Effects of Different Biological Amendments on Rice Physiology, Yield, Quality, and Soil Microbial Community of Rice–Crab Co-Culture in Saline–Alkali Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Experimental Implementation
2.4. Observation Items and Methods
2.4.1. Soil Sampling and Analysis
2.4.2. Measurements of Growth, Photosynthesis, Yield, and Quality
2.4.3. Microbial Community Diversity Analysis
2.4.4. Statistical Analysis and Data Processing
3. Results and Analysis
3.1. Effects of Biological Amendments on Chlorophyll and Photosynthesis
3.2. Effects of Biological Amendments on Yield, Quality, and Water Use Efficiency
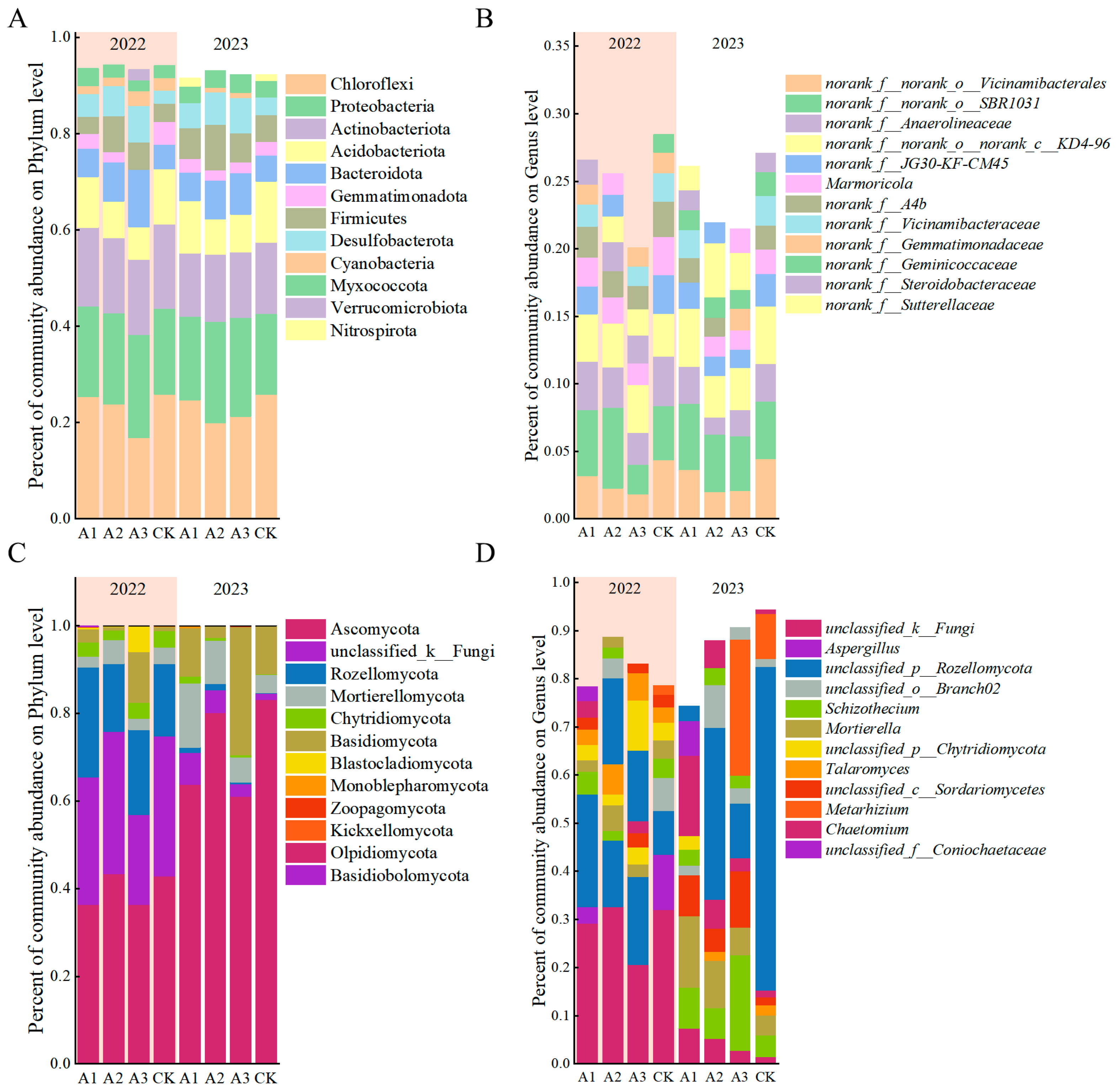
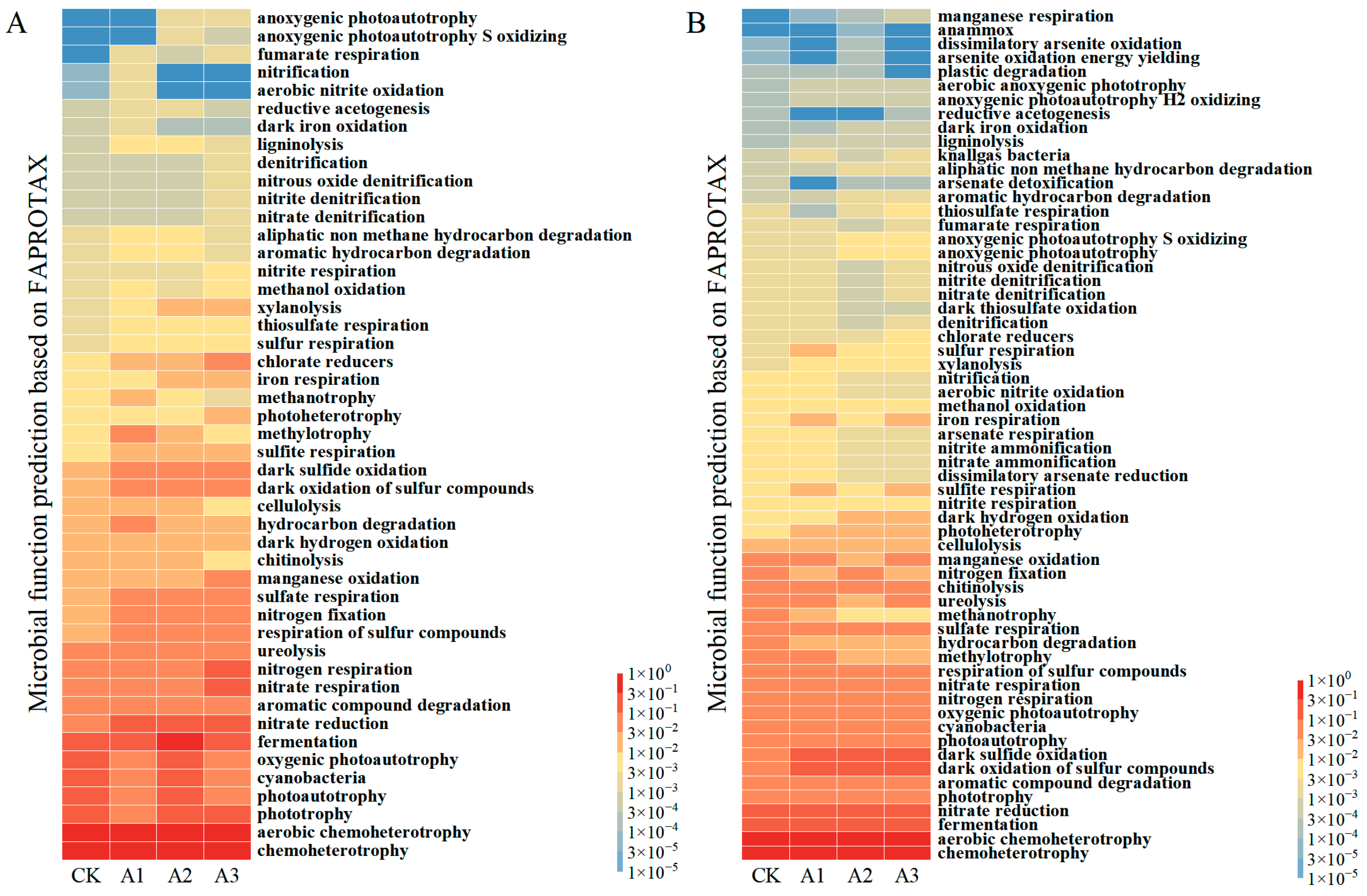
3.3. Effects of Biological Amendments on Microbial Community
4. Discussion
4.1. Response of Microbial Inoculants to Rice Physiology, Yield, Quality, and Soil Microorganisms
4.2. Differences in the Response of Biological Amendments to Rice Physiology, Yield, Quality, and Soil Microorganisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.J.; Guo, K.; Feng, X.H.; Sun, H.Y. Discussion on the agricultural efficient utilization of saline-alkali land resources. Chin. J. Eco-Agric. 2023, 31, 345–353. [Google Scholar]
- Liu, S.Y.; Yang, L.B.; Duan, D.X.; Gu, H.L.; Zhang, J.L. Using integrated fish farming to reclaim low-lying, saline-alkali land along the Yellow River in China. In Integrated Fish Farming; Taylor & Francis: Abingdon, UK, 2020; pp. 359–368. [Google Scholar]
- Ibrahim, L.A.; Shaghaleh, H.; Abu-Hashim, M.; Elsadek, E.A.; Hamoud, Y.A. Exploring the integration of rice and aquatic species: Insights from global and national experiences. Water 2023, 15, 2750. [Google Scholar] [CrossRef]
- Wang, C.; Shi, X.; Qi, Z.; Xiao, Y.; Zhao, J.; Peng, S.; Chu, Q. How does rice-animal co-culture system affect rice yield and greenhouse gas? A meta-analysis. Plant Soil 2023, 493, 325–340. [Google Scholar] [CrossRef]
- Riaz, U.; Mehdi, S.M.; Iqbal, S.; Khalid, H.I.; Qadir, A.A.; Anum, W.; Ahmad, M.; Murtaza, G. Bio-fertilizers: Eco-friendly approach for plant and soil environment. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Springer: Cham, Switzerland, 2020; pp. 189–213. [Google Scholar]
- Xue, W.; Yang, S.; Liu, X.; Qian, M.; Wang, H.; Yang, H.; Liu, X.; Shen, Y.; Li, J.; Sun, Z. Enhanced Sweet Sorghum Growth and Soil Quality in Coastal Saline–Alkali Soils Through Organic Acid-Containing Bio-Based Materials and Microbial Synergy. Agronomy 2025, 15, 56. [Google Scholar] [CrossRef]
