Alkaline Extraction in Air Enhances Antioxidant and Biological Activities of Humic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Humic Acid Preparations
2.2. Extraction of Humic Acids
2.3. Physicochemical Properties of HAC
2.4. Antioxidant Properties of Humic Acids
2.5. Biological Activity Tests
2.6. Statistical Data Treatment
3. Results
3.1. Physicochemical Properties of HAC Extracted in Air and Under Nitrogen
3.1.1. Elemental Composition and Optical Properties
3.1.2. Infrared Spectra
3.1.3. 13C-NMR and EPR Spectroscopy Data
3.1.4. Potentiometric Titration Data
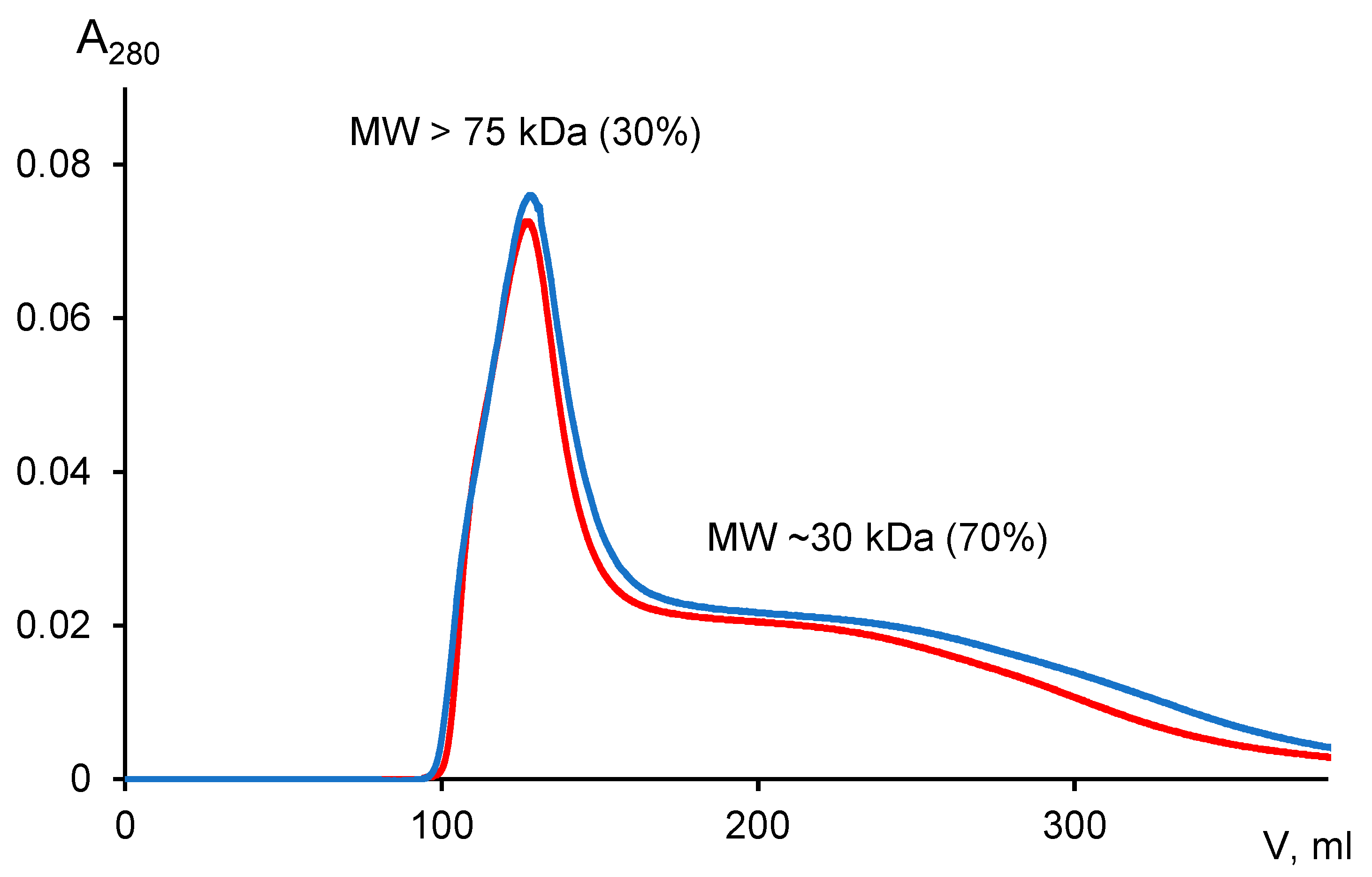
3.1.5. Molecular Weight Distributions
3.2. Antioxidant Properties of HAs
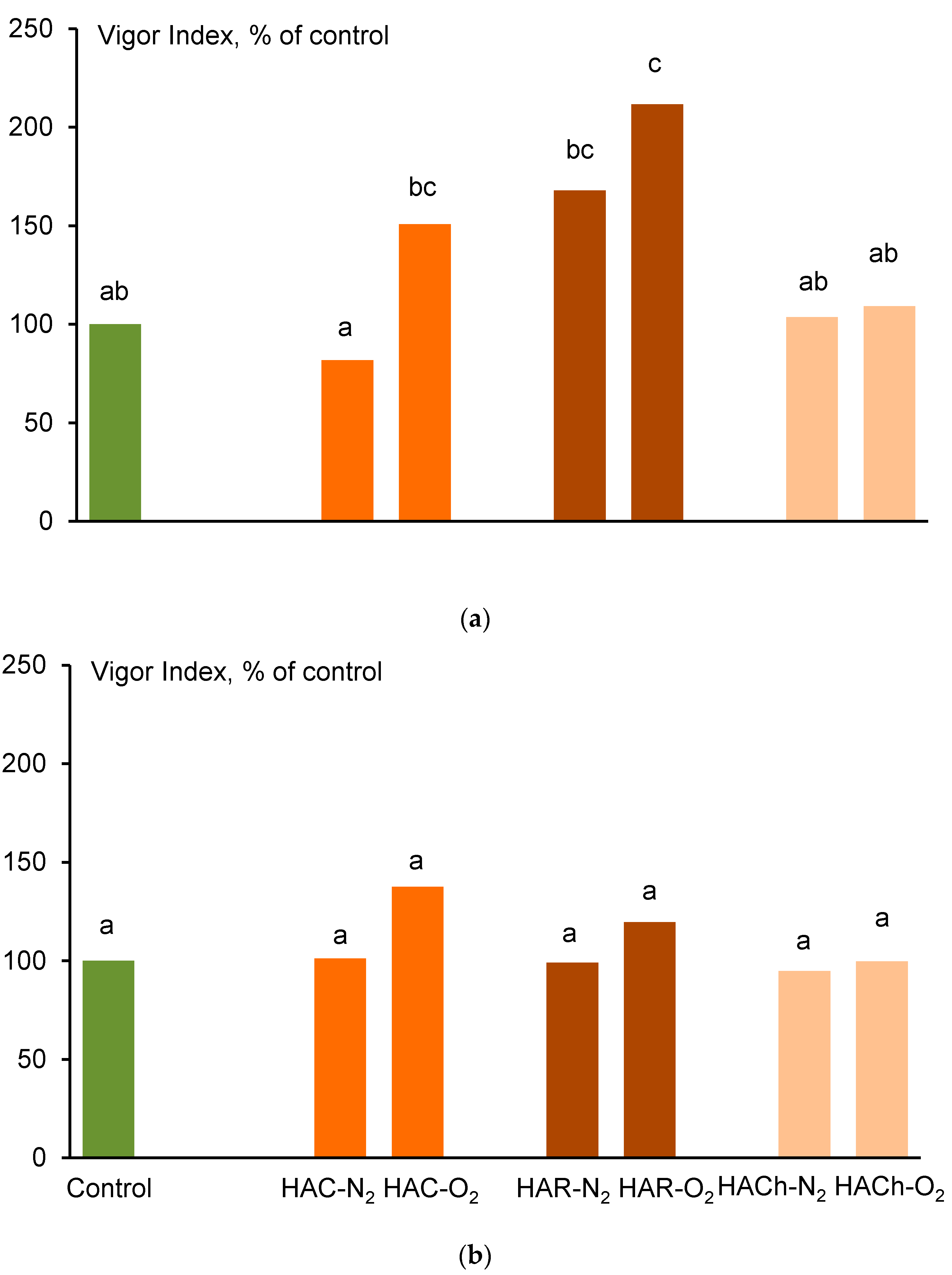
3.3. Biological Activity of HAs
4. Discussion
4.1. Physicochemical Properties of HAC and Antioxidant Activities of HAs
4.2. Antioxidant Properties of HAs
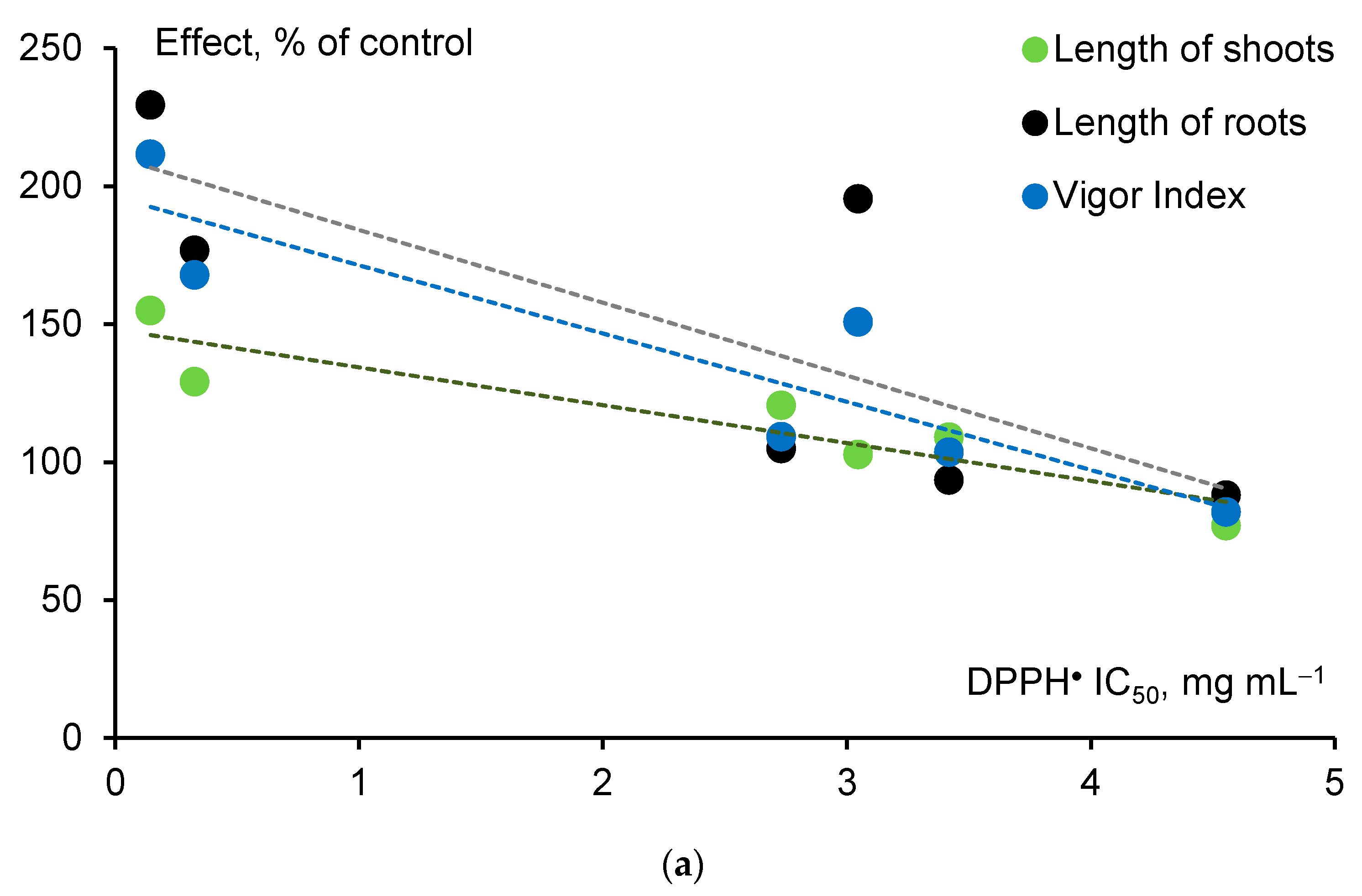
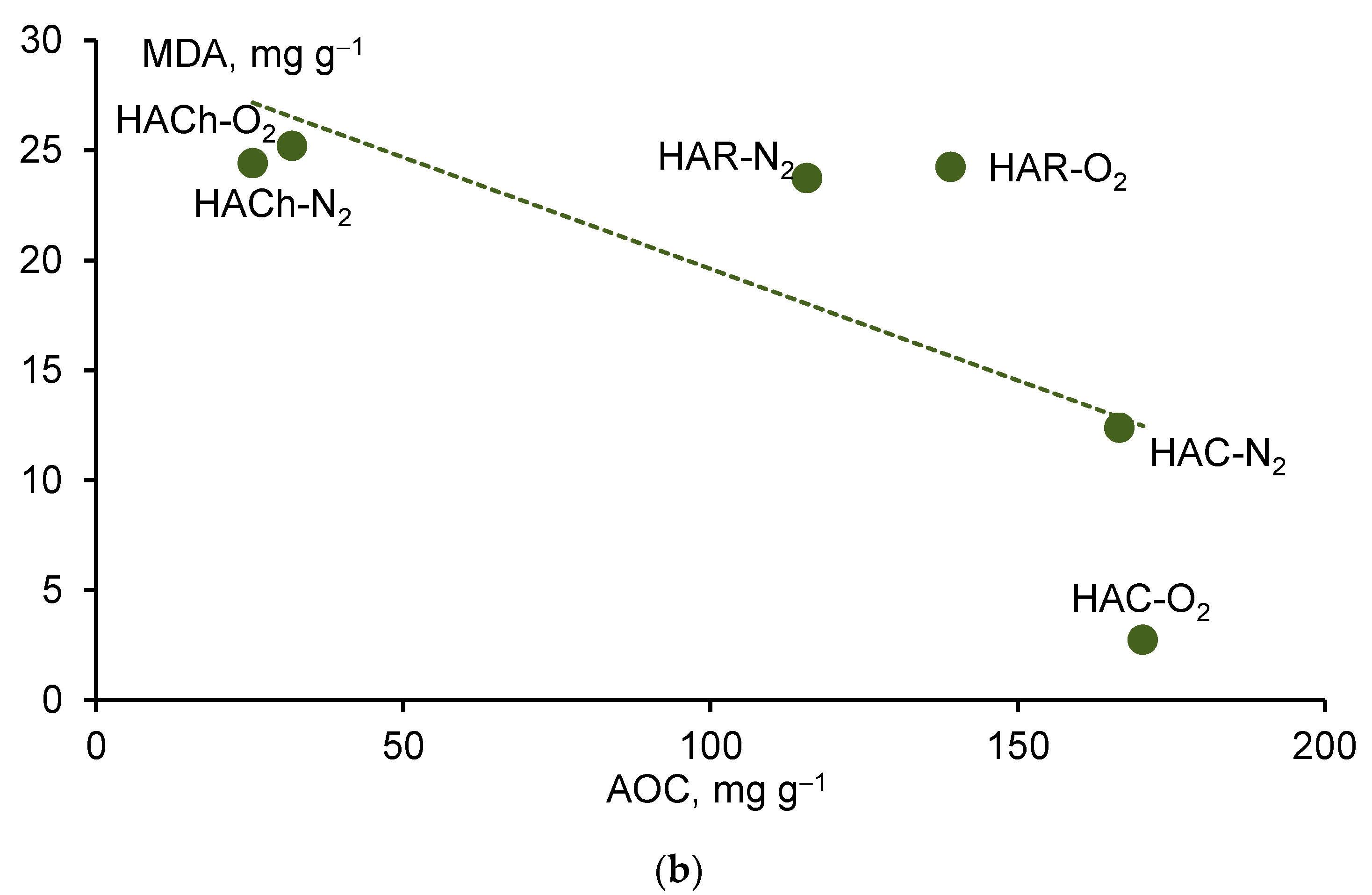
4.3. Biological Activity of Humic Acids in Relation to Their Antioxidant Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlov, D.S. Humic Acids of Soils; Amerind: New Delhi, India, 1985. [Google Scholar]
- Stevenson, F.J. Humic Substances: Genesis, Composition, Reactions; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Perminova, I.V.; Hatfield, K. Remediation chemistry of humic substances: Theory and implications for technology. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; Perminova, I.V., Ed.; Springer: Amsterdam, The Netherlands, 2005; pp. 3–36. [Google Scholar]
- Zhou, P.; Yan, H.; Gu, B. Competitive complexation of metal ions with humic substances. Chemosphere 2005, 58, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Bin Huang, B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468–469, 1014–1027. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cheng, X.; Zhang, Y. The impact of humic acid fertilizers on crop yield and nitrogen use efficiency: A meta-analysis. Agronomy 2024, 14, 2763. [Google Scholar] [CrossRef]
