Abstract
Plant biostimulants (PBs) have been considered the new wave for ecological intensification and sustainability, but are they sustainable? They increase nutrient use efficiency and reduce the impact of abiotic stress in plants. However, commercially available PBs based on humic substances are obtained using non-renewable sources of organic matter. At the same time, the microbial inoculants include a discussion of the properties of microorganisms and formulation design, as well as standards of purity and process control. Farmers depend on biological inputs like others to generate additional income for agribusiness. We produced a composite PB using humic substances isolated from vermicompost with KOH 5% and microbial consortia of plant growth-promoting bacteria (H. seropedicae, G. diazotrophicus, Bacillus spp.) grown in a simple medium with molasses and fishmeal as sources of C and N, respectively, in a homemade reactor at 37 °C for 36 h. The on-farm PB was applied directly in a passion fruit trial, and plant health and yield were monitored. The plants treated with the PB showed decreased visual symptoms of pests and diseases concurrent to higher activities of the enzymes used to monitor the induction of the plant resistance system (1,3-β glucanase, peroxidase, and phenylalanine ammonia-lyase). Plants treated with the PB yielded more than 50% more passion fruit than the control in soil with natural low fertility, fertilized with vermicompost. It is possible to produce PBs directly on the farm, leveraging locally available resources and simple technologies to sustainably enhance plant health and productivity.
1. Introduction
Industrial agriculture has polluted soils and water, reduced biodiversity, and caused pest outbreaks. Input substitution strategies, such as replacing chemical pesticides with biological inputs instead of synthetic fertilizers, are considered the first step towards agroecological transition [1]. Plant biostimulants (PBs) have been identified as one of the environmentally friendly approaches to regulate/modify physiological processes in plants to stimulate growth, mitigate stress-induced limitations, and increase yield [2]. In addition to some reservations about the environmental and social acceptability of the use of PBs compared to those they replace, they may also perpetuate farmers’ dependence on purchased inputs. The market size for PBs is estimated at USD 1.59 billion in 2024 and is expected to reach USD 2.34 billion by 2029, growing at a CAGR of 8–12% over the period 2024–2029 [3]. PBs have been defined as containing substance(s) and/or microorganisms whose function, when applied to plants or the rhizosphere, is to stimulate natural processes to improve/enhance nutrient uptake, nutrient efficiency, tolerance to abiotic stress, and crop quality [4]. Humic substances (HS) and plant growth-promoting bacteria (PGPB) are among the most common non-microbial and microbial PBs, respectively. HS are one of the most studied categories of PB, which can increase nutrient uptake, and promote primary and secondary plant metabolism [5]. They also help plants cope with stressful conditions by inducing the up-regulation of enzymatic and non-enzymatic antioxidant systems [5]. Non-renewable resources, such as peat and coals including lignite, leonardite [i.e., oxidized lignite], and oxidized sub-bituminous coals are being explored for the production of commercial humic products (HPs) for agricultural use promoting depletion of natural non-renewable resources [6]. Extraction from these sources has a negative impact on ecosystems. The recycling of organic waste as PBs is historically a common practice, and HS obtained from different types of compost, including vermicompost, have high bioactivity [7]. HS extracted from recyclable organic matter can be applied directly to plant surfaces mostly as liquid potassium (K-humate), allowing farmers to produce their own HS-PB.
Microbial inoculants, mainly based on bacteria and fungi, are applied to soil as alternatives to conventional inorganic fertilizers (biofertilizers) or to perform specific functions, including the biocontrol of pests and diseases (biopesticides) or for bioremediation and improvement of soil properties [8]. Plant growth-promoting bacteria (PGPB) are a diverse group of bacteria applied directly to the plant surface that can enhance the growth and yield of many crops as a result of multiple effects on the host, which can be broadly summarized as follows: (i) improving plant nutrient availability and (ii) alleviating both biotic and abiotic stresses on plants [2]. Some experiments have been carried out in which farmers cultivate microbial communities through open fermentation. However, the inoculants market is dominated by large companies. Currently, the leading producers of pesticides and chemical inputs have biostimulant products in their portfolio. This dominance by large companies raises concerns about the appropriation of biological technologies, potentially creating a new era of dependency for farmers. While these companies have the resources to develop and market advanced biostimulant products, their control of the market can limit accessibility and affordability for smallholder farmers. The proprietary nature of these products often leads to a lack of transparency and limited farmer autonomy; farmers become dependent on patented formulations and specific application protocols provided by these companies. As a result, the promise of sustainable agriculture through biostimulants may be undermined by the consolidation of market power in the hands of a few large companies, replicating the dependency problems seen with traditional chemical inputs. This situation highlights the need for policies and initiatives that support the open-source development of PBs and the empowerment of farmers to produce and use their own biological inputs independently.
Previously, we evaluated a composite PB prepared with soluble HS from vermicompost and PGPB consortia grown under laboratory conditions to support agroecological transitions of tropical fruits, including banana, pineapple, papaya, and passion fruit [9]. We observed a benefit of the proposed technology of the combined use of HS and PGPB, but the dependence on the growth of beneficial microbial strains selected in the laboratory in a commercial growth medium under controlled conditions represents a significant barrier to the direct use of this technology in traditional family-based agricultural practices. To address these challenges, we developed a simplified reactor for the growth of bacterial consortia using a simple growth medium. We also constructed an extractor for HS extraction. A field trial was conducted to assess the efficacy of the farm-produced composite PB on a passion fruit crop.