- Alharbi, K.; Osman, H.S.; Rashwan, E.; Hafez, E.M.; Omara, A.E.-D. Stimulating the Growth, Anabolism, Antioxidants, and Yield of Rice Plants Grown under Salt Stress by Combined Application of Bacterial Inoculants and Nano-Silicon. Plants 2022, 11, 3431. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, S.; Liu, S.; Zhang, X.; Dong, H.; Dai, S.; Chai, L.; Li, H.; Lv, Y.; Li, T.; et al. Trade-offs of organic amendment input on soil quality and crop productivity in saline-alkali land globally: A meta-analysis. Eur. J. Agron. 2025, 164, 127471. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Bio Technol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Elnahal, A.S.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.T.; Mandava, N.B.; Menossi, M. Brassinosteroids, the Sixth Class of Phytohormones: A Molecular View from the Discovery to Hormonal Interactions in Plant Development and Stress Adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, D.; Geng, Z.; Gao, W.; Tong, M.; Chu, J.; Yao, X. Waterlogging faced by bulbil expansion improved the growth of Pinellia ternata and its effect reinforced by brassinolide. Plant Physiol. Biochem. 2024, 207, 108377. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Halder, K.; Abdin, M.Z.; Majee, M.; Datta, A. Abiotic stress tolerance in plants: Brassinosteroids navigate competently. Int. J. Mol. Sci. 2022, 23, 14577. [Google Scholar] [CrossRef] [PubMed]
- Vriet, C.; Russinova, E.; Reuzeau, C. From squalene to brassinolide: The steroid metabolic and signaling pathways across the plant kingdom. Mol. Plant 2013, 6, 1738–1757. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Gong, B.; Wen, D.; Qiao, P.; Guo, H.; Shi, Q. Brassinosteroid Enhances Cucumber Stress Tolerance to NaHCO3 by Modulating Nitrogen Metabolism, Ionic Balance and Phytohormonal Response. Plants 2025, 14, 80. [Google Scholar] [CrossRef]
- Ali, A.M.; Bijay-Singh. Silicon: A crucial element for enhancing plant resilience in challenging environments. J. Plant Nutr. 2024, 48, 486–521. [Google Scholar] [CrossRef]
- Rao, G.B.; Susmitha, P.J.P.J. Silicon uptake, transportation and accumulation in Rice. J. Pharmacogn. Phytochem. 2017, 6, 290–293. [Google Scholar]
- He, C.; Wang, L.; Liu, J.; Liu, X.; Li, X.; Ma, J.; Lin, Y.; Xu, F. Evidence for ‘silicon’ within the cell walls of suspension-cultured rice cells. New Phytol. 2013, 200, 700–709. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Danilovtseva, E.N.; Pal’shin, V.A.; Ol’ga, N.V.; Zelinskiy, S.N.; Krishnan, U.M. Silicic acid condensation under the influence of water-soluble polymers: From biology to new materials. RSC Adv. 2017, 7, 20995–21027. [Google Scholar] [CrossRef]
