A Bacterial Endophyte Bacillus amyloliquefaciens W10 Enhances the Tomato Resistance Against Tuta absoluta
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Insects
2.2. The Cultivation and Inoculation of B. amyloliquefaciens W10
2.3. Determination of Tomato Growth Parameters
2.4. Analysis of Biological Parameters in T. absoluta
2.5. Oviposition Choice Test
2.6. Determination of Electrical Conductivity and MDA
2.7. Determination of H2O2 and O2− Content
2.8. Determination of Defense-Related Enzymes Activities
2.9. Determination of JA and SA Content
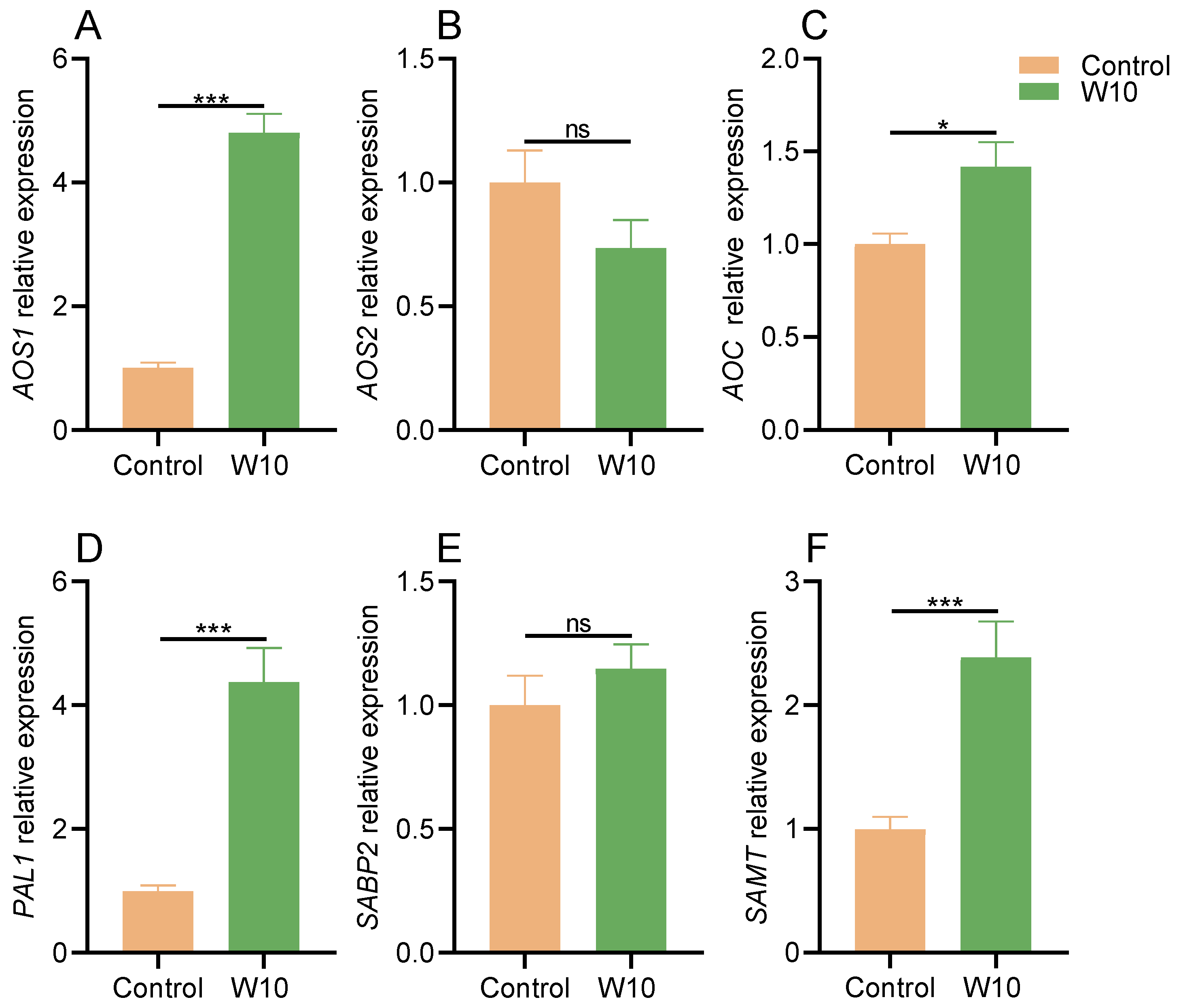
2.10. Gene Expression Analysis
2.11. Statistical Analysis
3. Results
3.1. Effects of B. amyloliquefaciens W10 on the Growth of Tomato Seedlings, the Survival Rate and Fecundity of T. absoluta
3.2. Effects of B. amyloliquefaciens W10 on the Biological Parameters of T. absoluta
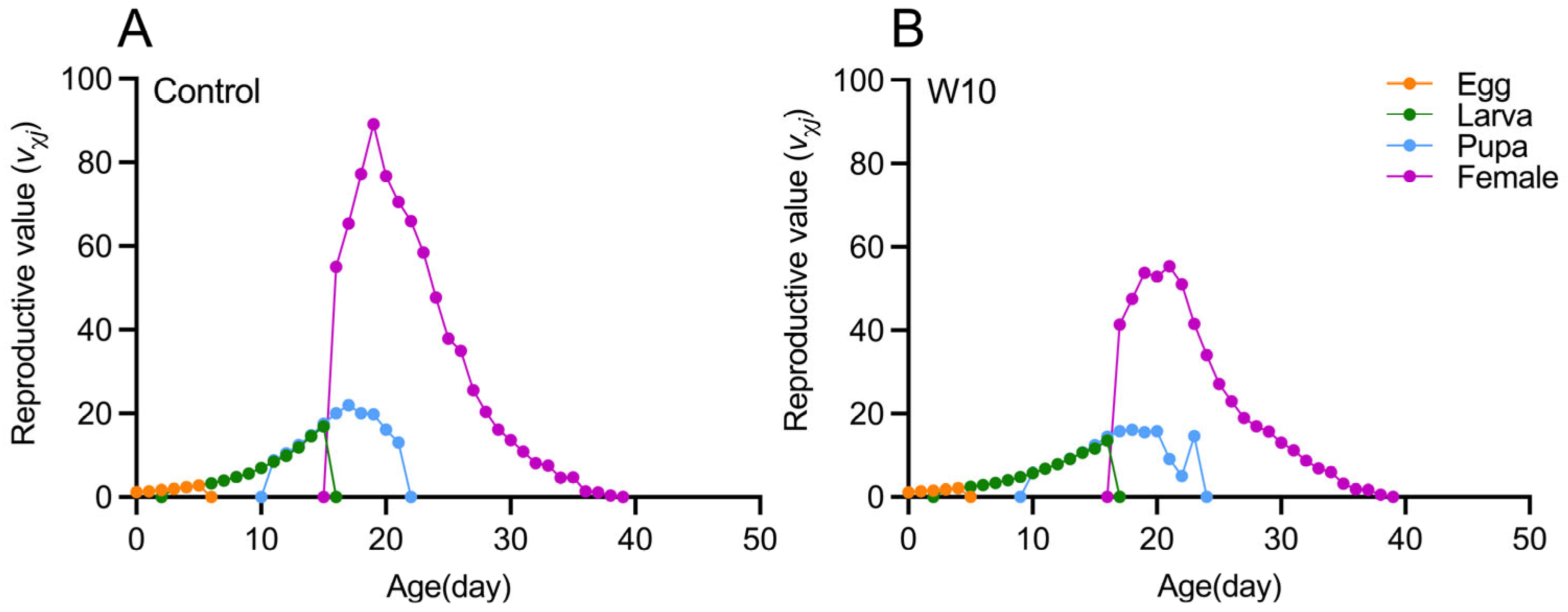
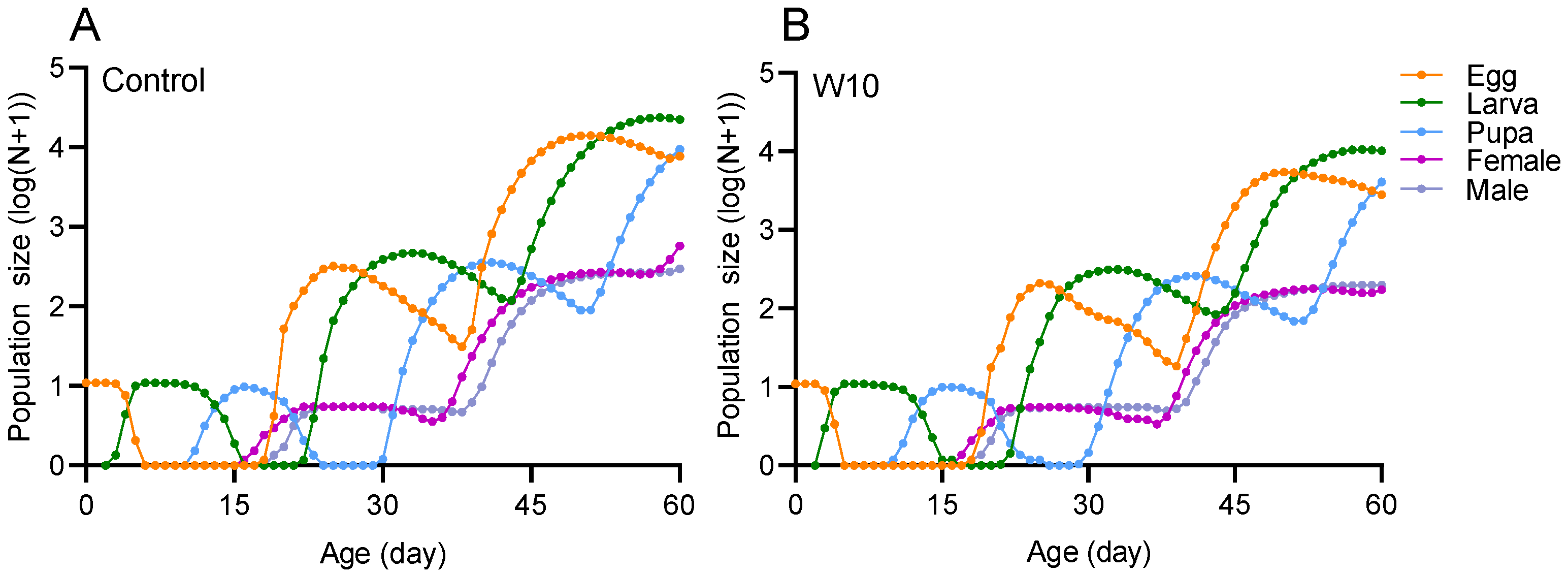
3.3. Effects of B. amyloliquefaciens W10 on the Population Life Table Parameters of T. absoluta
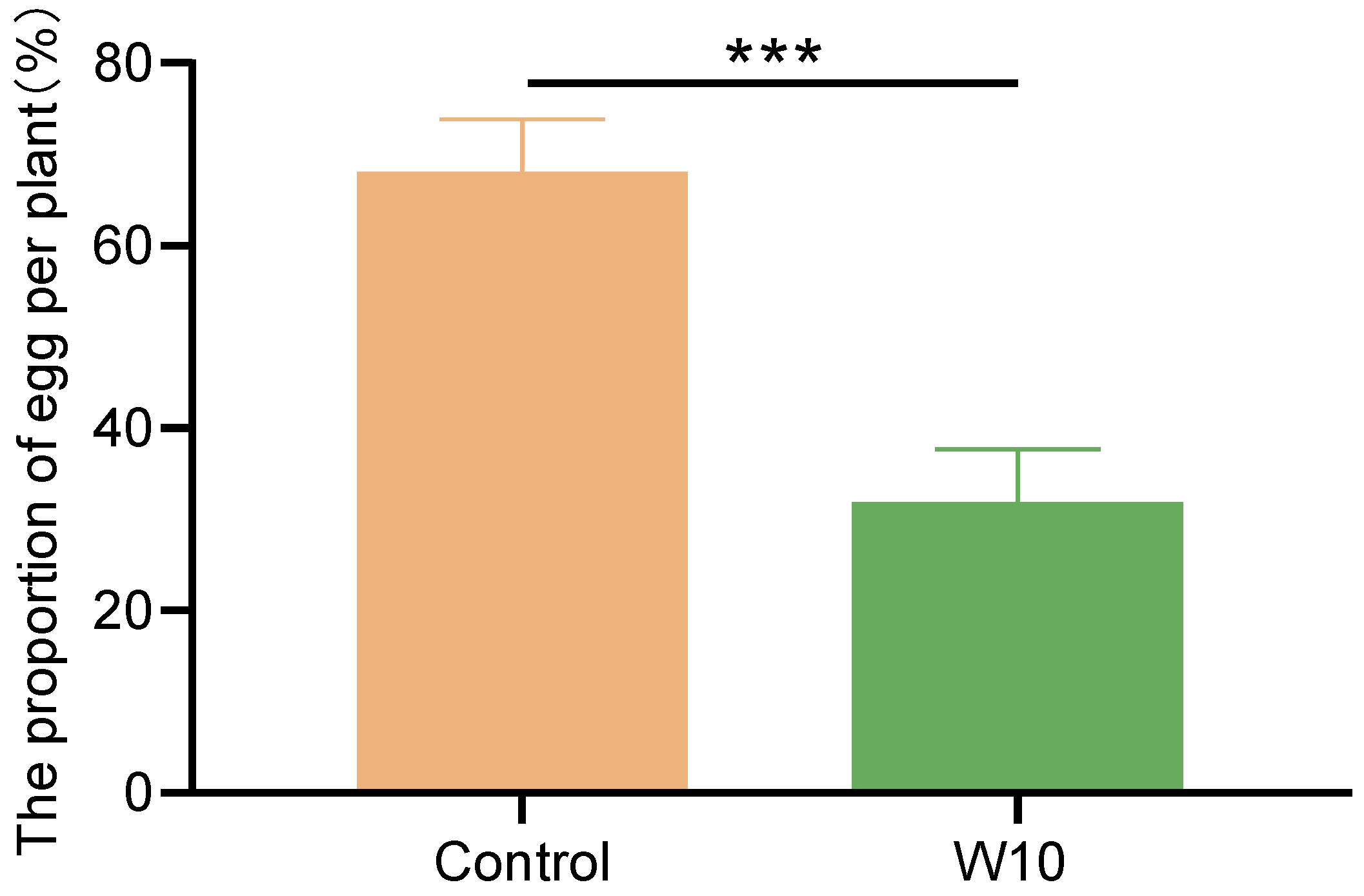
3.4. Effects of B. amyloliquefaciens W10 on the Oviposition Preference of T. absoluta
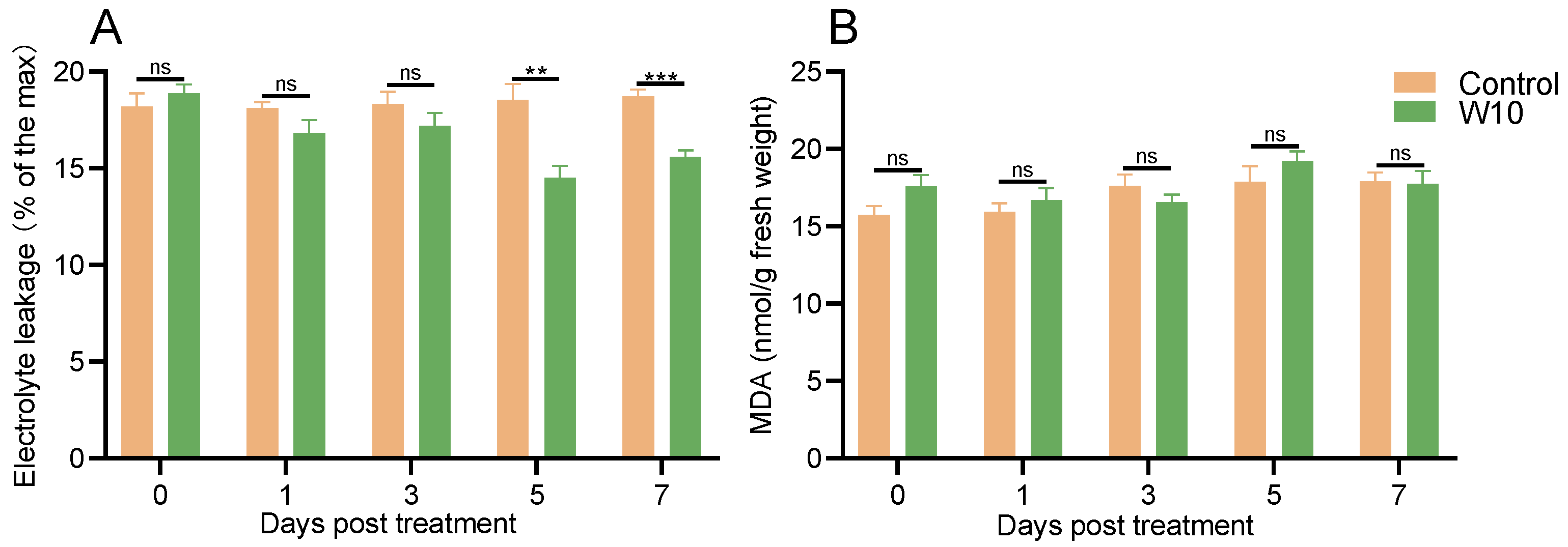
3.5. Effects of B. amyloliquefaciens W10 on Electrical Conductivity, MDA and ROS Accumulation in the Tomato Leaves
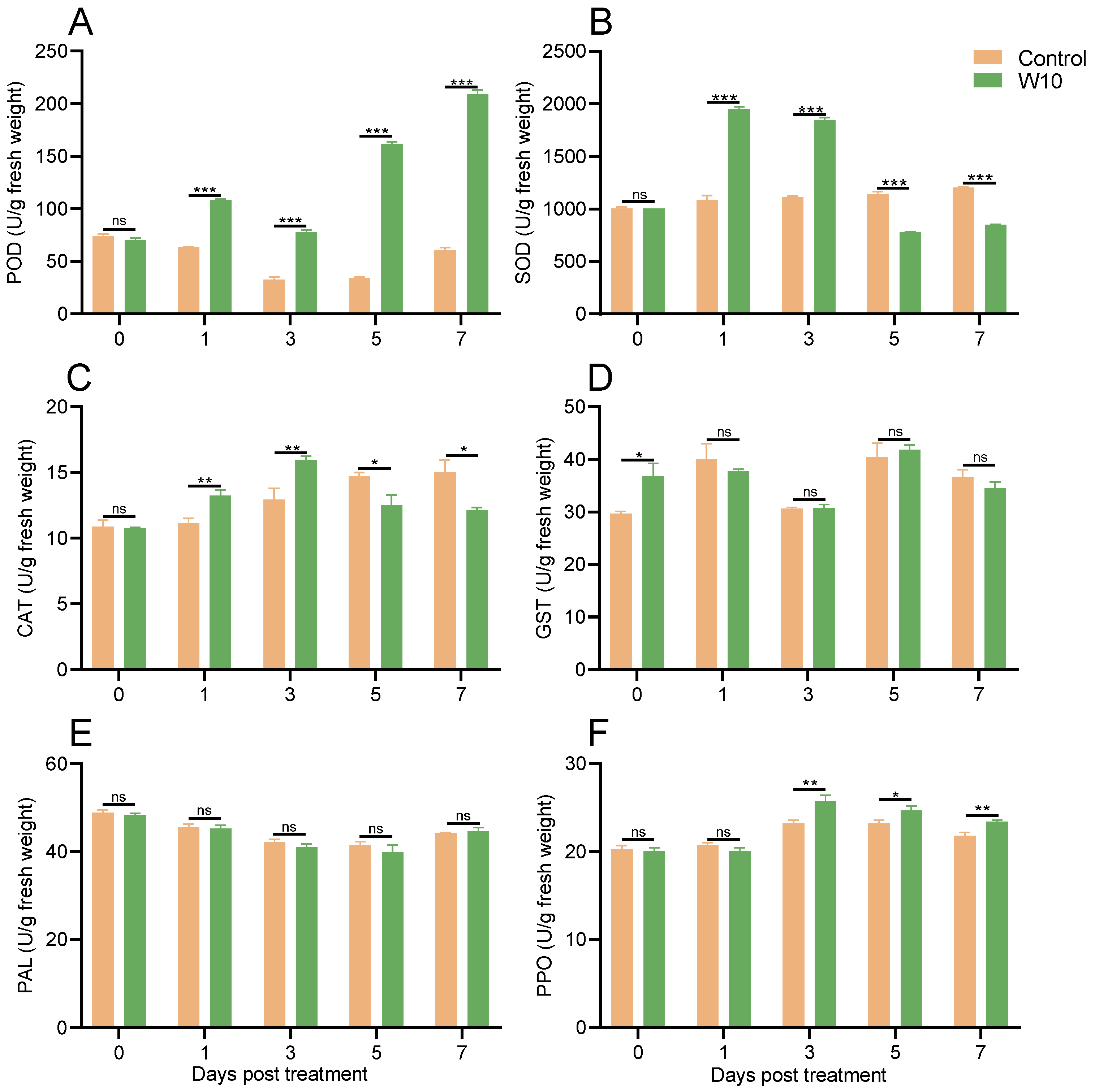
3.6. Effects of B. amyloliquefaciens W10 on the Activities of Defense-Related Enzymes in Tomato Leaves
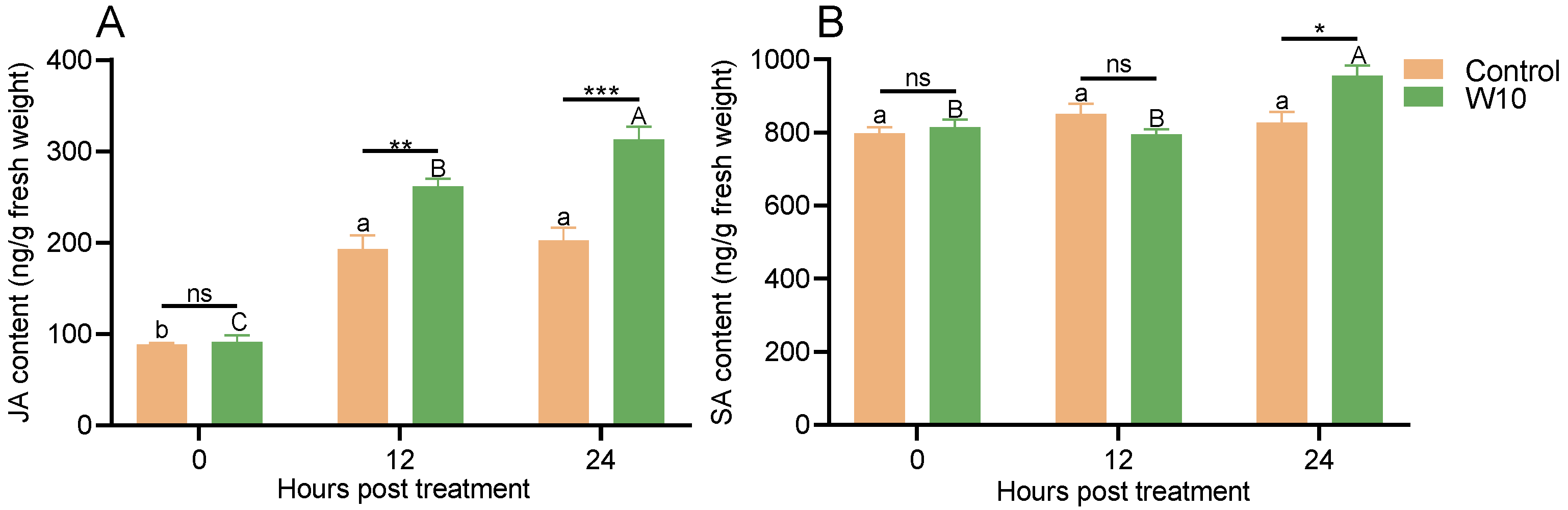
3.7. Effects of B. amyloliquefaciens W10 on JA and SA in Tomato Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Jabamo, T.; Ayalew, G.; Goftishu, M.; Wakgari, M. Integrated effect of insecticide and sex pheromone on the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Crop Prot. 2023, 171, 106285. [Google Scholar] [CrossRef]
- Wang, M.H.; Ismoilov, K.; Liu, W.X.; Bai, M.; Bai, X.S.; Chen, B.; Chen, H.L.; Chen, H.S.; Dong, Y.C.; Fang, K.; et al. Tuta absoluta management in China: Progress and prospects. Entomol. Gen. 2024, 44, 269–278. [Google Scholar] [CrossRef]
- Hassan, E.M.; Mesbah, I.I.; Ali, F.A. Prevalence, population dynamics and associated natural enemies of Tomato Leafminer, Tuta absoluta, in Egypt. Int. J. Trop. Insect Sci. 2022, 42, 143–162. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Sohrabi, F.; Nooryazdan, H.R.; Gharati, B.; Saeidi, Z. Plant resistance to the moth Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in tomato cultivars. Neotrop. Entomol. 2017, 46, 203–209. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Y.B.; Arnó, J.; Mansour, R. Research toward enhancing integrated management of Tuta absoluta, an ongoing invasive threat in Afro-Eurasia. Entomol. Gen. 2024, 44, 263–267. [Google Scholar] [CrossRef]