- Hassett, D.J.; Bise, M.S.I.; Hartenstein, R. Bactericidal action of humic acids. Soil Biol. Biochem. 1987, 19, 111–113. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.P.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Gomes de Melo, B.A.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mat. Sci. Engin. 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Perminova, I.V. Interactions between humic substances and microorganisms and their implications for nature-like bioremediation technologies. Molecules 2020, 29, 2706. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. An appreciation of the contribution of Frank Stevenson to the advancement of studies of soil organic matter and humic substances. J. Soils Sediments 2018, 18, 1212–1231. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Giannakopoulos, E.; Drosos, M.; Deligiannakis, Y. A humic-acid-like polycondensate produced with no use of catalyst. J. Colloid Interface Sci. 2009, 336, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Drosos, M.; Jerzykiewicz, M.; Louloudi, M.; De Ligiannakis, E. Progress towards synthetic modelling of humic acid: Peering into the physicochemical polymerization mechanism. Colloids Surf. A 2011, 389, 254–265. [Google Scholar] [CrossRef]
- Kishikawa, N.; Ohkubo, N.; Ohyama, K.; Nakashima, K.; Kuroda, N. Chemiluminescence assay for quinones based on generation of reactive oxygen species through the redox cycle of quinone. Anal. Bioanal. Chem. 2009, 393, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Waksman, S. Humus: Origin, Chemical Composition, and Importance in Nature; Williams and Wilkins Company: Baltimore, MD, USA, 1936. [Google Scholar]
- Kleber, M.; Lehmann, J. Humic substances extracted by alkali are invalid proxies for the dynamics and functions of organic matter in terrestrial and aquatic ecosystems. J. Environ. Qual. 2019, 48, 207–216. [Google Scholar] [CrossRef]
