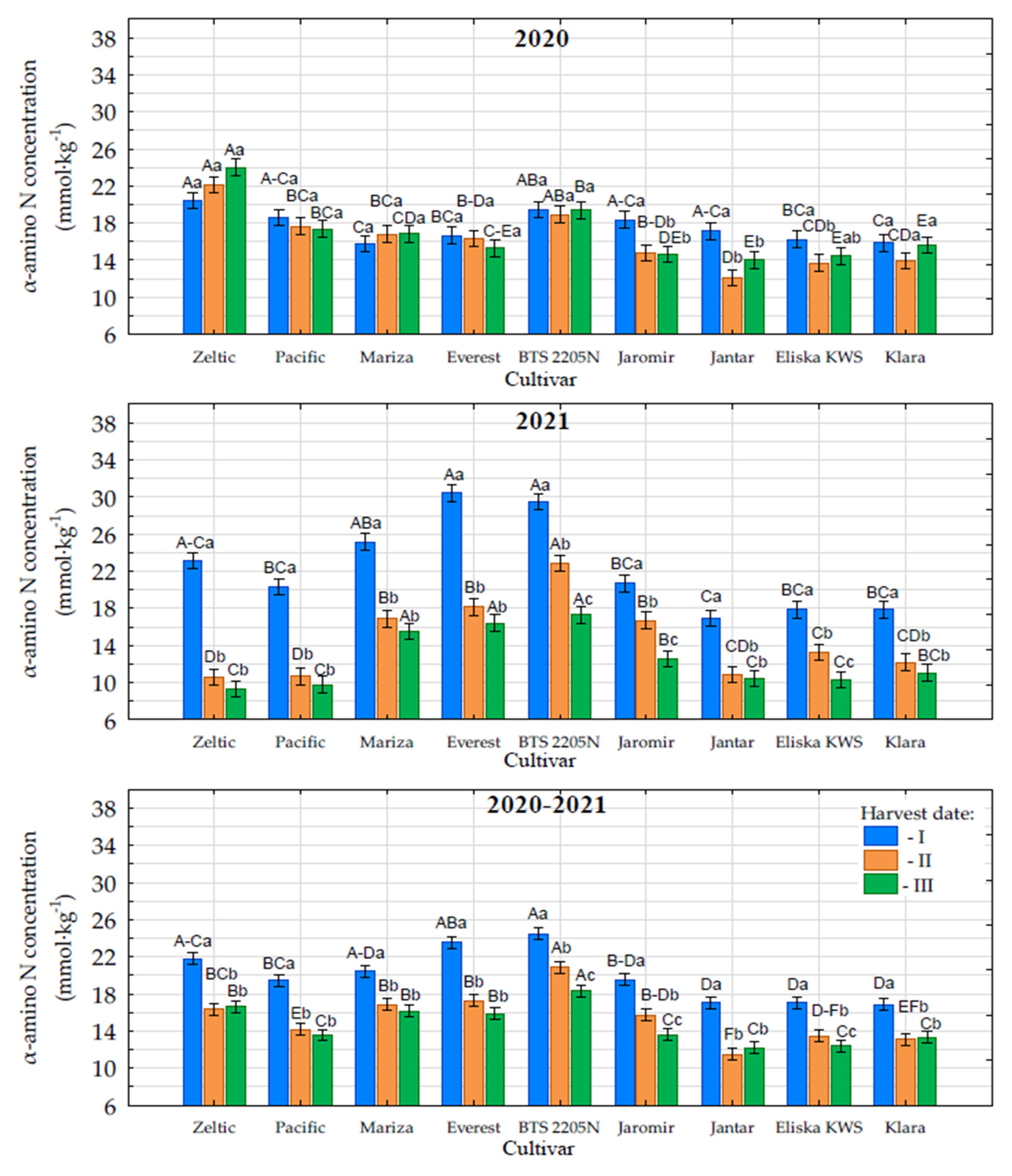
3.6. Content of α-Amino Nitrogen
3.7. Correlations Among Yield and Quality Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neves, M.F.; Gray, A.W.; Bourquard, B.A. Copersucar: A world leader in sugar and ethanol. Int. Food Agribus. Man. Rev. 2016, 19, 207–240. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 February 2025).