- Aboutalebian-Soureshjani, A.; Rafiee-Dastjerdi, H.; Naseri, B.; Hassanpour, M.; Khajehali, J. Indoxacarb resistance in Iranian populations of Tuta absoluta (Lepidoptera: Gelechiidae): Cross-resistance, biochemical and molecular mechanisms. Pestic. Biochem. Physiol. 2023, 196, 105633. [Google Scholar] [CrossRef]
- Zang, L.S.; Akhtar, Z.R.; Ali, A.; Tairq, K.; Campos, M.R. Flubendiamide resistance and its mode of inheritance in tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Insects 2022, 13, 1023. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Salazar-Mendoza, P.; Magalhes, D.M.; Pec, M.; Azevedo, K.E.X.; Delalibera, I.; Bento, J.M.S. Endophytic entomopathogenic fungus, individually and in combination with rhizobacteria, enhances resistance in wild and cultivated tomatoes to Tuta absoluta. J. Pest Sci. 2024. [Google Scholar] [CrossRef]
- Damme, V.M.V.; Beck, B.K.; Berckmoes, E.; Moerkens, R.; Wittemans, L.; De, V.R.; Nuyttens, D.; Casteels, H.F.; Maes, M.; Tirry, L.; et al. Efficacy of entomopathogenic nematodes against larvae of Tuta absoluta in the laboratory. Pest Manag. Sci. 2016, 72, 1702–1709. [Google Scholar] [CrossRef]
- Zhivko, M.; Beatriz, R.S.; Laura, D.; Ana, S.L.D.; Guadalupe, Z.H.; Dimitri, O.; Haymanti, S.; Dimitra, P.; Juan, M.G.; Alica, G.C.; et al. Beneficial soil fungi enhance tomato crop productivity and resistance to the leaf-mining pest Tuta absoluta in agronomic conditions. Agron. Sustain. Dev. 2024, 44, 55. [Google Scholar]
- Zhu, Y.X.; Zhang, Y.Y.; Wang, X.Y.; Yin, Y.; Du, Y.Z. Wolbachia modify host cell metabolite profiles in response to short-term temperature stress. Environ. Microbiol. Rep. 2024, 16, e70013. [Google Scholar] [CrossRef]
- Panwar, N.; Szczepaniec, A. Endophytic entomopathogenic fungi as biological control agents of insect pests. Pest Manag. Sci. 2024, 80, 6033–6040. [Google Scholar] [CrossRef]
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. 2022, 13, 933017. [Google Scholar] [CrossRef]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants 2022, 11, 384. [Google Scholar] [CrossRef]
- Zhang, J.M.; Huang, X.Q.; Yang, S.D.; Huang, A.R.; Ren, J.; Luo, X.G.; Feng, S.; Li, P.H.; Li, Z.G.; Dong, P.; et al. Endophytic Bacillus subtilis H17-16 effectively inhibits Phytophthora infestans, the pathogen of potato late blight, and its potential application. Pest Manag. Sci. 2023, 79, 5073–5086. [Google Scholar] [CrossRef]