In Brazil, passion fruit is grown on plots rarely larger than 5 hectares. It is a valuable alternative for production and income, especially for small producers who use a short marketing chain. In addition, there is a growing demand for pesticide-free fresh fruit [10]. However, passion fruit is subject to significant phytosanitary problems, including fusariosis (Fusarium oxysporum f.sp. passiflorae—Fop), collar rot (F. solani), anthracnose (Colletotrichum gloeosporioides), and wart disease. The crop is also susceptible to Cladosporium herbarum, bacteriosis (Xanthomonas axonopodis pv. passiflorae), and fruit smut (Cowpea aphid-borne mosaic virus—CABMV) [11]. Viruses and fusariosis are the main causes of significantly reduced productivity and production longevity. The cultivation of passion fruit has become an almost annual event in areas with a high disease incidence. To date, no cultivars have been developed that are resistant to fusariosis and viruses. It is recommended to develop plant nutrition management practices to reduce disease incidence and increase the productivity and longevity of the passion fruit plant [12]. The aim of this study was to evaluate the effect of homemade compost PBs using HS and PGPB on the health and yield of passion fruit.
2. Materials and Methods
2.1. On-Farm Biostimulant Production
2.1.1. Vermicompost Production
The vermicompost was produced in a bedding system using cattle manure and earthworms (E. Andrei). Each bed was filled with 0.3 m3 of cattle manure and 2 kg of adult earthworms (Figure 1a). After 120 days, mature vermicompost was collected, and one compost sample of each bed was analyzed according to the methodology of the Brazilian Ministry of Agriculture, Livestock and Supply [13] and showed the following results: EC: 1.46 mS/cm; pHH2O: 6.87; humidity: 45%; CEC: 350 mmol kg−1; organic carbon: 13.9%; N: 1.17%; C/N ratio: 12; P: 0.20%; K: 0.43%; Ca: 10.4 g kg−1; Mg: 6.7 g kg−1; S: 2.7 g kg−1; B: 6 mg kg−1; Cu: 36 mg kg−1; Mn: 594 mg kg−1; Zn: 77 mg kg−1, and Fe: 5834 mg kg−1.

Figure 1.
(a) Bed system of vermicomposting; (b) on-farm devices for extracting soluble humic substances (arrow 1), and simplified reactor for growth of bacteria consortia (arrow 2).
2.1.2. Humic Substances Extraction
We built an extractor in the farm workshop using an electric motor coupled to a reduction shaft to shake the vermicompost and the solvent in a 100 L stainless steel tank (Figure 1b). A 5% KOH solution was used in the extractor using a 1:20 (v:v) ratio of vermicompost to extractor. The mixture was stirred for four hours and left overnight to decant, and the soluble HS was separated using a tap attached to the extractor. The content of total organic carbon was determined in the laboratory using a TOC analyzer (Shimadzu, Tokyo, Japan), and the alkaline extract was diluted in water to produce a suspension with 60 mg C L−1. The pH of the suspension was not adjusted.
2.1.3. Plant Growth-Promoting Bacteria (PGPB)
Four plant growth-promoting bacteria (PGPB) were used: Herbaspirillum seropedicae strain HRC54, Bacillus safensis strain 77, Bacillus pumilus strain J1.1, and Gluconacetobacter diazotrophicus strain PR2. We built a reactor on the farm for the consortium growth (Figure 1b). The reactor consisted of a 50 L polypropylene tank with a conical bottom and side outlet. It had a heating and temperature control system and a forced air intake. The air was filtered and pumped through a compressor. The growth medium consisted of molasses (40 g/L) (Usina São Luís, Ourinhos, Brazil) and commercial fishmeal (20 g/L) (FAPESA, Anchieta, Brazil) diluted in tap water that was previously boiled. Before adding the inoculum, the medium was heated to 60 °C for 15 min. After lowering the temperature (36–37 °C), 100 mL of each bacterial strain with 1010–1011 cells/mL, assessed by an optical density curve, were added simultaneously and grown for 36 h. After this period, agitation and aeration were stopped, and after 4 h, the side outlet was opened to collect the bacterial consortia. An aliquot of the microbial consortium was taken and sent to the Cell and Tissue Biology Laboratory at UENF for counting, which showed 105 to 106 cells/mL.
2.1.4. Biostimulant Preparation
The microbial growth medium was filtered through a cloth and diluted in the humic suspension at 1:20 (v:v). The concentration of each bacterium in the PB suspension was 5 × 104 to 5 × 105 cells/mL.