- Harveson, R.M.; Hein, G.L.; Smith, J.A.; Wilson, R.G. An integrated approach to cultivar evaluation and selection for improving sugar beet profitability: A successful case study for the central high plains. Plant Dis. 2002, 86, 192–200. [Google Scholar] [CrossRef]
- Misra, V.; Mall, A.K.; Singh, D. Rhizoctonia root-rot diseases in sugar beet: Pathogen diversity, pathogenesis and cutting-edge advancements in management research. Microbe 2023, 1, 100011. [Google Scholar] [CrossRef]
- Hassani, M.; Heidari, B.; Dadkhodaie, A.; Stevanato, P. Genotype by environment interaction components underlying variations in root, sugar and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica 2018, 214, 79. [Google Scholar] [CrossRef]
- Stevanato, P.; Chiodi, C.; Broccanello, C.; Concheri, G. Sustainability of the sugar beet crop. Sugar Tech. 2019, 21, 703–716. [Google Scholar] [CrossRef]
- Alami, L.; Terouzi, W.; Otmani, M.; Abdelkhalek, O.; Salmaoui, S.; Mbarki, M. Effect of sugar beet harvest date on its technological quality parameters by exploratory analysis. J. Food Qual. 2021, 2021, 6639612. [Google Scholar] [CrossRef]
- Ćurčić, Ž.; Danojević, D.; Čačić, N.; Nagl, N.; Taški-Ajduković, K.; Kovačev, L. Influence of harvest dates on sugar beet quantitative traits. Ratar. Povrt. 2012, 49, 141–145. [Google Scholar] [CrossRef]
- Al-Sayed, H.; El-Razek, U.A.; Sarhan, H.; Fateh, H. Effect of harvest dates on yield and quality of sugar beet varieties. Aust. J. Basic Appl. Sci. 2012, 6, 525–529. [Google Scholar]
- Radivojevic, S.; Grbić, J.; Jevtić-Mučibabić, R.; Filipović, V. Importance of cultivar and harvest date on the yield and processing quality of sugar beet. Acta Period. Technol. 2011, 42, 123–129. [Google Scholar] [CrossRef]
- Aly, E.; Al-Labbody, A.; Aly, M. Effect of harvesting dates on quality and yield characteristics of some sugar beet varieties. Fayoum J. Agric. Res. Dev. 2011, 25, 230–237. [Google Scholar] [CrossRef]
- Pavlů, K.; Chochola, J.; Pulkrábek, J.; Urban, J. Influence of sowing and harvest dates on production of two different cultivars of sugar beet. Plant Soil Environ. 2017, 63, 76–81. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Maslaris, N. The effect of temperature, water input and length of growing season on sugar beet yield in five locations in Greece. J. Agric. Sci. 2014, 152, 177–187. [Google Scholar] [CrossRef]
- Javaheri, M.A. Effect of sowing and harvesting dates on root yield and some quality characteristics of sugar beet. Crop Sci. Res. Arid Reg. 2023, 4, 321–331. [Google Scholar] [CrossRef]
- El-Geddawy, D.I.; Abd Elsalam, K.A.; Abd Elateef, I.A.B. Impact of delaying harvesting dates for sugar beet varieties under recent environmental changes. Int. J. Agric. Appl. Sci. 2023, 4, 86–93. [Google Scholar] [CrossRef]
- Pačuta, V.; Rašovský, M.; Černý, I.; Michalska-Klimczak, B.; Wyszyński, Z.; Leśniewska, J.; Buday, M. Influence of weather conditions, variety and sea algae-based biopreparations on root yield, sugar content and polarized sugar yield of sugar beet. List. Cukrov Řepařské 2018, 134, 368–371. [Google Scholar]
- Hoffmann, C.M.; Kenter, C. Yield potential of sugar beet–have we hit the ceiling? Front. Plant Sci. 2018, 9, 289. [Google Scholar] [CrossRef]
- Mostoufi, N.; Faridkhou, A.; Gharebagh, R.S. Dynamic modelling of the sugar extraction process from sugar beet. Food Manuf. 2010, 3, 49–56. [Google Scholar] [CrossRef]