- González-Cabrera, J.; Mollá, O.; Montón, H.; Urbaneja, A. Efficacy of Bacillus thuringiensis (Berliner) in controlling the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Biocontrol 2011, 56, 71–80. [Google Scholar] [CrossRef]
- Li, H.Y.; Soares, M.A.; Torres, M.S.; Bergen, M.; White, J.F., Jr. Endophytic bacterium, Bacillus amyloliquefaciens, enhances ornamental hosta resistance to diseases and insect pests. J. Plant Interact. 2015, 10, 224–229. [Google Scholar] [CrossRef]
- Guo, S.; Pan, R.B.; Zhang, Y.; Gu, Q.W.; Shen, Q.R.; Yang, J.; Huang, L.Q.; Shen, Z.Z.; Li, R. Plant-microbe interactions influence plant performance via boosting beneficial root-endophytic bacteria. Environ. Microbiome 2025, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Miljakovic, D.; Marinkovic, J.; Balesevic-Tubic, S. The significance of Bacillus spp. In disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil. Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Do, Q.T. The endophytic bacterium Bacillus subtilis R8 as a prospective biocontrol agent for managing tea blister blight and enhancing tea yield. BioControl 2025. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Wees, S.C.V.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Koul, B.; Singh, S.; Dhanjal, D.S.; Singh, J. Plant Growth-Promoting Rhizobacteria (PGPRs): A Fruitful Resource; Springer: Singapore, 2019. [Google Scholar]
- Xing, Z.X.; Liu, D.; Luo, M.; Yang, Z.L.; Pang, W.Y.; Feng, Y.X.; Yan, J.N.; He, F.M.; Feng, X.; Yuan, Q.; et al. Analysis of the control effect of Bacillus amyloliquefaciens C4 wettable powder on potato bacterial wilt caused by Ralstonia solanacearum. Agronomy 2025, 15, 206. [Google Scholar] [CrossRef]
- Pires, L.M.; Marques, E.J.; Oliveira, J.V.; Alves, S.B. Selection of isolates of entomopathogenic fungi for controlling Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and their compatibility with insecticides used in tomato crop. Neotrop. Entomol. 2010, 39, 977–984. [Google Scholar] [CrossRef]
- Liang, M.; Feng, A.; Wang, C.; Zhu, X.; Su, J.; Xu, Z.; Yang, J.; Wang, W.; Chen, K.; Chen, B.; et al. Bacillus amyloliquefaciens LM-1 affects multiple cell biological processes in Magnaporthe oryzae to suppress rice blast. Microorganisms 2024, 12, 1246. [Google Scholar] [CrossRef]