- Zavarzina, A.G.; Kravchenko, E.G.; Konstantinova, A.I.; Perminova, I.V.; Chukov, S.N.; Demin, V.V. Comparison of the properties of humic acids extracted from soils by alkali in the presence and absence of oxygen. Euras. Soil Sci. 2019, 52, 880–891. [Google Scholar] [CrossRef]
- Siddiqui, Y.; Meon, S.; Ismail, R.; Rahmani, M.; Ali, A. In vitro fungicidal activity of humic acid fraction from oil palm compost. Int. J. Agric. Biol. 2009, 1, 448–452. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed priming: New comprehensive approaches for an old empirical technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Araújo, S., Ed.; IntechOpen Limited: London, UK, 2016; pp. 1–46. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Dong, Q.; An, J.; Song, W.; Guan, Y.; He, F.; Huang, Y.; Hu, J. Regulation of ZnO nanoparticles-induced physiological and molecular changes by seed priming with humic acid in Oryza sativa seedlings. Plant Growth Regul. 2017, 83, 27–41. [Google Scholar] [CrossRef]
- Adetumbi, J.A.; Orimadegun, I.O.; Akinyosoye, S.T.; Akintayo, O.T.; Agbeleye, O.A. Enhancing planting value of rice seed through priming with humic substance. J. Exp. Agric. Int. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Abu-Ria, M.; Shukry, W.; Abo-Hamed, S.; Albaqami, M.; Almuqadam, L.; Ibraheem, F. Humic acid modulates ionic homeostasis, osmolytes content, and antioxidant defense to improve salt tolerance in rice. Plants 2023, 12, 1834. [Google Scholar] [CrossRef]
- Zavarzina, A.G.; Demin, V.V. Acid-base properties of humic acids of different origin as seen from the potentiometric titration data. Euras. Soil Sci. 1999, 32, 1115–1122. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Khabibrakhmanova, V.R.; Rassabina, A.Y.; Khayrullina, A.F.; Minibayeva, F.V. Physico-chemical characteristics and antioxidant properties of melanins extracted from Leptogium furfuraceum (Harm.). Khimija Rastit. Syr’ja (Chem. Plant Raw Mater.) 2022, 4, 115–125. [Google Scholar] [CrossRef]