2.2. Plant Assay
Hybrid H09–110/111 purple passion fruit plants (P. edulis) developed by The Brazilian Agricultural Research Corporation (EMBRAPA, Cruz das Almas, Brazil) were grown in a plant substrate (1/3 vermiculite, 1/3 earthworm humus, and 1/3 washed sand) under tropical area, natural light, and temperature conditions in Seropédica, Rio de Janeiro State, Brazil. After two months, the seedlings were transferred to field conditions in the Campos dos Goytacazes, Rio de Janeiro State, Brazil. The seedlings were transplanted to the field and placed in an espalier on 6 wires fixed at 2.0 m from the ground. In each line, wooden posts were fixed every 6.0 m. The soil used for planting was classified as Ultisol (US Soil Survey) located in the Lagoa de Cima district of Campos dos Goytacazes, Rio de Janeiro, Brazil (21°46′19” S 41°30′56” W; altitude 14 m) showing the following characteristics: pH = 4.6; C = 10.4 g kg−1; N = 1.1 g kg−1; C: N = 9.54; organic matter (OM) = 20.10 g dm−3; P = 4.45 mg dm−3, Al+3 = 0.10 Cmolc dm−3; H+Al = 3.18 cmolc dm−3; Ca = 0.80 cmolc dm−3; Mg = 1.20 cmolc dm−3; sum of bases (SB) = 2.11 Cmolc dm−3; saturation of bases (V) = 39.89 (%); saturation by Al3+ = 4.52%; and cation exchange capacity (CEC) = 5.29 Cmolc dm−3. All characteristics were measured according to the methodology proposed by EMBRAPA [13]. The experiment was set up in a strip design to avoid contamination of the control by inoculant treatment via foliar spray with three replications, one line per strip, containing three replicates with five plants per parcel, where the three central plants were used for evaluation. Holes (0.40 × 0.40 × 0.40 m) were drilled in rows spaced 3.5 m apart with five plants per plot. The holes were fertilized with 20 L of cattle manure showing 1% N, 0.8% P2O5, 0.4% K2O, 100 g of dolomitic limestone, and 200 g of natural rock phosphate (9% P2O5 soluble in citric acid) one month before transplanting the seedlings. Two treatments were imposed to intercalated lines consisting of foliar pulverization of on-farm inoculant suspension sprayed at a rate equivalent to 400 L/ha with an electric knapsack sprayer or water, used as the control. We used a plastic curtain to protect the adjacent row from spray drift. Cover fertilization was carried out every two months by superficial application of 2.5 L of vermicompost around the plant, providing approximately 150 g N per year plant−1 with four applications. Two-month-old H09–110/11 passion fruit plants were transplanted to the field, and two days after transplant, 2 L of biostimulant produced as described above were applied around the plant. Water was used in the control treatments. Two weeks after the initial treatment, using an electric sprayer, the biostimulant was re-applied once to the drip point on the leaves.
Three central plants of the five-plant plots were marked to monitor the appearance of disease symptoms. Two days after foliar application of the treatment, one leaf from each marked plant was collected for analysis of enzyme activity, and evaluation was carried out on five leaves of the three central plants in the parcel during the initial flowering stage based on an arbitrary severity scale using notes where 5 corresponded to all leaves with presence of pest and disease of fungal (fusariosis, anthracnose), bacterial (Xanthomonas axonopodis pv. Passiflorae), and viral (Cowpea aphid-borne mosaic virus—CABMV) symptoms.
Phenylalanine ammonia-lyase assay PAL: (EC 4.3.1.5): The extraction of the PAL enzyme was carried out following the method described by Pascholati et al. [14], with modifications. With an extracting solution of 0.1 M sodium borate buffer, pH 8.8 (added with 5% w/v of polyvinylpyrrolidone (PVP), and 1.2 mL L−1 of β-mercaptoethanol), 100 mg of leaves were macerated in N2 and homogenized and subsequently centrifuged for 25 min at 12,000 rpm at 4 °C. After centrifugation and separation of the enzymatic extract, the enzymatic reaction was composed of 1 mL of crude enzymatic extract + 1 mL of 0.1 M phenylalanine solution + 1 mL of 0.2 M sodium borate solution (without PVP and β-mercaptoethanol). The samples were incubated in a water bath (36 °C) in the dark for 60 min, after which the reaction was stopped with 6M HCL, and enzyme activity readings were taken on a spectrophotometer at 290 nm in quartz cuvettes. The enzyme was expressed in µmol transcinnamic acid. min−1. g−1 FW.
Peroxidase (POX, E.C.1.11.1.7): Leaf extract: Approximately 1 g of fine powder using N2 was transferred to a 15 mL falcon tube with 1% (v/v) polyvinylpyrrolidone (PVP), 5 mL of sodium acetate buffer (0.1 M, pH 5), and 1 mL of EDTA (1mM). The extracts were centrifuged at 10,000× g for 10 min at 4 °C, and the supernatant was transferred to Eppendorf tubes and stored at −20 °C. The supernatants will evaluate β-1,3-glucanase, POX, and soluble protein content. POX activity was determined at 30 °C according to the method described by Hammerschmidt et al. [15]. The reaction medium was composed of 50 µL of guaiacol (0.02 M), 0.5 mL of hydrogen peroxide (0.38 M), and 2.0 mL of phosphate buffer (0.2 M pH 5.8). After being gently shaken, 50 µL of the enzyme extract was added, gently shaken again, and read at a wavelength of 470 nm. The results are expressed in μmol of H2O2 decomposition. min−1. g−1 FW.
