Current Progress in Microbial Biocontrol of Banana Fusarium Wilt: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
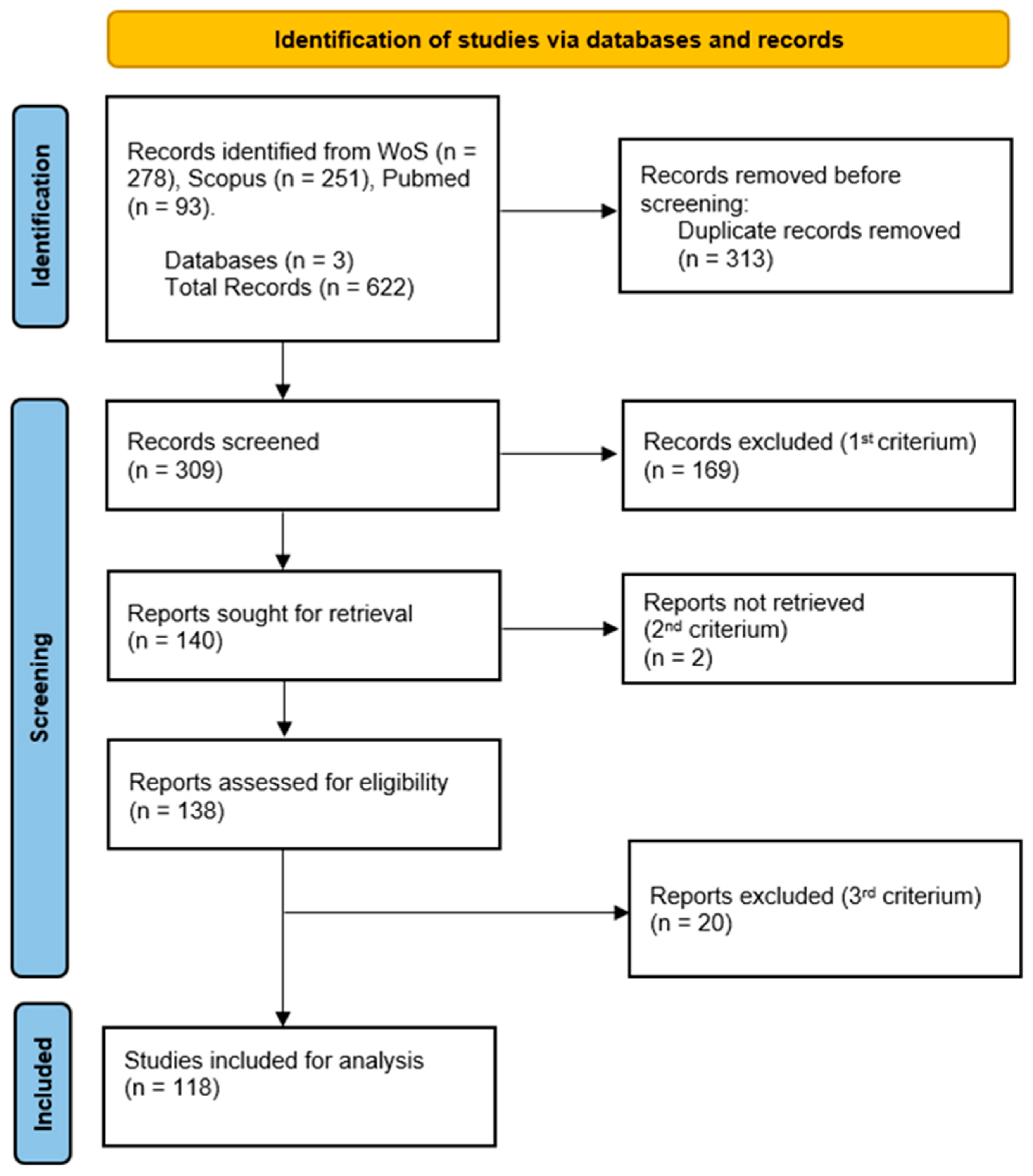
2.1. Literature Search and Dataset Construction
2.2. Article Selection and Exclusion
2.3. Data Extraction and Analysis
2.4. Graphical Summary
3. Results and Discussion
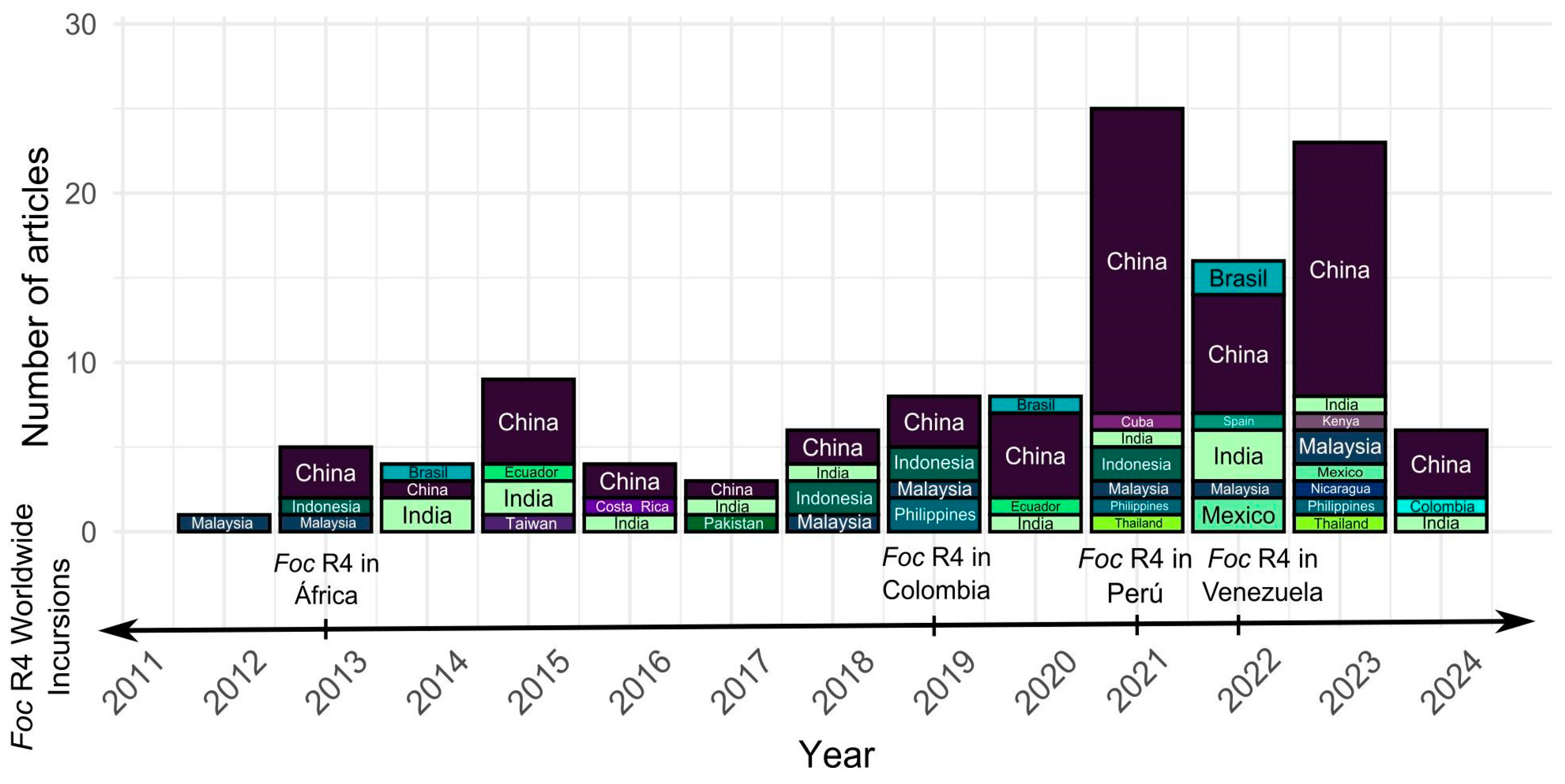
3.1. Asian Leadership in Antagonistic Microorganism Research and the Approach on Foc TR4
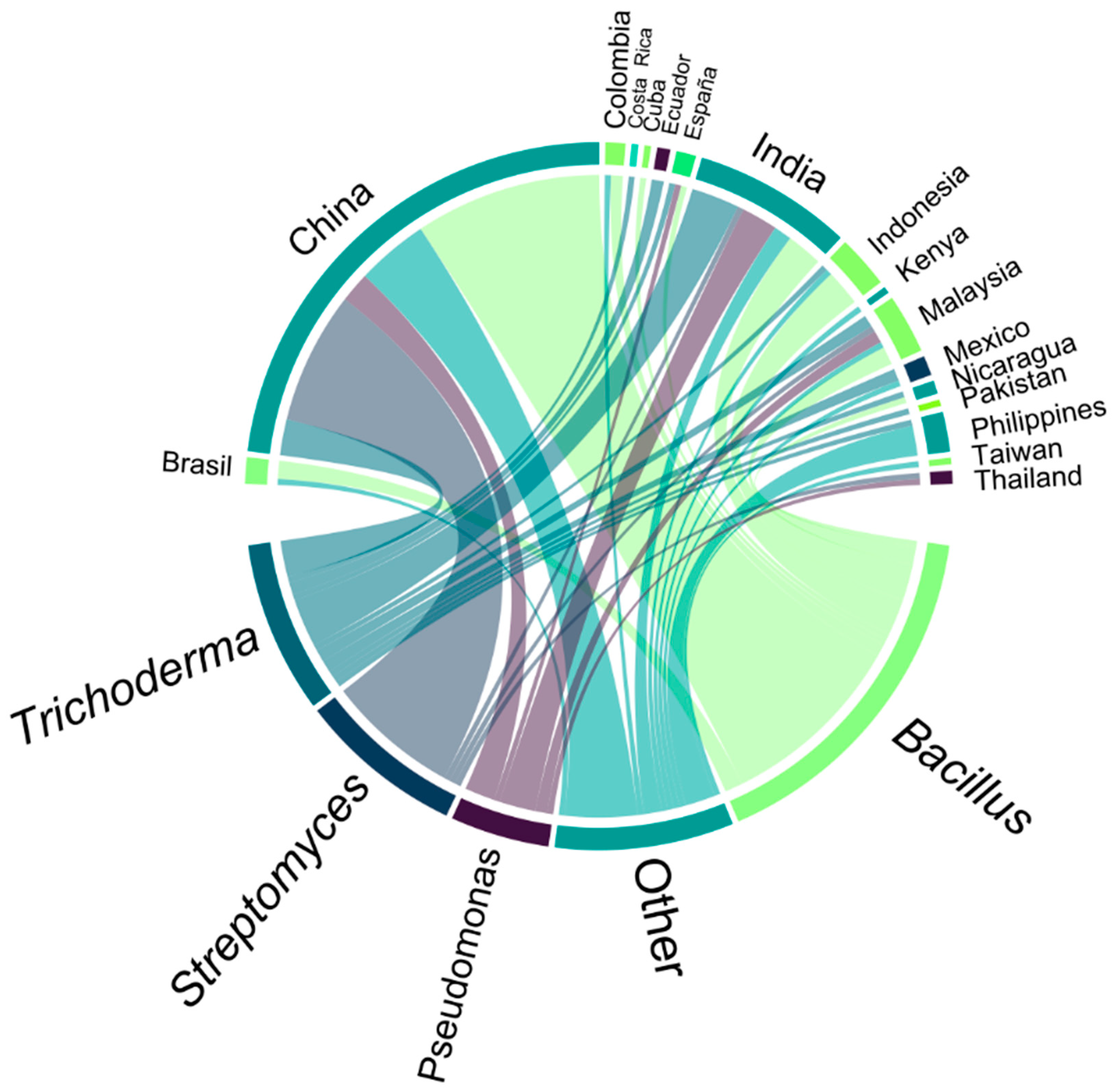
3.2. Preference for Antagonistic Microorganisms Studied in the Scientific Community
3.3. General Efficacy and Action Mechanisms of Microbial Biological-Control Agents (MBCAs)
3.3.1. Mechanisms of Microbial Action in the Biocontrol of Foc
3.3.2. General Efficacy of Microbial Biological-Control Agents (MBCAs)
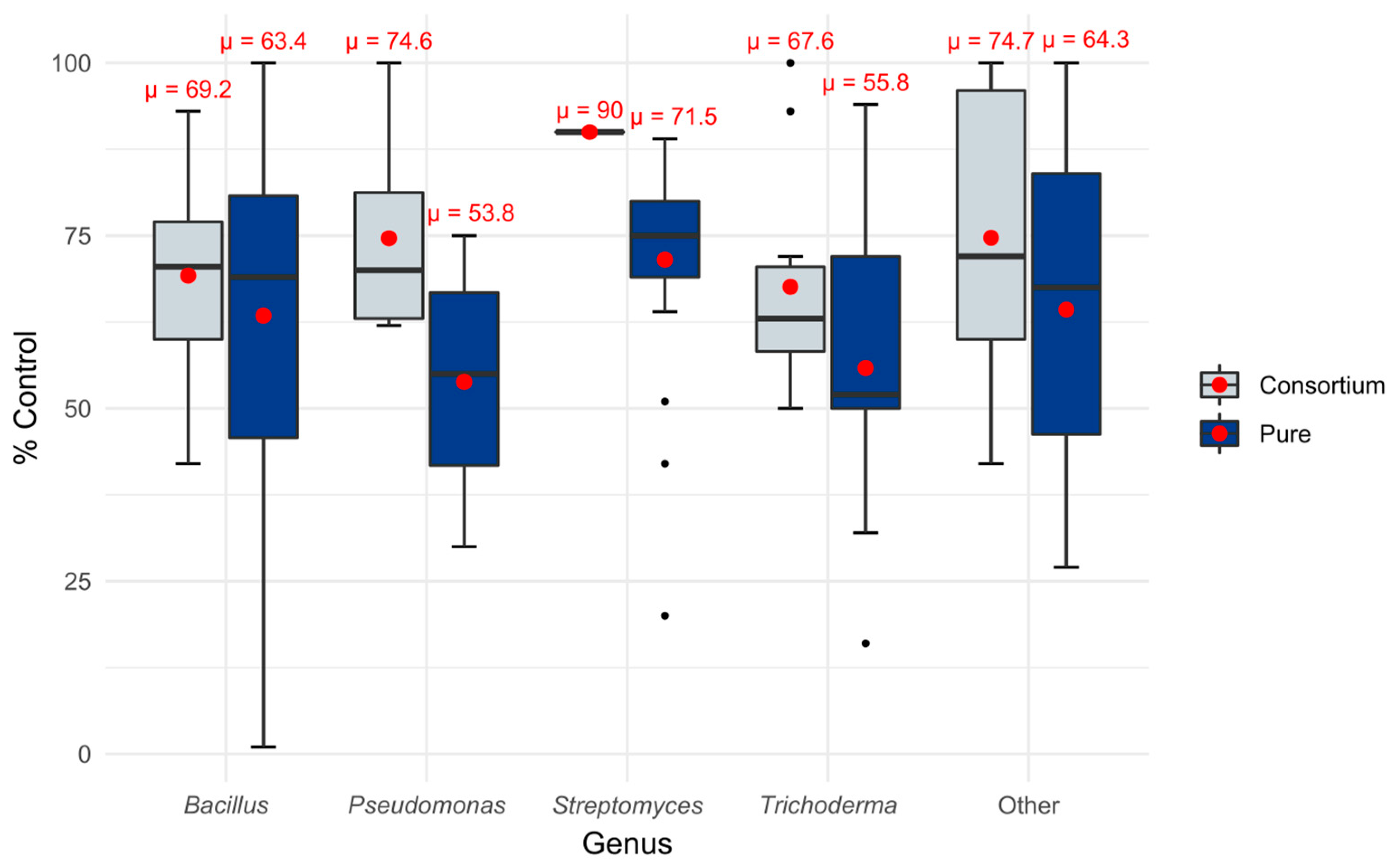
3.4. Efficacy of MBCAs in Consortia
3.5. Efficacy of MBCAs Associated with Their Origin
3.6. Efficacy of MBCAs at Different Trial Levels: Dual Culture, Pots, and Plots
3.6.1. Dual Culture in the Laboratory
3.6.2. Seedling Trials
3.6.3. Experimental-Plot Trials
3.7. Banana and Plantain Varieties Tested
3.8. Is There an Effective Microorganism for Controlling Foc?
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ploetz, R.C. Management of Fusarium Wilt of Banana: A Review with Special Reference to Tropical Race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Food and Agriculture Organizations of United Nations. Preventing the Spread and Introduction of Banana Fusarium Wilt Disease Tropical Race 4 (TR4): Guide for Travelers; Food and Agriculture Organizations of United Nations: Roma, Italy, 2020; Available online: https://openknowledge.fao.org/items/ceafec1c-8fa3-42a5-9a1f-7ae4cf02ff71 (accessed on 18 November 2024).
- Altendorf, S. Strengthening the Resilience of Agricultural Supply Chains—The Case of Fresh Fruits and Vegetables; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023. [Google Scholar]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of Banana: Current Knowledge on Epidemiology and Research Needs Toward Sustainable Disease Management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Zhan, N.; Kuang, M.; He, W.; Deng, G.; Liu, S.; Li, C.; Roux, N.; Dita, M.; Yi, G.; Sheng, O. Evaluation of Resistance of Banana Genotypes with AAB Genome to Fusarium Wilt Tropical Race 4 in China. J. Fungi 2022, 8, 1274. [Google Scholar] [CrossRef] [PubMed]
- Organización de las Naciones Unidas para la Alimentación y la Agricultura. SPOTLIGHT: Building Resilience against Fusarium Tropical Race 4 (TR4) in Africa; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Roma, Italy, 2023; Available online: https://www.fao.org/tr4gn/news/news-detail/en/c/1653646/ (accessed on 18 November 2024).
- Viljoen, A.; Mostert, D.; Chiconela, T.; Beukes, I.; Fraser, C.; Dwyer, J.; Murray, H.; Amisse, J.; Matabuana, E.L.; Tazan, G.; et al. Occurrence and Spread of the Banana Fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020, 116, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blomme, G.; Mahuku, G.; Kearsley, E.; Dita, M. Towards the Integrated Management of Fusarium Wilt of Banana. J. Fungi 2024, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, T.; Vargas, J.; Teixeira, L.; Staver, C.; Dita, M. Fusarium Tropical Race 4 in Latin America and the Caribbean: Status and Global Research Advances towards Disease Management. Front. Plant Sci. 2024, 15, 1397617. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Aitken, E. Effect of in Planta Treatment of ‘Cavendish’ Banana with Herbicides and Fungicides on the Colonisation and Sporulation by Fusarium oxysporum f.sp. cubense Subtropical Race 4. J. Fungi 2021, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Australian Pesticides and Veterinary Medicines Authority Online Services Portal. Available online: https://portal.apvma.gov.au/pubcris (accessed on 18 November 2024).
- Fungicide Resistance Action Committee FRAC Code List ©*2024: Fungal Control Agents Sorted by Cross-Resistance Pattern and Mode of Action. Available online: https://www.frac.info/knowledge-database/knowledge-database (accessed on 18 November 2024).
- Ploetz, R.C.; Konkol, J.L.; Pérez-Martínez, J.M.; Fernandez, R. Management of Laurel Wilt of Avocado, Caused by Raffaelea lauricola. Eur. J. Plant Pathol. 2017, 149, 133–143. [Google Scholar] [CrossRef]
- Warman, N.M.; Aitken, E.A.B. The Movement of Fusarium oxysporum f.sp. cubense (Sub-Tropical Race 4) in Susceptible Cultivars of Banana. Front. Plant Sci. 2018, 9, 1748. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhang, W.; Zhou, G.-D.; Li, S.; Wang, Y.; Yang, B.; Bai, T.; Fan, H.; He, P.; Zheng, S.-J. A Biological Product of Bacillus amyloliquefaciens QST713 Strain for Promoting Banana Plant Growth and Modifying Rhizosphere Soil Microbial Diversity and Community Composition. Front. Microbiol. 2023, 14, 1216018. [Google Scholar] [CrossRef] [PubMed]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological Control Agents Against Fusarium Wilt of Banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Arévalo, T. Alternatives for the Biocontrol of Fusarium oxysporum f. sp. cubense, Causal Agent of Fusarium Wilt or Panama Disease in Guineo (Musa balbisiana ABB) Under Field Conditions. Sch. J. Agric. Vet. Sci. 2023, 10, 12–18. [Google Scholar] [CrossRef]
- Wang, X.; Du, Z.; Chen, C.; Guo, S.; Mao, Q.; Wu, W.; Wu, R.; Han, W.; Xie, P.; Zeng, Y.; et al. Antifungal Effects and Biocontrol Potential of Lipopeptide-Producing Streptomyces against Banana Fusarium Wilt Fungus Fusarium oxysporum f. sp. cubense. Front. Microbiol. 2023, 14, 1177393. [Google Scholar] [CrossRef]
- Du, C.; Yang, D.; Jiang, S.; Zhang, J.; Ye, Y.; Pan, L.; Fu, G. Biocontrol Agents Inhibit Banana Fusarium Wilt and Alter the Rooted Soil Bacterial Community in the Field. J. Fungi 2024, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First Report of Fusarium Wilt Tropical Race 4 in Cavendish Bananas Caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- Acuña, R.; Rouard, M.; Leiva, A.M.; Marques, C.; Olortegui, J.A.; Ureta, C.; Cabrera-Pintado, R.M.; Rojas, J.C.; Lopez-Alvarez, D.; Cenci, A.; et al. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 Causing Fusarium Wilt in Cavendish Bananas in Peru. Plant Dis. 2022, 106, 2268. [Google Scholar] [CrossRef] [PubMed]
- Mejías Herrera, R.; Hernández, Y.; Magdama, F.; Mostert, D.; Bothma, S.; Paredes Salgado, E.M.; Terán, D.; González, E.; Angulo, R.; Angel, L.; et al. First Report of Fusarium Wilt of Cavendish Bananas Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 in Venezuela. Plant Dis. 2023, 107, 3297. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Olivares, B.O.; Rey, J.C.; Rojas, J.; Cardenas, J.; Muentes, C.; Dawson, C. The Advance of Fusarium Wilt Tropical Race 4 in Musaceae of Latin America and the Caribbean: Current Situation. Pathogens 2023, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Urrútia, G.; Bonfill, X. Declaración PRISMA: Una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med. Clínica 2010, 135, 507–511. [Google Scholar] [CrossRef]
- Kavino, M.; Manoranjitham, S.K.; Balamohan, T.N.; Kumar, N.; Karthiba, L.; Samiyappan, R. Enhancemenet of Growth and Panama Wilt Resistance in Banana by in Vitro Culturing of Banana Plantets with PGPR and Edophytes. Acta Hortic. 2014, 1024, 277–282. [Google Scholar] [CrossRef]
- Thangavelu, R.; Gopi, M. Field Suppression of Fusarium Wilt Disease in Banana by the Combined Application of Native Endophytic and Rhizospheric Bacterial Isolates Possessing Multiple Functions. Phytopathol. Mediterr. 2015, 54, 241–252. [Google Scholar] [CrossRef]
- Thangavelu, R.; Gopi, M. Combined Application of Native Trichoderma Isolates Possessing Multiple Functions for the Control of Fusarium Wilt Disease in Banana Cv. Grand Naine. Biocontrol Sci. Technol. 2015, 25, 1147–1164. [Google Scholar] [CrossRef]