- Sun, S.; Yang, Z.; Song, Z.; Wang, N.; Guo, N.; Niu, J.; Liu, A.; Bai, B.; Ahammed, G.J.; Chen, S. Silicon enhances plant resistance to Fusarium wilt by promoting antioxidant potential and photosynthetic capacity in cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 13, 1011859. [Google Scholar] [CrossRef]
- Mosa, W.F.A.E.G.; Sas-Paszt, L.; Frąc, M.; Trzciński, P. Microbial products and biofertilizers in improving growth and productivity of apple-a review. Pol. J. Microbiol. 2016, 65, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.; Baskar, B.; Srinath, S.; Usha, B. Plant-growth-promoting rhizobacteria Bacillus subtilis RR4 isolated from rice rhizosphere induces malic acid biosynthesis in rice roots. Can. J. Microbiol. 2018, 64, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.F.; Wang, X.H.; Wang, Q.; Li, M.; Ma, L.J.; Li, Y.Y.; Li, X.M.; Wang, L.L. Photosynthesis, stomatal conductance, endogenous hormones and organic acid synergistic regulation in leaves of rice (Oryza sativa L.) under elevated CO2. Appl. Ecol. Environ. Res. 2021, 19, 3773–3787. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Dhurakit, P.; Khaksar, G.; Thiravetyan, P. Effect of microorganisms on reducing cadmium uptake and toxicity in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2018, 25, 25690–25701. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.; Kumar, R.M.; Ilango, K.; Rex, A.; Usha, B. Transcriptome profiling of rice roots in early response to Bacillus subtilis (RR4) colonization. Botany 2018, 96, 749–765. [Google Scholar] [CrossRef]
- Jamily, A.S.; Koyama, Y.; Win, T.A.; Toyota, K.; Chikamatsu, S.; Shirai, T.; Uesugi, T.; Murakami, H.; Ishida, T.; Yasuhara, T. Effects of inoculation with a commercial microbial inoculant Bacillus subtilis C-3102 mixture on rice and barley growth and its possible mechanism in the plant growth stimulatory effect. J. Plant Prot. Res. 2019, 59, 193–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Lang, L.; Sun, Z.; Li, M. Potential application of Paenibacillus sp. C1 to the amelioration of soda saline-alkaline soil. Geomicrobiol. J. 2023, 40, 172–182. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, H.; Zhang, X.; Geng, S.; Zhang, Y.; Miao, Y.; Li, L.; Wang, Y. Optimized Phosphorus Application Enhances Canopy Photothermal Responses, Phosphorus Accumulation, and Yield in Summer Maize. Agronomy 2025, 15, 514. [Google Scholar] [CrossRef]
- GB/T 1354-2018; Milled rice. State Administration for Market Regulation of the People’s Republic of China. Chinese Standards Press: Beijing, China, 2018.