1,3 β-glucanase (E.C.3.2.1.29): The β-1,3 -glucanase activity in the samples was determined by the colorimetric quantification of glucose released from laminarin using p-hydroxybenzoic acid hydrazide (HAPHB) [16]. The reaction consisted of 250 µL of enzymatic extract and 250 µL of laminarin (4.0 mg/mL) incubated at 40 °C for 90 min. After this time, 1.5 mL of p-hydroxybenzoic acid hydrazide (1 g dissolved in 20 mL of 0.5 M HCl plus 80 mL of 0.5 M NaOH) was added and heated at 100 °C for 5 min. Afterward, the reaction was cooled to 30 °C on ice, and the absorbance was determined at 410 nm against the blank (500 uL extraction buffer + p-hydroxybenzoic acid hydrazide heated at 100 °C for 5 min). Finally, each sample was subtracted from the control value (corresponding to a mixture identical to that of the sample but without prior incubation). Absorbance readings were plotted on a standard curve for glucose, and the results were expressed in μg glucose/min/mg protein.
2.2.1. Crop Production
Fruits were collected weekly after the start of production, harvested from the ground, and weighed immediately. The values were accumulated and the sum was divided by three to calculate the average production per plant.
2.2.2. Data Analysis
Treatments were randomized by individual treatment strips within each replication. Therefore, the experimental designs are treatments nested within treatment strips, and R-4.2.0 for Windows was adjusted accordingly to provide the appropriate degrees of freedom for this design. A one-way ANOVA was used to test whether the observed differences in means between the control and biostimulant treatments were statistically significant. The Bartlett and Shapiro–Wilk tests were used to test the homogeneity of variance and normality of the data, respectively. The severity of pest and disease symptoms was analyzed using non-parametric statistics; the Friedman and Mann–Whitney (p < 0.05) tests were used to compare the control vs. inoculated treatments using R programme version 4.2.0 for Windows.
3. Results
After eight months of transplanting, the passionfruit started the flowering and fructification stage and the healthier appearance of the leaves was more noticeable on inoculated plants, with fewer disease symptoms and less insect leaf damage. The scale rating of leaf damage is shown in Figure 2. On an arbitrary rating scale, visual damage to the leaves of pre-marked plants was 2.2 in the inoculated plants and 3.6 in the control treatment (Table 1). This difference was significant by the non-parametric Mann–Whitney test for mean comparison (Table 1). Insect attacks were visibly higher in the control treatments, particularly caterpillars, stink bugs, and grasshoppers.

Figure 2.
Visual rating of passion fruit leaf damage from diseases and insects. 2: Leaf with some damage without chlorosis; 3: virus, bacteria, and fungi presence with increased chlorosis; 4: disease and insect damage; and 5: high incidence of disease and insect damage with all surfaces characterized by chlorosis.

Table 1.
Non-parametric analysis and statistical tests comparing the score of symptoms of pest and disease in passion fruit leaves treated with plant biostimulant (PB) or not (control = CT).
A visual reduction of pest and disease symptoms on the leaves (Table 1) was accompanied by a notable increase in the activity of enzymes utilized to monitor the induction of the plant’s defense system. The one-way ANOVA showed a normal distribution of the data, high homogeneity of the variance, and significance between treatments (Table S1 in the Supplementary File). Two days following the application of the inoculant, the leaves were collected, and the activities of 1,3-β glucanase (GLUC), peroxidase (POX), and phenylalanine ammonia-lyase (PAL) were analyzed. The results are shown in Figure 3. The GLUC activity enhanced 2-fold in inoculated plants, i.e., from 21 to 42 (ng min−1 mg prot−1), while the PAL increased 1.5-fold and POX 1.8-fold compared to the control plants.
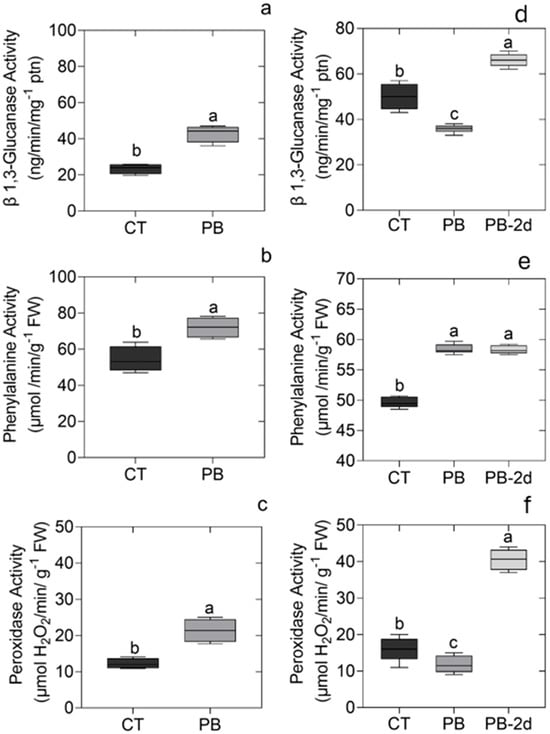
Figure 3.
