Abstract
The trend of global warming is becoming increasingly evident, with frequent extreme high-temperature events posing a severe challenge to food security. Rice (Oryza sativa L.), the world’s primary food crop, is highly susceptible to the adverse effects of high-temperature stress throughout its growth cycle. High temperatures, defined as ambient temperatures exceeding 35 °C during reproductive stages and 33 °C during vegetative stages, can impair seed germination, reduce tillering, disrupt pollination, and diminish grain quality. Notably, heat stress during the grain-filling stage accelerates grain maturation, leading to increased chalkiness, a higher degree of chalky formation, deteriorated cooking and eating quality, and decreased grain weight. To cope with high-temperature stress, rice activates a series of complex physiological and biochemical responses, including heat-related signaling pathways and transcriptional regulatory networks. Although some agronomic practices and genetic improvement methods have been developed to enhance rice’s heat tolerance, the regulatory mechanisms of rice’s response to high-temperature stress, especially the molecular mechanisms during the grain-filling stage, remain poorly understood. This review identifies knowledge gaps in understanding rice’s response mechanisms, emphasizing molecular pathways during the grain-filling stage and provides an outlook on future rice high-temperature defense measures.
1. Introduction
With the continuous growth of the population and the development of industry, the phenomenon of global warming is becoming increasingly severe, posing a significant threat to crop production. It is estimated that the yield of rice, wheat, and corn will decrease by 3–8% for every 1 °C increase in temperature [1]. Among these, temperature, as a key factor affecting rice growth, development, and yield and quality formation, is particularly concerning when it abnormally rises [2]. High temperatures above 35 °C during reproductive stages cause severe physiological disruptions [3]. Previous reviews have broadly addressed heat stress in rice but lack focus on the grain-filling stage and recent molecular advances. This review aims to fill that gap, comparing the recent literature, highlighting novel genes, and addressing multi-stress scenarios. This review summarizes the hazards of high-temperature stress on rice at different growth stages and the recent progress in molecular biology research on this issue. It systematically summarizes measures for defending against high-temperature hazards in rice and offers perspectives on future research directions, aiming to establish a theoretical foundation for mitigating heat stress in rice.
2. Effects of High-Temperature Stress on Rice
2.1. Effects of High Temperature During the Vegetative Growth Stage
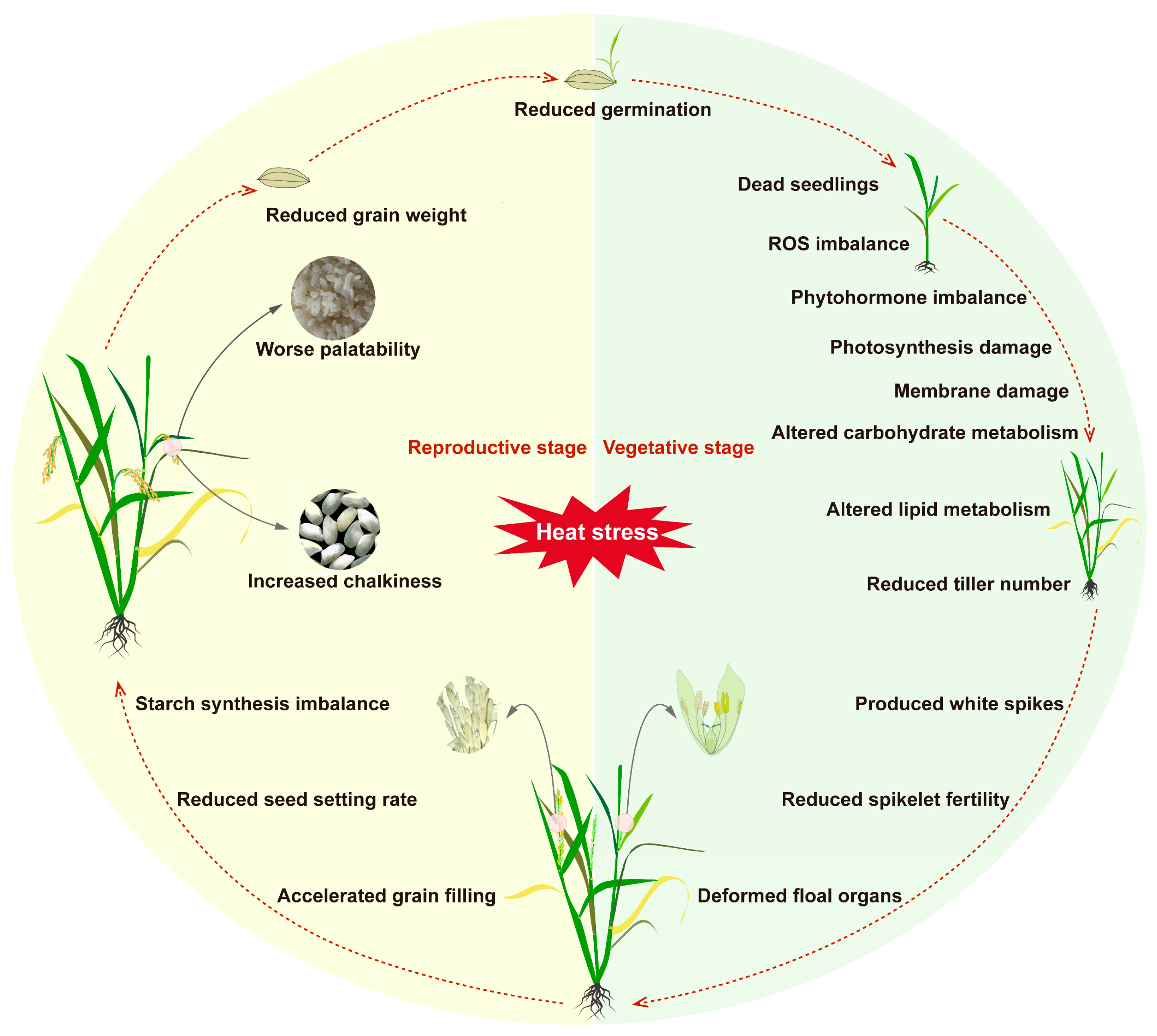
The vegetative stage of rice includes seed germination, early seedling growth, and tillering. The optimum growth temperature of rice at seed germination stage is 18–40 °C [4]. During the seed germination stage, prolonged exposure to high temperatures can delay the germination process of rice seeds and, in severe cases, significantly reduce the germination rate or even prevent germination altogether [3]. In the seedling stage, high temperatures accelerate water evaporation, damaging root growth and increasing seedling mortality [5]. During the tillering stage, high-temperature stress can damage the nutritional tissues of rice, manifested as leaf whitening, curling, and premature senescence, as well as affecting normal stem growth and reducing the number of tillers [6]. In addition to these visible morphological changes, high temperatures can also trigger a series of complex physiological responses (Figure 1). High temperatures can disrupt cell membrane integrity, inhibit photosynthetic efficiency, and exacerbate oxidative damage. Moreover, high temperatures can interfere with leaf transpiration, thereby affecting the absorption and transport of water and inorganic salts, which adversely impact root vitality and extension ability. More seriously, high temperatures can impede photosynthesis, destabilize thylakoid membranes, and interfere with the electron transfer process in the light reaction, further affecting rice’s energy metabolism and growth and development [7] (Table 1).
