Molecular Characterization of an EMS-Induced Ab-γg-Rich Saponin Mutant in Soybean (Glycine max (L.) Merr.)
Abstract
1. Introduction
2. Materials and Methods
2.1. EMS-Induced Mutant Population
2.2. Saponin Composition Determination in F2 Seeds by Thin-Layer Chromatography
2.3. Saponin Composition Quantification in the Two Parental Lines by LC-PDA-MS/MS
2.4. Mapping Analysis and Whole-Genome Resequencing
2.5. Sg-3 Gene Sequencing and Genotyping
2.6. Development of an Sg-3b Allele Genotyping Assay
2.7. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.8. Statistical Analysis and cis-Elements Analysis
3. Results
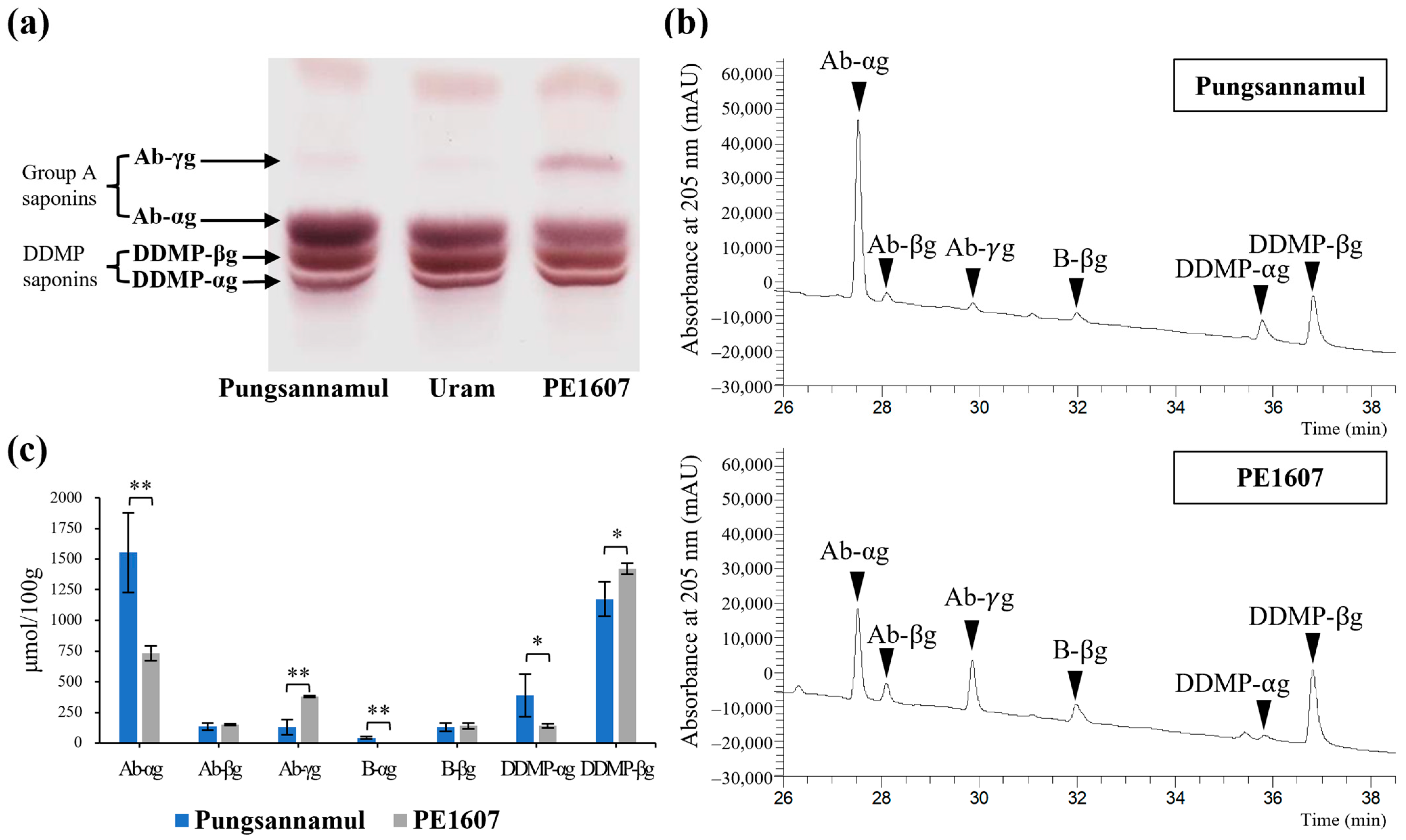
3.1. Isolation of an Ab-γg Saponin Rich Mutant Line
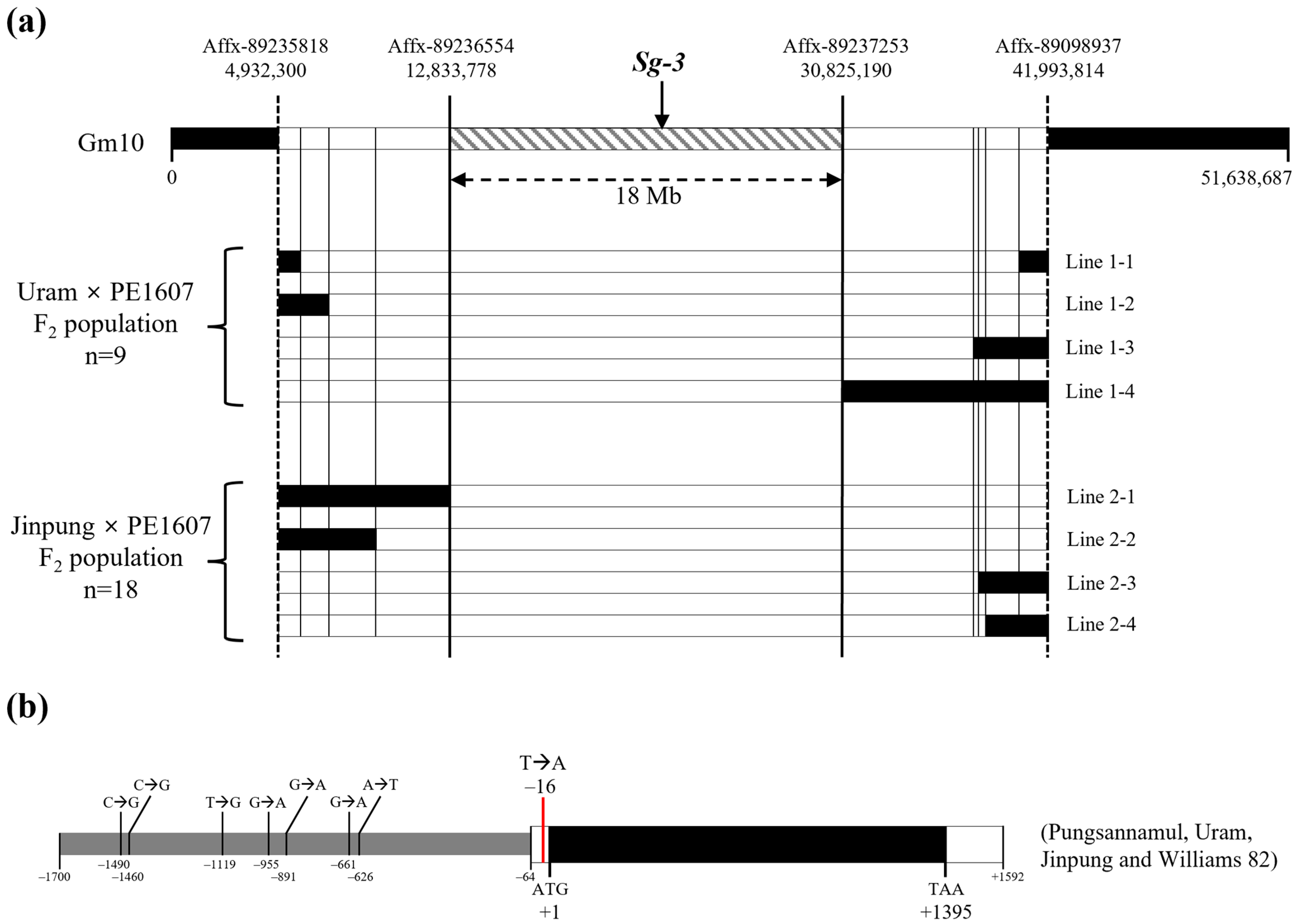
3.2. Mapping Analysis of the PE1607 Mutant
3.3. Segregation of the Ab-γg Saponin-Rich Phenotype Among F2 Seeds of Two Segregating Populations
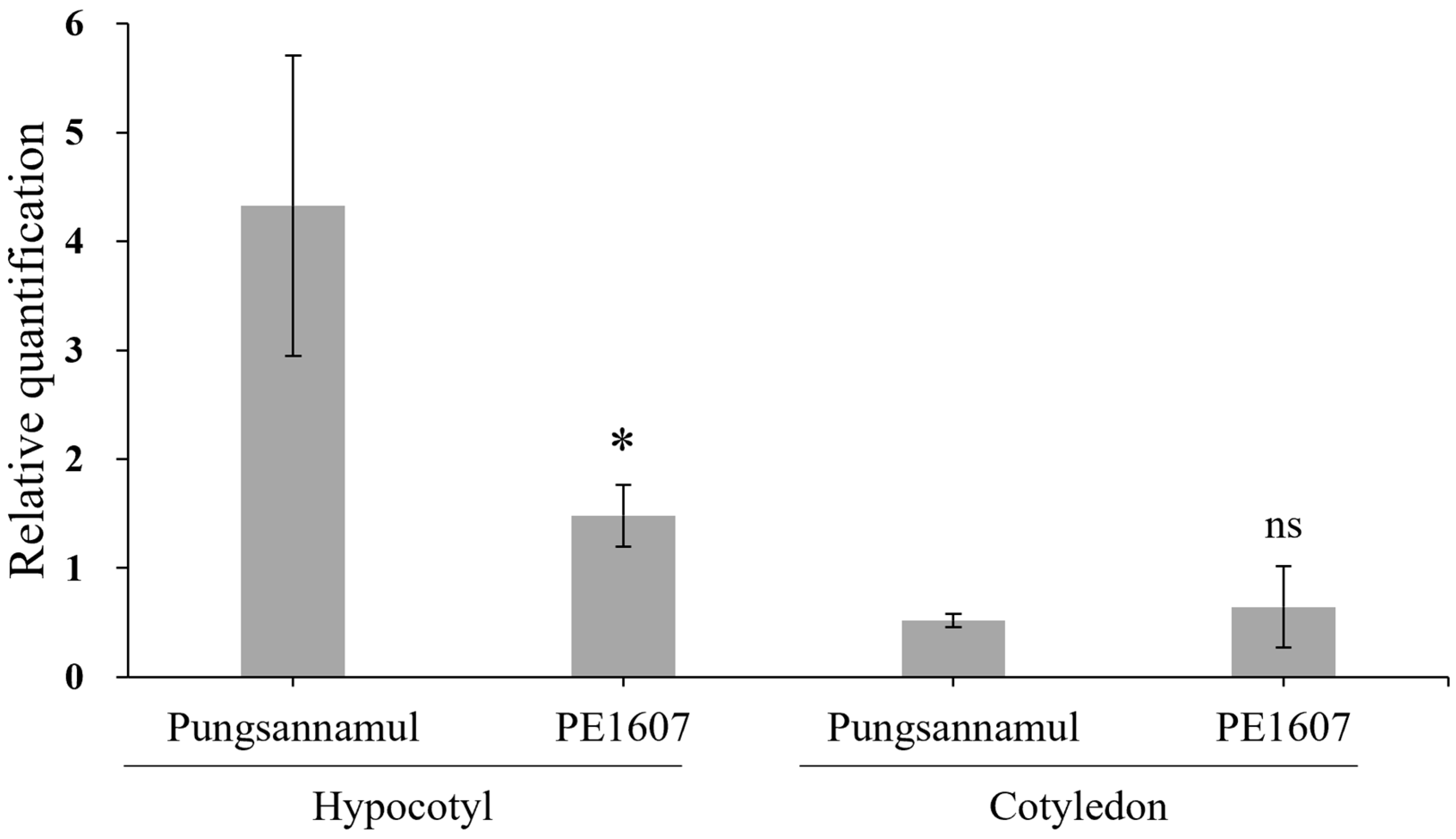
3.4. Molecular Analysis of the Sg-3 Mutant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Riaz, M.N. Soy Applications in Food; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Sugano, M. Soy in Health and Disease Prevention; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Rotundo, J.L.; Marshall, R.; McCormick, R.; Truong, S.K.; Styles, D.; Gerde, J.A.; Gonzalez-Escobar, E.; Carmo-Silva, E.; Janes-Bassett, V.; Logue, J. European soybean to benefit people and the environment. Sci. Rep. 2024, 14, 7612. [Google Scholar] [CrossRef] [PubMed]
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current knowledge in soybean composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Harada, K.; Okubo, K. Composition and content of saponins in soybean seed according to variety, cultivation year and maturity. Agric. Biol. Chem. 1991, 55, 323–331. [Google Scholar]
- Kim, S.-L.; Berhow, M.A.; Kim, J.-T.; Chi, H.-Y.; Lee, S.-J.; Chung, I.-M. Evaluation of soyasaponin, isoflavone, protein, lipid, and free sugar accumulation in developing soybean seeds. J. Agric. Food Chem. 2006, 54, 10003–10010. [Google Scholar] [CrossRef]