- Mitelut, A.C.; Popa, M.E. Seed germination bioassay for toxicity evaluation of different composting biodegradable materials. Rom. Biotechnol. Lett. 2011, 16, 121–129. [Google Scholar]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture. 2019, 9, 201. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of malondialdehyde (MDA) by thiobarbituric acid (TBA) assay. In Plant-Microbe Interactions; Springer Protocols Handbooks; Humana: New York, NY, USA, 2021; pp. 103–106. [Google Scholar] [CrossRef]
- Senesi, N.; D’Orazio, V.; Ricca, G. Humic acids in the first generation of EUROSOILS. Geoderma 2003, 116, 325–344. [Google Scholar] [CrossRef]
- Samilova, O.A.; Aizenshtadt, A.M.; Bogolitsin, K.G.; Kosyakov, D.S.; Gorbova, N.S. Acid-base properties of Bjerkman lignin. Lesnoy Zhurnal 2004, 98–105. (In Russian) [Google Scholar]
- Watanabe, A.; McPhail, D.B.; Maie, N.; Kawasaki, S.; Anderson, H.A.; Cheshire, M.V. Electron spin resonance characteristics of humic acids from a wide range of soil types. Org. Geochem. 2005, 36, 981–990. [Google Scholar] [CrossRef]
- Lodygin, E.D.; Beznosikov, V.A.; Chukov, S.N. 2007. Paramagnetic properties of humus acids of podzolic and bog-podzolic soils. Eurasian Soil Sci. 2007, 40, 726–728. [Google Scholar] [CrossRef]
- Lodygin, E.; Abakumov, E. The use of spectroscopic methods to study organic matter in virgin and arable soils: A scoping review. Agronomy 2024, 14, 1003. [Google Scholar] [CrossRef]
- Jezierskit, A.; Czechowski, F.; Jerzykiewicz, M.; Drozd, J. EPR investigations of structure of humic acids from compost, soil, peat and soft brown coal upon oxidation and metal uptake. Appl. Magn. Reson. 2000, 18, 127–136. [Google Scholar] [CrossRef]
- Debska, B.; Spychaj-Fabisiak, E.; Szulc, W.; Gaj, R.; Banach-Szott, M. EPR Spectroscopy as a Tool to Characterize the Maturity Degree of Humic Acids. Materials 2021, 14, 3410. [Google Scholar] [CrossRef]