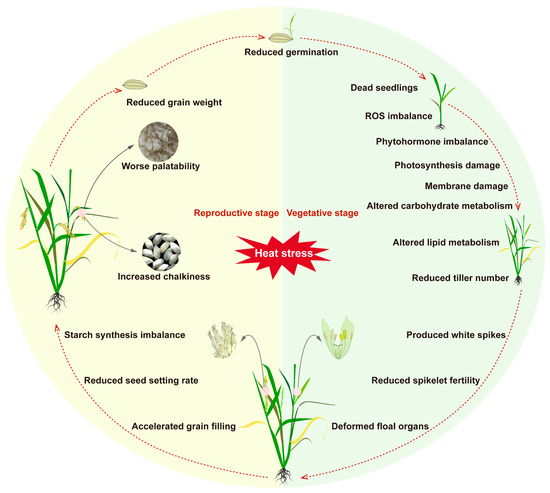
Figure 1.
The ambient temperature exceeds the critical value of normal growth of rice, which causes irreversible damage to the growth and development of rice.

Table 1.
High-temperature threshold for the development of the rice plant at different growth stages.
High temperature had different effects on different growth stages of rice: (1) vegetative growth period delayed seed germination, water loss, and reduced germination rate. Damage to vegetative growth makes leaves white, curl, causes premature senescence, and affects stem growth and plant height. It affects transpiration, which further affects the absorption and transport of water and inorganic salts in rice. It affects the stability of plasma membrane, inhibits photosynthesis, causes ROS accumulation, plant hormone imbalance, carbohydrate changes, lipid metabolism changes, and decreases the number of tillers in dead seedlings and vegetative stages. (2) Reproductive growth period: it affects the reproductive development of rice, resulting in deformity of flower organs, reduction in spikelet fecundity and quantity, abnormal pollen development, and changes in flowering time. (3) Maturity stage: promotes the senescence of functional leaves, speeds up the grain filling of rice, reduces grain filling, reduces starch biosynthesis, reduces starch accumulation, increases the chalkiness rate of grains at maturity, affects the appearance quality and taste of rice, and reduces rice yield.
2.2. Effects of High Temperature During the Reproductive Growth Stage
The reproductive growth stage of rice includes panicle initiation, development of male and female gametophytes, flowering, pollination, and fertilization [8,9]. Compared to the vegetative growth stage, rice’s reproductive growth stage is more sensitive to high-temperature stress, particularly during the heading and flowering period, which are highly sensitive to temperature fluctuations [10]. During the panicle initiation and development stage, high-temperature stress can significantly reduce photosynthetic efficiency, thereby affecting dry matter accumulation and normal panicle development [11]. In the heading and flowering stage, which is a critical period for grain formation, exposure to high temperatures can lead to pollen sterility, impaired pollen tube elongation, and disrupted fertilization, ultimately resulting in reduced panicle grain numbers, decreased seed-setting rates, and significant yield losses. The severity of these negative impacts is positively correlated with the intensity and duration of high temperatures [12,13,14,15]. Studies have shown that just three consecutive days of temperatures above 38 °C are sufficient to cause pollen sterility and poor fertilization, leading to the appearance of white panicles [16,17]. After double fertilization, continuous exposure to 39 °C for two days can also cause abnormal early endosperm development and severely damage subsequent endosperm development [18,19]. It is worth noting that different rice varieties have varying degrees of heat tolerance. Generally, indica rice has stronger heat tolerance than japonica rice. For example, during the heading and flowering stage, rice exposed to high (38 °C) and control (29 °C) temperatures were analyzed morphologically and proteomically in panicles and spikelets, and it was found that high temperatures (38 °C) reduced the number of pollen grains on the stigma of the aus-type heat-tolerant variety N22 by 55%, while the number of pollen grains on the stigma of the japonica-type heat-sensitive variety Mianhui101 decreased by 86%, but there was no reduction in the indica-type IR64 [20]. In addition, high temperatures can also lead to reduced pollen protein content [21]. In-depth proteomic analysis of anthers further revealed that high temperatures promote the degradation of ribosomal proteins in Mianhui101, but this phenomenon was not observed in N22. At the same time, high temperatures also induced the expression of heat shock proteins, expansions, and lipid transfer proteins in the anthers of N22, which may play an important role in enhancing rice’s heat tolerance [21].
2.3. Effects of High Temperature During the Grain-Filling Stage
Grain filling and maturation of rice grains are crucial for determining grain yield and quality. This process mainly involves the conversion of sucrose to starch and the effective transfer of photosynthates to the grains, involving the synthesis and precise allocation of carbohydrates, proteins, and lipids within the seeds [22,23]. The grain-filling stage is a critical period for rice’s sensitivity to high temperatures, and exposure to high-temperature stress can significantly impact both quality and yield. High temperatures during the grain-filling stage can first impede the accumulation of dry matter in the grains, leading to an increase in the proportion of shriveled grains. Additionally, excessively high temperatures can accelerate grain filling, resulting in incomplete filling. This causes starch granules to be loosely arranged with gas interspaces, ultimately affecting key quality indicators such as chalkiness, chalky grain rate, and milled rice rate, thereby reducing rice yield and quality [24,25] (Figure 1). Studies have reported that five consecutive days of temperatures above 35 °C can cause premature grain maturity in rice, significantly reducing thousand-grain weight and increasing chalkiness [26,27]. Moreover, high temperatures can also affect the expression and biological activity of key enzymes involved in the conversion of sucrose to starch in the endosperm, thereby slowing starch biosynthesis and influencing total starch content and starch accumulation patterns, especially the amylose content in the endosperm [28,29,30]. High temperatures can also damage the structure and function of the plasma membrane, alter the ratio of saturated to unsaturated fatty acids, cause protein denaturation, and impair the activity of fatty acid desaturase enzymes, leading to a decrease in the degree of unsaturation of fatty acid chains and further affecting rice’s adaptability to high temperatures [31,32]. For example, Ca2+ channels, such as CNGCs and the Ca2+-permeable transporters-linked proteins (ANNs), play an important role in rice heat stress by regulating Ca2+ levels [33]. OsCNGC14 and OsCNGC16 have been shown to be involved in heat response and heat stress tolerance in rice, and they exhibited a significant heat-sensitive phenotype after loss of function [34]. OsANN1 is a calcium-binding protein with ATPase activity, which is sensitive to heat stress after knockout of this gene, resulting in a severe redox imbalance in vivo [35]. It is also worth noting that high temperatures can weaken leaf assimilation capacity and accelerate leaf senescence, thereby inhibiting photosynthesis in other nutritional organs such as stems and leaf sheaths. This results in a significant reduction in fixed carbon sources provided to the panicles by these nutritional organs, leading to reduced grain weight [36]. Therefore, the hazards of high-temperature stress during the grain-filling stage are multidimensional, not only directly affecting the developmental process of this stage but also impacting multiple key developmental stages in both vegetative and reproductive growth, posing a serious threat to rice’s overall growth and final yield.