- Dimopoulou, A.; Theologidis, I.; Varympopi, A.; Papafotis, D.; Mermigka, G.; Tzima, A.; Panopoulos, N.J.; Skandalis, N. Shifting perspectives of translational research in bio-bactericides: Reviewing the Bacillus amyloliquefaciens paradigm. Biology 2021, 10, 1202. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, R.; Zhang, R.; Jiang, W.; Chen, X.; Yin, C.; Mao, Z. Isolation, identification, and antibacterial mechanisms of Bacillus amyloliquefaciens QSB-6 and its effect on plant roots. Front. Microbiol. 2021, 12, 746799. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. J. Basic. Microbiol. 2013, 54, 448–456. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, P.; Liu, W.S.; Bernier, M.C.; Zhang, C.Q.; Adhikari, A.; Opiyo, S.O.; Zhao, L.J.; Banks, F.; Xia, Y.; et al. Plant growth promotion and plant disease suppression induced by Bacillus amyloliquefaciens strain GD4a. Plants 2024, 13, 672. [Google Scholar] [CrossRef]
- Luan, P.Y.; Yi, Y.J.; Huang, Y.F.; Cui, L.Q.; Hou, Z.P.; Zhu, L.J.; Liu, Y. Biocontrol potential and action mechanism of Bacillus amyloliquefaciens DB2 on Bipolaris sorokiniana. Front. Microbiol. 2023, 14, 1149363. [Google Scholar] [CrossRef]
- Khan, R.; He, P.B.; Chen, X.J.; He, P.F.; Ahmed, A.; Wu, Y.X.; Tang, G.W.; Tang, P.; Li, X.Y.; Munir, S.; et al. Bacillus endophytes for sustainable management of tomato spotted wilt virus and yield production. Pest Manag. Sci. 2024. [Google Scholar] [CrossRef]
- Uwaremwe, C.; Yue, L.; Wang, Y.; Tian, Y.; Zhao, X.; Liu, Y.; Zhou, Q.; Zhang, Y.B.; Wang, R.Y. An endophytic strain of Bacillus amyloliquefaciens suppresses Fusarium oxysporum infection of Chinese wolfberry by altering its rhizosphere bacterial community. Front. Microbiol. 2021, 12, 782523. [Google Scholar] [CrossRef]
- Zhao, K.B.; Ma, R.Y.; Cheng, M.; Guo, T.; Wu, W.J.; Song, Y.X.; Xu, H.; Tan, A.P.; Qin, B.F.; Wei, S.P.; et al. Isolation of Macrolactin A from a new Bacillus amyloliquefaciens and its aphicidal activity against Rhopalosiphum padi. Pest Manag. Sci. 2024. [Google Scholar] [CrossRef]
- Sellappan, R.; Thangavel, K. Bacillus amyloliquefaciens MZ895491: An endophytic bacterium as potential biocontrol agent in integrated pest management of fall armyworm in maize. Biocontrol Sci. Technol. 2024, 34, 1150–1164. [Google Scholar] [CrossRef]
- Qian, S.; Ahmed, A.; He, P.; He, P.; Munir, S.; Xia, M.; Tang, C.; Tang, P.; Wang, Z.; Khan, R.; et al. Bacillus amyloliquefaciens AK-12 helps rapeseed establish a protection against Brevicoryne brassicae. Int. J. Mol. Sci. 2023, 24, 15893. [Google Scholar] [CrossRef]
- Qi, H.Y.; Wang, D.; Han, D.F.; Song, J.; Ali, M.; Dai, X.F.; Zhang, X.J.; Chen, J.Y. Unlocking antagonistic potential of Bacillus amyloliquefaciens KRS005 to control gray mold. Front. Microbiol. 2023, 14, 1189354. [Google Scholar] [CrossRef]
- Ji, Z.L.; Tang, L.J.; Zhang, Q.X.; Xu, J.Y.; Chen, X.J.; Tong, Y.H. Isolation, purification and characterization of antifungal protein from Bacillus licheniformis W10 strain. Acta Phytophy. Sin. 2007, 3, 38–42. [Google Scholar]