- GB/T 5009.5-2016; Determination of Protein in Food. National Health and Family Planning Commission of the People’s Republic of China. Chinese Standards Press: Beijing, China, 2016.
- Biswas, T.; Bandyopadhyay, P.K.; Nandi, R.; Mukherjee, S.; Kundu, A.; Reddy, P.; Mandal, B.; Kumar, P. Impact of mulching and nutrients on soil water balance and actual evapotranspiration of irrigated winter cabbage (Brassica oleracea var. capitata L.). Agric. Water Manag. 2022, 263, 107456. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, X.; Lv, T.; Bai, M.; Wang, Z.; Niu, J. Effects of Water Stress on Fluorescence Parameters and Photosynthetic Characteristics of Drip Irrigation in Rice. Water 2020, 12, 289. [Google Scholar] [CrossRef]
- Han, J.; Dong, Y.; Zhang, M. Chemical fertilizer reduction with organic fertilizer effectively improve soil fertility and microbial community from newly cultivated land in the Loess Plateau of China. Appl. Soil Ecol. 2021, 165, 103966. [Google Scholar] [CrossRef]
- Yang, G.; Juncang, T.; Zhi, W. Composition and functional diversity of soil and water microbial communities in the rice-crab symbiosis system. PLoS ONE 2025, 20, e0316815. [Google Scholar] [CrossRef]
- Li, N.; Li, J.; Zhang, S.; Lan, X.; Zhou, H. Unveiling the Microbial Mysteries of Mulberry Rhizosphere in Saline-Alkaline Soils. Rhizosphere 2025, 33, 101040. [Google Scholar] [CrossRef]
- Chen, J.; Zang, Y.; Yang, Z.; Qu, T.; Sun, T.; Liang, S.; Zhu, M.; Wang, Y.; Tang, X. Composition and functional diversity of epiphytic bacterial and fungal communities on marine macrophytes in an intertidal zone. Front. Microbiol. 2022, 13, 839465. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Huang, J.; Gao, K.; Yang, L.; Lu, Y. Successional action of Bacteroidota and Firmicutes in decomposing straw polymers in a paddy soil. Environ. Microbiome 2023, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Prazdnova, E.V.; Minkina, T.M.; Gorovtsov, A.V. Sulfate-reducing bacteria unearthed: Ecological functions of the diverse prokaryotic group in terrestrial environments. Appl. Environ. Microbiol. 2024, 90, e01390-23. [Google Scholar] [CrossRef]
- Petersen, J.F.; Valk, L.C.; Verhoeven, M.D.; Nierychlo, M.A.; Singleton, C.M.; Dueholm, M.K.; Nielsen, P.H. Diversity and physiology of abundant Rhodoferax species in global wastewater treatment systems. Syst. Appl. Microbiol. 2024, 48, 126574. [Google Scholar] [CrossRef]
- Li, X.; Ding, L.; Li, X.; Zhu, Y. Abundance, diversity, and structure of Geobacteraceae community in paddy soil under long-term fertilization practices. Appl. Soil Ecol. 2020, 153, 103577. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Pei, M.; Fu, J.; Ji, H.; Zhao, L.; Xiao, X. Enhanced rice yields are related to pronounced shifts in soil resident bacterial community structures in response to Rhodopseudomonas palustris and Bacillus subtilis inoculation. J. Soils Sediments 2021, 21, 2369–2380. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Liu, X.; Yao, L.; Chen, Z.; Han, H. Exopolysaccharide-Producing Bacteria Regulate Soil Aggregates and Bacterial Communities to Inhibit the Uptake of Cadmium and Lead by Lettuce. Microorganisms 2024, 12, 2112. [Google Scholar] [CrossRef]