- Shimoyamada, M.; Harada, K.; Okubo, K. Saponin composition in developing soybean seed (Glycine max (L.) Merrill, cv. Mikuriyaao). Agric. Biol. Chem. 1991, 55, 1403–1405. [Google Scholar]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef]
- Yano, R.; Takagi, K.; Takada, Y.; Mukaiyama, K.; Tsukamoto, C.; Sayama, T.; Kaga, A.; Anai, T.; Sawai, S.; Ohyama, K. Metabolic switching of astringent and beneficial triterpenoid saponins in soybean is achieved by a loss-of-function mutation in cytochrome P450 72A69. Plant J. 2017, 89, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, J.; Park, G.T.; Komagamine, K.; Tsukamoto, C.; Chang, J.H.; Lee, J.D.; Kim, J.H.; Seo, H.S.; Song, J.T. Biosynthesis of DDMP saponins in soybean is regulated by a distinct UDP-glycosyltransferase. New Phytol. 2019, 222, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Sawai, S.; Saito, K. Triterpenoid biosynthesis and engineering in plants. Front. Plant Sci. 2011, 2, 25. [Google Scholar] [CrossRef]
- Yoshiki, Y.; Kudou, S.; OKuBo, K. Relationship between chemical structures and biological activities of triterpenoid saponins from soybean. Biosci. Biotechnol. Biochem. 1998, 62, 2291–2299. [Google Scholar] [CrossRef]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological functionality of soyasaponins and soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef]
- Okubo, K.; Iijima, M.; Kobayashi, Y.; Yoshikoshi, M.; Uchida, T.; Kudou, S. Components responsible for the undesirable taste of soybean seeds. Biosci. Biotechnol. Biochem. 1992, 56, 99–103. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Jackson, C.-J.C.; Poysa, V.; Di Berardo, C.; Bewley, J.D.; Jenkinson, J. Soyasapogenol A and B distribution in soybean (Glycine max L. Merr.) in relation to seed physiology, genetic variability, and growing location. J. Agric. Food Chem. 2003, 51, 5888–5894. [Google Scholar] [CrossRef]