- Xu, G.; Fu, L.R.; Wu, L.; Lu, J.; Xu, M.Q.; Qian, R.H.; Shao, C.J.; Qian, M.S.; Zhang, Y.Y.; Yang, G.Q. A tyramine receptor gene LsTAR2 is involved in reproduction and feeding in the small brown planthopper Laodelphax striatellus. Pestic. Biochem. Physiol. 2025, 209, 106335. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Salih Özgökçe, M.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Roya, T. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Ozgokce, M.S. Agestage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.W.; Xue, L.W.; Du, J.B.; Shang, J.; Xu, F.; Yuan, S.; Lin, H.H. Lack of salicylic acid in Arabidopsis protects plants against moderate salt stress. Z. Naturforsch. C 2009, 64, 231–238. [Google Scholar] [CrossRef]
- Imahori, Y.; Takemura, M.; Bai, J. Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage. Postharvest Biol. Technol. 2008, 49, 54–60. [Google Scholar] [CrossRef]
- Zhang, L.; Oh, Y.; Li, H.Y.; Baldwin, I.T.; Galis, I. Alternative oxidase in resistance to biotic stresses: Nicotiana attenuata AOX contributes to resistance to a pathogen and a piercing-sucking insect but not Manduca sexta larvae. Plant Physiol. 2012, 160, 1453–1467. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Qian, M.S.; Shao, C.J.; Fu, L.R.; Wu, L.; Qian, R.H.; Xu, M.Q.; Lu, J.; Xu, G.; Yang, G.Q. Functional characterization of β-adrenergic-like octopamine receptors in planthopper reproduction and feeding. Int. J. Biol. Macromol. 2025, 288, 138722. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 10320. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Huang, Z.Y.; Li, X.; Ma, L.M.; Gu, Q.; Wu, H.J.; Liu, J.; Borriss, R.; Wu, Z.; Gao, X.W. Stomatal closure and SA-, JA/ET-signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 2018, 9, 9847. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Q.; Liu, Y.L.; Zhang, J.Y.; Liu, X.L.; Ju, Z.G.; Shi, H.X.; Zhou, W.W. Dual role of endophytic entomopathogenic fungi: Induce plant growth and control tomato leafminer Phthorimaea absoluta. Pest Manag. Sci. 2023, 79, 4557–4568. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Salih Özgökçe, M.; Güncan, A. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–732. [Google Scholar] [CrossRef]
- Gadhave, K.; Gange, A. Plant-associated Bacillus spp. alter life-history traits of the specialist insect Brevicoryne brassicae L. Agr. Forest Entomol. 2016, 18, 35–42. [Google Scholar] [CrossRef]