- Sumathi, S.; Thangavelu, R. Co-Inoculation of Arbuscular Mycorrhizal Fungi (AMF) and Their Mycorrhizae Helper. Plant Arch. 2016, 16, 365–375. [Google Scholar]
- Chaves, N.P.; Staver, C.; Dita, M.A. Potential of Trichoderma Asperellum for Biocontrol of Fusarium Wilt in Banana. Acta Hortic. 2016, 1114, 261–265. [Google Scholar] [CrossRef]
- Kavino, M.; Manoranjitham, S.K. In Vitro Bacterization of Banana (Musa spp.) with Native Endophytic and Rhizospheric Bacterial Isolates: Novel Ways to Combat Fusarium Wilt. Eur. J. Plant Pathol. 2018, 151, 371–387. [Google Scholar] [CrossRef]
- Vieira, L.C.S.; Costa, S.N.; Borges, C.V.; Gonçalves, Z.S.; Haddad, F. Fusarium oxysporum f. sp. cubense Biocontrol Mediated by Bacillus spp. in Prata-Anã Banana. Agraria 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Mahachai, P.; Meesungnoen, O.; Wattanachaiyingcharoen, W.; Subsoontorn, P. Bacterial Biocontrol against Fusarium Wilt in Pisang Awak (Namwa) Banana. Asia-Pac. J. Sci. Technol. 2021, 26, APST-26. [Google Scholar]
- Cruz-Martín, M.; Leyva, L.; Acosta-Suárez, M.; Pichardo, T.; Bermúdez-Caraballoso, I.; Alvarado-Capó, Y. Antifungal Activity of Bacillus Amyloliquefaciens against Fusarium oxysporum f. sp. cubense Race 1. Agron. Mesoam. 2021, 32, 466–478. [Google Scholar] [CrossRef]
- Hernández-Melchor, D.J.; Ferrera-Cerrato, R.; López-Pérez, P.A.; Ferrera-Rodríguez, M.R.; de Jesús García-Ávila, C.; Alarcón, A. Qualitative and Quantitative Enzymatic Profile of Native Trichoderma Strains and Biocontrol Potential Against Fusarium oxysporum f. sp. cubense Race 1. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e3264. [Google Scholar] [CrossRef]
- Martins, M.J.; Xavier, A.A.; Cardoso, I.C.; Silveira, D.F.; Ribeiro, R.C.F.; Pimenta, S.; Nietsche, S. Autochthonous Endophytic Bacteria from Musa sp. Controls Fusarium oxysporum f. sp. cubense under in Vitro Conditions. An. Acad. Bras. Ciências 2022, 94, e20210835. [Google Scholar] [CrossRef]
- Vijayasanthi, S.; Akila, R.; Ayyandurai, M.; Kannan, R. Survey, Identification and Management of Fusarium Wilt of Banana in Tamirabarani Tract of Southern Districts of Tamil Nadu. J. Biol. Control 2022, 36, 64–70. [Google Scholar] [CrossRef]
- Hernández-Melchor, D.J.; Guerrero-Chávez, A.C.; Ferrera-Rodríguez, M.R.; Ferrera-Cerrato, R.; Larsen, J.; Alarcón, A. Cellulase and Chitinase Activities and Antagonism against Fusarium oxysporum f. sp. cubense Race 1 of Six Trichoderma Strains Isolated from Mexican Maize Cropping. Biotechnol. Lett. 2023, 45, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Kawicha, P.; Nitayaros, J.; Saman, P.; Thaporn, S.; Thanyasiriwat, T.; Somtrakoon, K.; Sangdee, K.; Sangdee, A. Evaluation of Soil Streptomyces spp. for the Biological Control of Fusarium Wilt Disease and Growth Promotion in Tomato and Banana. Plant Pathol. J. 2023, 39, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-García, L.F.; Carmona-Gutiérrez, S.L.; Moreno-Velandia, C.A.; Villarreal-Navarrete, A.d.P.; Burbano-David, D.M.; Quiroga-Mateus, R.Y.; Gómez-Marroquín, M.R.; Rodríguez-Yzquierdo, G.A.; Betancourt-Vásquez, M. Microbial-Based Biofungicides Mitigate the Damage Caused by Fusarium oxysporum f. sp. cubense Race 1 and Improve the Physiological Performance in Banana. J. Fungi 2024, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.S.Y.; Mah, S.W.; Tee, C.S. Evaluating the Feasibility of Induced Host Resistance by Endophytic Isolate Penicillium citrinum BTF08 as a Control Mechanism for Fusarium Wilt in Banana Plantlets. Biol. Control 2012, 61, 155–159. [Google Scholar] [CrossRef]
- Zacky, F.A.; Ting, A.S.Y. Investigating the Bioactivity of Cells and Cell-Free Extracts of Streptomyces Griseus towards Fusarium oxysporum f. sp. cubense Race 4. Biol. Control 2013, 66, 204–208. [Google Scholar] [CrossRef]
- Yuan, J.; Ruan, Y.; Wang, B.; Zhang, J.; Waseem, R.; Huang, Q.; Shen, Q. Plant Growth-Promoting Rhizobacteria Strain Bacillus amyloliquefaciens Njn-6-Enriched Bio-Organic Fertilizer Suppressed Fusarium Wilt and Promoted the Growth of Banana Plants. J. Agric. Food Chem. 2013, 61, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Asih Nawangsih, A.; Purba, F. Isolation of Fluorescent Pseudomonads, Heat Tolerant and Chitinolytic Bacteria in Banana Rhizosphere with Antagonistic Activities against Fusarium oxysporum f. sp. cubense in Vitro and Molecular Identification of Selected Isolates. J. Int. Soc. Southeast Asian Agric. Sci. 2013, 19, 30–40. [Google Scholar]
- Souza, A.; Cruz, J.C.; Sousa, N.R.; Procopio, A.R.L.; Silva, G.F. Endophytic Bacteria from Banana Cultivars and Their Antifungal Activity. Genet. Mol. Res. 2014, 13, 8661–8670. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, B.; Lv, N.; Sun, Y.; Jiang, X.; Li, R.; Ruan, Y.; Shen, Q. Effect of the Combination of Bio-Organic Fertiliser with Bacillus amyloliquefaciens NJN-6 on the Control of Banana Fusarium Wilt Disease, Crop Production and Banana Rhizosphere Culturable Microflora. Biocontrol Sci. Technol. 2015, 25, 716–731. [Google Scholar] [CrossRef]
- Ho, Y.-N.; Chiang, H.-M.; Chao, C.-P.; Su, C.-C.; Hsu, H.-F.; Guo, C.; Hsieh, J.-L.; Huang, C.-C. In Planta Biocontrol of Soilborne Fusarium Wilt of Banana through a Plant Endophytic Bacterium, Burkholderia cenocepacia 869T2. Plant Soil 2015, 387, 295–306. [Google Scholar] [CrossRef]
- Xue, C.; Ryan Penton, C.; Shen, Z.; Zhang, R.; Huang, Q.; Li, R.; Ruan, Y.; Shen, Q. Manipulating the Banana Rhizosphere Microbiome for Biological Control of Panama Disease. Sci. Rep. 2015, 5, 11124. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, L.; Ling, N.; Raza, W.; Shen, Q.; Huang, Q. Plant-Growth-Promoting Traits and Antifungal Potential of the Bacillus amyloliquefaciens YL-25. Biocontrol Sci. Technol. 2014, 25, 276–290. [Google Scholar] [CrossRef]
- Tan, D.; Fu, L.; Han, B.; Sun, X.; Zheng, P.; Zhang, J. Identification of an Endophytic Antifungal Bacterial Strain Isolated from the Rubber Tree and Its Application in the Biological Control of Banana Fusarium Wilt. PLoS ONE 2015, 10, e0131974. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ruan, Y.; Tao, C.; Li, R.; Shen, Q. Continous Application of Bioorganic Fertilizer Induced Resilient Culturable Bacteria Community Associated with Banana Fusarium Wilt Suppression. Sci. Rep. 2016, 6, 27731. [Google Scholar] [CrossRef]
- Zhou, D.; Jing, T.; Qi, D.; Feng, R.; Duan, Y.; Chen, Y.; Wang, F.; Zhang, X.; Xie, J. Isolation and Identification of Streptomyces lunalinharesii and Its Control Effect on the Banana Fusarium Wilt Disease. Acta Hortic. Sin. 2017, 44, 664–674. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates Against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Qi, D.; Zou, L.; Zhou, D.; Chen, Y.; Gao, Z.; Feng, R.; Zhang, M.; Li, K.; Xie, J.; Wang, W. Taxonomy and Broad-Spectrum Antifungal Activity of Streptomyces sp. SCA3-4 Isolated From Rhizosphere Soil of Opuntia stricta. Front. Microbiol. 2019, 10, 1390. [Google Scholar] [CrossRef]
- Huang, J.; Pang, Y.; Zhang, F.; Huang, Q.; Zhang, M.; Tang, S.; Fu, H.; Li, P. Suppression of Fusarium Wilt of Banana by Combining Acid Soil Ameliorant with Biofertilizer Made from Bacillus velezensis H-6. Eur. J. Plant Pathol. 2019, 154, 585–596. [Google Scholar] [CrossRef]
- Shen, Z.; Xue, C.; Penton, C.R.; Thomashow, L.S.; Zhang, N.; Wang, B.; Ruan, Y.; Li, R.; Shen, Q. Suppression of Banana Panama Disease Induced by Soil Microbiome Reconstruction through an Integrated Agricultural Strategy. Soil Biol. Biochem. 2019, 128, 164–174. [Google Scholar] [CrossRef]
- Puig, C.G.; Cumagun, C.J.R. Rainforest Fungal Endophytes for the Bio-Enhancement of Banana toward Fusarium oxysporum f. sp. cubense Tropical Race 4. Arch. Phytopathol. Plant Prot. 2019, 52, 776–794. [Google Scholar] [CrossRef]