3. Molecular Mechanisms of Rice Grain-Filling Stage Under Heat Stress
3.1. Molecular Mechanisms for Maintaining Starch Synthesis-Related Enzyme Activity in the Endosperm Under High Temperature
The endosperm, as the core component of rice seeds, is responsible for providing the necessary energy and material basis for seed germination. During the early stages of seed development, continuous exposure to high temperatures (35 °C) for 72 h can severely impede the normal development of the embryo and endosperm. Regarding the impact of high-temperature stress on endosperm development during the grain-filling stage, studies have shown that the Polycomb Repressive Complex 2 (PRC2) plays a crucial role in the early endosperm development of both Arabidopsis and rice, especially during the transition from the syncytial to cellular stage [37]. Genome-wide association analysis has revealed that FIE1, which encodes a PRC2 component, can regulate seed sensitivity to nighttime high temperatures during grain filling, thereby ensuring stable rice yield under high-temperature conditions [38]. Additionally, some MADS-box proteins are also involved in PRC2-mediated early endosperm development in rice. For example, MADS87 positively regulates seed size but negatively regulates heat tolerance, demonstrating its complex regulatory role between rice endosperm development and heat tolerance [18,39]. High temperatures can also affect the development of amyloplasts in rice endosperm. For instance, OsHsp70cp-2 promotes protein import into amyloplasts, and its mutant exhibits a more pronounced chalky phenotype under high-temperature stress compared to the wild type, although no such phenomenon is observed under normal conditions [40,41]. On the other hand, amylase, a starch-degrading enzyme, is responsible for regulating the formation of starch granules in the endosperm during seed maturation. Studies have shown that the expression and activity of amylases such as Amy1C/3A/3D/3E increase in response to heat stress during seed development. Inhibiting the expression of these genes in developing seeds can reduce chalkiness under high-temperature conditions, indicating that these genes contribute to chalkiness by degrading starch accumulated during seed development [42,43]. The expression of OsbZIP58 responds to high temperature and produces alternative splicing under high temperature. The osbzip58 mutant is particularly sensitive to high temperature and has been shown to significantly reduce the contents of starch, lipid, and storage protein, especially under high temperature. This is related to its negative regulation of the expression of α-amylase Amy1C/3A/3D/3E [44,45].
The grain-filling process is a critical stage in rice seed development, involving the synthesis, transport, and allocation of carbohydrates, proteins, and lipids. Under high-temperature stress, ensuring that these nutrients in rice seeds can be continuously supplied from the source to the sink and maintaining the normal accumulation of storage materials (mainly starch) in the endosperm is crucial for maintaining rice quality and yield. The endosperm, as the main component of rice seeds, determines the quality of rice through its starch content and quality. High-temperature environments can negatively affect rice quality by reducing amylose content during the grain-filling process. The Wx (waxy) gene is the primary regulator of amylose content, and high temperatures promote the splicing of its pre-mRNA, increasing the percentage of the Wx large subunit. This subunit has higher enzyme activity, which helps stabilize amylose content and improve grain quality under high-temperature conditions [46]. Additionally, inhibiting the high-temperature-responsive MADS7 gene in the endosperm can increase Wx expression and amylose content [47]. Moreover, the activity and expression of various key enzymes and transcription factors involved in the synthesis, transport, or allocation of storage materials are also affected by high temperatures [48]. Studies have found that high temperatures during the grain-filling stage leads to reduced activity of starch branching enzymes and amylose content [49]. Starch-synthesis-related enzymes such as AGPL1, AGPL3, and PHO1 interact with FLO24 to affect starch biosynthesis in rice endosperm, especially under high-temperature stress [50]. NAC transcription factors OsNAC127 and OsNAC129 mainly influence grain quality formation by affecting sugar transport under heat stress [51]. High temperatures can also alter starch pasting properties, including peak viscosity, holding viscosity, final viscosity, breakdown value, setback value, and pasting temperature, leading to deteriorated eating quality and reduced commercial value of rice [52,53,54]. These studies not only reveal the complex effects of high temperatures on rice endosperm development but also provide an important theoretical basis and potential targets for improving rice heat tolerance through genetic improvement.
3.2. Molecular Mechanisms for Maintaining Reactive Oxygen Species and Phytohormone Balance in the Endosperm Under High Temperature
High-temperature stress has a profound impact on the physiological functions of rice, most notably leading to the imbalance of reactive oxygen species (ROS) homeostasis and causing excessive accumulation of ROS. This accumulation not only triggers a series of physiological responses such as DNA damage, protein denaturation, pigment degradation, carbohydrate oxidation, and lipid peroxidation but also severely affects rice reproductive development, manifested as increased chalky grain rate [55]. In response to excessive ROS accumulation, white-core rate 1 (WCR1), which encodes an F-box protein, plays a key role. WCR1 interacts with the metallothionein gene MT2b, which encodes a metallothionein that binds metal ions and removes reactive oxygen species (ROS). WCR1 regulates MT2b transcription levels and inhibits 26S proteasome-mediated MT2b protein degradation, thereby promoting ROS clearance and delaying endosperm cell programmed death (PCD), ultimately increasing the accumulation of storage substances and reducing chalky grain rate. Additionally, allelic variation in the WCR1 promoter enhances the binding activity of OsDOF17, which, in turn, promotes WCR1 expression and significantly reduces chalkiness, improving grain quality [56]. Under high-temperature stress, phosphorylation and ubiquitination modifications jointly regulated the synthesis pathway of phenolic compounds, ultimately affecting the ability of phenolic compounds to scavenge reactive oxygen species and plant resistance to stress. The metabolism of rice endosperm depends on the precise regulation of several post-translational modification (PTM)-based signaling networks, including phosphorylation and ubiquitination modifications. Several enzymes related to starch metabolism possessed both phosphorylation and ubiquitination modifications, and 18 pairs of sites with different modification types appeared adjacent to the same protein, revealing the crosstalk mechanism between different modifications and the synergistic regulation of starch metabolism by phosphorylation and ubiquitination modifications under high-temperature stress [57].
Phytohormones, as key signaling molecules, play a central role in regulating the developmental processes of higher plants and their responses to environmental stimuli. Gene expression profiling of early endosperm development has shown that genes related to hormone synthesis and signaling exhibit distinct dynamic changes at different stages of endosperm development. Using the endosperm-specific promoter TFSE1 to drive the expression of genes related to auxin, gibberellin, brassinosteroid, and cytokinin synthesis, it was found that changes in hormone content in the endosperm affect grain shape and seed quality. Auxin and gibberellin promote grain elongation, while cytokinin leads to longer and narrower seeds. Brassinosteroid has the most significant effect, as it can promote both the length and width of rice grains and reduce the chalkiness and chalky grain rate of milled rice [58]. These findings provide valuable insights for molecular breeding studies aimed at regulating early endosperm development and improving yield. Grain filling defect 1 (DG1) participates in the transport of leaf-derived ABA to the caryopsis in a temperature-dependent manner. Under high-temperature stress, DG1 knockout mutants exhibit poor grain filling and severe chalkiness [59]. OsHTAS encodes a RING-finger E3 ubiquitin ligase that regulates intracellular hydrogen peroxide accumulation through both ABA-dependent and DST-dependent pathways, thereby positively enhancing rice heat tolerance under heat stress [60]. Studies have shown that exogenous application of ABA can inhibit ROS overaccumulation and increase the activity of starch synthase in rice grains under high-temperature stress, thereby promoting grain filling in rice [61]. In addition, the interaction between hormones also plays a regulatory role in the development of rice endosperm under high temperature. At high temperature, OsIAA21 and OsIAA29 competitively bind OsARF17 to enhance the transcriptional activity of OsARF17, which regulates the expression of several genes related to starch and protein synthesis (such as OsPDIL1-1, OsSS1, OsNAC20, OsSBE1, and OsC2H2). It mediates the auxin signaling pathway in rice, thereby regulating the development of rice grains under high temperature [62]. Therefore, in-depth exploration of the functions of ROS and phytohormones within cells and their involvement in high-temperature stress responses is of great significance for identifying heat-tolerant genes during the grain-filling stage and breeding heat-tolerant varieties.