(a) β 1,3-Glucanase (GLUC), (b) phenylalanine ammonia-lyase (PAL), and (c) peroxidase (POX) activities on leaf extracts in plants treated or not (control = CT) with the composite plant biostimulant (PB) formulated with humic substances and plant growth-promoting bacterial consortia. Leaves were collected two days after inoculation at the time of transplanting. (d) GLUC; (e) PAL; and (f) POX activity six months after the transplantation (control and PB) and two days after the new application of the treatments by foliar spray (PB-2d). Data represents the mean (n = 3 plants per plot; three plots per line and three lines per treatment followed by standard deviation). ANOVA is shown in Table S1 in Supplementary File. Different letters represent significant differences by the LSD test (p < 0.05).
Six months later the PAL activity remained higher in the treated plants in comparison with the control. However, the GLUC and POX activity decreased with time (Figure 3). After two days of re-inoculation of the PB by spray foliar, we observed a significant increase in all activities of the enzymes showing the transient effect of PB to GLUC and POX stimulation.
The yield of passionfruit was significantly larger in the treated plants than in the control (Figure 4). The increase was around 50% (9080 kg ha−1 vs. 13,874 kg ha−1) without changes in fruit characteristics such as brix, total acidity, and juice pH.
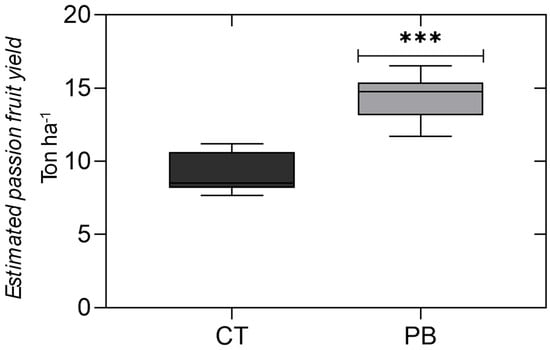
Figure 4.
The estimated passion fruit yield in kilograms per hectare in the control (CT) and plots treated with the plant biostimulant (PB). *** is significant by F test (p < 0.0001). ANOVA is shown in the Table S3 in Supplementary File.
4. Discussion
Input substitution is a key aspect of system transformation. The substitution of an external industrial input for another organic one has a minimal impact on social and economic relations, despite the less negative impact on human health. Technological independence and the use of bioproducts that can be produced locally with renewable sources of humified organic matter and PGPB are important aspects of the small-scale circular economy. Agro-ecological practices are now being used to generate rents for agribusiness linked to international PB markets associated with environmentally friendly products to promote plant growth and increase crop productivity. The current landscape of PB production and application reveals significant inequalities in access to organic technologies, knowledge, and local resources. While large agribusinesses use their resources to dominate the market with advanced, patented PB products, smallholder and traditional farmers often face barriers to adopting these technologies due to cost, accessibility, and knowledge gaps. The estimated cost of inputs (KOH 5%, molasses, fishmeal, electricity, and labor) to produce the PB to be applied to 1 ha (20 L SH, 50 L bacterial consortium), excluding the reactor and extractor devices, is approximately US$ 10.1 per ha. The estimate is based on local prices and then indexed to dollars. The difference in production obtained with the use of PBs of around 4 tonnes ha−1 would generate an additional income of $3900 in direct sales at street markets in the capitals of south-eastern Brazil (Vitória, Rio de Janeiro, Belo Horizonte, and São Paulo), with an average price of $0.985 per kilogram of fresh fruit according to supply centers (Ceasa) from Campinas (São Paulo), Belo Horizonte (Minas Gerais)—São Paulo (São Paulo), January–February 2025. This difference is more than enough to cover the cost of cheap equipment made in non-specialized local workshops.
The sustainability discourse surrounding PBs [17] is not sustainable as most commercial products based on HS are derived from non-renewable sources [6] and often overlook the importance of using locally available resources. Promoting the development and use of PBs made from locally sourced, renewable materials can reduce environmental impacts and increase the resilience of farming communities. Farmers can be encouraged to develop on-farm PBs using locally available organic matter and microbial consortia, thereby reducing dependence on external inputs.
The prevailing view is that traditional farmers’ local preparations of microbial inoculants are considered old technology with questionable bioefficacy. Health issues are often raised while promoting the market for highly selected products that combine elite microbial strains and patented formulations with industrial secrets. The marginalization of traditional farmers’ practices in favor of commercially developed PBs undermines the potential benefits of local knowledge and innovation. Traditional methods of preparing microbial inoculants, while sometimes questioned for their bioefficacy, provide a foundation that could be strengthened with scientific support and research. Farmers’ empowerment through education and extension services can bridge the gap between traditional practices and modern scientific advances, ensuring that local innovations are recognized and improved upon, rather than dismissed. The current state of affairs leads us to consider whether it is possible to produce PBs with high bioactivity in small-scale farming conditions.
Two simple devices were constructed using components typically found in a mechanical workshop (motor, reducer, electrical resistor, and diodes for temperature control) to extract HS and bacterial growth (Figure 1). The growth medium used (4% molasses, 2% fish meal) has been previously studied and has shown to be an effective means of sustaining microbial growth [18]. The inoculum was obtained from the university microbiology laboratory and this is our Achilles heel, as there are several challenges to producing high-quality microbial inoculants on farms, but with the right approach and resources farmers can effectively achieve this goal.