- Zhou, Y.; Sang, T.; Tian, M.; Jahan, M.S.; Wang, J.; Li, X.; Guo, S.; Liu, H.; Wang, Y.; Shu, S. Effects of Bacillus cereus on Photosynthesis and Antioxidant Metabolism of Cucumber Seedlings under Salt Stress. Horticulturae 2022, 8, 463. [Google Scholar] [CrossRef]
- Shi, S.; Wang, E.; Li, C.; Zhou, H.; Cai, M.; Cao, C.; Jiang, Y. Comprehensive evaluation of 17 qualities of 84 types of rice based on principal component analysis. Foods 2021, 10, 2883. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fu, T. Characteristics of rhizosphere and bulk soil microbial community of Chinese cabbage (Brassica campestris) grown in Karst area. Front. Microbiol. 2023, 14, 1241436. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, L.; Jiang, P.; Yang, Z.; Chen, Z.; Xu, F.; Guo, X.; Sun, Y.; Ma, J. Grain Chalkiness Is Decreased by Balancing the Synthesis of Protein and Starch in Hybrid Indica Rice Grains under Nitrogen Fertilization. Foods 2024, 13, 855. [Google Scholar] [CrossRef]
- Sun, B.O.; Gu, L.; Bao, L.; Zhang, S.; Wei, Y.; Bai, Z.; Zhuang, G.; Zhuang, X. Application of biofertilizer containing Bacillus subtilis reduced the nitrogen loss in agricultural soil. Soil Biol. Biochem. 2020, 148, 107911. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, J.; Tong, Z.; Deng, Z.; Wang, Y.; Wang, J. Response of microbial community diversity and the abundance of nitrogen-cycling genes to Bacillus subtilis application in mulberry field soil. Soil Res. 2024, 62, SR23210. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to microbiome: A paradigm shift in the application of microor-ganisms for sustainable agriculture. Front. Microbiol. 2020, 11, 622926. [Google Scholar] [CrossRef]
- Kaviya, N.; Upadhayay, V.K.; Singh, J.; Khan, A.; Panwar, M.; Singh, A.V. Role of microorganisms in soil genesis and functions. In Mycorrhizosphere Pedogenesis; Springer: Singapore, 2019; pp. 25–52. [Google Scholar]
- Poppeliers, S.W.M.; Sánchez-Gil, J.J.; de Jonge, R. Microbes to support plant health: Understanding bioinoculant success in complex conditions. Curr. Opin. Microbiol. 2023, 73, 102286. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Thussagunpanit, J.; Jutamanee, K.; Sonjaroon, W.; Kaveeta, L.; Chai-Arree, W.; Pankean, P.; Suksamrarn, A. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica 2015, 53, 312–320. [Google Scholar] [CrossRef]