3.3. Molecular Mechanisms for Maintaining Protein Homeostasis in the Endosperm Under High Temperature
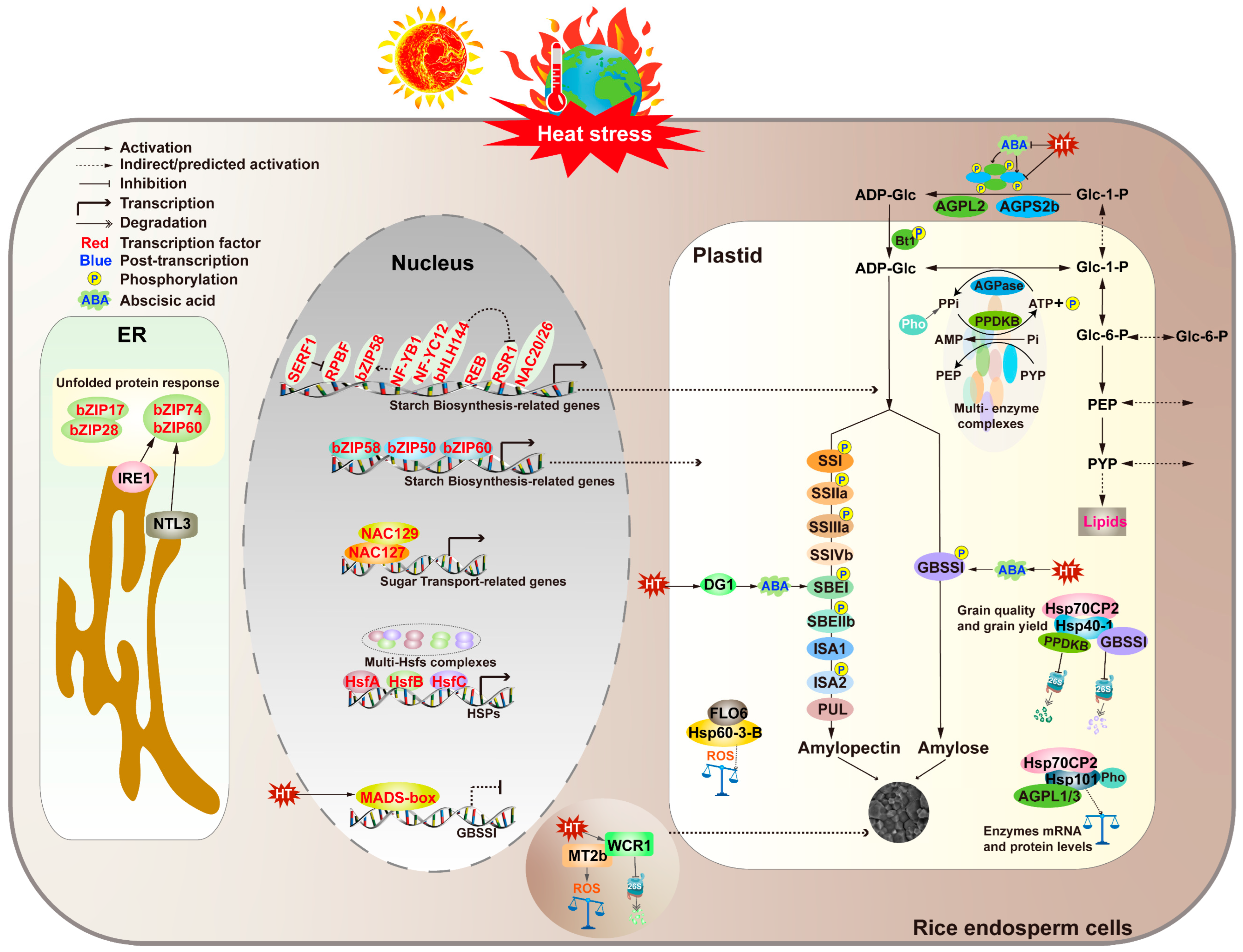
The disruption of protein homeostasis in plants by high temperatures is a complex process involving multiple aspects of protein synthesis, folding, quality control, and subcellular localization. This disruption leads to the accumulation of unfolded or misfolded proteins, which can exert toxic effects within plant cells [63]. However, plants have evolved a series of regulatory mechanisms to cope with such adverse environments, ensuring the correct folding and maturation of proteins within organelles. In the endoplasmic reticulum, the accumulation of misfolded or damaged proteins can trigger the unfolded protein response (UPR). This response rebuilds protein homeostasis through mechanisms such as increasing protein folding capacity, enhancing proteasome-related protein degradation, and autophagy [64,65,66,67]. In rice, the UPR pathway plays an important role in regulating the synthesis and secretion of seed storage proteins (SSPs). Under high-temperature conditions, the loss of function of UPR genes such as bZIP60 can lead to increased chalkiness in the endosperm [66]. Additionally, the upregulation of other UPR genes such as OsbZIP50 and BiP1/2/3 in the bzip60 mutant endosperm further indicates that the UPR pathway plays a significant role in regulating chalkiness formation in grains, especially under heat stress conditions [68]. In addition to the UPR pathway, heat shock proteins (Hsps) also play a regulatory role in protein stability in rice endosperm under high temperatures. Plants have a rich family of Hsps, which, as molecular chaperones, assist in protein folding, assembly, translocation, and degradation processes to maintain protein and cell membrane stability. Under stress conditions, Hsps can reshape misfolded proteins, restore their normal conformations, and maintain cellular homeostasis, thereby protecting plants from damage caused by abiotic stresses [69,70]. In recent years, the role of Hsps in rice endosperm development has gradually attracted attention. Studies have found that the seed endosperm of Oshsp70cp-2 mutants exhibits different chalky phenotypes under high- and low-temperature stress conditions, but the specific regulatory mechanisms still need further exploration. It has been reported that overexpression of HSP70 genes is positively correlated with enhanced heat tolerance, thereby increasing plant tolerance to salt, water, and heat stresses [71]. Further research has shown that the interaction between OsHsp70-2 and OsHsp40-1 can significantly enhance the ATPase activity of OsHsp70-2. Overexpression of OsHsp70-2 and OsHsp40-1 can improve the appearance quality and yield of rice under natural high-temperature conditions [72]. As a molecular module, OsHsp70-2 and OsHsp40-1 regulate starch biosynthesis in rice endosperm by controlling the activity and protein stability of key enzymes such as GBSSI and PPDKB. This finding provides a new molecular mechanism for the development of rice endosperm during the grain-filling stage, helps us better understand the regulatory mechanisms of rice quality under high-temperature stress, and provides theoretical support and practical guidance for the future breeding of heat-tolerant varieties [72] (see Figure 2 and Table 2).
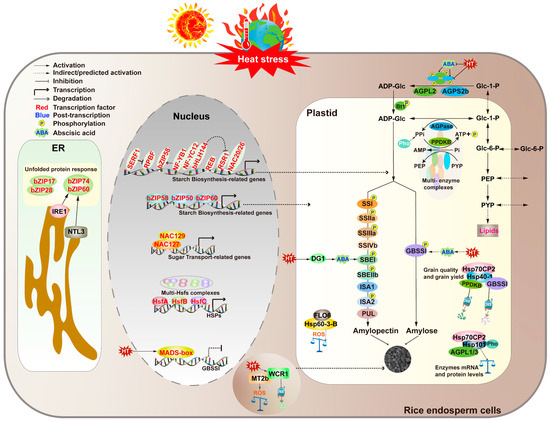
Figure 2.