- Wong, C.K.F.; Saidi, N.B.; Vadamalai, G.; Teh, C.Y.; Zulperi, D. Effect of Bioformulations on the Biocontrol Efficacy, Microbial Viability and Storage Stability of a Consortium of Biocontrol Agents against Fusarium Wilt of Banana. J. Appl. Microbiol. 2019, 127, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.G.; Puig, C.G.; Cumagun, C.J.R. Non-Synergistic Effect of Trichoderma harzianum and Glomus spp. in Reducing Infection of Fusarium Wilt in Banana. Pathogens 2019, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Zhang, Y.; Hao, X.; Zhang, Z.; Lai, Z. The Root Endophytic Fungus Serendipita indica Improves Resistance of Banana to Fusarium oxysporum f. sp. cubense Tropical Race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, J.; He, W.; Chen, J.; Pang, Z.; Hu, H.; Xie, J. Fermentation Optimization and Disease Suppression Ability of a Streptomyces ma. FS-4 from Banana Rhizosphere Soil. BMC Microbiol. 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-Organic Fertilizers Stimulate Indigenous Soil Pseudomonas Populations to Enhance Plant Disease Suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Damodaran, T.; Rajan, S.; Muthukumar, M.; Gopal, R.; Yadav, K.; Kumar, S.; Ahmad, I.; Kumari, N.; Mishra, V.K.; Jha, S.K. Biological Management of Banana Fusarium Wilt Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Antagonistic Fungal Isolate CSR-T-3 (Trichoderma reesei). Front. Microbiol. 2020, 11, 595845. [Google Scholar] [CrossRef]
- Jing, T.; Zhou, D.; Zhang, M.; Yun, T.; Qi, D.; Wei, Y.; Chen, Y.; Zang, X.; Wang, W.; Xie, J. Newly Isolated Streptomyces sp. JBS5-6 as a Potential Biocontrol Agent to Control Banana Fusarium Wilt: Genome Sequencing and Secondary Metabolite Cluster Profiles. Front. Microbiol. 2020, 11, 602591. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shan, Y.; Li, Y.; Li, Q.; Wu, C. The Soil Nutrient Environment Determines the Strategy by Which Bacillus velezensis HN03 Suppresses Fusarium Wilt in Banana Plants. Front. Plant Sci. 2020, 11, 599904. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Bo, B.; Malec, P.; Fu, P. The Effect of a Consortium of Penicillium sp. and Bacillus spp. in Suppressing Banana Fungal Diseases Caused by Fusarium sp. and Alternaria sp. J. Appl. Microbiol. 2021, 131, 1890–1908. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Bo, B.; Malec, P.; Khan, S.; Fu, P. Newly Isolated Strain of Trichoderma asperellum from Disease Suppressive Soil Is a Potential Bio-Control Agent to Suppress Fusarium Soil Borne Fungal Phytopathogens. J. Plant Pathol. 2021, 103, 549–561. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, D.; Qi, D.; Gao, Z.; Xie, J.; Luo, Y. Growth Promotion and Disease Suppression Ability of a Streptomyces sp. CB-75 from Banana Rhizosphere Soil. Front. Microbiol. 2018, 8, 2704. [Google Scholar] [CrossRef]
- Qi, D.; Zou, L.; Zhou, D.; Zhang, M.; Wei, Y.; Zhang, L.; Xie, J.; Wang, W. Identification and Antifungal Mechanism of a Novel Actinobacterium Streptomyces huiliensis sp. Nov. Against Fusarium oxysporum f. sp. cubense Tropical Race 4 of Banana. Front. Microbiol. 2021, 12, 722661. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Jing, T.; Zhou, D.; Zhang, M.; Zhao, Y.; Li, K.; Zang, X.; Zhang, L.; Xie, J.; Wang, W. Potential Biological Control of Endophytic Streptomyces sp. 5-4 Against Fusarium Wilt of Banana Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4. Phytopathology® 2022, 112, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Maulidah, N.I.; Tseng, T.-S.; Chen, G.-H.; Hsieh, H.-Y.; Chang, S.-F.; Chuang, H. Transcriptome Analysis Revealed Cellular Pathways Associated with Abiotic Stress Tolerance and Disease Resistance Induced by Pseudomonas Aeruginosa in Banana Plants. Plant Gene 2021, 27, 100321. [Google Scholar] [CrossRef]
- Tian, D.; Song, X.; Li, C.; Zhou, W.; Qin, L.; Wei, L.; Di, W.; Huang, S.; Li, B.; Huang, Q.; et al. Antifungal Mechanism of Bacillus amyloliquefaciens Strain GKT04 against Fusarium Wilt Revealed Using Genomic and Transcriptomic Analyses. Microbiol. Open 2021, 10, e1192. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, B.; Hong, S.; Xiong, W.; Shen, Z.; Ruan, Y.; Li, R.; Shen, Q.; Dini-Andreote, F. Promoting Soil Microbial-Mediated Suppressiveness against Fusarium Wilt Disease by the Enrichment of Specific Fungal Taxa via Crop Rotation. Biol. Fertil. Soils 2021, 57, 1137–1153. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Wang, T.; Zhang, Y.; Zhang, S.; Luo, Y. Plant Growth-Promoting Rhizobacteria HN6 Induced the Change and Reorganization of Fusarium Microflora in the Rhizosphere of Banana Seedlings to Construct a Healthy Banana Microflora. Front. Microbiol. 2021, 12, 685408. [Google Scholar] [CrossRef]
- Zou, N.; Zhou, D.; Chen, Y.; Lin, P.; Chen, Y.; Wang, W.; Xie, J.; Wang, M. A Novel Antifungal Actinomycete Streptomyces sp. Strain H3-2 Effectively Controls Banana Fusarium Wilt. Front. Microbiol. 2021, 12, 706647. [Google Scholar] [CrossRef]
- Zhu, Z.; Tian, Z.; Li, J. A Streptomyces Morookaensis Strain Promotes Plant Growth and Suppresses Fusarium Wilt of Banana. Trop. Plant Pathol. 2021, 46, 175–185. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Huang, Y.; Peng, J.; Xie, J.; Wang, W. Isolation and Evaluation of Rhizosphere Actinomycetes with Potential Application for Biocontrolling Fusarium Wilt of Banana Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4. Front. Microbiol. 2021, 12, 763038. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-P.; Ho, Y.-C. Beneficial Microbes and Basal Fertilization in Antagonism of Banana Fusarium Wilt. Agronomy 2021, 11, 2043. [Google Scholar] [CrossRef]
- Catambacan, D.G.; Cumagun, C.J.R. Weed-Associated Fungal Endophytes as Biocontrol Agents of Fusarium oxysporum f. sp. cubense TR4 in Cavendish Banana. J. Fungi 2021, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, P.; Fan, H.; Liu, L.; Yin, K.; Yang, B.; Li, Y.; Huang, S.-M.; Li, X.; Zheng, S.-J. A Real-Time Fluorescent Reverse Transcription Quantitative PCR Assay for Rapid Detection of Genetic Markers’ Expression Associated with Fusarium Wilt of Banana Biocontrol Activities in Bacillus. J. Fungi 2021, 7, 353. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Li, S.; Zeng, L.; He, P.; Xu, S.; Bai, T.; Huang, Y.; Guo, Z.; Zheng, S.-J. Biological Control of Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Natively Isolated Bacillus spp. YN0904 and YN1419. J. Fungi 2021, 7, 795. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, T.; Muthusamy, M.; Marimuthu, T. Development of Integrated Approach to Manage the Fusarial Wilt of Banana. Crop Prot. 2003, 22, 1117–1123. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Zhou, D.; Zhang, M.; Qi, D.; Jing, T.; Zang, X.; Qi, C.; Wang, W.; Xie, J. Biological Control of Banana Wilt Disease Caused by Fusarium oxysporum f. sp. cubense Using Streptomyces sp. H4. Biol. Control 2021, 155, 104524. [Google Scholar] [CrossRef]
- Ullas Prasanna, S.; Krishna, V.; Ravi Kumar, S.; Vinay Kumar, N.M.; Nayaka, S.S.; Raagavalli, K.; Ajith, S. Trichoderma Spp. Intervened Activation of Defensive Enzymes in Musa paradisiaca cv. Malnad Rasbale Plantlets. J. Biol. Control 2022, 36, 101–111. [Google Scholar] [CrossRef]
- Qi, D.; Zou, L.; Zhou, D.; Zhang, M.; Wei, Y.; Li, K.; Zhao, Y.; Zhang, L.; Xie, J. Biocontrol Potential and Antifungal Mechanism of a Novel Streptomyces sichuanensis against Fusarium oxysporum f. sp. cubense Tropical Race 4 in Vitro and in Vivo. Appl. Microbiol. Biotechnol. 2022, 106, 1633–1649. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Y.; Cai, B.; Zhou, D.; Qi, D.; Zhang, M.; Zhao, Y.; Li, K.; Wedge, D.E.; Pan, Z.; et al. Discovery of Niphimycin C from Streptomyces yongxingensis sp. nov. as a Promising Agrochemical Fungicide for Controlling Banana Fusarium Wilt by Destroying the Mitochondrial Structure and Function. J. Agric. Food Chem. 2022, 70, 12784–12795. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, B.; Li, K.; Zhao, Y.; Li, C.; Liu, S.; Xiang, D.; Zhang, L.; Xie, J.; Wang, W. Biological Control of Fusarium oxysporum f. sp. cubense Tropical Race 4 in Banana Plantlets Using Newly Isolated Streptomyces sp. WHL7 from Marine Soft Coral. Plant Dis. 2022, 106, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Zhang, M.; Zhou, D.; Jing, T.; Zang, X.; Qi, D.; Chen, Y.; Li, K.; Zhao, Y.; Tang, W.; et al. Anti-Foc RT4 Activity of a Newly Isolated Streptomyces sp. 5–10 From a Medicinal Plant (Curculigo capitulata). Front. Microbiol. 2021, 11, 610698. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Li, S.; Li, S.; Zhang, H.; Jiang, M. The Siderophore-Producing Bacterium, Bacillus Siamensis Gxun-6, Has an Antifungal Activity against Fusarium oxysporum and Promotes the Growth of Banana. Egypt J. Biol. Pest Control 2022, 32, 34. [Google Scholar] [CrossRef]