- Al-Mansour, R.; Azzam, H.; Al Jbawi, E. Response of two sugar beet (Beta vulgaris L.) genotypes to different harvest dates in winter time. Jordan J. Agric. Sci. 2013, 9, 630–639. [Google Scholar]
- Elwan, A.; Helmy, S.A.M. Yield and technological characteristics of sugar beet as affected by sowing date, nitrogen level and harvesting age. J. Plant Prod. 2018, 9, 345–352. [Google Scholar] [CrossRef]
- Lauer, J. Sugar beet performance and interactions with planting date, genotype, and harvest date. Agron. J. 1997, 89, 469–475. [Google Scholar] [CrossRef]
- Scott, R.K.; English, S.; Wood, D.W.; Unsworth, M. The yield of sugar beet in relation to weather and length of growing season. J. Agric. Sci. 1973, 81, 339–347. [Google Scholar] [CrossRef]
- Nasr, M.; El-Razek, A.A.A. Sugar beet performance under newly reclaimed soils conditions of Sinai, Egypt. Sugar Tech. 2008, 10, 210–218. [Google Scholar] [CrossRef]
- Ada, R.; Akinerdem, F. Determination of the yield, quality and losses of mechanized harvesting of sugar beet harvested at different dates. Turk. J. Agric. For. 2011, 25, 17–25. [Google Scholar]
- Bastaubayeva, S.O.; Tabynbayeva, L.K.; Yerzhebayeva, R.S.; Konusbekov, K.; Abekova, A.M.; Bekbatyrov, M.B. Climatic and agronomic impacts on sugar beet (Beta vulgaris L.) production. SABRAO J. Breed. Genet. 2022, 54, 141–152. [Google Scholar] [CrossRef]
- Schnepel, K.; Hoffmann, C.M. Effect of extending the growing period on yield formation of sugar beet. J. Agron. Crop Sci. 2016, 202, 530–541. [Google Scholar] [CrossRef]
- Jokić, A.; Zavargo, Z.; Gyura, J.; Radivojević, S.; Šereš, Z. An artificial neural network approach to prediction of sugar beet yield and quality in Serbia. In Sugar Beet Crops: Growth, Fertilization and Yield; Hertsburg, C.T., Ed.; Nova Science Publisher: Hauppauge, NY, USA, 2010; pp. 153–166. [Google Scholar]
- Singh, A.K.; Rai, A.C.; Rai, A.; Singh, M. Applications in post-harvest management of vegetable crops. In Advances in Postharvest Technologies of Vegetable Crops; Apple Academic Press: Palm Bay, FL, USA, 2018; 558p. [Google Scholar] [CrossRef]
- Cook, D.F.; Dadour, I.R.; Voss, S.C. Management of stable fly and other nuisance flies breeding in rotting vegetable matter associated with horticultural crop production. Int. J. Pest Manag. 2011, 57, 315–320. [Google Scholar] [CrossRef]
- Kolarić, L.; Popović, V.; Paunović, J.; Živanović, L.; Ikanović, J.; Sikora, V. Sugar beet yield and quality in the agroecological conditions of Central Banat, Serbia. Agric. For. 2015, 61, 33–41. [Google Scholar] [CrossRef]
- Heidarian, F.; Rokhzadi, A.; Mirahmadi, F. Response of sugar beet to irrigation interval, harvesting time and integrated use of farmyard manure and nitrogen fertilizer. Environ. Exp. Biol. 2018, 16, 169–175. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources (WRB). World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; FAO: Rome, Italy, 2015; Volume 106. [Google Scholar]
- ICUMSA Method GS6-3; Polarimetric Sucrose Content of Sugar Beet by the Macerator or Cold Aqueous Digestion Method Using Aluminium Sulphate as Clarifying Agent—Official. Verlag Dr. Albert Bartens KG: Berlin, Germany, 1994.
- ICUMSA Method GS6-7; Determination of Potassium and Sodium in Sugar Beet by Flame Photometry—Official. Verlag Dr. Albert Bartens KG: Berlin, Germany, 2007.
- ICUMSA Method GS6-5; α-Amino Nitrogen in Sugar Beet by the Copper Method (‘Blue Number’) After Defecation with Aluminium Sulphate—Official. Verlag Dr. Albert Bartens KG: Berlin, Germany, 2007.