In this study, each bacterial strain was cultured separately in an optimal growth medium under precisely controlled conditions. One possibility is to create a spin-off/start-up at the university to produce the starting inoculum and deliver it to farmers packaged in vermiculite.
It is well known that the higher the soil organic matter content, the healthier the agroecosystem and the higher the plant productivity and crop quality. On the other hand, HS can be an effective alternative for alleviating symptoms and improving tolerance to various plant diseases [19]. Systemic acquired resistance (SAR) and induced systemic resistance (ISR) are two forms of resistance in plants [20]. SAR is induced by pathogen-related proteins (PR proteins) and can alter plant morphology and anatomy using salicylate as a cell signal. Β-1,3 glucanase activity is a marker of PR induction [21], and PAL is associated with phenylpropanoid metabolism and salicylic acid biosynthesis [22]. Schiavon et al. [23] showed that the activity of PAL was significantly higher in maize seedlings treated with HS than in the control, and the increase in PAL activities was followed by an increase in PAL expression in the level of total phenolic acids and flavonoids. Peroxidases are oxidoreductive enzymes that are involved in processes to restore and maintain the functionalities of cell wall polysaccharides such as phenol oxidation, suberization, auxin metabolism, phytoalexin synthesis, cross-linking of cell wall components, and lignification of host plant cell during the defense reaction against pathogens [24]. GLUC, PAL, and POX activities were found at higher levels in treated plants than in the control (Figure 3). The stimulation of GLUC and POX activity by PBs appears to be transient, as evidenced by the observation that six months after application, the activity levels were similar to those found in the control (Figure 3). However, the level of PAL activity remains higher in treated plants than in the control at all assessment times. Activation of the SAR system by composite PBs was previously observed in passion fruit by Santos-Jimenez et al. [25], confirming a transient effect. We did not monitor any markers of the ISR that mobilize signaling pathways involving ethylene and jasmonates [26]. However, we observed less leaf damage from insect attacks in treated plants than in the control. Silva et al. [27] observed that tomato plants treated with humic acids showed higher transcript levels of genes encoding jasmonic acid synthesis (LOX, OPR3) and signaling genes (JAZ and JAR), and the increase in jasmonic acid concentration in plants treated with humic acids was clearly reported by genes fused to a synthetic promoter responsive to jasmonic acid (JERE::GUS).
The biocontrol of plant diseases by microbial inoculants has been widely reported in the scientific literature, especially those related to Bacillus spp. genera. [28]. The actions of PGPB in the biocontrol of plant pathogens include antibiotic production, nutrient competition, parasitism, pathogen toxin inhibition, and induced resistance. Beneduzi et al. [29] found that PGPR can induce ISR as a strategy to improve plant disease resistance. Previous studies have shown that ISR stimulates PGPR via the SA-dependent pathway rather than the JA/ET-dependent pathway [30]. Despite the mechanism induced, the general effect of PGPB on plants includes PGPR and can enhance nutrient availability, including phosphate solubilization and nitrogen (N2) fixation, and synthesis of phytohormones that alter root architecture, resulting in increased root surface area and improved root performance, favoring plant growth. In addition, HS-treated plants exhibit a significant increase in root hair length and density, suggesting that these substances stimulate a ‘nutrient acquisition response’ that promotes nutrient uptake in plants by increasing the absorptive surface area through hormone-like activities [31]. Furthermore, the direct effect of HS on plant nutrition is mediated by modulating the synthesis and functionality of the plasma membrane (PM) H+-ATPase [31]. This enzyme plays a central role in plant growth and mineral nutrition by establishing an electrochemical proton gradient across the PM that modulates the primary active transport by plant cells [32]. Both nitrate and phosphate uptake at the PM occurs by active co-transport, with symport ratios of 2 H+:1 NO3− and 1 H+:1 PO3−, respectively, against the electrochemical gradient. This process is energized by H+-ATPase activity. Numerous studies have been carried out on the effects of HS on PM H+-ATPase activity [31]. It was previously observed that HS promoted significant expression of high-affinity nitrate transporters (PeNRT2.2) and plasma membrane H+-ATPase (PeMHA), thereby improving the nutritional status of passion fruit and increasing the efficiency use of organic fertilizer use [33]. The effect of HS combined with PGPB on plant-rhizosphere ion fluxes and transcription of cell membrane ion transporters showed a high promotion of PB-induced H+ influx [34]. These electrochemical changes have a direct impact on the soil microbiota, which is the main mechanism used by plants to recruit beneficial microorganisms to the rhizosphere. As mentioned above, HS can induce both SAR and ISR in a complex manner, likely including changes in hormonal balance and cell signaling events. However, the eliciting effect of composite PBs also appears to be relatively short-lived, as evidenced by an analysis of transcript levels of the pathogenesis-related protein 3 (PR-3) and LOX genes in passion fruit [25]. These genes are involved in both SAR and ISR [35,36]. After two days of HS foliar spraying, a significant increase in transcript levels of both genes was observed. Six weeks later, a decrease was observed. This behavior was also observed in the present work, showing that it is necessary to maintain the induction of plant resistance systems in order to repeat the application of PBs in plants with cycles longer than six months. The composite PB application promoted a passion fruit yield of more than 50% greater than that of the control plants (Figure 4). The productivity level was 10% lower than that achieved with industrial agriculture (irrigation, chemical fertilizers, and pesticides) in Brazil, estimated at 15,300 kg ha−1 [37]. In addition, the market analysis showed that the public is willing to pay a small premium for food that is perceived as healthier, such as that grown without pesticides [38], thus increasing economic return.