- Du, C.; Yang, D.; Ye, Y.; Pan, L.; Zhang, J.; Jiang, S.; Fu, G. Construction of a Compound Microbial Agent for Biocontrol against Fusarium Wilt of Banana. Front. Microbiol. 2022, 13, 1066807. [Google Scholar] [CrossRef] [PubMed]
- Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J.; Bubici, G. Designing a Synthetic Microbial Community Devoted to Biological Control: The Case Study of Fusarium Wilt of Banana. Front. Microbiol. 2022, 13, 967885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Wang, Y.; Zhang, J.; Wan, S.; Huang, Y.; Yun, T.; Xie, J.; Wang, W. Biocontrol Potential of Endophytic Streptomyces malaysiensis 8ZJF-21 From Medicinal Plant Against Banana Fusarium Wilt Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4. Front. Plant Sci. 2022, 13, 874819. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Thomashow, L.S.; Ou, Y.; Tao, C.; Wang, J.; Xiong, W.; Liu, H.; Li, R.; Shen, Q.; Kowalchuk, G.A. Shared Core Microbiome and Functionality of Key Taxa Suppressive to Banana Fusarium Wilt. Research 2022, 2022, 9818073. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, Y.R.; Yong, C.S.Y.; Othman, S.N.; Mohd Zainudin, N.A.I.; Mustafa, M. Isolation and Molecular Identification of a Siderophore Producing Bacterium and Its Antagonistic Effect against Fusarium oxysporum f. sp. cubense Tropical Race 4. Pertanika J. Trop. Agric. Sci. 2022, 45, 187–206. [Google Scholar] [CrossRef]
- Yang, D.; Du, C.-j.; Pan, L.-f.; Ye, Y.-f.; Fu, G. Screening, Identification and Control Efficiency of Antagonistic Bacteria for Banana Fusarium Wilt. J. South. Agric. 2023, 54, 414–423. [Google Scholar] [CrossRef]
- Yadav, K.; Damodaran, T.; Dutt, K.; Singh, A.; Muthukumar, M.; Rajan, S.; Gopal, R.; Sharma, P.C. Effective Biocontrol of Banana Fusarium Wilt Tropical Race 4 by a Bacillus Rhizobacteria Strain with Antagonistic Secondary Metabolites. Rhizosphere 2021, 18, 100341. [Google Scholar] [CrossRef]
- Long, W.; Chen, Y.; Wei, Y.; Feng, J.; Zhou, D.; Cai, B.; Qi, D.; Zhang, M.; Zhao, Y.; Li, K.; et al. A Newly Isolated Trichoderma parareesei N4-3 Exhibiting a Biocontrol Potential for Banana Fusarium Wilt by Hyperparasitism. Front. Plant Sci. 2023, 14, 1289959. [Google Scholar] [CrossRef] [PubMed]
- Taping, J.M.F.; Borja, B.T.; Bretaña, B.L.P.; Tanabe, M.E.N.; Cabasan, M.T.N. Fungal Endophytes as Potential Biocontrol Agent of Panama Disease of Banana. Egypt J. Biol. Pest Control 2023, 33, 84. [Google Scholar] [CrossRef]
- Ruan, Y.-N.; Nong, C.; Jintrawet, A.; Fan, H.; Fu, L.; Zheng, S.-J.; Li, S.; Wang, Z.-Y. A Smooth Vetch (Vicia villosa Var.) Strain Endogenous to the Broad-Spectrum Antagonist Bacillus siamensis JSZ06 Alleviates Banana Wilt Disease. Front. Plant Sci. 2024, 15, 1410197. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, G.; Deng, R.; Hu, X.; Tan, H.; Chen, Y.; Tian, Z.; Li, J. Spatiotemporal Biocontrol and Rhizosphere Microbiome Analysis of Fusarium Wilt of Banana. Commun. Biol. 2023, 6, 27. [Google Scholar] [CrossRef]
- Lv, N.; Tao, C.; Ou, Y.; Wang, J.; Deng, X.; Liu, H.; Shen, Z.; Li, R.; Shen, Q. Root-Associated Antagonistic Pseudomonas spp. Contribute to Soil Suppressiveness against Banana Fusarium Wilt Disease of Banana. Microbiol. Spectr. 2023, 11, e03525-22. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Yang, X.; Liu, B.; Chu, Y.; Liu, S.; Li, C. Bio-Priming of Banana Tissue Culture Plantlets with Endophytic Bacillus velezensis EB1 to Improve Fusarium Wilt Resistance. Front. Microbiol. 2023, 14, 1146331. [Google Scholar] [CrossRef]
- Yun, T.; Jing, T.; Zang, X.; Zhou, D.; Li, K.; Zhao, Y.; Wang, W.; Xie, J. Antimicrobial Mechanisms and Secondary Metabolite Profiles of Streptomyces hygroscopicus subsp. hygroscopicus 5–4 against Banana Fusarium Wilt Disease Using Metabolomics. Front. Microbiol. 2023, 14, 1159534. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; He, P.; Xu, S.; Li, S.; Wang, Y.; Zhang, W.; Li, X.; Shang, H.; Zeng, L.; Zheng, S.-J. Banana Disease-Suppressive Soil Drives Bacillus Assembled to Defense Fusarium Wilt of Banana. Front. Microbiol. 2023, 14, 1211301. [Google Scholar] [CrossRef]
- Luo, M.; Chen, Y.; Qing-yun, H.; Huang, Z.; Song, H.; Dong, Z. Trichoderma koningiopsis Tk905: An Efficient Biocontrol, Induced Resistance Agent against Banana Fusarium Wilt Disease and a Potential Plant-Growth-Promoting Fungus. Front. Microbiol. 2023, 14, 1301062. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Pang, Z.; Yin, S.; Xiao, W.; Hu, H.; Xie, J. Screening and Analysis of Antifungal Strains Bacillus subtilis JF-4 and B. Amylum JF-5 for the Biological Control of Fusarium Wilt of Banana. J. Fungi 2023, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Singh, P.; Qi, Y.; Singh, R.K.; Qin, Q.; Jin, C.; Wang, B.; Fang, W. Pseudomonas aeruginosa Strain 91: A Multifaceted Biocontrol Agent against Banana Fusarium Wilt. J. Fungi 2023, 9, 1047. [Google Scholar] [CrossRef]
- Nthuku, B.M.; Kahariri, E.W.; Kinyua, J.K.; Nyaboga, E.N. Fungal Endophytes of Moringa (Moringa oleifera L.), Neem (Azadirachta indica) and Lavender (Lavandula angustifolia) and Their Biological Control of Fusarium Wilt of Banana. Microbiol. Res. 2023, 14, 2113–2132. [Google Scholar] [CrossRef]
- Zakaria, M.A.T.; Sakimin, S.Z.; Ismail, M.R.; Ahmad, K.; Kasim, S.; Baghdadi, A. Biostimulant Activity of Silicate Compounds and Antagonistic Bacteria on Physiological Growth Enhancement and Resistance of Banana to Fusarium Wilt Disease. Plants 2023, 12, 1124. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Y.; Guo, L.; Yang, L.; Wang, J.; Liang, C.; Huang, J. The Effect of Banana Rhizosphere Chemotaxis and Chemoattractants on Bacillus velezensis LG14-3 Root Colonization and Suppression of Banana Fusarium Wilt Disease. Sustainability 2023, 15, 351. [Google Scholar] [CrossRef]
- Zakaria, M.A.T.; Sakimin, S.Z.; Ismail, M.R.; Ahmad, K.; Kasim, S. A Field Evaluation of Sodium Silicate and Bacillus subtilis on the Growth and Yield of Bananas Following Fusarium Wilt Disease Infection. Sustainability 2023, 15, 3141. [Google Scholar] [CrossRef]
- Sadarahalli Ullas, P.; Venkatarangaiah, K.; Ravi, K.S.; Sheshagiri, A.; Somashekar, N.S. Antagonistic Potential of Trichoderma strains Isolated from Musa paradisiaca cv. Malnad Rasbale Grown Farmyards against Foc Race 4 Pathogen. Res. J. Biotechnol. 2024, 19, 120–127. [Google Scholar]
- Chen, Y.; Li, X.; Zhou, D.; Wei, Y.; Feng, J.; Cai, B.; Qi, D.; Zhang, M.; Zhao, Y.; Li, K.; et al. Streptomyces-Secreted Fluvirucin B6 as a Potential Bio-Fungicide for Managing Banana Fusarium Wilt and Mycotoxins and Modulating the Soil Microbial Community Structure. J. Agric. Food Chem. 2024, 72, 17890–17902. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xie, J.; Qi, Y.; Wang, B.; Fang, W.; Tao, G.; Jiang, X. Screening and evaluation of the biocontrol efficacy of a Trichoderma brevicompactum strain and its metabolite trichodermin against banana Fusarium wilt. Shengwu Gongcheng Xuebao/Chin. J. Biotechnol. 2024, 40, 211–225. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Xu, S.; Li, S.; Zeng, L.; Shang, H.; Li, X.; Fan, H.; Zheng, S.-J. Biological Control of the Native Endophytic Fungus Pochonia chlamydosporia from the Root Nodule of Dolichos lablab on Fusarium Wilt of Banana TR4. Front. Microbiol. 2024, 15, 1371336. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Q.; Zou, L.; Zhang, M.; Li, K.; Zhao, Y.; Chen, Y.; Feng, J.; Zhou, D.; Wei, Y.; et al. Taxonomic Identification and Antagonistic Activity of Streptomyces luomodiensis sp. nov. against Phytopathogenic Fungi. Front. Microbiol. 2024, 15, 1402653. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yuan, J.; Zhang, J.; Shen, Z.; Zhang, M.; Li, R.; Ruan, Y.; Shen, Q. Effects of Novel Bioorganic Fertilizer Produced by Bacillus amyloliquefaciens W19 on Antagonism of Fusarium Wilt of Banana. Biol. Fertil. Soils 2013, 49, 435–446. [Google Scholar] [CrossRef]