- Nakashima, H.; Okubo, K.; Honda, Y.; Tamura, T.; Matsuda, S.; Yamamoto, N. Inhibitory effect of glycosides like saponin from soybean on the infectivity of HIV in vitro. Aids 1989, 3, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, H.; Hiraoka, N.; Ikeshiro, Y. Inhibitory activity of soyasaponin II on virus replication in vitro. Planta Medica 1997, 63, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, J.; Yokomizo, K.; Hirakawa, T.; SHII, Y.; NOHARA, T.; UYEDA, M. Anti-herpes virus activity of fabaceous triterpenoidal saponins. Biol. Pharm. Bull. 2000, 23, 887–889. [Google Scholar] [CrossRef]
- Ikeda, T.; Yokomizo, K.; Okawa, M.; Tsuchihashi, R.; Kinjo, J.; Nohara, T.; Uyeda, M. Anti-herpes virus type 1 activity of oleanane-type triterpenoids. Biol. Pharm. Bull. 2005, 28, 1779–1781. [Google Scholar] [CrossRef]
- Ellington, A.A.; Berhow, M.; Singletary, K.W. Induction of macroautophagy in human colon cancer cells by soybean B-group triterpenoid saponins. Carcinogenesis 2005, 26, 159–167. [Google Scholar] [CrossRef]
- Ellington, A.A.; Berhow, M.A.; Singletary, K.W. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis 2006, 27, 298–306. [Google Scholar] [CrossRef]
- Shibuya, M.; Nishimura, K.; Yasuyama, N.; Ebizuka, Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max. FEBS Lett. 2010, 584, 2258–2264. [Google Scholar] [CrossRef]
- Sayama, T.; Ono, E.; Takagi, K.; Takada, Y.; Horikawa, M.; Nakamoto, Y.; Hirose, A.; Sasama, H.; Ohashi, M.; Hasegawa, H. The Sg-1 glycosyltransferase locus regulates structural diversity of triterpenoid saponins of soybean. Plant Cell 2012, 24, 2123–2138. [Google Scholar] [CrossRef]
- Takagi, K.; Yano, R.; Tochigi, S.; Fujisawa, Y.; Tsuchinaga, H.; Takahashi, Y.; Takada, Y.; Kaga, A.; Anai, T.; Tsukamoto, C. Genetic and functional characterization of Sg-4 glycosyltransferase involved in the formation of sugar chain structure at the C-3 position of soybean saponins. Phytochemistry 2018, 156, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Yano, R.; Takagi, K.; Tochigi, S.; Fujisawa, Y.; Nomura, Y.; Tsuchinaga, H.; Takahashi, Y.; Takada, Y.; Kaga, A.; Anai, T. Isolation and characterization of the soybean Sg-3 gene that is involved in genetic variation in sugar chain composition at the C-3 position in soyasaponins. Plant Cell Physiol. 2018, 59, 797–810. [Google Scholar] [CrossRef]
- Takada, Y.; Tayama, I.; Sayama, T.; Sasama, H.; Saruta, M.; Kikuchi, A.; Ishimoto, M.; Tsukamoto, C. Genetic analysis of variations in the sugar chain composition at the C-3 position of soybean seed saponins. Breed. Sci. 2012, 61, 639–645. [Google Scholar] [CrossRef]
- Ko, J.; Sundaramoorthy, J.; Tsukamoto, C.; Park, J.; Lee, J.; Jo, H.; Lee, J.-D.; Seo, H.S.; Song, J.T. Genetic and Molecular Characterization of a New EMS-Induced Mutant without the Third Glucose Moiety at the C-3 Sugar Chain of Saponin in Glycine max (L.) Merr. Agronomy 2022, 12, 1598. [Google Scholar] [CrossRef]
- Chae, J.-H.; Dhakal, K.H.; Asekova, S.; Song, J.T.; Lee, J.-D. Variation of fatty acid composition in soybean’Pungsannamul’mutation population from EMS treatment. Curr. Res. Agric. Life Sci. 2013, 31, 45–50. [Google Scholar]
- Suh SugKee, S.S.; Kim HagSin, K.H.; Oh YoungJin, O.Y.; Kim KyungHo, K.K.; Cho SangKyun, C.S.; Kim YoungJin, K.Y.; Kim SooDong, K.S.; Park HoKi, P.H.; Park MoonSoo, P.M.; Cho SooYeon, C.S. A new soyabean variety for sprouts with small seeds and high yields, “Pungsan-namulkong”. RDA J. Crop Sci. 1997, 39, 120–124. [Google Scholar]
- Lee, C.; Choi, M.-S.; Kim, H.-T.; Yun, H.-T.; Lee, B.; Chung, Y.-S.; Kim, R.W.; Choi, H.-K. Soybean [Glycine max (L.) Merrill]: Importance as a crop and pedigree reconstruction of Korean varieties. Plant Breed. Biotechnol. 2015, 3, 179–196. [Google Scholar] [CrossRef]
- Jong Min, K.; Won Young, H.; Hyun Tae, K.; Young Hoon, L.; Man Soo, C.; Byong Won, L.; Sang Uk, S.; Jeong Hyun, S.; Ki Won, O.; Hong Tae, Y.; et al. Soybean cultivar for soy-paste, ‘Uram’ with mechanization harvesting, large seed, disease resistance and high yield. Korean J. Breed. Sci. 2016, 48, 301–306. [Google Scholar] [CrossRef]