- Zykova, M.V.; Brazovskii, K.S.; Bratishko, K.A.; Buyko, E.E.; Logvinova, L.A.; Romanenko, S.V.; Konstantinov, A.I.; Krivoshchekov, S.V.; Perminova, I.V.; Belousov, M.V. Quantitative structure-activity relationship, ontology-based model of the antioxidant and cell protective activity of peat humic acids. Polymers 2022, 14, 3293. [Google Scholar] [CrossRef]
- Shukry, W.M.; Abu-Ria, M.E.; Abo-Hamed, S.A.; Anis, G.B.; Ibraheem, F. The efficiency of humic acid for improving salinity tolerance in salt sensitive rice (Oryza sativa): Growth responses and physiological mechanisms. Gesunde Pflanzen. 2023, 75, 2639–2653. [Google Scholar] [CrossRef]
- Poomani, S.; Yadav, S.; Choudhary, R.; Singh, D.; Dahuja, A.; Yadav, S.K. Seed priming with humic acid modifies seedling vigor and biochemical response of lentil under heat stress conditions. Turk. J. Agric. For. 2023, 47, 1043–1057. [Google Scholar] [CrossRef]
- Rao, D.; Yadav, S.; Choudhary, R.; Singh, D.; Bhardwaj, R.; Barthakur, S.; Yadav, S.K. Silicic and humic acid priming improves micro- and macronutrient uptake, salinity stress tolerance, seed quality, and physio-biochemical parameters in lentil (Lens culinaris spp. culinaris). Plants 2023, 12, 3539. [Google Scholar] [CrossRef]
- Forni, C.; Borromeo, I. The utilization of seed priming as a tool to overcome salt and drought stresses: Is still a long way to go? Seeds 2023, 2, 406–420. [Google Scholar] [CrossRef]
- Kaushal, K.; Rajani, K.; Kumar, R.R.; Ranjan, T.; Kumar, A.; Ahmad, M.F.; Kumar, V.; Kumar, V.; Kumar, A. Physio-biochemical responses and crop performance analysis in chickpea upon botanical priming. Sci. Rep. 2024, 14, 9342. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xin, X.; Yin, G.; Zhou, J.; Zhou, Y.; Lu, X. Timing for antioxidant-priming against rice seed ageing: Optimal only in non-resistant stage. Sci. Rep. 2020, 10, 13294. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Corbineau, F.; Taskiran-Özbingöl, N.; El-Maarouf-Bouteau, H. Improvement of seed quality by priming: Concept and biological basis. Seeds 2023, 2, 101–115. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.A.; da S. Irineu, L.E.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 12. [Google Scholar] [CrossRef]


| HA Source | pH | C, % | N, % | CHA/CFA 1 | CHA/Corg 2 |
|---|---|---|---|---|---|
| Compost | 6.8 | 15.0 | 1.6 | 2.2 | 0.27 (medium) |
| Retisol | 4.4 | 5.1 | 0.3 | 0.6 | 0.28 (medium) |
| Chernozem | 5.2 | 4.7 | 0.3 | 2.6 | 0.65 (very high) |
| HA | Ash, % | Content, Mass and Atomic, % | Atomic Ratios | E465 3 | E4/E6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | H:C | O:C | ||||
| HAC-N2 | 1.9 | 1 53.11 ± 0.0 2 40.7 ± 0.2 | 3.6 ± 0.0 33.5 ± 0.0 | 4.9 ± 0.1 3.2 ± 0.4 | 1.7 ± 0.0 0.5 ± 0.0 | 38.5 ± 0.1 22.1 ± 0.2 | 0.8 | 0.5 | 0.03 | 4.7 |
| HAC-O2 | 2.1 | 1 52.8 ± 0.2 2 42.7 ± 0.1 | 3.1 ± 0.1 29.8 ± 0.0 | 4.5 ± 0.1 3.1 ± 0.1 | 1.5 ± 0.0 0.5 ± 0.0 | 40.2 ± 0.1 24.2 ± 0.0 | 0.7 | 0.6 | 0.04 | 5.5 |
| Absorption Maxima, cm−1 | Group and Oscillations |
|---|---|
| 3300 w 1 | O–H stretching, N–H stretching (trace), intermolecular hydrogen bonds |
| 3050 sh 2 | Aromatic C–H stretching |
| 2930 m 3, 2845 sh | C–H stretching in aliphatic CH2, CH3 groups |
| 1710 m | C=O stretching of COOH, aldehydes and ketones |
| 1650 s 4 | C=O stretching of amide groups (amide I), C=O of quinones and/or H–bonded conjugated ketones |
| 1535 w | N–H deformation and C=N stretching (amide II) |
| 1510 w | aromatic C=C stretching |
| 1450 w | C–H in CH2 (or CH3) deformation |
| 1420 m | C=N stretching of primary amides (amide III) |
| 1260 sh, 1220 m | C–O stretching of COOH, O–H deformation of COOH C–O stretching of aryl ethers and phenols |
| 1080, 1030 w | C–O stretching of polysaccharides |