- Huangfu, N.B.; Shang, J.; Guo, L.X.; Zhu, X.Z.; Zhang, K.X.; Niu, R.C.; Li, D.Y.; Gao, X.K.; Wang, L.; Ji, J.C.; et al. Life table analysis and RNA-Seq reveal hormesis and transgenerational effects of deltamethrin on Aphis gossypii. Pest Manag. Sci. 2025, 81, 477–489. [Google Scholar] [CrossRef]
- Huangfu, N.B.; Guo, L.X.; Shang, J.; Wang, L.; Zhang, K.X.; Li, D.Y.; Gao, X.K.; Zhu, X.Z.; Ji, J.C.; Luo, J.Y.; et al. Hormetic dose response induced by sublethal-dose sulfoxaflor leads to reproductive stimulation of Aphis gossypii. Pestic. Biochem. Physiol. 2024, 204, 106061. [Google Scholar] [CrossRef]
- Xu, G.; She, S.Y.; Gui, W.; Ma, C.; Zhang, Y.Y.; Qian, M.S.; Yang, G.Q. Seed priming of rice varieties with decoyinine improve their resistance against the brown planthopper Nilaparvata lugens. Agronomy 2023, 13, 72. [Google Scholar] [CrossRef]
- Xu, G.; Li, C.T.; Gui, W.; Xu, M.Q.; Lu, J.; Qian, M.S.; Zhang, Y.Y.; Yang, G.Q. Colonization of Piriformospora indica enhances rice resistance against the brown planthopper Nilaparvata lugens. Pest Manag. Sci. 2024, 80, 4386–4398. [Google Scholar] [CrossRef]
- Disi, J.; Simmons, J.; Zebelo, S. Plant growth-promoting rhizobacteria-induced defense against insect herbivores. In Field Crops: Sustainable Management by PGPR; Maheshwari, D.K., Dheeman, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 385–410. [Google Scholar]
- Bertea, C.M.; Casacci, L.P.; Bonelli, S.; Zampollo, A.; Barbero, F. Chemical, physiological and molecular responses of host plants to Lepidopteran egg-laying. Front. Plant Sci. 2020, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Arturo, R.O.; Hexon Angel, C.C.; Nuvia, O.C.; Alfonso, L.C. Robert Winkler, Lourdes Macías-Rodríguez. Volatiles released by Beauveria bassiana induce oviposition behavior in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). FEMS Microbiol. Ecol. 2022, 98, 114. [Google Scholar]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Chen, G.; Hu, W.Y.; Xie, F.D.; Zhang, L.J. Solvent for extracting malondialdehyde in plant as an index of senescence. Plant Physiol. J. 1991, 1, 44–46. [Google Scholar]
- Zhao, Z.C.; Yu, M.X.; Wei, Y.Y.; Xu, F.; Jiang, S.; Chen, Y.; Ding, P.; Shao, X.F. Cinnamon essential oil causes cell membrane rupture and oxidative damage of Rhizopus stolonifer to control soft rot of peaches. Food Control 2025, 170, 111039. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, L.S.; Meng, Y.; Lv, R.; Zhou, Z.; Zhu, L.; Lin, H.H.; Xi, D.H. Alpha-momorcharin enhances Tobacco mosaic virus resistance in tobaccoNN by manipulating jasmonic acid-salicylic acid crosstalk. J. Plant Physiol. 2018, 223, 116–126. [Google Scholar] [CrossRef]