- Takada, Y.; Sasama, H.; Sayama, T.; Kikuchi, A.; Kato, S.; Ishimoto, M.; Tsukamoto, C. Genetic and chemical analysis of a key biosynthetic step for soyasapogenol A, an aglycone of group A saponins that influence soymilk flavor. Theor. Appl. Genet. 2013, 126, 721–731. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Lee, Y.G.; Jeong, N.; Kim, J.H.; Lee, K.; Kim, K.H.; Pirani, A.; Ha, B.K.; Kang, S.T.; Park, B.S.; Moon, J.K. Development, validation and genetic analysis of a large soybean SNP genotyping array. Plant J. 2015, 81, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Subgroup, G.P.D.P. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Libault, M.; Thibivilliers, S.; Bilgin, D.; Radwan, O.; Benitez, M.; Clough, S.; Stacey, G. Identification of four soybean reference genes for gene expression normalization. Plant Genome 2008, 1, 44–54. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Sundaramoorthy, J.; Palaniswamy, S.; Park, G.T.; Son, H.R.; Tsukamoto, C.; Lee, J.-D.; Kim, J.H.; Seo, H.S.; Song, J.T. Characterization of a new sg-5 variant with reduced biosynthesis of group A saponins in soybean (Glycine max (L.) Merr.). Mol. Breed. 2019, 39, 144. [Google Scholar] [CrossRef]
- Park, C.W.; Kulkarni, K.P.; Kim, M.; Mukaiyama, K.; Tsukamoto, C.; Chung, G.; Song, J.T.; Lee, J.-D. Characterization of an EMS-induced soybean mutant with an increased content of Af saponin and a new component Ab-δ in the seed hypocotyl. Euphytica 2018, 214, 163. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Fujisawa, Y.; Takahashi, Y.; Abe, H.; Yamane, K.; Mukaiyama, K.; Son, H.-R.; Hiraga, S.; Kaga, A.; Anai, T. High-throughput screening and characterization of a high-density soybean mutant library elucidate the biosynthesis pathway of triterpenoid saponins. Plant Cell Physiol. 2019, 60, 1082–1097. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, S.H.; Choi, S.-B.; Won, S.-K.; Heo, Y.-K.; Cho, M.; Park, Y.-I.; Cho, H.-T. Functional conservation of a root hair cell-specific cis-element in angiosperms with different root hair distribution patterns. Plant Cell 2006, 18, 2958–2970. [Google Scholar] [CrossRef]