- Ostertagova, E.; Ostertag, O. Methodology and application of one-way ANOVA. Am. J. Med. Eng. 2013, 1, 256–261. [Google Scholar]
- Dzieżyc, J.; Nowak, L.; Panek, K. Dekadowe wskaźniki potrzeb opadowych roślin uprawnych w Polsce. Zesz. Probl. Post. Nauk Roln. 1987, 314, 11–33. [Google Scholar]
- Lebedeva, M.G.; Lupo, A.R.; Solovyov, A.B.; Chendev, Y.G.; Rankoth, L.M. Sugar beet harvests under modern climatic conditions in the Belgorod Region (southwest Russia). Climate 2020, 8, 46. [Google Scholar] [CrossRef]
- Rezaei, H.; Paknejad, F.; Ilkaee, M.N.; Habibi, D.; Sadeghi-Shoae, M. Investigation of methanolic sodium nanosilicate and glycine on yield and quality of sugar beet (Beta vulgaris L.). Iran. J. Field Crops Res. 2025, 22, 385–399. [Google Scholar] [CrossRef]
- Bönecke, E.; Breitsameter, L.; Brüggemann, N.; Chen, T.-W.; Feike, T.; Kage, H.; Kersebaum, K.; Piepho, H.; Stützel, H. Decoupling of impact factors reveals the response of German winter wheat yields to climatic changes. Glob. Change Biol. 2020, 26, 3601–3626. [Google Scholar] [CrossRef] [PubMed]
- Rempelos, L.; Barański, M.; Sufar, E.; Gilroy, J.; Shotton, P.; Leifert, H.; Średnicka-Tober, D.; Hasanaliyeva, G.; Rosa, E.; Hajšlová, J.; et al. Effect of Climatic Conditions, and Agronomic Practices Used in Organic and Conventional Crop Production on Yield and Nutritional Composition Parameters in Potato, Cabbage, Lettuce and Onion; Results from the Long-Term NFSC-Trials. Agronomy 2023, 13, 1225. [Google Scholar] [CrossRef]
- Macholdt, J.; Gyldengren, J.G.; Diamantopoulos, E.; Styczen, M. How will future climate depending agronomic management impact the yield risk of wheat cropping systems? A regional case study of Eastern Denmark. J. Agric. Sci. 2020, 158, 660–675. [Google Scholar] [CrossRef]
- Fitters, T.F.J.; Mooney, S.; Sparkes, D. Impact of Water Availability on Root Growth of Sugar Beet Varieties. Soil Use Manag. 2020, 38, 1033–1043. [Google Scholar] [CrossRef]
- Fasahat, P.; Aghaeezadeh, M.; Kakueinezhad, M.; Jabbari, L. A Meta-Analysis of Genotype × Environment Interaction on Sugar Beet Performance. Biom. Lett. 2020, 57, 221–236. [Google Scholar] [CrossRef]
- Ebmeyer, H.; Fiedler-Wiechers, K.; Hoffmann, C. Drought Tolerance of Sugar Beet—Evaluation of Genotypic Differences in Yield Potential and Yield Stability under Varying Environmental Conditions. Eur. J. Agron. 2021, 125, 126262. [Google Scholar] [CrossRef]
- Dang, X.; Hu, X.; Ma, Y.; Li, Y.; Kan, W.; Dong, X. AMMI and GGE Biplot Analysis for Genotype × Environment Interactions Affecting the Yield and Quality Characteristics of Sugar Beet. PeerJ 2024, 12, e16882. [Google Scholar] [CrossRef]
- Crivcianschi, G. Plasticity and stability of some sugar beet varieties to root rot. Rev. Ştiinţă Inovare Cult. Artă Akad. 2023, 2, 62–66. [Google Scholar] [CrossRef]
- Mohammadi-Ahmadmahmoudi, H.; Deihimfard, R.; Koocheki, A. Yield Gap Analysis Simulated for Sugar Beet-Growing Areas in Iran. Agron. J. 2020, 112, 1234–1245. [Google Scholar] [CrossRef]
- Hemayati, S.S.; Hamdi, F.; Saremirad, A.; Hamze, H. Genotype by environment interaction and stability analysis for harvest date in sugar beet cultivars. Sci. Rep. 2024, 14, 16015. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.I.M.; El-Naggar, A.A.A.; Yassin, M.A. Effect of Cercospora leaf spot disease on sugar beet yield. Am. J. Agric. For. 2022, 10, 138–143. [Google Scholar] [CrossRef]