- Mukami, A.; Ngetich, A.; Mweu, C.; Oduor, R.O.; Muthangya, M.; Mbinda, W.M. Differential characterization of physiological and biochemical responses during drought stress in finger millet varieties. Physiol. Mol. Biol. Plants 2019, 25, 837–846. [Google Scholar] [CrossRef]
- Ji, Z.L.; Peng, S.; Zhu, W.; Dong, J.P.; Zhu, F. Induced resistance in nectarine fruit by Bacillus licheniformis W10 for the control of brown rot caused by Monilinia fructicola. Food Microbiol. 2020, 92, 103558. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van, B.F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Chen, J. Production and role of reactive oxygen species in plant defense responsesly. Chin. Bull. Bot. 1999, 16, 107–111. [Google Scholar]
- Deng, X.G.; Zhu, T.; Zou, L.J.; Han, X.Y.; Zhou, X.; Xi, D.H.; Zhang, D.W.; Lin, H.H. Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J. 2016, 85, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Yan, S.Y.; Li, Y.J.; Li, G.S.; Zhao, Y.J.; Sun, S.Q.; Su, J.; Cui, Z.Q.; Huo, J.F.; Sun, Y.; et al. Defense-related enzyme activities and metabolomic analysis reveal differentially accumulated metabolites and response pathways for sheath blight resistance in rice. Plants 2024, 13, 3554. [Google Scholar] [CrossRef]
- Poór, P.; Ördög, A.; Czékus, Z.; Borbély, P.; Takács, Z.; Kovács, J.; Tari, I. Regulation of the key antioxidant enzymes by developmental processes and environmental stresses in the dark. Biol. Plant. 2018, 62, 201–210. [Google Scholar] [CrossRef]
- Ren, A.T.; Zhu, Y.; Chen, Y.L.; Ren, H.X.; Li, J.Y.; Abbott, L.K.; Xiong, Y.C. Arbuscular mycorrhizal fungus alters root-sourced signal (abscisic acid) for better drought acclimation in Zea mays L. seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Hu, S.S.; Hu, B.; Chen, Z.B.; Vosátka, M.; Vymazal, J. Antioxidant response in arbuscular mycorrhizal fungi inoculated wetland plant under Cr stress. Environ. Res. 2020, 191, 110203. [Google Scholar] [CrossRef]
- Kurniawan, A.; Chuang, H.W. Rhizobacterial Bacillus mycoides functions in stimulating the antioxidant defence system and multiple phytohormone signalling pathways to regulate plant growth and stress tolerance. J. Appl. Microbiol. 2022, 132, 1260–1274. [Google Scholar] [CrossRef]

| Treatment | Stem Diameter/cm | Stem Height/cm | Shoot Fresh Weight/g | Root Fresh Weight/g |
|---|---|---|---|---|
| Control | 0.31 ± 0.01 a | 27.88 ± 0.92 c | 7.68 ± 0.31 b | 1.41 ± 0.11 d |
| 108 CFU/mL (once) | 0.28 ± 0.01 a | 29.68 ± 1.01 bc | 8.20 ± 0.27 ab | 1.58 ± 0.09 cd |
| 2 × 108 CFU/mL (once) | 0.30 ± 0.01 a | 30.02 ± 0.88 bc | 8.12 ± 0.28 ab | 1.74 ± 0.10 bcd |