- Selvaraj, S.; Ganeshamoorthi, P.; Anand, T.; Raguchander, T.; Seenivasan, N.; Samiyappan, R. Evaluation of a Liquid Formulation of Pseudomonas fluorescens against Fusarium oxysporum f. sp. cubense and Helicotylenchus multicinctus in Banana Plantation. BioControl 2014, 59, 345–355. [Google Scholar] [CrossRef]
- Zhang, N.; He, X.; Zhang, J.; Raza, W.; Yang, X.-M.; Ruan, Y.-Z.; Shen, Q.-R.; Huang, Q.-W. Suppression of Fusarium Wilt of Banana with Application of Bio-Organic Fertilizers. Pedosphere 2014, 24, 613–624. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Ruan, Y. Effects of Bio-Organic Fertilizers Produced by Four Bacillus amyloliquefaciens strains on Banana Fusarium Wilt Disease. Compos. Sci. Util. 2015, 23, 185–198. [Google Scholar] [CrossRef]
- Wang, B.; Shen, Z.; Zhang, F.; Raza, W.; Yuan, J.; Huang, R.; Ruan, Y.; Li, R.; Shen, Q. Bacillus amyloliquefaciens Strain W19 Can Promote Growth and Yield and Suppress Fusarium Wilt in Banana Under Greenhouse and Field Conditions. Pedosphere 2016, 26, 733–744. [Google Scholar] [CrossRef]
- Khan, B.; Akash, Z.; Asad, S.; Javed, N.; Rajput, N.A.; Jabbar, A.; Din, W.U.; Atif, R.M. Antagonistic Potential of Trichoderma harzianum against Fusarium oxysporum f. sp. cubense Associated with Panama Wilt of Banana. Pak. J. Phytopathol. 2017, 29, 111–116. [Google Scholar] [CrossRef]
- Ivayani, I.; Ginting, C.; Yusnita, Y.; Dirmawati, S.R. Effectiveness of the Application of Organic Matter and Trichoderma viride from Suppresive Soil to Contol Fusarium Wilt on Banana Plant. J. Trop. Plant Pests Dis. 2018, 18, 119–126. [Google Scholar] [CrossRef][Green Version]
- Din, S.; Sakimin, S.; Sijam, K.; Baghdadi, A.; Zakaria, M. Potential of Bacillus subtilis Inoculated on Biorichar Amended Soil for Suppression of Fusarium Wilt, Biochemical Changes and Leaf Gas Exchange under Water Stress Condition of Banana (Musa acuminata) Cv. Berangan. Fundam. Appl. Agric. 2018, 4, 515. [Google Scholar] [CrossRef]
- Proboningrum, A.; Hadiwiyono; Widono, S. Sholahuddin Effectivity and Compatibility of Azotobacter and Bacillus for Biological Control Agents of Fusarium Wilt on Banana Seedlings. IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012003. [Google Scholar] [CrossRef]
- Rahman, S.S.M.S.A.; Zainudin, N.A.I.M.; Aziz, N.A.A. Evaluation of Trichoderma asperellum B1902 in Controlling Fusarium Wilt of Cavendish Banana Cultivar. Sains Malays. 2021, 50, 2549–2561. [Google Scholar] [CrossRef]
- Tao, C.; Wang, Z.; Liu, S.; Lv, N.; Deng, X.; Xiong, W.; Shen, Z.; Zhang, N.; Geisen, S.; Li, R.; et al. Additive Fungal Interactions Drive Biocontrol of Fusarium Wilt Disease. New Phytol. 2023, 238, 1198–1214. [Google Scholar] [CrossRef]
- Gonzalez, M.F.; Magdama, F.; Galarza, L.; Sosa, D.; Romero, C. Evaluation of the Sensitivity and Synergistic Effect of Trichoderma reesei and Mancozeb to Inhibit under in Vitro Conditions the Growth of Fusarium oxysporum. Commun. Integr. Biol. 2020, 13, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Masrukhin; Putri, A.L.; Sulistiyani, T.R.; Ilyas, M.; Purnaningsih, I.; Saskiawan, I.; Niam, M.Y. Antifungal Activity of Bacterial Isolates from Straw Mushroom Cultivation Medium against Phytopathogenic Fungi. J. Trop. Biodivers. Biotechnol. 2021, 6, 59235. [Google Scholar] [CrossRef]
- Hadi, A.E.; Khalisha, A.; Pambudi, A.; Effendi, Y. Potential of Bacteria Consortium as Growth Controller of Pathogenic Fungi Fusarium oxysporum f. sp. cubense (Foc). IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 012029. [Google Scholar] [CrossRef]
- Ravi, S.; Sevugapperumal, N.; Nallusamy, S.; Shanmugam, H.; Mathiyazhagan, K.; Rangasamy, A.; Akkanna Subbiah, K.; Varagur Ganesan, M. Differential Bacterial Endophytome in Foc-resistant Banana Cultivar Displays Enhanced Antagonistic Activity against Fusarium oxysporum f. sp. cubense (Foc). Environ. Microbiol. 2022, 24, 2701–2715. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Cai, J.; Liu, X.; Huang, G. Antifungal Activity of Volatile Compounds Generated by Endophytic Fungi Sarocladium brachiariae HND5 against Fusarium oxysporum f. sp. cubense. PLoS ONE 2021, 16, e0260747. [Google Scholar] [CrossRef]
- Singh, P.; Xie, J.; Qi, Y.; Qin, Q.; Jin, C.; Wang, B.; Fang, W. A Thermotolerant Marine Bacillus amyloliquefaciens S185 Producing Iturin A5 for Antifungal Activity against Fusarium oxysporum f. sp. cubense. Mar. Drugs 2021, 19, 516. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, G.M.; Marinho-Prado, J.S.; Assalin, M.R.; Martins, L.G.; Braga, E.S.; Tasic, L.; Dita, M.; Lopes, R.B. Natural Occurrence of Beauveria caledonica, Pathogenicity to Cosmopolites Sordidus and Antifungal Activity against Fusarium oxysporum f. sp. cubense. Pest Manag. Sci. 2022, 78, 4458–4470. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Lin, B.; Zhang, R. A Novel Antifungal Protein of Bacillus subtilis B25. SpringerPlus 2013, 2, 543. [Google Scholar] [CrossRef] [PubMed]
- Galarza, L.; Akagi, Y.; Takao, K.; Kim, C.S.; Maekawa, N.; Itai, A.; Peralta, E.; Santos, E.; Kodama, M. Characterization of Trichoderma Species Isolated in Ecuador and Their Antagonistic Activities against Phytopathogenic Fungi from Ecuador and Japan. J. Gen. Plant Pathol. 2015, 81, 201–210. [Google Scholar] [CrossRef]
- Khushboo; Gangwar, M. Antifungal Activity of Endophytic Actinomyetes against Fusarium Wilt (Fusarium Oxysporum) of Banana Trees (Musa acuminata). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 328–337. [Google Scholar] [CrossRef][Green Version]
- Karim, H.; Hamka, L.; Kurnia, N.; Junda, M. Effectivity of Anatagonistic Bacteria in Controlling of Fusarium Wilt Diseases of Banana (Musa paradisiaca) by In Vitro. J. Phys. Conf. Ser. 2018, 1028, 012014. [Google Scholar] [CrossRef]
- Widyantoro, A.; Hadiwiyono; Subagiya. Antagonism and Compatibility of Biofertilizer Bacteria toward Fusarium oxysporum f. sp. cubense. Asian J. Agric. Biol. 2019, 7, 263–268. [Google Scholar]
- Florencio-Anastasio, J.G.; García-Ávila, C.d.J.; Alarcón, A.; Ferrera-Cerrato, R.; Quezada-Salinas, A.; Almaraz-Suárez, J.J.; Moreno-Velázquez, M.; Hernández-Ramos, L. Effectiveness of Antagonistic Bacteria, Commercial Fungicides, and Fourth Generation Quaternary Ammonium Salts, against Fusarium oxysporum f. sp. cubense Race “1 or 2”. Eur. J. Plant Pathol. 2022, 163, 719–731. [Google Scholar] [CrossRef]
- Aguayo, J.; Cerf-Wendling, I.; Folscher, A.B.; Fourrier-Jeandel, C.; Ioos, R.; Mathews, M.C.; Mostert, D.; Renault, C.; Wilson, V.; Viljoen, A. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 (TR4) Causing Banana Wilt in the Island of Mayotte. Plant Dis. 2021, 105, 219. [Google Scholar] [CrossRef]
- Viljoen, A. The Status of Fusarium Wilt (Panama Disease) of Banana in South Africa. S. Afr. J. Sci. 2002, 98, 341–344. [Google Scholar]
- Li, C.; Zuo, C.; Beukes, I.; Yang, Q.; Sheng, Q.; Kuang, R.; Wei, Y.; Hu, C.; Rose, L.; Karangwa, P.; et al. Diversity and Distribution of the Banana Wilt Pathogen Fusarium oxysporum f. sp. cubense in China. Fungal Genom. Biol. 2013, 3, 2. [Google Scholar] [CrossRef]
- Damodaran, T.; Mishra, V.K.; Jha, S.K.; Gopal, R.; Rajan, S.; Ahmed, I. First Report of Fusarium Wilt in Banana Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 in India. Plant Dis. 2019, 103, 1022. [Google Scholar] [CrossRef]
- Food and Agriculture Organization Crops and Livestock Products Statistics. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 November 2024).