We have demonstrated that farmers can produce effective microbial inoculants on-farm using simple mechanical equipment, effective growth media, access to high-quality inoculum, and rigorous quality control. Establishing a university spin-off to supply the initial inoculum can bridge the gap between academic research and practical agricultural application, enabling farmers to adopt sustainable and productive farming practices.
To promote a truly sustainable agricultural system, it is essential to democratize access to biological technologies, strengthen the knowledge base of smallholder farmers, and promote local resource use. This approach increases the bio-efficiency of on-farm PB production and promotes economic independence and environmental stewardship. Supporting smallholder farmers to produce high-quality, bioactive PBs within their local conditions can create a more resilient and equitable agricultural ecosystem, in line with the true principles of sustainability.
5. Conclusions
Plant biostimulants can improve agronomic outcomes by enhancing nutrient use efficiency and the adaptive response to biotic and abiotic stresses. In this case study with passion fruit, we observed a significant increase in crop yield with a decrease in pest and disease symptoms, likely due to the promotion of SAR as revealed by the enhancement of transcription levels of GLU, POX, and PAL. Most of the humic-based biostimulants available on the market come from non-renewable sources of organic matter while growth-promoting bacteria are sold separately. It is possible to obtain bioactive HS from vermicompost and to grow an efficient microbial consortium on the farm where the passion fruit is produced. Technological independence is as important as ensuring production conditions for future generations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15030681/s1, Table S1. Analysis of variance (ANOVA) of passion fruit enzymatic activity after transplanting. Table S2. Analysis of variance (ANOVA) of passion fruit enzymatic activity six months after transplanting. Table S3. Analysis of variance (ANOVA) of passion fruit yield (kg/ha).
Author Contributions
Conceptualization, L.P.C. and F.L.O.; methodology, N.A.C., D.M.-B. and R.M.S.; investigation, R.C.C.R.; resources, L.P.C., F.L.O. and R.C.C.R.; writing—original draft preparation, L.P.C.; writing—review and editing, F.L.O.; project administration and funding acquisition, L.P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) grant E-26/210.097/2022.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Acknowledgments
Thanks to Rich Lamar of huma.us for suggestions on the text.
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Glissman, S.R. The Ecology of Sustainable Food Systems, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 405. [Google Scholar]
- Li, J.; Van Gerrewey, T.; Geelen, D.A. Meta-Analysis of biostimulant yield effectiveness in field trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef] [PubMed]
- EBIC. Economic Overview of the European Biostimulants Market. 2024. Available online: https://biostimulants.eu/highlights/economic-overview-of-the-european-biostimulants-market/ (accessed on 21 July 2024).
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Lamar, R.T.; Gralian, J.; Hockaday, W.C.; Jerzykiewicz, M.; Monda, H. Investigation into the role of carboxylic acid and phenolic hydroxyl groups in the plant biostimulant activity of a humic acid purified from an oxidized sub-bituminous coal. Front. Plant Sci. 2024, 15, 1328006. [Google Scholar] [CrossRef]
- Scotti, R.; Pane, C.; Spaccini, R.; Palese, A.M.; Piccolo, A.; Celano, G.; Zaccardelli, M. On-farm compost: A useful tool to improve soil quality under intensive farming systems. Appl. Soil Ecol. 2016, 107, 13–23. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Canellas, N.A.; Jindo, K.; Rosa, R.C.C.; Piccolo, A. Challenge of transition: The history of a case study involving tropical fruits polyculture stimulated by humic acids and plant-growth promoting bacteria. Chem. Biol. Technol. Agric. 2022, 9, 76. [Google Scholar] [CrossRef]
- van der Ploeg, J.A. O Sistema Alimentar em Tempos de COVID-19: Ensinamentos Para o Futuro CADERNOS PARA DEBATE n. 3. Available online: https://outraspalavras.net/wp-content/uploads/2021/10/211001-VanderPloegCriseAgricola.pdf (accessed on 7 September 2022).