| 4 × 108 CFU/mL (once) | 0.30 ± 0.01 a | 31.31 ± 0.95 abc | 8.44 ± 0.34 ab | 2.16 ± 0.11 abc |
| 108 CFU/mL (twice) | 0.29 ± 0.01 a | 29.31 ± 1.02 bc | 8.81 ± 0.35 ab | 2.26 ± 0.19 ab |
| 2 × 108 CFU/mL (twice) | 0.29 ± 0.01 a | 32.98 ± 1.08 ab | 9.42 ± 0.22 a | 2.41 ± 0.14 a |
| 4 × 108 CFU/mL (twice) | 0.32 ± 0.01 a | 35.24 ± 0.84 a | 9.40 ± 0.44 a | 2.40 ± 0.19 a |
| Treatment | Larval Stage | Pupal Stage | Pre-Mature Stage | Fecundity (Eggs) |
|---|---|---|---|---|
| Control | 0.91 ± 0.01 a | 0.86 ± 0.01 a | 0.78 ± 0.01 a | 124.25 ± 12.85 a |
| 108 CFU/mL (once) | 0.91 ± 0.01 a | 0.85 ± 0.01 a | 0.78 ± 0.02 a | 126.67 ± 9.71 a |
| 2 × 108 CFU/mL (once) | 0.90 ± 0.01 a | 0.87 ± 0.01 a | 0.79 ± 0.02 a | 95.64 ± 8.59 ab |
| 4 × 108 CFU/mL (once) | 0.90 ± 0.01 a | 0.87 ± 0.01 a | 0.78 ± 0.02 a | 110.23 ± 12.88 ab |
| 108 CFU/mL (twice) | 0.91 ± 0.01 a | 0.85 ± 0.01 a | 0.77 ± 0.02 a | 122.26 ± 10.79 ab |
| 2 × 108 CFU/mL (twice) | 0.91 ± 0.01 a | 0.86 ± 0.01 a | 0.78 ± 0.02 a | 79.79 ± 6.50 b |
| 4 × 108 CFU/mL (twice) | 0.89 ± 0.01 a | 0.87 ± 0.01 a | 0.77 ± 0.01 a | 87.78 ± 7.96 ab |
| Parameters | Control | W10 (2 × 108 CFU/mL) | p |
|---|---|---|---|
| Net reproductive rate R0 (offspring) | 67.017 ± 10.687 | 41.898 ± 7.012 | 0.049 |
| Intrinsic rate of increase rm (days−1) | 0.172 ± 0.008 | 0.148 ± 0.008 | 0.037 |
| Mean generation time T (days) | 24.500 ± 0.430 | 25.148 ± 0.460 | 0.304 |
| Finite capacity of increase λ (days−1) | 1.187 ± 0.009 | 1.160 ± 0.009 | 0.036 |
| Doubling time DT (days) | 4.039 ± 0.189 | 4.667 ± 0.254 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, M.; Sheng, C.; Zheng, M.; Zhu, K.; Yu, Y.; Xu, G.; Yang, G. A Bacterial Endophyte Bacillus amyloliquefaciens W10 Enhances the Tomato Resistance Against Tuta absoluta. Agronomy 2025, 15, 695. https://doi.org/10.3390/agronomy15030695
Qian M, Sheng C, Zheng M, Zhu K, Yu Y, Xu G, Yang G. A Bacterial Endophyte Bacillus amyloliquefaciens W10 Enhances the Tomato Resistance Against Tuta absoluta. Agronomy. 2025; 15(3):695. https://doi.org/10.3390/agronomy15030695
Chicago/Turabian StyleQian, Mingshi, Chaoqi Sheng, Mingying Zheng, Ke Zhu, Youxin Yu, Gang Xu, and Guoqing Yang. 2025. "A Bacterial Endophyte Bacillus amyloliquefaciens W10 Enhances the Tomato Resistance Against Tuta absoluta" Agronomy 15, no. 3: 695. https://doi.org/10.3390/agronomy15030695
APA StyleQian, M., Sheng, C., Zheng, M., Zhu, K., Yu, Y., Xu, G., & Yang, G. (2025). A Bacterial Endophyte Bacillus amyloliquefaciens W10 Enhances the Tomato Resistance Against Tuta absoluta. Agronomy, 15(3), 695. https://doi.org/10.3390/agronomy15030695