- Liu, J.; Lai, R.; He, L.; Xing, P.; Luo, H.; Yang, S.; Zou, Y.; Tang, X. Foliar application of brassinolide induced regulation of grain yield and quality, antioxidant responses and aroma in fragrant rice. Phyton-Int. J. Exp. Bot. 2022, 91, 1061–1071. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pradhan, J.; Kumar, S.; Pramanik, K.; Kastury, C.; Kumari, G.; Jaiswal, A.; Jena, C. Root responses under water deficit stress: Unraveling the impact on wheat crop and the ameliorating role of brassinolide. J. Environ. Biol. 2024, 45, 87–95. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 2022, 3, 100228. [Google Scholar] [CrossRef] [PubMed]
- Lacal, J. The potential of hydrocarbon chemotaxis to increase bioavailability and biodegradation efficiency. In Cellular Ecophysiology of Microbe: Hydrocarbon and Lipid Interactions; Springer International Publishin: Cham, Switzerland, 2018; pp. 241–254. [Google Scholar]
- San, N.S.; Suzuki, K.; Soda, K.; Adachi, S.; Kasahara, H.; Yamamoto, T.; Ikka, T.; Kondo, K.; Yamanouchi, U.; Sugimoto, K.; et al. Semi-dwarf 1 (sd1) gene enhances light penetration into the canopy through regulating leaf inclination angle in rice. Field Crops Res. 2020, 246, 107694. [Google Scholar] [CrossRef]
- Muchlisyiyah, J.; Shamsudin, R.; Kadir Basha, R.; Shukri, R.; How, S.; Niranjan, K.; Onwude, D. Parboiled Rice Processing Method, Rice Quality, Health Benefits, Environment, and Future Perspectives: A Review. Agriculture 2023, 13, 1390. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Yang, Q.; Chang, S.; Zhang, X.; Luo, F.; Li, W.; Ren, J. The fate of dietary polysaccharides in the digestive tract. Trends Food Sci. Technol. 2024, 150, 104606. [Google Scholar] [CrossRef]
- Diao, M.; Dyksma, S.; Koeksoy, E.; Ngugi, D.K.; Anantharaman, K.; Loy, A.; Pester, M. Global diversity and inferred ecophysiology of microorganisms with the potential for dissimilatory sulfate/sulfite reduction. FEMS Microbiol. Rev. 2023, 47, fuad058. [Google Scholar] [CrossRef] [PubMed]
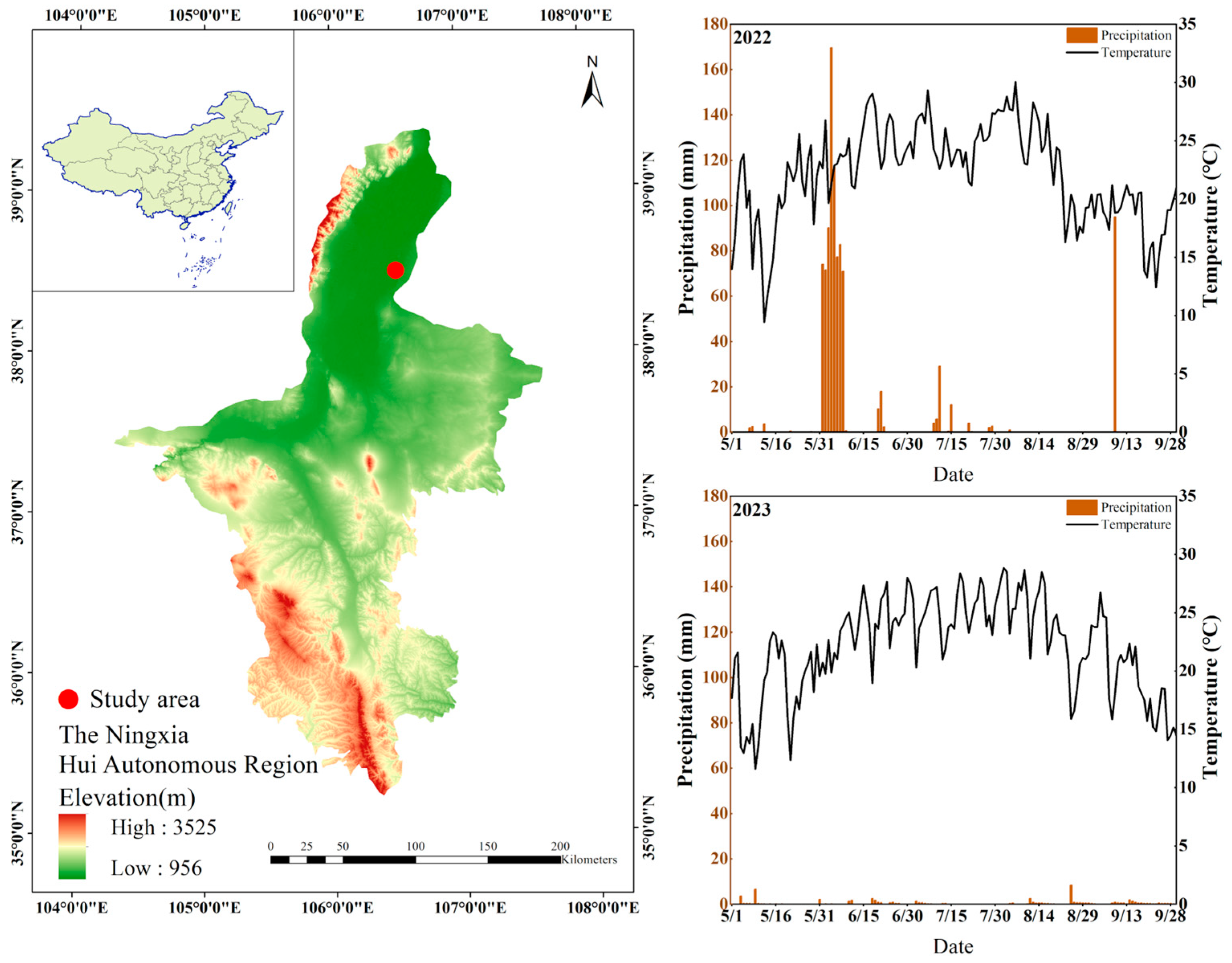


| Year | pH | Organic Matter (g·kg−1) | Total N (g·kg−1) | Olsen Phosphorus (mg·kg−1) | Available Potassium (mg·kg−1) |
|---|---|---|---|---|---|
| 2022 | 8.46 | 12.1 | 1.2 | 23.0 | 220.0 |
| 2023 | 8.17 | 16.9 | 1.0 | 39.0 | 214.8 |
| Years | pH | Total Nitrogen mg·L−1 | Total Phosphorus mg·L−1 | Ammonium Nitrogen mg·L−1 | Mineralization Degreemg·L−1 | CO32− g·L−1 | HCO3− g·L−1 | SO42− g·L−1 | Cl− g·L−1 | Ca2+ g·L−1 | Mg2+ g·L−1 | K+ g·L−1 | Na+ g·L−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | 7.48 | 0.28 | 0.044 | 0.055 | 397 | 0.0061 | 0.13 | 0.21 | 0.057 | 0.056 | 0.050 | 0.27 | 0.26 |
| 2023 | 7.77 | 9.07 | 0.283 | 0.230 | 394 | 0.0086 | 0.32 | 0.17 | 0.080 | 0.060 | 0.079 | 0.004 | 0.085 |
| 2022 | Treatments | 6–16 | 6–30 | 7–9 | 7–13 | 7–25 | 8–5 | 8–15 | 8–24 | 9–4 | 9–14 | Mean |
| A1 | 32.4 ± 0.5 a | 40.5 ± 1.1 a | 43.0 ± 0.4 a | 44.7 ± 0.9 a | 42.3 ± 1.2 a | 44.0 ± 1.5 a | 42.1 ± 0.5 a | 42.9 ± 0.9 a | 42.7 ± 1.1 a | 17.8 ± 0.3 a | 39.2 ± 8.0 a | |
| A2 | 32.2 ± 2.4 a | 40.7 ± 0.9 a | 43.1 ± 0.8 a | 44.8 ± 1.8 a | 42.1 ± 1.1 a | 41.8 ± 0.5 ab | 42.9 ± 0.6 a | 41.7 ± 0.5 a | 43.0 ± 1.1 a | 17.8 ± 0.6 a | 39.0 ± 8.0 a | |