- Clement, W.K.F.; Vadamalai, G.; Saidi, N.B.; Zulperi, D. Research Progress, Challenges, Future Perspectives on the Management of Fusarium Wilt of Banana in Malaysia: A Review. Malays. J. Sci. 2019, 38, 47–66. [Google Scholar] [CrossRef]
- Köberl, M.; Dita, M.; Martinuz, A.; Staver, C.; Berg, G. Members of Gammaproteobacteria as Indicator Species of Healthy Banana Plants on Fusarium Wilt-Infested Fields in Central America. Sci. Rep. 2017, 7, 45318. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Caraballoso, I.; Cruz-Martín, M.; Concepción-Hernández, M. Biotechnological Tools for the Development of Foc TR4-Resistant or -Tolerant Musa spp. Cultivars. In Agricultural, Forestry and Bioindustry Biotechnology and Biodiscovery; Chong, P.A., Newman, D.J., Steinmacher, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 403–431. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, N.; Huang, Q.; Raza, W.; Li, R.; Vivanco, J.M.; Shen, Q. Organic Acids from Root Exudates of Banana Help Root Colonization of PGPR Strain Bacillus amyloliquefaciens NJN-6. Sci. Rep. 2015, 5, srep13438. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Nakkeeran, S.; Saranya, N.; Kavino, M.; Ragapriya, V.; Varanavasiappan, S.; Raveendran, M.; Krishnamoorthy, A.S.; Malathy, V.G.; Haripriya, S. Biohardening of Banana Cv. Karpooravalli (ABB; Pisang Awak) with Bacillus velezensis YEBBR6 Promotes Plant Growth and Reprograms the Innate Immune Response Against Fusarium oxysporum f. sp. cubense. Front. Sustain. Food Syst. 2022, 6, 845512. [Google Scholar] [CrossRef]
- Prigigallo, M.I.; De Stradis, A.; Anand, A.; Mannerucci, F.; L’Haridon, F.; Weisskopf, L.; Bubici, G. Basidiomycetes Are Particularly Sensitive to Bacterial Volatile Compounds: Mechanistic Insight Into the Case Study of Pseudomonas protegens Volatilome Against Heterobasidion abietinum. Front. Microbiol. 2021, 12, 684664. [Google Scholar] [CrossRef]
- He, P.; Li, S.; Xu, S.; Fan, H.; Wang, Y.; Zhou, W.; Fu, G.; Han, G.; Wang, Y.-Y.; Zheng, S.-J. Monitoring Tritrophic Biocontrol Interactions Between Bacillus spp., Fusarium oxysporum f. sp. cubense, Tropical Race 4, and Banana Plants in Vivo Based on Fluorescent Transformation System. Front. Microbiol. 2021, 12, 754918. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, J.; Li, S.; Chen, F.; Song, C.; Zhang, H.; Jiang, M.; Shen, N. Comparative Transcriptome Analysis Unravels the Response Mechanisms of Fusarium oxysporum f. sp. cubense to a Biocontrol Agent, Pseudomonas aeruginosa Gxun-2. Int. J. Mol. Sci. 2022, 23, 15432. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.F.; Zulperi, D.; Saidi, N.B.; Vadamalai, G. A Consortium of Pseudomonas aeruginosa and Trichoderma harzianum for Improving Growth and Induced Biochemical Changes in Fusarium Wilt Infected Bananas. Trop. Life Sci. Res. 2021, 32, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Yang, P. The Gene Task1 Is Involved in Morphological Development, Mycoparasitism and Antibiosis of Trichoderma asperellum. Biocontrol Sci. Technol. 2017, 27, 620–635. [Google Scholar] [CrossRef]
- Utami, U.; Nisa, C.; Putri, A.Y.; Rahmawati, E. The Potency of Secondary Metabolites Endophytic Fungi Trichoderma sp. as Biocontrol of Colletotrichum sp. and Fusarium oxysporum Causing Disease in Chili. AIP Conf. Proc. 2019, 2120, 080020. [Google Scholar] [CrossRef]
- Troppens, D.M.; Morrissey, J.P. Metabolite-Mediated Interactions Between Bacteria and Fungi. In Biocommunication of Fungi; Witzany, G., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 207–218. [Google Scholar] [CrossRef]
- Rabari, A.; Ruparelia, J.; Jha, C.K.; Sayyed, R.Z.; Mitra, D.; Priyadarshini, A.; Senapati, A.; Panneerselvam, P.; Das Mohapatra, P.K. Articulating Beneficial Rhizobacteria-Mediated Plant Defenses through Induced Systemic Resistance: A Review. Pedosphere 2023, 33, 556–566. [Google Scholar] [CrossRef]
- Segarra, G.; Van Der Ent, S.; Trillas, I.; Pieterse, C.M.J. MYB72, a Node of Convergence in Induced Systemic Resistance Triggered by a Fungal and a Bacterial Beneficial Microbe. Plant Biol. 2009, 11, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Fountaine, J.M. Practical Application of Induced Resistance to Plant Diseases: An Appraisal of Effectiveness under Field Conditions. J. Agric. Sci. 2009, 147, 523–535. [Google Scholar] [CrossRef]
- Karthika, S.; Remya, M.; Varghese, S.; Dhanraj, N.D.; Sali, S.; Rebello, S.; Jose, S.M.; Jisha, M.S. Bacillus tequilensis PKDN31 and Bacillus licheniformis PKDL10–As Double Headed Swords to Combat Fusarium oxysporum f. sp. lycopersici Induced Tomato Wilt. Microb. Pathog. 2022, 172, 105784. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, J.; Małolepsza, U. Diversity in Plant Systemic Resistance Induced by Trichoderma. Biol. Control 2013, 67, 149–156. [Google Scholar] [CrossRef]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic Mycoparasites and Their Genomes. Microbiol. Spectr. 2017, 5, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, M. Fungal Enzymes in Biocontrol of Phytopathogens. In Progress in Mycology; Satyanarayana, T., Deshmukh, S.K., Deshpande, M.V., Eds.; Springer Nature: Singapore, 2021; pp. 327–356. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, X.; Ran, W.; Shen, Q. Fusarium oxysporum Induces the Production of Proteins and Volatile Organic Compounds by Trichoderma harzianum T-E5. FEMS Microbiol. Lett. 2014, 359, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bekkar, A.A.; Belabid, L.; Zaim, S. Biocontrol of Phytopathogenic Fusarium spp. by Antagonistic Trichoderma. Biopest. Int. 2016, 12, 37–45. [Google Scholar]
- Wood, J.; Ashby, B. Hyperparasitism and the Evolution of Parasite Virulence. Evolution 2023, 77, 2631–2641. [Google Scholar] [CrossRef]
- Mohammad, A.; Hadi, G.; Masoud, A. Evaluation of Different Combinations of Trichoderma Species for Controlling Fusarium Rot of Lentil. Afr. J. Biotechnol. 2011, 10, 2653–2658. [Google Scholar] [CrossRef]
- Yusnawan, E.; Inayati, A.; Baliadi, Y. Isolation of Antagonistic Fungi from Rhizospheres and Its Biocontrol Activity against Different Isolates of Soil Borne Fungal Pathogens Infected Legumes. Biodiversitas 2019, 20, 2048–2054. [Google Scholar] [CrossRef]
- Woodward, S.; Boddy, L. Interactions between Saprotrophic Fungi. In British Mycological Society Symposia Series; Woodward, S., Boddy, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 28, pp. 125–141. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Wang, B.; Zhong, S.; Su, L.; Li, R.; Shen, Q. Effect of Biofertilizer for Suppressing Fusarium Wilt Disease of Banana as Well as Enhancing Microbial and Chemical Properties of Soil under Greenhouse Trial. Appl. Soil Ecol. 2015, 93, 111–119. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D Produced by Bacillus amyloliquefaciens Is Involved in the Antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075-17. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Huang, Q.; Shen, Q. The Ultrasound-assisted Extraction and Identification of Antifungal Substances from B. amyloliquefaciens Strain NJN-6 Suppressing Fusarium oxysporum. J. Basic Microbiol. 2012, 52, 721–730. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Xu, H.; Zhang, F.; Zhang, X.; Yan, Y.; He, L.; Liu, J. Isolation of Lipopeptide Antibiotics from Bacillus siamensis: A Potential Biocontrol Agent for Fusarium Graminearum. Can. J. Microbiol. 2022, 68, 403–411. [Google Scholar] [CrossRef]
- Báez-Astorga, P.A.; Cázares-Álvarez, J.E.; Cruz-Mendívil, A.; Quiroz-Figueroa, F.R.; Sánchez-Valle, V.I.; Maldonado-Mendoza, I.E. Molecular and Biochemical Characterisation of Antagonistic Mechanisms of the Biocontrol Agent Bacillus cereus B 25 Inhibiting the Growth of the Phytopathogen Fusarium Verticillioides P03 during Their Direct Interaction in Vitro. Biocontrol Sci. Technol. 2022, 32, 1074–1094. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Al-Ani, L.K.T.; Bekbayeva, L.; Salleh, B. Biological Control of Fusarium oxysporum f. sp. cubense by Pseudomonas fluorescens and BABA in Vitro. World Appl. Sci. J. 2011, 15, 189–191. [Google Scholar]
- Gleeson, O.; O’Gara, F.; Morrissey, J.P. The Pseudomonas Fluorescens Secondary Metabolite 2,4 Diacetylphloroglucinol Impairs Mitochondrial Function in Saccharomyces cerevisiae. Antonie Van Leeuwenhoek 2010, 97, 261–273. [Google Scholar] [CrossRef]
- Sharma, D.; Gupta, M.; Gupta, S.; Jaglan, S.; Mallick, S.A. Characterization of Secondary Metabolites Produced during Interaction of Pseudomonas fluorescens with Fusarium Oxysporum. Indian J. Agric. Sci. 2019, 89, 998–1004. [Google Scholar] [CrossRef]
- Quecine, M.C.; Kidarsa, T.A.; Goebel, N.C.; Shaffer, B.T.; Henkels, M.D.; Zabriskie, T.M.; Loper, J.E. An Interspecies Signaling System Mediated by Fusaric Acid Has Parallel Effects on Antifungal Metabolite Production by Pseudomonas protegens strain Pf-5 and Antibiosis of Fusarium spp. Appl. Environ. Microbiol. 2016, 82, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Thi Thanh Dang, T.; Thi Thanh Nguyen, M.; Thi Nguyen, T.; Hong Pham, H.; Tran, V.-T.; Tran, D.T.; Nguyen, C.X. Characterisation of Streptomyces sp. VNUA116 with Strong Antifungal Activity against Fusarium oxysporum f. sp. cubense Tropical Race 4. Arch. Phytopathol. Plant Prot. 2024, 57, 315–330. [Google Scholar] [CrossRef]
- Patel, S.; Ahmed, S.; Eswari, J.S. Therapeutic Cyclic Lipopeptides Mining from Microbes: Latest Strides and Hurdles. World J. Microbiol. Biotechnol. 2015, 31, 1177–1193. [Google Scholar] [CrossRef]
- Sharma, M.; Manhas, R.K. Purification and Characterization of Salvianolic Acid B from Streptomyces sp. M4 Possessing Antifungal Activity against Fungal Phytopathogens. Microbiol. Res. 2020, 237, 126478. [Google Scholar] [CrossRef] [PubMed]
- Sanó, L.; Oliveira, L.L.B.D.; Leão, M.D.M.; Santos, J.E.D.Á.D.; Medeiros, S.C.D.; Schneider, F.; Sousa, A.B.O.D.; Taniguchi, C.A.K.; Muniz, C.R.; Grangeiro, T.B.; et al. Trichoderma longibrachiatum as a Biostimulant of Micropropagated Banana Seedlings under Acclimatization. Plant Physiol. Biochem. 2022, 190, 184–192. [Google Scholar] [CrossRef]
- Hazarika, T.K.; Nautiyal, B.P.; Bhattacharyya, R.K. Conjunctive Use of Bio-Fertilizers and Organics for Improving Growth, Yield and Quality of Banana Cv. Grand Naine. Indian J. Hortic. 2015, 72, 461. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; De Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Uma Shaanker, R. Inhibition of Plant Pathogenic Fungi by Endophytic Trichoderma spp. through Mycoparasitism and Volatile Organic Compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, B.; Verma, S.; Pal, J.; Mukesh; Akanksha; Chauhan, P. Unveiling the Biocontrol Potential of Trichoderma. Eur. J. Plant Pathol. 2023, 167, 569–591. [Google Scholar] [CrossRef]
- Silva, R.N.; Monteiro, V.N.; Steindorff, A.S.; Gomes, E.V.; Noronha, E.F.; Ulhoa, C.J. Trichoderma/Pathogen/Plant Interaction in Pre-Harvest Food Security. Fungal Biol. 2019, 123, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Najeeb, S.; Chen, J.; Wang, R.; Zhang, J.; Hou, J.; Liu, T. Insights into the Molecular Mechanism of Trichoderma Stimulating Plant Growth and Immunity against Phytopathogens. Physiol. Plant. 2023, 175, e14133. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Sharma, A.; Kaur, R.; Sharma, R.; Sharma, V. The Riddles of Trichoderma Induced Plant Immunity. Biol. Control 2022, 174, 105037. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Zhou, D.; Qi, D.; Li, K.; Tang, W.; Chen, Y.; Jing, T.; Zang, X.; Xie, J.; et al. A Newly Isolated Streptomyces sp. YYS-7 with a Broad-Spectrum Antifungal Activity Improves the Banana Plant Resistance to Fusarium oxysporum f. sp. cubense Tropical Race 4. Front. Microbiol. 2020, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.M.; Cairo, P.A.R.; Borges, A.L.; Silva, L.D.D.; Haddad, F. Investigating the Ideal Mixture of Soil and Organic Compound with Bacillus sp. and Trichoderma asperellum Inoculations for Optimal Growth and Nutrient Content of Banana Seedlings. S. Afr. J. Bot. 2021, 137, 249–256. [Google Scholar] [CrossRef]