Heat stress signaling and responses in rice endosperm. Rice is more sensitive to heat stress at grain-filling stages than the vegetative stage. High temperature (HT) downregulates the expression of starch-synthesis-related genes (SSRGs) and the stability of their encoded enzymes, especially those encoding AGPase and GBSSI, leading to defective chalkiness and impaired starch accumulation. Several key transcription factors (highlighted in red) are involved in the stimulation or inhibition of SSRGs at the transcriptional level. At the post-translational level, phosphorylation affects starch synthesis by promoting the formation of multi-enzyme complexes. Abscisic acid (ABA) synthesized in rice leaves directly activated the expression of most of the SSRGs and multiple hub transcription factors in rice caryopsis after long-distance transport from leaves to caryopsis. Grain filling defect 1 (DG1)-mediated long-distance transport of ABA is sensitive to high temperatures during seed development. Heat stress transcriptionally activates HS, alters membrane fluidity and permeability, affects the activity of membrane proteins, and causes chloroplast and mitochondrial dysfunction. This leads to an increase in intracellular ROS and ROS second messengers, rapidly activating downstream regulatory networks. OsHsp60-3B interacted with FLO6 to regulate the biosynthesis of starch granules in rice pollen at HT, reduce the level of ROS in anthers, and ensure the normal development of male gametophytes in rice. The WCR1 interacted with MT2b to maintain its protein stability, thereby reducing ROS accumulation, reducing chalkiness, and improving grain quality. HSF triggers a transcriptional cascade to activate the expression of downstream genes including HSP, HSF, and HSP, which can help to maintain the normal physiological metabolism by stabilizing unfolded proteins and promoting the renaturation of aggregated proteins induced under heat stress. By regulating ONAC127/129, MADS box, bZIP60, bZIP58, Hsp70cp-2, and Hsp40cp-1, these factors maintain the normal development of endosperm, promote endosperm starch biosynthesis, reduce grain chalkiness, and improve grain quality.

Table 2.
List of genes conferring high-temperature tolerance in rice.
4. Strategies for Defending Against High-Temperature Effects in Rice
4.1. Identification of Heat-Tolerant Genes and Varieties
Global warming and frequent extreme high-temperature events pose a serious threat to food security. Therefore, it is crucial to identify heat-tolerant genes and breed heat-tolerant rice varieties. Using genomic techniques, we should conduct in-depth analyses of genetic variations in rice varieties, especially those genes closely related to heat tolerance. For example, NAT1 regulates rice heat tolerance by controlling wax formation in rice, and favorable variations have been identified from natural populations to obtain heat-tolerant materials [73]. The heat tolerance key regulator TT1 encodes the α2 subunit of the 26S proteasome. TT1 is a natural allele found in African rice, which enhances heat tolerance by promoting the effective degradation of heat-stress-induced cytotoxic proteins in various species [74]. TT2 is a pleiotropic gene that simultaneously regulates rice grain shape, alkali tolerance, and heat tolerance. TT2 is a typical gene differentiated between indica and japonica, and the indica genotype confers heat tolerance to rice [75]. TT3.1 encodes a RING-type E3 ubiquitin ligase, and the TT3 from African cultivated rice CG14 is more heat-tolerant than that from Asian cultivated rice WYJ. It has been found that there are two antagonistic genes regulating heat tolerance at the TT3 locus: TT3.1 and TT3.2, with TT3.1 positively regulating resistance and TT3.2 negatively regulating resistance. TT3.1 functions upstream of TT3.2 [76]. Through functional validation experiments, the core role of these genes in enhancing rice’s heat tolerance can be clarified. With modern molecular biology techniques such as marker-assisted breeding, genetic transformation, and gene editing, scientists can accurately identify heat-tolerant genes and introduce them into high-quality, stress-resistant rice varieties. New heat-tolerant varieties can be bred through hybrid breeding and their performance verified in the field to promote widespread application.
4.2. Rational Agronomic Management Practices
Rational agronomic management can effectively mitigate the impact of high temperatures on rice. For example, increasing irrigation can lower soil and surface temperatures. Previous studies have shown that the method of deep irrigation in the field combined with spraying can significantly reduce panicle temperature and mitigate the damage of high temperatures to rice yield [77,78]. It is important to note that field water level management should be rational. While excessive water can lower temperatures, it can also lead to oxygen deficiency in rice. Therefore, rational water level management should both retain water to cool down and prevent oxygen deficiency. In terms of fertilization, it is recommended to apply fertilizers in stages to maintain stable rice growth. Studies have shown that the combined application of biochar and phosphate fertilizer can mitigate the high-temperature damage to rice during flowering. Under high-temperature conditions, spraying a mixture of NaB4O7·10H2O and KH2PO4 or silicon fertilizer can significantly alleviate the damage of high temperatures to rice [79,80]. In addition, interventions in the physiological processes of rice under high temperatures, such as spraying inhibitors or adding elements to maintain photosynthetic rates, focusing on ion balance, ROS scavenging, and carbon metabolism, can also help. Adjusting plant architecture, such as selecting varieties with short stature, thick stems, and thick leaves, or using hormone treatments to promote stem development can also mitigate the impact of high temperatures [81,82]. Moreover, the cross-talk of proline with other osmoprotectants and signaling molecules, such as glycine betaine, abscisic acid, nitric oxide, hydrogen sulfide, and soluble sugars, helps to strengthen the protective mechanism in stressful environments. By controlling the biosynthesis of proline through genetic engineering, varieties that are less responsive to temperature can be developed [83].
5. Conclusions and Future Perspectives
Global warming is an undeniable reality, and heat stress has become a significant limiting factor for safe rice production in China. Scientific research aimed at enhancing the heat tolerance of rice is crucial for ensuring food security. Future research on rice responses to heat stress should focus on the following areas:
- (1)
- Heat-Tolerant Genetic Breeding:
While traditional breeding and marker-assisted selection have made progress, CRISPR gene-editing technology offers higher efficiency. Identifying key genes associated with heat tolerance in rice, exploring heat-resistant gene resources, and utilizing genomic technologies (such as GWAS and QTL mapping) to screen for heat-related genes (e.g., heat shock proteins (HSPs), HSFA family transcription factors, and ROS-scavenging genes) are essential. Efforts should be made to develop heat-tolerant genes from wild rice and local varieties and to improve cultivars precisely through gene-editing techniques like CRISPR/Cas9. Synthetic biology can be employed to construct modular gene circuits for heat tolerance, leading to the development of climate-resilient rice that responds intelligently to heat stress. Additionally, it is vital to explore the synergistic regulatory mechanisms between heat tolerance and other stress resistances (such as drought and disease resistance) to cultivate multi-resistant varieties.
- (2)
- Regulatory Mechanisms:
Although substantial research has been conducted on heat damage to rice production and rice responses to heat stress, the impact of heat stress on rice seed development warrants further investigation. Key areas include:
Heat-sensitive stages of rice: focus on the reproductive growth stages (e.g., pollen development, flowering, and grain filling) to elucidate response mechanisms, and analyze the physiological and molecular bases of pollen sterility, grain emptiness, and chalky endosperm formation.
Organelle interactions and signal transduction: study how high temperatures impair chloroplast and mitochondrial functions and explore the regulatory networks of ROS signal transmission and energy metabolism between organelles under heat stress.
Epigenetic regulation: investigate how DNA methylation, histone modifications, and other epigenetic changes under heat stress influence heat tolerance and develop epigenetic editing technologies.
- (3)
- Environmental Interactions and Ecological Adaptability:
It is necessary to explore the synergistic responses to heat combined with other stresses, such as drought, intense light, high humidity, and salinity, to understand cross-adaptation mechanisms. The adaptability of heat-tolerant varieties across different climatic regions (e.g., tropical and subtropical zones) should be evaluated and geographic information systems (GIS) used to predict their potential for widespread adoption under future climate scenarios.