| HA | Distribution of Structural Groups by Spectral Region (ppm), % of the Total Area of NMR Spectrum | Paramagnetic Properties, EPR Data | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Aliphatic Fragments | Sugars | Aromatic Fragments | Carboxyls, Ketones, Quinones | Intensity, Spin g−1 | Line- Width, Gauss | ||||
| CHn 5–48 | CH3–O, N CH2-O 48–92 | OC–O, N 92–110 | Car 110–144 | CarO 144–164 | COO 164–185 | C=O 185–220 | |||
| HAC-N2 | 22.9 | 16.7 | 5.9 | 22.8 | 9.4 | 17.6 | 4.7 | 7.08 × 1016 | 4.02 |
| HAC-O2 | 17.3 | 20.1 | 8.2 | 23.6 | 9.3 | 17.3 | 4.2 | 9.11 × 1016 | 4.87 |
| HA Preparation | Backward Titration (0.1 M HCl), pH Intervals | Forward Titration (0.1 M NaOH), pH Intervals | |||
|---|---|---|---|---|---|
| 10.5–7.7 | 7.7–5.1 | 5.1–3.7 | 3.8–6.4 | 6.4–10.4 | |
| Functional group content, mmol g−1 | |||||
| HAC-N2 | 4.55 ± 0.25 | 1.65 ± 0.34 | 1.55 ± 0.44 | 1.90 ± 0.10 | 10.4 ± 0.10 |
| HAC-O2 | 8.47 ± 0.50 | 6.73 ± 1.15 | 1.30 ± 0.26 | 1.67 ± 0.10 | 10.5 ± 0.10 |
| pKa values | |||||
| HAC-N2 | 9.4 ± 0.0 | 6.4 ± 0.2 | 4.4 ± 0.1 | 4.9 ± 0.1 | 9.6 ± 0.1 |
| HAC-O2 | 9.5 ± 0.3 | 6.5 ± 0.5 | 4.4 ± 0.2 | 5.0 ± 0.1 | 9.7 ± 0.1 |
| Compost | Retisol | Chernozem | |||
|---|---|---|---|---|---|
| HAC-N2 | HAC-O2 | HAR-N2 | HAR-O2 | HACh-N2 | HACh-O2 |
| Antiradical activity (DPPH assay), IC-50 | |||||
| 4.55 ± 0.39 | 3.05 ± 0.03 | 7.33 ± 0.33 | 4.65 ± 0.15 | 3.42 ± 0.05 | 2.73 ± 0.03 |
| Total antioxidant capacity (phosphomolybdenum assay), mg AAE g−1 | |||||
| 166.6 ± 6.5 | 170.4 ± 1.0 | 115.7 ± 0.4 | 139.1 ± 9.4 | 25.5 ± 0.6 | 31.9 ± 1.4 |
| Chelating capacity (ferrous ion chelation), IC-50 | |||||
| 1.00 ± 0.14 | 1.04 ± 0.05 | 1.38 ± 0.08 | 1.40 ± 0.05 | 1.37 ± 0.09 | 1.39 ± 0.02 |
| Variant | Germination, % | Shoots | Roots |
|---|---|---|---|
| Length, % of Control | |||
| Radish | |||
| Control | 93 a 1 | 100 ab | 100 a |
| HAC-N2 | 95 a | 77 a | 88 a |
| HAC-O2 | 93 a | 103 ab | 195 ab |
| HAR-N2 | 100 a | 129 bc | 177 ab |
| HAR-O2 | 100 a | 155 c | 229 b |
| HACh-N2 | 100 a | 109 ab | 93 a |
| HACh-O2 | 100 a | 121 bc | 105 a |
| Wheat | |||