| A3 | 32.5 ± 3.7 a | 40.8 ± 0.5 a | 43.1 ± 0.5 a | 44.6 ± 0.9 a | 42.0 ± 0.5 a | 44.0 ± 0.7 a | 42.2 ± 0.9 a | 41.8 ± 0.4 a | 42.8 ± 1.6 a | 19.4 ± 1.5 a | 39.3 ± 7.6 a | |
| CK | 32.2 ± 0.6 a | 40.0 ± 0.6 a | 42.9 ± 0.6 a | 44.5 ± 1.7 a | 42.0 ± 0.4 a | 40.4 ± 0.6b | 42.5 ± 1.0 a | 42.2 ± 1.1 a | 42.7 ± 0.4 a | 17.7 ± 1.0 a | 38.7 ± 7.9 a | |
| 2023 | Treatments | 6–13 | 6–23 | 7–3 | 7–13 | 7–23 | 8–2 | 8–12 | 8–22 | 9–3 | 9–13 | Mean |
| A1 | 28.8 ± 0.7 a | 36.3 ± 0.5 a | 39.6 ± 1.5 a | 44.2 ± 1.9 a | 38.3 ± 0.8 a | 42.6 ± 1.6 a | 40.2 ± 0.7 a | 40.0 ± 1.4 a | 38.6 ± 0.6 ab | 29.3 ± 1.6 a | 37.8 ± 5.0 a | |
| A2 | 29.2 ± 1.0 a | 36.0 ± 1.4 a | 39.7 ± 0.7 a | 43.9 ± 0.2 a | 38.2 ± 0.9 a | 42.7 ± 0.8 a | 40.2 ± 0.3 a | 40.1 ± 1.5 a | 38.1 ± 0.8 ab | 29.1 ± 2.0 a | 37.7 ± 5.0 a | |
| A3 | 29.2 ± 0.6 a | 35.4 ± 2.0 a | 40.9 ± 1.0 a | 43.4 ± 1.1 a | 38.5 ± 0.8 a | 43.0 ± 1.8 a | 40.5 ± 0.3 a | 40.5 ± 0.8 a | 39.6 ± 2.3 a | 30.4 ± 0.7 a | 38.1 ± 4.9 a | |
| CK | 29.3 ± 0.5 a | 33.7 ± 1.2 a | 39.5 ± 0.7 a | 43.8 ± 1.3 a | 39.1 ± 1.7 a | 40.4 ± 0.7 a | 39.6 ± 0.7 a | 39.1 ± 1.7 a | 35.4 ± 1.9b | 30.1 ± 1.2 a | 37.0 ± 4.7 a |
| Years | Treatment | Yield kg·hm−2 | Panicle Length cm | Panicle Weight g | 100-Grain Weight g | Seed-Setting Rate % | WUE kg/m3 | Crab Yield |
|---|---|---|---|---|---|---|---|---|
| 2022 | A1 | 9154.6 ± 200.1 a | 21.5 ± 1.4 a | 2.9 ± 0.4 a | 3.1 ± 0.2 a | 91.2 ± 4.0 a | 0.64 ± 0.01 a | 361.5 ± 18.0 a |
| A2 | 8937.8 ± 305.7 ab | 20.5 ± 0.5 a | 2.8 ± 0.2 a | 3.0 ± 0.2 a | 92.8 ± 1.4 a | 0.63 ± 0.02 a | 345.0 ± 23.6 a | |
| A3 | 9087.9 ± 275.5 a | 20.4 ± 0.7 a | 2.9 ± 0.4 a | 3.1 ± 0.0 a | 93.1 ± 1.2 a | 0.64 ± 0.02 a | 355.5 ± 21.3 a | |
| CK | 8320.8 ± 605.1 b | 20.4 ± 0.8 a | 2.7 ± 0.2 a | 3.0 ± 0.1 a | 92.7 ± 2.3 a | 0.60 ± 0.04 a | 309.5 ± 7.4 b | |
| 2023 | A1 | 7976.2 ± 369.2 ab | 20.8 ± 1.8 a | 3.1 ± 0.1 ab | 3.0 ± 0.2 ab | 92.3 ± 1.2 bc | 0.64 ± 0.03 ab | 343.0 ± 15.1 a |
| A2 | 8276.4 ± 459.2 a | 20.4 ± 2.9 a | 3.1 ± 0.2 ab | 2.9 ± 0.1 b | 94.8 ± 1.5 ab | 0.65 ± 0.04 a | 352.5 ± 18.2 a | |
| A3 | 8520.9 ± 371.4 a | 20.3 ± 1.7 a | 3.3 ± 0.1 a | 3.2 ± 0.1 a | 94.9 ± 1.8 a | 0.67 ± 0.03 a | 370.5 ± 13.7 a | |
| CK | 7353.7 ± 280.5 b | 20.1 ± 1.2 a | 2.8 ± 0.1 b | 2.9 ± 0.0 ab | 90.1 ± 0.6 c | 0.58 ± 0.02 b | 311.5 ± 8.5 b |
| Years | Treatments | Chalkiness Degree % | Chalkiness Rate % | Protein Content % | Viscosity mm | Taste Score | Amylose Content % |
|---|---|---|---|---|---|---|---|
| 2022 | A1 | 6.5 ± 0.6 a | 19.8 ± 2.3 a | 5.5 ± 0.3 b | 135.8 ± 4.1 a | 69.0 ± 1.0 ab | 14.3 ± 0.6 b |
| A2 | 5.2 ± 0.3 bc | 13.6 ± 0.9 b | 5.9 ± 0.4 b | 113.5 ± 11.5 b | 67.0 ± 2.0 b | 16.9 ± 0.4 a | |
| A3 | 5.7 ± 0.6 ab | 14.2 ± 0.7 b | 7.6 ± 0.4 a | 111.0 ± 9.6 b | 75.0 ± 2.6 a | 14.1 ± 0.5 b | |
| CK | 4.5 ± 0.4 b | 10.7 ± 1.1 c | 5.3 ± 0.3 b | 134.3 ± 3.3 a | 71.0 ± 6.1 ab | 16.0 ± 0.8 a | |
| 2023 | A1 | 7.1 ± 0.4 a | 14.6 ± 0.6 a | 6.9 ± 0.2 b | 127.5 ± 4.8 ab | 69.0 ± 2.0 b | 14.4 ± 0.3 ab |