- Roszak, A. Lista Opisowa Odmian Roślin Rolniczych; Burak 2022; COBORU: Słupia Wielka, Poland, 2022; pp. 8–29. [Google Scholar]
- Bhuyian, M.Z.R.; Solanki, S.; del Río Mendoza, L.E.; Borowicz, P.; Lashman, D.; Qi, A.; Ameen, G.; Khan, M.F.R. Histopathological Investigation of Varietal Responses to Cercospora beticola Infection Process on Sugar Beet Leaves. Plant Dis. 2023, 107, 3906–3912. [Google Scholar] [CrossRef]
- Studnicki, M.; Lenartowicz, T.; Noras, K.; Wójcik-Gront, E.; Wyszyński, Z. Assessment of Stability and Adaptation Patterns of White Sugar Yield from Sugar Beet Cultivars in Temperate Climate Environments. Agronomy 2019, 9, 405. [Google Scholar] [CrossRef]
- Varga, I.; Markulj Kulundžić, A.; Tkalec Kojić, M.; Antunović, M. Does the Amount of Pre-Sowing Nitrogen Fertilization Affect Sugar Beet Root Yield and Quality of Different Genotypes? Nitrogen 2024, 5, 386–408. [Google Scholar] [CrossRef]
- Baryga, A.; Polec, B. Wpływ terminu zbioru i sposobu przechowywania korzeni buraków cukrowych na wartość przerobową surowca. Post. Nauk. Techn. Przem. Rol.-Spoż. 2020, 75, 34–55. [Google Scholar]
- Đulaković, V.; Glamoclija, N.; Filipović, V.; Ugrenovic, V. Mineral nutrition plants in function of stable sugar beet production. Sel. I Semen. 2015, 21, 39–49. [Google Scholar] [CrossRef]
- Aly, E.F.A.; El-Bakary, H.M.Y.; Mekhaile, N.E.G. Yield and quality response of some sugar beet varieties to nitrogen and boron fertilization in sandy soil. Egypt. J. Agric. Sci. 2020, 71, 95–105. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Maslaris, N. Nitrogen effects on yield, quality, and K/Na selectivity of sugar beets grown on clays under semi-arid, irrigated conditions. Int. J. Plant Prod. 2013, 7, 355–372. [Google Scholar] [CrossRef]
- Kiniec, A.; Piszczek, J.; Miziniak, W.; Sitarski, A. Impact of the variety and severity of Cercospora beticola infection on the qualitative and quantitative parameters of sugar beet yields. Pol. J. Agron. 2020, 41, 29–37. [Google Scholar] [CrossRef]
- Gouda, M.I.M.; El-Naggar, A.A.A. Efficacy of some fungicides on controlling cercospora leaf spot and their impact on sugar beet yield components. J. Plant Prot. Pathol. 2014, 5, 79–87. [Google Scholar] [CrossRef]
- Fugate, K.K.; Khan, M.F.; Eide, J.D.; Hakk, P.C.; Lafta, A.M. Sugar beet root storage properties are unaffected by Cercospora leaf spot. Plant Dis. 2023, 107, 1649–1958. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M. Response of three sugar beet varieties to planting density and potassium fertilizer levels under sandy soil conditions. J. Agric. Res. 2017, 44, 449–463. [Google Scholar] [CrossRef]
- Ata, A. Influence of Cercospora leaf spot on sugar beet yield components in Egypt. Egypt. J. Phytopathol. 2015, 42, 11–24. [Google Scholar] [CrossRef]
- Bocianowski, J.; Jakubowska, M.; Zawada, D.; Dobosz, R. The effect of acaricide control of the two-spotted spider Mite Tetranychus urticae Koch on the cultivation of cugar beet (Beta vulgaris L.) and on the Size and Quality of the Yield. App. Sci. 2022, 12, 12139. [Google Scholar] [CrossRef]
- Maslaris, N.; Tsialtas, I.; Ouzounidis, T. Soil factors affecting yield, quality, and response to nitrogen of sugar beets grown on light-textured soils in Northern Greece. Commun. Soil Sci. Plant Anal. 2010, 41, 1551–1564. [Google Scholar] [CrossRef]