- González-Arriagada, M.P.; Heck, D.W.; Silva, R.A.; Santos, A.; Alves, G.; Del Ponte, E.M.; Mizubuti, E.S.G. Spatiotemporal Dynamics of Fusarium Wilt of Banana Caused by Subtropical Race 4. Trop. Plant Pathol. 2024, 49, 886–897. [Google Scholar] [CrossRef]
| MCBA | Origin of MCBAs | Level | Doses | Efficacy (%) | Measure | Mechanism | Variety | Reference |
|---|---|---|---|---|---|---|---|---|
| Pseudomonas fluorescens Bacillus spp. | Pseudomonas fluorescens: ND Bacillus spp.: banana stems | Seedling trial | 3 mL/plant (3∙108 CFU/mL) | 74.05 | Control | Resistance induction | Red Banana (AAA) | [25] |
| Achromobacter Bacillus cereus | Rhizosphere and banana plants | Experimental-plot trial | 50 g/plant (108 cells/g) | 4.2 | Incidence | Antibiosis | Musa acuminata AAA cv. Grand Naine | [26] |
| Trichoderma sp. Trichoderma asperellum | Banana rhizosphere | Experimental-plot trial | 50 g/plant (106 spores/mL) | 47.63 | Incidence | Antibiosis | Musa acuminata AAA cv. Grand Naine | [27] |
| Glomus mossae Pseudomonas spp. | Banana rhizosphere | Seedling trial | Glomus: 250 g/plant (80 spores/100 g of soil); Pseudomonas: 15 mL/plant (108 cells/mL) | 100 | Severity | Competitive inhiitibon | Musa acuminata AAA cv. Grand Naine | [28] |
| Trichoderma asperellum | Banana plant | Seedling trial | ND (106 CFU/mL) | 50 | Control | Antibiosis, mycoparasitism | Gros Michel (AAA) | [29] |
| Bacillus subtilis Pseudomonas fluorescens | Pseudomonas fluorescens: Rice plants Bacillus subtilis: Banana plants | Experimental-plot trial | ND (3∙1010 CFU/mL) | 78 | Control | Resistance induction | Red Banana (AAA) | [30] |
| Bacillus spp. | Bank | Seedling trial | ND | 100 | Severity | Antibiosis | Prata-Anã | [31] |
| Pseudomonas fluorescens | Bacillus siamensis: Banana plant Pseudomonas fluorescens: Bank | Seedling trial | 200 mL/plant (108 CFU/mL) | 30 | Incidence | Antibiosis, systemic resistance induction | Pisang Awak (Namwa) banana (Musa spp. ABB) | [32] |
| Bacillus amyloliquefaciens | Banana plant | Seedling trial | ND | 25 | Inhibition | Antibiosis and Enzyme production | Gros Michel (AAA) | [33] |
| Trichoderma sp. | Maize rhizosphere | Dual-culture trial | NA | 52.2 | inhibition | Enzyme production | NA | [34] |
| Bacillus sp. | Banana plant | Dual-culture trial | NA | 96 | Inhibition | Antibiosis, enzyme production, systemic resistance induction | NA | [35] |
| Trichoderma sp. Pseudomonas fluorescens | Banana rhizosphere | Seedling trial | 15 g/pot | Trichoderma sp. + P. fluorescens 63.43 T. asperellum + P. fluorescens: 63.43 | Control | Mycoparasitism, antibiosis, resistance induction | Rasthali (Silk-AAB) | [36] |
| Trichoderma harzianum Trichoderma tomentosum | Maize roots and rhizosphere | Dual-culture trial | NA | 54 | Inhibition | Mycoparasitism, enzyme production, antibiosis | NA | [37] |
| Streptomyces spp. | Rhizosphere of tomato plants | Seedling trial | 10 mL/plant | 78.1 | Control | Enzyme production | Musa (ABB Group) ‘Pakchong 50’ | [38] |
| Bacillus spp. Trichoderma spp. | Commercial products in Colombia | Seedling trial | 100 mL/plant (106 conidia of Trichoderma/107 CFU of Bacillus) | 73.9 | Incidence | Antibiosis, enzyme production, systemic resistance induction | Gros Michel (AAA) and Cavendish cv. Williams (as a control) | [39] |
| MCBA | Origin of MCBAs | Level | Doses | Efficacy (%) | Measure | Mechanism | Variety | Reference |
|---|---|---|---|---|---|---|---|---|
| Penicillium citrinum | Wild banana plant | Seedling trial | 50 mL/plant (106 CFU/mL) | 27.05 | Incidence | Systemic resistance induction | Berangan | [40] |
| Streptomyces griseus | Soil | Plate assay | 5 mL/200 g of soil (108 CFU/mL) | 66 | Abundance | Mycoparasitism | NA | [41] |
| Bacillus amyloliquefaciens | Banana rhizosphere | Seedling trial | 1.5% soil weight (109 CFU/g) | 28 | Incidence | Antibiosis | Musa AAA Cavendish cv. Brazil | [42] |
| Bacillus pumilus | Banana rhizosphere | Dual-culture trial | NA | 42.47 | Inhibition | Antibiosis, enzyme production | NA | [43] |
| Bacillus amyloliquefaciens | Banana plant | Dual-culture trial | NA | 30 | Inhibition | ND | NA | [44] |
| Bacillus amyloliquefaciens | ND | Seedling trial | 450 g/pot (109 CFU/g) | 75 | Incidence | Antibiosis | Musa acuminata AAA Cavendish cv. Brazil. | [45] |
| Burkholderia cenocepacia | Roots of Chrysopogon zizanioides | Experimental-plot trial | 10 mL/plant (6–7 OD 60 nm of cells) | 86.32 | Incidence | Antibiosis | Musa acuminata AAA Cavendish cv. Pei-Chiao | [46] |
| Bacillus amyloliquefaciens | Banana rhizosphere | Experimental-plot trial | In seedlings: 4% w/w in pots. After transplanting: 500 units/plant | 68.5 | Incidence | Antibiosis | Musa AAA Cavendish cv. Brazil | [47] |
| Bacillus amyloliquefaciens | Banana rhizosphere | Seedling trial | 2% w/w (109 CFU/g) | 64.97 | Incidence | Antibiosis | Musa AAA Cavendish cv. Brazil | [48] |
| Serratia marcescens | Rubber tree rhizosphere | Experimental-plot trial | 100 mL/plant (108 CFU/mL) | 70 | Severity | Enzyme production | Williams (Cavendish subgroup) | [49] |
| Bacillus amyloliquefaciens | Banana rhizosphere | Experimental-plot trial | 8 a 12 tn/ha per year (108 CFU/g) | 70 | Incidence | ND | Musa acuminata AAA Cavendish cv. Brazil | [50] |
| Streptomyces lunalinharesii | Soil of the banana crop | Seedling trial | 100 mL/plant (of the diluted ferment 1/50) | 72.72 | Control | ND | “Nantian Huang” and “Brazilian bananas” | [51] |
| Bacillus velezensis | Tomato rhizosphere | Seedling trial | 50 mL/seedling (~106 cells per gram of soil) | 44 | Incidence | Antibiosis | Cavendish banana seedling ‘Brazilian’ | [52] |
| Streptomyces sp. | Rhizosphere of Opuntia stricta | Dual-culture trial | Crude ethanolic extract of Streptomyces at 100 µg/mL | 67.59 | Inhibition | Antibiosis | NA | [53] |
| Bacillus velezensis | Banana rhizosphere | Seedling trial | 0.5% w/w (108 CFU/g) | 66.7 | Control | Competition | Musa AAA Cavendish cv. Brazil | [54] |
| Bacillus amyloliquefaciens | Banana rhizosphere | Seedling trial | 180 g/seedling 109 CFU/g) | 55 | Control | Antibiosis, competition | Musa acuminata Cavendish cv. Brazil. | [55] |
| Schizophyllum | Plant ND | Seedling trial | 20 mL suspension (5∙104 spores/plant) | 78.57 | Incidence | Antibiosis, mycoparasitism, competition | Musa acuminata AAA Cavendish cv. Gran Naine and GCTCV 219 | [56] |
| Pseudomonas aeruginosa Trichoderma harzianum | Bank | Seedling trial | 50 g/plant | 66.67 | Severity | ND | Musa acuminata AAA Berangan | [57] |
| Trichoderma harzianum, Glomus spp. | Trichoderma harzianum: Commercial inoculant; Glomus spp.: commercial mycorrhiza | Seedling trial | Trichoderma harzianum: 3∙105 conidia/g; Glomus spp.: 5 g/plant (does not specify cubic centimeters (cc) | 100 | Control | ND | Lakatan banana seedlings | [58] |
| Serendipita indica | ND | Seedling trial | 100 mL/kg of soil (105 chlamydospores/mL) | ND | Severity | Systemic resistance induction | Musa acuminata cv. Tianbaojiao | [59] |
| Streptomyces manipurensis | Banana rhizosphere | Seedling trial | 50 mL/plant | 78.95 | Incidence | Antibiosis | NA | [60] |
| B. amyloliquefaciens Pseudomonas spp. | Banana rhizosphere | Seedling trial | 108 CFU/g of substrate per plant | 91.66 | Abundance | Antibiosis | Cavendish cv. Brazil | [61] |
| Trichoderma reesei, Trichoderma asperellum, Trichoderma koningiopsis | Trichoderma reesei: banana rhizosphere. Trichoderma asperellum: banana rhizosphere. Trichoderma koningiopsis: rhizosphere of Saccharam spontaneum | Experimental-plot trial | 500 mL of a 3% formulation (108 spores/mL) | 85.19 | Inhibition | Mycoparasitism, resistance induction | Musa acuminata AAA Cavendish cv. Grand Naine (G-9) | [62] |
| Streptomyces violaceusniger | Soil of the banana crop | Seedling trial | 100 mL/plant (ND concentration) | 64.94 | Control | Enzyme production | Musa acuminata AAA Cavendish cv. Brazilian | [63] |
| Bacillus velezensis | Banana rhizosphere | Seedling trial | 500 mL (50 times diluted from 108 CFU/mL of Bacillus) | ND | Incidence | Resistance induction, microbial community modification | Musa acuminata cv Cavendish | [64] |
| Consorcio: Bacillus subtilis, Bacillus velezensis, Penicillium sp. | Banana rhizosphere | Seedling trial | Bacillus: 5 mL per 1.5∙108 CFU/mL Penicillium: 1.8∙103 spore/mL | 60.4 | Control | Enzyme production, mycoparasitism, antibiosis | Musa acuminata William B6 | [65] |
| Trichoderma asperellum | Banana rhizosphere | Seedling trial | 5 mL; 2.4∙103 spore/mL per planta | 32.38 | Severity | Antibiosis, mycoparasitism | Williams B6 | [66] |
| Streptomyces sp. | Soil of the banana crop | Seedling trial | 100 mL/700 g of soil (of the filtered and diluted ferment 1/50) | 83.12 | Control | Antibiosis | Not mentioned (the paper does not specify the variety of banana used in the experiments) | [67] |
| Streptomyces huiliensis | Rhizosphere of Opuntia stricta | Dual-culture trial | NA | 62.55% (Foc R4) 44.51% (Foc R1) | Inhibition | Antibiosis | NA | [68] |
| Streptomyces hygroscopicus | Roots of Piper austrosinense | Seedling trial | ND (106 CFU/mL) | 71.36 | Control | Enzyme production, systemic resistance induction | Musa AAA Cavendish ‘Brazil’ | [69] |
| Pseudomonas aeruginosa | Compost | Dual-culture trial | ND | 75 | Inhibition | Systemic resistance induction, enzyme production | NA | [70] |
| Bacillus amyloliquefaciens | Banana plant | Dual-culture trial | NA | 85.72 | Inhibition | Antibiosis | NA | [71] |
| Talaromyces pinophilus, Clonostachys rossmaniae | Soil | Seedling trial | 1.5 kg of soil (105 CFU/g) | ND | ND | Antibiosis and competition for resources | Musa acuminata AAA Cavendish cv. Brazilian | [72] |
| Streptomyces aureoverticillatus | ND | Seedling trial | 200 mL/plant (105 CFU/mL) | 86.09 | Control | Antibiosis | Cavendish banana subgroup cv. Brazil | [73] |
| Streptomyces sp. | Banana rhizosphere | Seedling trial | 100 mL/plant (107 CFU/mL) | 89.4 | Control | Antibiosis | Cavendish banana | [74] |
| Streptomyces morookaensis | Bank | Seedling trial | 100 mL/plant (106 spores/mL) | 78.12 | Incidence | Antibiosis | Banana variety Brazilian (Musa sp., AAA, Cavendish subgroup) | [75] |
| Streptomyces sp. nov. | Rhizosphere of Machilus pingii | Dual-culture trial | NA | 80.48 | Inhibition | Antibiosis | NA | [76] |
| Bacillus mycoies | Bank | Dual-culture trial | NA | 61.1 | Inhibition | Antibiosis, systemic resistance induction | NA | [77] |
| Ceratobasidium sp. | Banana crop weeds | Seedling trial | 50 mL/plant | 28.94 | Severity | Mycoparasitism, antibiosis, competition for nutrients | Grand Nine GCTCV 218 | [78] |
| Bacillus | Banana cultivation in vitro tissue seedlings | Dual-culture trial | NA | 79.63 | Inhibition | Antibiosis | NA | [79] |
| B. amyloliquefascens | Infected banana plants | Seedling trial | 40 mL/plant | 85.61 | Control | Mycoparasitism, antibiosis, systemic resistance induction | Cavendish | [80] |
| Bacillus velezencis | Banana plant | Seedling trial | 1% (108 CFU/mL) | 100 | Incidence | Antibiosis, resistance induction, hyperparasitism | Karpooravalli | [81] |
| Streptomyces sp. | Coral Dichotella gemmacea | Seedling trial | 1 kg of soil (106 CFU/g of soil) | ND | Incidence | Antibiosis | Banana (Baxi Jiao, Musa acuminata AAA genotype cv. Cavendish) | [82] |