| A2 | 6.8 ± 0.4 a | 13.2 ± 0.3 b | 7.5 ± 0.2 a | 120.5 ± 3.4 ab | 68.0 ± 1.7 b | 14.8 ± 0.3 a | |
| A3 | 6.6 ± 0.5 a | 13.8 ± 0.5 ab | 7.4 ± 0.4 ab | 129.7 ± 1.4 a | 73.0 ± 1.0 a | 14.0 ± 0.3 b | |
| CK | 5.5 ± 0.3 b | 13.4 ± 0.4 b | 7.4 ± 0.2 ab | 116.6 ± 11.4 b | 73.0 ± 1.0 a | 14.6 ± 0.3 a |
| Years | Sample Name | OTUs | Simpson | Ace | Coverage | |
|---|---|---|---|---|---|---|
| Bacteria | 2022 | A1 | 3822.00 ± 389.75 a | 0.002 ± 0.0001 b | 4863.64 ± 249.71 a | 0.974 ± 0.0098 |
| A2 | 3266.33 ± 102.32 c | 0.003 ± 0.0003 a | 4405.56 ± 50.98 b | 0.966 ± 0.0071 | ||
| A3 | 3420.33 ± 36.61 ab | 0.003 ± 0.0002 a | 4652.83 ± 49.03 ab | 0.96 ± 0.0004 | ||
| CK | 3497.33 ± 149.53 ab | 0.002 ± 0.0002 b | 4570.96 ± 52.77 b | 0.969 ± 0.0081 | ||
| 2023 | A1 | 4129.67 ± 44.96 a | 0.002 ± 0.0000 b | 5068.10 ± 15.53 a | 0.977 ± 0.0003 | |
| A2 | 4120.00 ± 176.03 a | 0.003 ± 0.0007 a | 5099.33 ± 290.32 a | 0.976 ± 0.0020 | ||
| A3 | 4388.67 ± 243.75 a | 0.003 ± 0.0002 b | 5399.45 ± 308.12 a | 0.975 ± 0.0016 | ||
| CK | 4118.33 ± 77.59 a | 0.002 ± 0.0001 b | 4965.81 ± 88.95 a | 0.978 ± 0.0004 | ||
| Fungus | 2022 | A1 | 341.33 ± 7.23 a | 0.021 ± 0.0025 b | 963.02 ± 70.66 ab | 0.998 ± 0.0012 |
| A2 | 258.33 ± 43.52 b | 0.054 ± 0.0161 ab | 731.98 ± 152.58 c | 0.998 ± 0.0006 | ||
| A3 | 352.33 ± 17.9 a | 0.074 ± 0.0385 a | 1062.19 ± 85.01 a | 0.997 ± 0.0004 | ||
| CK | 278.33 ± 41.19 b | 0.041 ± 0.0162 ab | 798.07 ± 135.72 bc | 0.998 ± 0.0008 | ||
| 2023 | A1 | 219.00 ± 10.00 c | 0.525 ± 0.2693 a | 233.91 ± 7.31 b | 1.000 ± 0.0001 | |
| A2 | 316.33 ± 64.84 b | 0.152 ± 0.0620 b | 339.89 ± 72.92 a | 0.999 ± 0.0002 | ||
| A3 | 401.33 ± 30.07 a | 0.168 ± 0.0805 b | 420.74 ± 35.27 a | 0.999 ± 0.0002 | ||
| CK | 337.00 ± 22.52 ab | 0.068 ± 0.0164 b | 352.02 ± 16.58 a | 1.000 ± 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Tian, J.; Wang, Z. Effects of Different Biological Amendments on Rice Physiology, Yield, Quality, and Soil Microbial Community of Rice–Crab Co-Culture in Saline–Alkali Soil. Agronomy 2025, 15, 649. https://doi.org/10.3390/agronomy15030649
Guo Y, Tian J, Wang Z. Effects of Different Biological Amendments on Rice Physiology, Yield, Quality, and Soil Microbial Community of Rice–Crab Co-Culture in Saline–Alkali Soil. Agronomy. 2025; 15(3):649. https://doi.org/10.3390/agronomy15030649
Chicago/Turabian StyleGuo, Yang, Juncang Tian, and Zhi Wang. 2025. "Effects of Different Biological Amendments on Rice Physiology, Yield, Quality, and Soil Microbial Community of Rice–Crab Co-Culture in Saline–Alkali Soil" Agronomy 15, no. 3: 649. https://doi.org/10.3390/agronomy15030649
APA StyleGuo, Y., Tian, J., & Wang, Z. (2025). Effects of Different Biological Amendments on Rice Physiology, Yield, Quality, and Soil Microbial Community of Rice–Crab Co-Culture in Saline–Alkali Soil. Agronomy, 15(3), 649. https://doi.org/10.3390/agronomy15030649