| Control | 100 a | 100 ab | 100 a |
| HAC-N2 | 95 a | 81 a | 111 a |
| HAC-O2 | 99 a | 124 b | 140 a |
| HAR-N2 | 98 a | 96 ab | 99 a |
| HAR-O2 | 100 a | 94 ab | 115 a |
| HACh-N2 | 98 a | 87 a | 102 a |
| HACh-O2 | 95 a | 97 ab | 104 a |
| Variant | Shoots | Roots |
|---|---|---|
| MDA, nmol g−1 | ||
| Radish | ||
| Control | 21.1 ± 1.0 c 1 | 26.2 ± 0.3 b |
| HAR-N2 | 23.7 ± 0.2 d | 26.8 ± 2.3 bc |
| HAR-O2 | 24.2 ± 0.8 de | 31.7 ± 1.2 d |
| HACh-N2 | 24.4 ± 1.0 de | 31.8 ± 0.4 d |
| HACh-O2 | 25.2 ± 1.3 e | 29.1 ± 0.8 c |
| HAC-N2 | 12.4 ± 0.3 a | 18.8 ± 1.3 a |
| HAC-O2 | 17.5 ± 0.1 b | 20.5 ± 1.6 a |
| Wheat | ||
| Control | 33.1 ± 1.3 a | 35.7 ± 1.7 ab |
| HAR-N2 | 41.2 ± 0.1 bc | 45.4 ± 7.6 c |
| HAR-O2 | 41.2 ± 1.9 bc | 43.3 ± 2.4 bc |
| HACh-N2 | 39.1 ± 0.6 b | 39.1 ± 1.1 abc |
| HACh-O2 | 43.2 ± 0.3 c | 34.5 ± 1.8 a |
| HAC-N2 | 40.0 ± 2.1 b | 34.6 ± 0.5 a |
| HAC-O2 | 40.4 ± 1.0 b | 41.2 ± 8.9 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavarzina, A.; Davydova, I.; Kulikova, N.; Nikolaeva, A.; Philippova, O. Alkaline Extraction in Air Enhances Antioxidant and Biological Activities of Humic Acids. Agronomy 2025, 15, 689. https://doi.org/10.3390/agronomy15030689
Zavarzina A, Davydova I, Kulikova N, Nikolaeva A, Philippova O. Alkaline Extraction in Air Enhances Antioxidant and Biological Activities of Humic Acids. Agronomy. 2025; 15(3):689. https://doi.org/10.3390/agronomy15030689
Chicago/Turabian StyleZavarzina, Anna, Irina Davydova, Natalia Kulikova, Anastasiya Nikolaeva, and Olga Philippova. 2025. "Alkaline Extraction in Air Enhances Antioxidant and Biological Activities of Humic Acids" Agronomy 15, no. 3: 689. https://doi.org/10.3390/agronomy15030689
APA StyleZavarzina, A., Davydova, I., Kulikova, N., Nikolaeva, A., & Philippova, O. (2025). Alkaline Extraction in Air Enhances Antioxidant and Biological Activities of Humic Acids. Agronomy, 15(3), 689. https://doi.org/10.3390/agronomy15030689