| Trichoderma harzianum and Trichoderma viride | Banana cultivation | Seedling trial | ND | ND | ND | Antibiosis and enzyme production | Musa paradisiaca cv. Malnad Rasbale | [83] |
| Streptomyces sichuanensis | Rhizosphere of Opuntia stricta | Seedling trial | 100 mL/plant | 51.01 | Control | Antibiosis | Cavendish cultivar ‘Brazilian’ (AAA) | [84] |
| Streptomyces yongxingensis | Coral | Dual-culture trial | NA | 75.42 | Inhibition | Antibiosis | NA | [85] |
| Streptomyces sp. | Coral | Seedling trial | 106 CFU/g of soil | 80 | Inhibition | Antibiosis | Not specified variety | [86] |
| Streptomyces sp. | Plant of Curculigo capitulata | Dual-culture trial | NA | 73.18 | Inhibition | Antibiosis | NA | [87] |
| Bacillus siamensis | Banana rhizosphere | Seedling trial | 25 mL/plant (107 CFU/mL) | 88.26 | Control | Antibiosis and hyperparasitism | Brazilian bananas (Musa acuminata AAA genotype cv. Cavendish) | [88] |
| Trichoderma harzianum, Burkholderia cepacia, Paenibacillus terrae, Bacillus amyloliquefaciens | Banana rhizosphere | Experimental-plot trial | 62.5 L/ha (109 CFU/mL) | 57.14 | Control | ND | Banana Cavendish subgroup cv. Williams | [89] |
| Pseudomonas chlororaphis, Bacillus velezensis, Trichoderma virens | Banana rhizosphere | Seedling trial | 50 mL/plant (107 cells or conidia/mL) | 62 | Incidence | Antibiosis and systemic resistance induction | Gran Enana | [90] |
| Streptomyces malaysiensis | Roots of Curculigo capitulata | Dual-culture trial | NA | 42.88 | Inhibition | Systemic resistance induction and antibiosis | Banana Cavendish subgroup cv. Brazil | [91] |
| Pseudomonas spp. | Banana cultivation | Seedling trial | 50 mL/plant (ND concentration) | 39.4 | Incidence | Antibiosis, biofilm formation, quorum sensing, and competition for resources | Musa acuminata Cavendish cv. Brazil | [92] |
| Pseudomonas aeruginosa | Soil in banana cultivation | Dual-culture trial | NA | 50.38 | Inhibition | Antibiosis | NA | [93] |
| Bacillus amyloliquefaciens + Burkholderia cepacia | Rhizosphere of various crops | Seedling trial | 100 mL/plant (108 CFU/mL) | 68.89 | Severity | Antibiosis, Parasitism | Williams B6 | [94] |
| Bacillus licheniformis | Banana rhizosphere | Seedling trial | 500 mL (108 cells/mL) | 77.59 | Inhibition | Antibiosis and systemic resistance induction | Musa acuminata AAA Cavendish cv. Grand Naine | [95] |
| Trichoderma parareesei | Banana rhizosphere | Seedling trial | 100 mL/plant (107 CFU/mL) | 72 | Control | Antibiosis, enzyme production, hyperparasitism | Musa acuminata L. AAA genotype cv. Cavendish | [96] |
| Macrophomina phaseolina and Xylaria feejeensis. | Banana plant | Dual-culture rial | NA | 96.56 | Inhibition | Antibiosis, mycoparasitism | NA | [97] |
| Bacillus siamensis | Stem of Vicia villosa | Seedling trial | 100 mL/plant (108 CFU/mL) | 79.25 | Control | Antibiosis | Baxi (Musa spp. AAA) | [98] |
| Piriformospore indica Streptomyces morookaensis | Piriformospora indica: Commercial Streptomyces malaysiensis: Native | Experimental-plot trial | 50 mL (106 chlamydospore /mL) | 90 | Incidence | Antibiosis and mycoparasitism | Musa acuminate AAA Cavendish cv. Brazilian | [99] |
| Pseudomonas spp. | Rhizosphere and banana plants | Seedling trial | 20 mL of broth 5.0∙107 CFU/g per plant | ND | ND | Antibiosis and systemic resistance induction | Musa, AAA Cavendish cv. Brazil | [100] |
| Bacillus velezensis | Banana plant | Seedling trial | 0.1 mL/plant (106 CFU/mL) | 80 | Severity | Antibiosis and systemic resistance induction | Musa acuminata AAA Cavendish cv. Brazilian | [101] |
| Streptomyces hygroscopicus | Roots of Piper austrosinense | Dual-culture trial | 50 µL (500 µg/mL) | 82.09 | Inhibition | Antibiosis | NA | [102] |
| Streptomyces sp. | Leaves of a tea plant | Seedling trial | 15 mL/plant (106 CFU/mL) | 87.7 | Control | Antibiosis | Musa acuminata AAA Cavendish | [18] |
| Bacillus velezensis | Isolated from banana suppressive bulk-soils | Seedling trial | 40 mL/plant (108 CFU/mL) | 81.67 | Control | Antibiosis and systemic resistance induction | Musa acuminata AAA Cavendish cv. Brazilian | [103] |
| Bacillus amyloliquefaciens | Banana rhizosphere, medicinal plants, commercial product | Seedling trial | 5 mL/plant | 1.86 | Severity | Systemic resistance induction | Musa acuminata AAA Cavendish cv. Brazilian, Yunjiao No. 1 (AAA) | [15] |
| Trichoderma koningiopsis | Roots of Dendrobium plants | Seedling trial | 10 g/plant | 52.92 | Control | Systemic resistance induction | Musa spp. AAA group Cavendish | [104] |
| Bacillus subtilis | Banana rhizosphere | Seedling trial | 107 CFU/g of soil | 48.3 | Control | Antibiosis | ND | [105] |
| Pseudomonas aeruginosa | Banana rhizosphere | Dual-culture trial | 100 µL per plate | 69 | Inhibition | Enzyme production | NA | [106] |
| Neofusicoccum parvum | Plant of Moringa oleifera, Azadirachta indica, and Lavandula angustifolia | Seedling trial | 25 mL/plant (106 spores/mL) | 100 | Inhibition | Antibiosis and systemic resistance induction | Grand Naine | [107] |
| Bacillus subtilis | Plant tissues of Moringa | Seedling trial | 25 mL/plant (108 CFU/mL) | 56.25 | Inhibition | Antibiosis and systemic resistance induction | Berangan | [108] |
| Bacillus velezensis | Bank | Seedling trial | 100 mL/plant (5∙107 CFU/mL) | 64.48 | Control | Antibiosis | Musa acuminata Cavendish cv. Brazilian | [109] |
| Bacillus subtilis | ND | Experimental-plot trial | 30 mL/plant (108 CFU/mL) | 63.05 | Control | Systemic resistance induction | Berangan | [110] |
| Trichoderma harzianum | Natives: Rhizosphere of Musa Paradisiaca cv. Malnad Rasbale Bank: Trichoderma harzianum and Trichoderma viride of the NFCCI-Agarkar Research Institute | Dual-culture trial | NA | ND | ND | Antibiosis and mycoparasitism | NA | [111] |
| Streptomyces solisilvae | Soft coral Menella woodin | Seedling trial | 106 CFU/g of soil | 73.46 | Severity | Antibiosis | Not mentioned (the paper does not specify the variety of banana) | [112] |
| Trichoderma brevicompactum | Rhizosphere of broad beans and coriander | Seedling trial | 50 mL/plant | 52.6 | Severity | Antibiosis, competition, mycoparasitism, systemic resistance induction | Cavendish (AAA) | [113] |
| Pochonia chlamydosporia | Root nodules of Dolichos lablab in banana fields | Seedling trial | ND (106 CFU/mL) | 96.87 | Control | Competition for nutrients, antibiosis, enzyme production | Musa spp. AAA Brazilian cultivar | [114] |
| Streptomyces luomodiensis | Soil from a hot–arid valley | Dual-culture trial | NA | 74.22 | Inhibition | Enzyme production | NA | [115] |
| MCBA | Origin of MCBAs | Level | Doses | Efficacy (%) | Measure | Mechanism | Variety | Foc race * | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Bacillus amyloliquefaciens | Banana plant | Seedling trial | 1.5% soil weight (109 CFU/g) | 77 | Control | Antibiosis | Musa AAA Cavendish cv. Brazil | R4 ** | [116] |
| Pseudomonas fluorescens | ND | Experimental-plot trial | 4 L/ha (9∙108 CFU/mL) | 60 | Incidence | Antibiosis | ND | ND | [117] |
| Bacillus amyloliquefaciens | Banana cultivation soil | Seedling trial | 5 g of biofertilizer/kg of soil | 84.94 | Incidence | Resistance induction, niche competition | ND | ND | [118] |
| Bacillus amyloliquefaciens | Banana rhizosphere | Seedling trial | 2% w/w soil (3∙108 CFU/g) | 24.3 | Incidence | Antibiosis | Musa AAA Cavendish subgroup cv. Brazil | R4 ** | [119] |
| Bacillus amyloliquefaciens | Bank | Experimental-plot trial | 6 kg/plant (109 CFU/g) | 88 | Incidence | Antibiosis | Musa AAA Cavendish cv. Brazil | R4 ** | [120] |
| Trichoderma harzianum | Bank | Seedling trial | 20% of the culture filtrate of T. harzianum | 16.66 | Incidence | Antibiosis, enzyme production | Dwarf Cavendish | R4 ** | [121] |
| Trichoderma viridae | Chili pepper roots | Seedling trial | Seedlings:10 g of rice (1.73∙108 spores/g) Soil: 10 g of rice (1.69∙108 spores/g) | 65 | Control | ND | Cavendish | R4 ** | [122] |
| Bacillus subtilis | Bank | Seedling trial | 60 mL (108 CFU/mL) | 45.08 | Incidence | Oxidative stress reduction | Musa acuminata cv. Berangan | R4 ** | [123] |
| Azotobacter and Bacillus sp. | Azotobacter sp.: reed rhizosphere; Bacillus sp.: sugar cane and banana rhizosphere | Seedling trial | 108 CFU/mL per plant | 60 | Inhibition | Antibiosis, resistance induction | ND | ND | [124] |
| Trichoderma asperellum | Soil | Seedling trial | 500 mL/plant (107 conidia/mL) | 94.44 | Severity | Mycoparasitism, enzyme production | Cavendish banana cultivar | R4 ** | [125] |
| Trichoderma spp., Bacillus subtilis | Trichoderma sp.: commercial Bacillus subtilis: commercial | Experimental-plot trial | 8 L (Trichoderma) + 250 g (Bacillus subtilis)/ha | 93.79 | Severity | Competitive exclusion | Musa balbisiana ABB | R2 * | [17] |
| Trichoderma guizhouense Humicola spp. | Banana cultivation soil | Seedling trial | 300 mL/plant | 72 | Incidence | Competition for nutrients and systemic resistance induction | Musa AAA Cavendish cv Brazil | R4 ** | [126] |
| Trichoderma reesei | Bank | Dual culture | NA | 36 | Inhibition | Mycoparasitism | NA | [127] | |
| Bacillus siamensis | Volvariella volvacea culture media | Dual culture | ND | ND | Antibiosis | NA | [128] | ||
| Bacillus vallismortis | Soil | Dual culture | NA | 26.17 | Inhibition | Antibiosis, systemic resistance induction | NA | [129] | |
| Brachybacterium paraconglomeratum | Banana plant | Dual culture | NA | 65.5 | Control | Antibiosis | NA | [130] | |
| Sarocladium brachiariae | Brachiaria brizantha leaves | Dual culture | ND | ND | ND | Antibiosis | ND | ND | [131] |
| Bacillus amyloliquefaciens | Costa marina | Dual culture | NA | 78 | Inhibition | Antibiosis | ND | ND | [132] |
| Beauveria caledonica | Banana weevils | Dual culture | NA | 47.68 | ET50 | Antibiosis | ND | ND | [133] |
| Bacillus subtilis | Banana rhizosphere | Dual culture | NA | 11.5 | Inhibition | Antibiosis | ND | ND | [134] |
| Trichoderma harzianum | Soils of Agricultural Fields, cocoa bark and Pleurotus spp. substrate | Dual culture | NA | 74.1 | Inhibition | Mycoparasitism | ND | ND | [135] |
| Streptomyces albosporus | Banco | Dual culture | NA | 76.33 | Inhibition | Antibiosis | ND | ND | [136] |
| Bacillus spp. | Rizósfera de banano | Dual culture | NA | 80.47 | Inhibition | Antibiosis, enzyme production | ND | ND | [137] |
| Bacillus sp. | NA | Dual culture | NA | 87.71 | Inhibition | Competition for nutrients | ND | ND | [138] |
| Paenibacillus sp. | Banco | Dual culture | NA | 46.6 | Inhibition | Antagonism | NA | ND | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solórzano, R.; Ramírez Maguiña, H.A.; Johnson, L.; Ureta Sierra, C.; Cruz, J. Current Progress in Microbial Biocontrol of Banana Fusarium Wilt: A Systematic Review. Agronomy 2025, 15, 619. https://doi.org/10.3390/agronomy15030619
Solórzano R, Ramírez Maguiña HA, Johnson L, Ureta Sierra C, Cruz J. Current Progress in Microbial Biocontrol of Banana Fusarium Wilt: A Systematic Review. Agronomy. 2025; 15(3):619. https://doi.org/10.3390/agronomy15030619
Chicago/Turabian StyleSolórzano, Richard, Héctor Andrés Ramírez Maguiña, Luis Johnson, Cledy Ureta Sierra, and Juancarlos Cruz. 2025. "Current Progress in Microbial Biocontrol of Banana Fusarium Wilt: A Systematic Review" Agronomy 15, no. 3: 619. https://doi.org/10.3390/agronomy15030619
APA StyleSolórzano, R., Ramírez Maguiña, H. A., Johnson, L., Ureta Sierra, C., & Cruz, J. (2025). Current Progress in Microbial Biocontrol of Banana Fusarium Wilt: A Systematic Review. Agronomy, 15(3), 619. https://doi.org/10.3390/agronomy15030619