- Rehab, I.; El Maghraby, S.S.; Kandil, E.E.; Ibrahim, N.Y. Productivity and quality of sugar beet in relation to humic acid and boron fertilization under nubaria conditions. Alex. Sci. Exch. J. 2019, 40, 115–126. [Google Scholar] [CrossRef]
- Maurya, S.K.; Kalhapure, A.; Verma, V.K.; Tiwari, A.; Chaubey, C.; Maurya, D.K.; Kumar, M. Irrigation Scheduling and Cultivar Management for Increasing Water Productivity under Dryland Condition: A Review. Int. J. Environ. Clim. Change 2024, 14, 461–470. [Google Scholar] [CrossRef]
- Ferweez, H.; Bashandy, T. Screening for Drought Tolerance and Molecular Variability among Some Sugar Beet Cultivars. SVU Int. J. Agric. Sci. 2021, 3, 20–29. [Google Scholar] [CrossRef]
- Laufer, D.; Kenter, C.; Ladewig, E. Einfluss von Fungizidstrategie und Sorte auf die Entwicklung der Cercospora-Blattfleckenkrankheit in Zuckerrüben. Sugar Ind. 2020, 145, 172–182. [Google Scholar] [CrossRef]
- Horáková, V.; Dvořáčková, O.; Mezlík, T. Seznam Doporu Čených Odrůd 2014. Pšenice Ozimá, Ječmen Jarní, Ječmen Ozimý, Tritikale Ozimé, Oves Setý (Pluchatý), Hrách Polní; Ústřední Kont Rolní a Zkušební Ústav Zemědělský Brno, Národní odrůdový úřad: Brno, Czech Republic, 2014. [Google Scholar]
- Boulineau, F.; Leclerc, C. Evolution des variétés au travers du catalogue officiel. Sélectionneur Français 2014, 64, 35–50. [Google Scholar]
- Esmaeili, R.; Mohammadian, R.; Heidari Sharif Abad, H.; Noor Mohammadi, G. Improving Quantity and Quality of Sugar Beet Yield Using Agronomic Methods in Summer Cultivation. Plant Soil Environ. 2022, 68, 347–357. [Google Scholar] [CrossRef]

| Harvest Date | 2020 | 2021 | ||||
|---|---|---|---|---|---|---|
| I | II | III | I | II | III | |
| Y × S | ns. | 0.4286 | ns. | ns. | ns. | ns. |
| Y × K | 0.3662 | 0.4648 | ns. | 0.5041 | ns. | ns. |
| Y × Na | ns. | −0.4457 | −0.5270 | ns. | ns. | ns. |
| Y × αN | ns. | ns. | ns. | ns. | ns. | ns. |
| S × K | ns. | ns. | −0.3833 | −0.5845 | −0.7283 | −0.8294 |
| S × Na | −0.5289 | −0.6068 | −0.4975 | −0.4878 | ns. | −0.4679 |
| S × αN | ns. | ns. | −0.4449 | ns. | ns. | ns. |
| K × Na | ns. | −0.3528 | ns. | 0.6288 | 0.6044 | 0.5401 |
| K × αN | 0.6965 | 0.8361 | 0.7916 | ns. | ns. | ns. |
| Na × αN | ns. | ns. | ns. | ns. | ns. | −0.6878 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicki, R.; Wilczewski, E.; Kłosowski, M. The Timing of Sugar Beet Harvesting Significantly Influences Roots Yield and Quality Characteristics. Agronomy 2025, 15, 704. https://doi.org/10.3390/agronomy15030704
Nowicki R, Wilczewski E, Kłosowski M. The Timing of Sugar Beet Harvesting Significantly Influences Roots Yield and Quality Characteristics. Agronomy. 2025; 15(3):704. https://doi.org/10.3390/agronomy15030704
Chicago/Turabian StyleNowicki, Radosław, Edward Wilczewski, and Michał Kłosowski. 2025. "The Timing of Sugar Beet Harvesting Significantly Influences Roots Yield and Quality Characteristics" Agronomy 15, no. 3: 704. https://doi.org/10.3390/agronomy15030704
APA StyleNowicki, R., Wilczewski, E., & Kłosowski, M. (2025). The Timing of Sugar Beet Harvesting Significantly Influences Roots Yield and Quality Characteristics. Agronomy, 15(3), 704. https://doi.org/10.3390/agronomy15030704