- Santos Filho, H.P.; Laranjeira, F.F.; Santos, C.C.F.; Barbosa, C.J. Doenças do maracujazeiro. In Maracujá: Produção e Qualidade na Passicultura; Lima, A.A., Cunha, M.A.P., Eds.; Embrapa Mandioca e Fruticultura: Cruz das Almas, BA, Brazil, 2004; pp. 240–280. [Google Scholar]
- de Jesus, O.N. (Ed.) Plano Estratégico para a Cultura do Maracujá 2017–2021 Documentos 231; Embrapa Mandioca e Fruticultura: Cruz das Almas, BA, Brazil, 2019; 28p. [Google Scholar]
- Embrapa. Manual de Métodos de Análise de Solo; CNPS: Rio de Janeiro, RJ, Brazil, 1997. [Google Scholar]
- Pascholati, S.F.; Nicholson, R.L.; Butler, L.G. Phenylalanine Ammonia-Lyase Activity and Anthocyanin Accumulation in Wounded Maize Mesocotyls. J. Phytopathol. 1986, 115, 165–172. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kué, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Lever, M. New reactions for colorimetric determination of carbohydrates. Anal. Biochem. 1972, 47, 273–279. [Google Scholar] [CrossRef]
- Hafez, M.; El-Nile, A.; Hassan, M.I.; Zeitar, E.M.; Mohammed, S.R.; Abdallah, A.M.; Zohir, W.A.; Popov, A.I.; Minkina, T.; Rashad, M. Humic Substances and Their Potential to Enhance Soil, Plants, and Animals’ Productivity: A New Concept for Sustainable Agriculture. Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Publisher: New York, NY, USA, 2023; Volume 44, Chapter 3. [Google Scholar]
- Hung, S.H.W.; Huang, T.C.; Lai, Y.C.; Wu, I.C.; Liu, C.H.; Huarng, Y.F.; Huang, C.C. Endophytic biostimulants for smart agriculture: Burkholderia seminalis 869T2 benefits heading leafy vegetables in-field management in Taiwan. Agronomy 2023, 13, 967. [Google Scholar] [CrossRef]
- Silva, R.M.; Canellas, L.P. Organic matter in the pest and plant disease control: A meta-analysis. Chem. Biol. Technol. Agric. 2022, 9, 70. [Google Scholar]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging roles of β-Glucanases in plant development and adaptative responses. Plants 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.; Vaccaro, S.; Francioso, O.; Nardi, S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef]
- Appua, M.; Ramalingamb, P.; Sathiyanarayananc, A.; Huanga, J. An overview of plant defense-related enzymes responses to biotic stresses. Plant Gene 2021, 27, 100302. [Google Scholar] [CrossRef]
- Santos-Jiménez, J.L.; Montebianco, C.B.; Olivares, F.L.; Caanellas, L.P.; Barreto-Bergter, E.; Rosa, R.C.C.; Vaslin, M.F.S. Passion fruit plants treated with biostimulants induce defense-related and phytohormone-associated genes. Plant Gene 2022, 30, 100357. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van Loon, L.C. Salicylic acid-independent plant defense pathways. Trends Plant Sci. 1999, 4, 52–58. [Google Scholar] [CrossRef]
- Silva, R.M.; Canellas, N.A.; Olivares, F.L.; Piccolo, A.; Canellas, L.P. Humic substances trigger plant immune responses. Chem. Biol. Technol. Agric. 2023, 10, 123. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant growth promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Takishita, Y.; Charron, J.B.; Smith, D.L. Biocontrol rhizobacterium Pseudomona sp. 23s induces systemic resistance in tomato (Solanum lycopersicum L.) against bacterial canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 2119. [Google Scholar] [CrossRef]
- Nardi, S.; Carletti, P.; Pizzeghello, D.; Muscolo, A. Biological activities of humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems, Part 1: Fundamentals and Impact of Mineral-Organic Biota Interactions on the Formation, Transformation, Turnover, and Storage of Natural Nonliving Organic Matter (NOM); Senesi, N., Xing, B., Huang, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2009; Volume 2, pp. 305–339. [Google Scholar]
- Quaggiotti, S.; Ruperti, B.; Pizzeghello, D.; Francioso, O.; Tugnoli, V.; Nardi, S. Effect of low molecular size humic substances on nitrate uptake and expression of genes involved in nitrate transport in maize [Zea mays L.]. J. Exp. Bot. 2004, 55, 803–813. [Google Scholar] [CrossRef]
- Cavalcanti Filho, P.F.M.; Baía, D.C.; Ribeiro, R.C.; Rosa, R.C.C.; Canellas, L.P. Humic acids induce the expression of nitrate transporters in passion-fruit seedlings. Rev. Bras. Frut. 2023, 45, e-941. [Google Scholar] [CrossRef]
- Azevedo, I.G.; Olivares, F.L.O.; Ramos, A.C.R.; Bertolazi, A.A.; Canellas, L.P. Humic acids and Herbaspirillum seropedicae change the extracellular H+ flux and gene expression in maize roots seedlings. Chem. Biol. Technol. Agric. 2019, 6, 8. [Google Scholar] [CrossRef]
- Zribi, I.; Ghorbel, M.; Brini, F. Pathogenesis Related Proteins (PRs): From Cellular Mechanisms to Plant Defense. Curr. Protein Pept. Sci. 2021, 22, 396–412. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- EMBRAPA. Embrapa Mandioca e Fruticultura. 2022. Available online: https://www.embrapa.br/mandioca-e-fruticultura/cultivos/maracuja (accessed on 10 March 2025).
- SEBRAE. O Mercado Para os Produtos Orgânicos Está Aquecido. Available online: https://sebrae.com.br/sites/PortalSebrae/artigos/o-mercado-para-os-produtos-organicos-esta-aquecido,5f48897d3f94e410VgnVCM1000003b74010aRCRD (accessed on 17 February 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).