- Loke, J.C.; Stahlberg, E.A.; Strenski, D.G.; Haas, B.J.; Wood, P.C.; Li, Q.Q. Compilation of mRNA polyadenylation signals in Arabidopsis revealed a new signal element and potential secondary structures. Plant Physiol. 2005, 138, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.D.; Kumagai, M.H.; Majumdar, A.; Huang, N.; Sutliff, T.D.; Rodriguez, R.L. The α-amylase genes in Oryza sativa: Characterization of cDNA clones and mRNA expression during seed germination. Mol. Gen. Genet. MGG 1990, 221, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Guhaniyogi, J.; Brewer, G. Regulation of mRNA stability in mammalian cells. Gene 2001, 265, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, N.J.; Furger, A.; Dye, M.J. Integrating mRNA processing with transcription. Cell 2002, 108, 501–512. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Kenigsbuch, D.; Sun, L.; Harel, E.; Ong, M.S.; Tobin, E.M. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell 1997, 9, 491–507. [Google Scholar]
- Sakai, H.; Honma, T.; Aoyama, T.; Sato, S.; Kato, T.; Tabata, S.; Oka, A. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 2001, 294, 1519–1521. [Google Scholar] [CrossRef]
- Mangas, S.; Bonfill, M.; Osuna, L.; Moyano, E.; Tortoriello, J.; Cusido, R.M.; Piñol, M.T.; Palazón, J. The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry 2006, 67, 2041–2049. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wu, X.; Liu, D.; Li, J.; Li, J.; Liu, S.; Gao, W. Jasmonic acid and methyl dihydrojasmonate enhance saponin biosynthesis as well as expression of functional genes in adventitious roots of Panax notoginseng FH Chen. Biotechnol. Appl. Biochem. 2017, 64, 225–238. [Google Scholar] [CrossRef]