- (4)
- Intelligent Technologies and Interdisciplinary Integration:
AI-Assisted breeding: leverage machine learning to integrate multi-omics data (genomics, transcriptomics, and metabolomics) for predicting heat-tolerant phenotypes and accelerating the breeding process.
Synthetic biology applications: design artificial synthetic pathways to enhance antioxidant systems and heat-protective protein expression, creating engineered rice varieties with improved heat tolerance.
- (5)
- Socio-Economic and Policy Support:
Variety promotion and farmer acceptance: assess the economic benefits of heat-tolerant varieties and optimize their traits (e.g., balancing yield and taste) based on farmer needs.
Climate adaptation policies: promote the inclusion of heat-tolerant rice in national climate-smart agriculture programs and establish disaster insurance and subsidy mechanisms. Advanced technologies such as remote sensing, GIS, and global positioning systems should be integrated to develop comprehensive monitoring, early warning, and impact assessment systems for rice heat damage.
Author Contributions
X.W. and F.L. designed the main conceptual ideas; F.L. and J.Q. wrote the manuscript; J.Q., L.C. and B.F. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32401922); the Zhejiang Provincial Natural Science Foundation of China (LZ25C130011, LQ24C130007); and The Agricultural Science and Technology Innovation Program (ASTIP).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Paul, P.; Dhatt, B.K.; Sandhu, J.; Hussain, W.; Irvin, L.; Morota, G.; Staswick, P.; Walia, H. Divergent phenotypic response of rice accessions to transient heat stress during early seed development. Plant Direct. 2020, 4, e00196. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, W.; Miguel, S.; Assefa, S.; Amri, A.; Bishaw, Z.; Francis, C.; Michael, B. Genetic gains in wheat breeding and its role in feeding the world. Crop Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar]
- Xu, Y.F.; Chu, C.C.; Yao, S.G. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Redfern, S.K.; Azzu, N.; Binamira, J.S. Rice in Southeast Asia: Facing risks and vulnerabilities to respond to climate change. Building resilience for adaptation to climate change in the agriculture sector. In Proceedings of the a Joint FAO/OECD WORKSHOP, Rome, Italy, 23–24 April 2012; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012. [Google Scholar]
- Bahuguna, R.N.; Jha, J.; Pal, M.; Shah, D.; Lawas, L.M.; Khetarpal, S.; Jagadish, K.S. Physiological and biochemical characterization of NERICA-L-44: A novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol. Plant. 2015, 154, 543–559. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathi, S.; Singh, S.P.; Prasad, A.; Akter, F.; Syed, M.A.; Badri, J.; Das, S.P.; Bhattarai, R.; Natividad, M.A.; et al. Rice breeding for yield under drought has selected for longer flag leaves and lower stomatal density. J. Exp. Bot. 2021, 72, 4981–4992. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, J.X. TT3.1: A journey to protect chloroplasts upon heat stress. Stress Biol. 2022, 2, 27. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Murty, M.V.R.; Quick, W.P. Rice responses to rising temperatures-challenges, perspectives and future directions. Plant Cell Environ. 2015, 38, 1686–1698. [Google Scholar] [CrossRef]
- Xie, Y.J.; Shen, Q.P.; Li, F.F.; Ni, S.; Yu, J.S. Chapter Three-Temperature response of plants and heat tolerance in Rice: A review. Adv. Agron. 2023, 179, 135–203. [Google Scholar]
- Matsui, T.; Omasa, K.; Horie, T. The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Product. Sci. 2015, 4, 90–93. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, L.; Zhou, J.X.; Hu, S.B.; Chen, H.Z. Research progress on heat stress of rice at flowering stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.R.; Gao, J.; Lin, H.X.; Lin, Y.S. The molecular basis of heat stress responses in plants. Mol. Plant. 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Gu, Z.D.; Ding, Y.F.; Wang, K.; Jiang, Q.; Zhu, C. Effect of high temperature stress on physiological characteristics of spikelet of rice during flowering stage. Chin. J. Rice Sci. 2016, 30, 637–646. [Google Scholar]
- Guo, J.M.; Wu, Y.; Yang, S.B.; Jiang, X.D.; Xie, X.Y.; Wang, J.J.; Shen, S.H. Yield different and its causes for one season rice under different sowing dates in typical high temperature year. Chin. J. Agrometeorol. 2017, 38, 121–130. [Google Scholar]
- Wei, J.L.; Pan, X.H.; Deng, Q.H. Effects of nighttime temperature increase at different growth stages on double season rice grain yield. Chin. J. Appl. Ecol. 2010, 21, 331–337. [Google Scholar]
- Wu, C.; Cui, K.H.; Wang, W.C.; Li, Q.; Fahad, S.; Hu, Q.Q.; Huang, J.L.; Nie, L.X.; Peng, S.B. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci. Rep. 2016, 6, 34978. [Google Scholar] [CrossRef]
- Gong, Z.Z.; Xiong, L.M.; Shi, H.Z.; Yang, S.H.; Herrera-Estrella, L.R.; Xu, G.H.; Chao, D.Y.; Li, J.R.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Folsom, J.J.; Begcy, K.; Hao, X.J.; Wang, D.; Walia, H. Rice Fertilization-Independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol. 2014, 165, 238–248. [Google Scholar] [CrossRef]
- Chen, C.; Begcy, K.; Kiu, K.; Folsom, J.J.; Wang, Z.; Zhang, C.; Walia, H. Heat stress yields a unique MADS box transcription factor in determining seed size and thermal sensitivity. Plant Physiol. 2016, 171, 606–622. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Muthurajan, R.; Oane, R.; Wheeler, T.R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef]
- Ren, H.M.; Bao, J.P.; Gao, Z.X.; Sun, D.Y.; Zheng, S.Z.; Bai, J.T. How rice adapts to high temperatures. Front. Plant Sci. 2023, 14, 1137923. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Butardo Jr, V.M.; Misra, G.; Cuevas, R.P.; Anacleto, R.; Kishor, P.B.K. Designing climate-resilient rice with ideal grain quality suited for high-temperature stress. J. Exp. Bot. 2015, 66, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, H.X.; Li, L.; Liu, X.; Chen, L.; Chen, W.Z.; Ding, Y.F. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct. Plant Biol. 2018, 45, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Sasaki, M.; Kuribayashi, N.; Susuki, H.; Sasuga, Y.; Shiraya, T.; Inomata, T.; Itoh, K.; Baslam, M.; Mitsui, T. Proteomic and glycomic characterization of rice chalky grains produced under moderate and high-temperature conditions in field system. Rice 2016, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, T.; Parween, S.; Saito, Y.; Shigemitsu, T.; Yamakawa, H.; Nakazono, M.; Masumura, T.; Nishizawa, N.K.; Kondo, M.; Screenivasulu, N. Laser Microdissection-Based Tissue-Specific Transcriptome Analysis Reveals a Novel Regulatory Network of Genes Involved in Heat-Induced Grain Chalk in Rice Endosperm. Plant Cell Physiol. 2019, 60, 626–642. [Google Scholar] [CrossRef]