| Parents and Their Progenies | Saponin Phenotype | Observed | (Expected) | χ2 Value | Probability | Saponin Phenotype | Observed | (Expected) | χ2 Value | Probability |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross 1 | ||||||||||
| P1: Uram | Wild type | Wild type | ||||||||
| P2: PE1607 | Ab-γg rich | Ab-γg rich | ||||||||
| F2 population | Wild type | 107 | (110) | 0.16 | 0.68 ns * | Wild type | 34 | (36) | 0.18 | 0.91 ns |
| Heterozygous | 73 | (74) | ||||||||
| Ab-γg rich | 39 | (36) | Ab-γg rich | 39 | (36) | |||||
| Cross 2 | ||||||||||
| P1: Jinpung | Wild type | Wild type | ||||||||
| P2: PE1607 | Ab-γg rich | Ab-γg rich | ||||||||
| F2 population | Wild type | 56 | (56) | 0 | 1 ns | Wild type | 19 | (18) | 0.04 | 0.98 ns |
| Heterozygous | 37 | (38) | ||||||||
| Ab-γg rich | 18 | (18) | Ab-γg rich | 18 | (18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Son, H.; Jo, H.; Tsukamoto, C.; Lee, J.; Lee, J.-D.; Seo, H.S.; Song, J.T. Molecular Characterization of an EMS-Induced Ab-γg-Rich Saponin Mutant in Soybean (Glycine max (L.) Merr.). Agronomy 2025, 15, 648. https://doi.org/10.3390/agronomy15030648
Park J, Son H, Jo H, Tsukamoto C, Lee J, Lee J-D, Seo HS, Song JT. Molecular Characterization of an EMS-Induced Ab-γg-Rich Saponin Mutant in Soybean (Glycine max (L.) Merr.). Agronomy. 2025; 15(3):648. https://doi.org/10.3390/agronomy15030648
Chicago/Turabian StylePark, Junbeom, Haereon Son, Hyun Jo, Chigen Tsukamoto, Jinwon Lee, Jeong-Dong Lee, Hak Soo Seo, and Jong Tae Song. 2025. "Molecular Characterization of an EMS-Induced Ab-γg-Rich Saponin Mutant in Soybean (Glycine max (L.) Merr.)" Agronomy 15, no. 3: 648. https://doi.org/10.3390/agronomy15030648
APA StylePark, J., Son, H., Jo, H., Tsukamoto, C., Lee, J., Lee, J.-D., Seo, H. S., & Song, J. T. (2025). Molecular Characterization of an EMS-Induced Ab-γg-Rich Saponin Mutant in Soybean (Glycine max (L.) Merr.). Agronomy, 15(3), 648. https://doi.org/10.3390/agronomy15030648