- Shi, W.J.; Yin, X.Y.; Struik, P.C.; Solis, C.; Xie, F.M.; Schimidt, R.C.; Huang, M.; Zou, Y.B.; Ye, C.R.; Jagadish, S.V.K. High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. J. Exp. Bot. 2017, 68, 5233–5245. [Google Scholar] [CrossRef]
- Begcy, K.; Sandhu, J.; Walia, H. Transient Heat Stress During Early Seed Development Primes Germination and Seedling Establishment in Rice. Front. Plant Sci. 2018, 9, 1768. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hakata, M. Atlas of rice grain filling-related metabolism under high temperature: Joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 2010, 51, 795–809. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Jiang, Y.Y.; Zhang, H.; Wang, S.Y.; Wang, F.L.; Zhu, Y. Genetic Control and High Temperature Effects on Starch Biosynthesis and Grain Quality in Rice. Front. Plant Sci. 2021, 12, 757997. [Google Scholar] [CrossRef]
- Shirdelmoghanloo, H.; Chen, K.F.; Paynter, B.H.; Angessa, T.T.; Westcott, S.; Khan, H.A.; Hill, C.B.; Li, C.D. Grain-Filling Rate Improves Physical Grain Quality in Barley Under Heat Stress Conditions During the Grain-Filling Period. Front. Plant Sci. 2022, 13, 858652. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 5, 100990. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Yang, C.; Xu, J.M.; Lu, H.P.; Liu, J.X. The hot science in rice research: How rice plants cope with heat stress. Plant. Cell Environ. 2023, 46, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.M.; Lu, S.; Li, Z.; Cheng, J.W.; Hu, P.; Zhu, T.Q.; Wang, X.; Jin, M.; Wang, X.X.; Li, L.Q.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhang, Q.; Liu, D.L.; Wang, H.Q.; Yin, J.Y.; Wang, R.; He, M.L.; Cui, M.; Shang, Z.L.; Wang, D.K.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain-filling problem in ‘super’ rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef]
- Tonosaki, K.; Kinoshita, T. Possible roles for polycomb repressive complex 2 in cereal endosperm. Front. Plant Sci. 2015, 6, 144. [Google Scholar] [CrossRef]
- Dhatt, B.K.; Paul, P.; Sandhu, J.; Hussain, W.; Irvin, L.; Zhu, F.Y.; Adviento-Borbe, M.A.; Lorence, A.; Staswick, P.; Yu, H.F.; et al. Allelic variation in rice Fertilization Independent Endosperm 1 contributes to grain width under high night temperature stress. New Phytol. 2021, 229, 335–350. [Google Scholar] [CrossRef]
- Paul, P.; Dhatt, B.K.; Miller, M.; Folsom, J.J.; Wang, Z.; Krassovskaya, I.; Liu, K.; Sandhu, J.; Yu, H.H.; Zhang, C.; et al. MADS78 and MADS79 are essential regulators of early seed development in rice. Plant Physiol. 2020, 182, 933–948. [Google Scholar] [CrossRef]
- Zhu, X.P.; Teng, X.; Wang, Y.L.; Hao, Y.Y.; Jing, R.N.; Wang, Y.F.; Liu, Y.; Zhu, J.P.; Wu, M.M.; Zhong, M.S.; et al. FLOURYENDOSPERM11 encoding a plastid heat shock protein 70 is essential for amyloplast development in rice. Plant Sci. 2018, 277, 89–99. [Google Scholar] [CrossRef]
- Tabassum, R.; Dosaka, T.; Ichida, H.; Morita, R.; Ding, Y.F.; Abe, T.; Katsube-Tanaka, T. FLOURY ENDOSPERM11-2 encodes plastid HSP70-2 involved with the temperature-dependent chalkiness of rice (Oryza sativa L.) grains. Plant J. 2020, 103, 604–616. [Google Scholar] [CrossRef]
- Hakata, M.; Kuroda, M.; Miyashita, T.; Yamaguchi, T.; Kojima, M.; Sakakibara, H.; Mitsui, T.; Yamakawa, H. Suppression of α-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnol. J. 2012, 10, 1110–1117. [Google Scholar] [CrossRef]
- Nakata, M.; Fukamatsu, Y.; Miyashita, T.; Hakata, M.; Kimura, R.; Nakata, Y.; Kuroda, M.; Yamaguchi, T.; Yamakawa, H. High Temperature-Induced Expression of Rice α-Amylases in Developing Endosperm Produces Chalky Grains. Front. Plant Sci. 2017, 8, 2089. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.F.; Zhang, H.; Wang, L.C.; Zhu, Z.G.; Gao, J.P.; Li, C.S.; Zhu, Y. High temperature inhibits the accumulation of storage materials by inducing alternative splicing of OsbZIP58 during filling stage in rice. Plant Cell Environ. 2020, 43, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Xu, H.; Zhu, Y.; Liu, Q.Q.; Cai, X.L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 2013, 64, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Duan, L.; Dai, J.S.; Zhang, C.Q.; Li, J.; Gu, M.H.; Liu, Q.Q.; Zhu, Y. Major QTLs reduce the deleterious effects of high temperature on rice amylose content by increasing splicing efficiency of Wx pre-mRNA. Theor. Appl. Genet. 2014, 127, 273–282. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Feng, M.J.; Zhu, Y. Suppression of OsMADS7 in ice endosperm stabilizes amylose content under high temperature tress. Plant Biotechnol. J. 2018, 16, 18–26. [Google Scholar] [CrossRef]
- Huang, L.C.; Tan, H.Y.; Zhang, C.Q.; Li, Q.F.; Liu, Q.Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef]
- Kato, K.; Suzuki, Y.; Hosaka, Y.; Takahashi, R.; Kodama, I.; Sato, K.; Kawamoto, T.; Kumamaru, T.; Fujita, N. Effect of high temperature on starch biosynthetic enzymes and starch structure in japonica rice cultivar ‘Akitakomachi’ (Oryza sativa L.) endosperm and palatability of cooked rice. J. Cereal Sci. 2019, 87, 209–214. [Google Scholar] [CrossRef]
- Wu, H.M.; Ren, Y.L.; Dong, H.; Xie, C.; Zhao, L.; Wang, X.; Zhang, F.L.; Zhang, B.L.; Jiang, X.K.; Huang, Y.S.; et al. FLOURY ENDOSPERM24, a heat shock protein 101 (HSP101), is required for starch biosynthesis and endosperm development in rice. New Phytol. 2024, 242, 2635–2651. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, Z.Q.; Jiang, H.; Wang, Z.; Wu, F.S.; Xiong, Y.F.; Yao, J.L. A heat stress responsive NAC transcription factor heterodimer plays key roles in rice grain filling. J. Exp. Bot. 2021, 72, 2947–2964. [Google Scholar] [CrossRef]
- Yao, D.P.; Wu, J.; Luo, Q.H.; Li, J.W.; Zhuang, W.; Xiao, G.; Deng, Q.Y.; Lei, D.Y.; Bai, B. Influence of high natural field temperature during grain filling stage on the morphological structure and physicochemical properties of rice (Oryza sativa L.) starch. Food Chem. 2020, 310, 125817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Zhou, L.H.; Zhu, Z.B.; Lu, H.W.; Zhou, X.Z.; Qian, Y.T.; Li, Q.F.; Gu, M.H.; Liu, Q.Q. Characterization of Grain Quality and Starch Fine Structure of Two Japonica Rice (Oryza sativa) Cultivars with Good Sensory Properties at Different Temperatures during the Filling Stage. J. Agric. Food Chem. 2016, 64, 4048–4057. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheng, F.; Zhong, L.; Sun, Z. Different of RVA profile among different early indica rice varieties and effect of temperature at grain filling stage on it. Chin. J. Rice Sci. 2003, 17, 39–43. [Google Scholar]
- Suriyasak, C.; Harano, K.; Tanamachi, K.; Matsuo, K.; Tamada, A.; Iwaya-Inoue, M.; Ishibashi, Y. Reactive oxygen species induced by heat stress during grain filling of rice (Oryza sativa L.) are involved in occurrence of grain chalkiness. J. Plant Physiol. 2017, 216, 52–57. [Google Scholar] [CrossRef]
- Wu, B.; Yun, P.; Zhou, H.; Xia, D.; Gu, Y.; Li, P.B.; Yao, J.L.; Zhou, Z.Q.; Chen, J.X.; Liu, R.J.; et al. Natural variation in WHITE-CORE RATE 1 regulates redox homeostasis in rice endosperm to affect grain quality. Plant Cell 2022, 34, 1912–1932. [Google Scholar] [CrossRef]
- Pang, Y.H.; Ying, Y.N.; Yu, F.F.; Bao, J.S. Integrated analysis of phosphoproteome and ubiquitinated proteome in rice endosperm under high temperature stress. J. Zhejiang Univ. Sci. (Agric. Life Sci. Ed.) 2024, 50, 382–392. [Google Scholar]
- Zhang, X.F.; Tong, J.H.; Bai, A.N.; Liu, C.M.; Xiao, L.T.; Xue, H.W. Phytohormone dynamics in developing endosperm influence rice grain shape and quality. J. Integr. Plant Biol. 2020, 62, 1625–1637. [Google Scholar] [CrossRef]
- Qin, P.; Zhang, G.H.; Hu, B.H.; Wu, J.; Chen, W.L.; Ren, Z.J.; Liu, Y.L.; Xie, J.; Yuan, H.; Tu, B.; et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci. Adv. 2021, 7, eabc8873. [Google Scholar] [CrossRef]
- Liu, J.P.; Zhang, C.C.; Wei, C.C.; Wang, M.G.; Yu, F.F.; Xie, Q.; Tu, J.M. The RING Finger Ubiquitin E3 Ligase OsHTAS Enhances Heat Tolerance by Promoting H2O2-Induced Stomatal Closure in Rice. Plant Physiol. 2016, 170, 429–443. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhong, X.; Liao, J.P.; Ji, P.; Yang, J.S.; Cao, Z.R.; Duan, X.M.; Xiong, J.R.; Wang, Y.; Xu, C.; et al. Exogenous abscisic acid improves grain filling capacity under heat stress by enhancing antioxidative defense capability in rice. BMC Plant Biol. 2023, 23, 619. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zhou, W.; Guo, X.Y.; Ling, S.; Li, W.; Wang, X.; Yao, J.L. Heat Stress Responsive Aux/IAA Protein, OsIAA29 Regulates Grain Filling Through OsARF17 Mediated Auxin Signaling Pathway. Rice 2024, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Mesihovic, A.; Hu, Y.J.; Schleiff, E. Unfolded protein response in pollen development and heat stress tolerance. Plant Reprod. 2016, 29, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.; Irvin, L.; Liu, K.; Staswick, P.; Zhang, C.; Walia, H. Endoplasmic reticulum stress pathway mediates the early heat stress response of developing rice seeds. Plant Cell Environ. 2021, 44, 2604–2624. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.J.; Zhao, S.L.; Jiao, G.A.; Duan, Y.Q.; Ma, L.Y.; Dong, N.N.; Lu, F.F.; Zhu, M.D.; Shao, G.N.; Hu, S.K.; et al. OPAQUE3, encoding a transmembrane bZIP transcription factor, regulates endosperm storage protein and starch biosynthesis in rice. Plant Commun. 2022, 3, 100463. [Google Scholar] [CrossRef]
- Yang, W.P.; Xu, P.K.; Zhang, J.C.; Zhang, S.; Li, Z.W.; Yang, K.; Chang, X.Y.; Li, Y.B. OsbZIP60-mediated unfolded protein response regulates grain chalkiness in rice. J. Genet. Genom. 2022, 49, 414–426. [Google Scholar] [CrossRef]
- Lu, S.J.; Yang, Z.T.; Sun, L.; Sun, L.; Song, Z.T.; Liu, J.X. Conservation of IRE1-regulated bZIP74 mRNA unconventional splicing in rice (Oryza sativa L.) involved in ER stress responses. Mol. Plant 2012, 5, 504–514. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Qing, T.; Shu, X.L.; Liu, J.X. Unfolded protein response and storage product accumulation in rice grains. Seed Biol. 2022, 1, 33–37. [Google Scholar] [CrossRef]
- Berka, M.; Kopecka, R.; Berkova, V.; Brzobohaty, B.; Cerny, M. Regulation of heat shock proteins 70 and their role in plant immunity. J. Exp. Bot. 2022, 73, 1894–1909. [Google Scholar] [CrossRef]
- De-Jong, W.W.; Caspers, G.J.; Leunissen, J.A. Genealogy of the alpha-crystallin-small heat-shock protein superfamily. Int. J. Biol. Macromol. 1998, 22, 151–162. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Lu, F.F.; Jiao, G.A.; Qiu, J.H.; Zhao, S.L.; Zhao, F.L.; Wang, P.; Chen, L.N.; Chen, P.F.; Li, X.W.; Don, N.N.; et al. A molecular module improves rice grain quality and yield at high temperature. Nat. Sci. Rev. 2025, 12, nwae416. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, X.; Wang, M.; Zhu, Q.; Lv, Y.; Xu, J.; Liu, J. The NAT1-bHLH110-CER1/CER1L module regulates heat stress tolerance in rice. Nat. Genet. 2025, 57, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Chao, D.Y.; Wu, Y.; Huang, X.H.; Chen, K.; Cui, L.G.; Su, L.; Ye, W.W.; Chen, H.; Chen, H.C.; et al. Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.R.; Zhang, H.; Gao, J.; Shan, J.X.; Ye, W.W.; Lin, H.X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plant 2022, 8, 53–67. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.F.; Kan, Y.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Guo, T.; Xiang, Y.H.; Yang, Y.B.; Li, Y.C.; et al. A gen module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef]
- Kan, Y.; Lin, H.X. A research progress of thermo-perception and thermo-responses in rice. Chin. J. Nat. 2022, 44, 411–421. [Google Scholar]
- Mir, M.S.; Raja, W.; Kanth, R.H.; Dar, E.A.; Shah, Z.A.; Bhat, M.A.; Mir, A.H.; Wani, F.J.; Bhat, T.A.; Bhat, J.A.; et al. Optimizing irrigation and nitrogen levels to achieve sustainable rice productivity and profitability. Sci. Rep. 2025, 15, 6675. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, H.; Zhang, F.; Wang, H.; Zhang, F.S. Effects of silicon on anther dehiscence and pollen shedding in rice under high-temperature stress. Acta Agron. Sin. 2005, 31, 134–136. [Google Scholar]
- Zhao, J. Effect of application quantity of N, P and K on resistant capability of rice against hot disaster of high temperature. Soils Fertil. 2005, 5, 13–16. [Google Scholar]
- Chen, T.T.; Li, G.Y.; Islam, M.R.; Fu, W.M.; Feng, B.H.; Tao, L.X.; Fu, G.F. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biol. 2019, 19, 525. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, P.H.; Fu, W.M.; Li, G.Y.; Feng, B.H.; Chen, T.T.; Li, H.B.; Tao, L.X.; Fu, G.F. Acid invertase confers heat tolerance in rice plants by maintaining energy homoeostasis of spikelets. Plant Cell Environ. 2020, 43, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of proline function in higher plants under extreme temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).