Fly High: Volatile Organic Compounds for the Early Detection of the Seed-Borne Pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
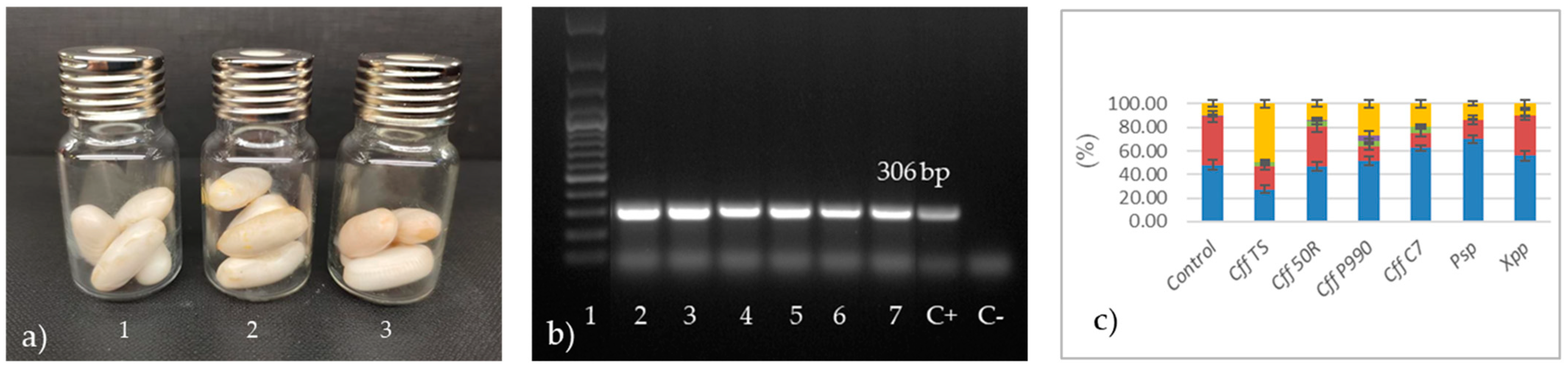
2.2. Bacterial Inoculation and Seed Infection
2.3. Analysis of Volatile Organic Compounds (VOCs)
2.4. Experimental Design and Statistics
3. Results
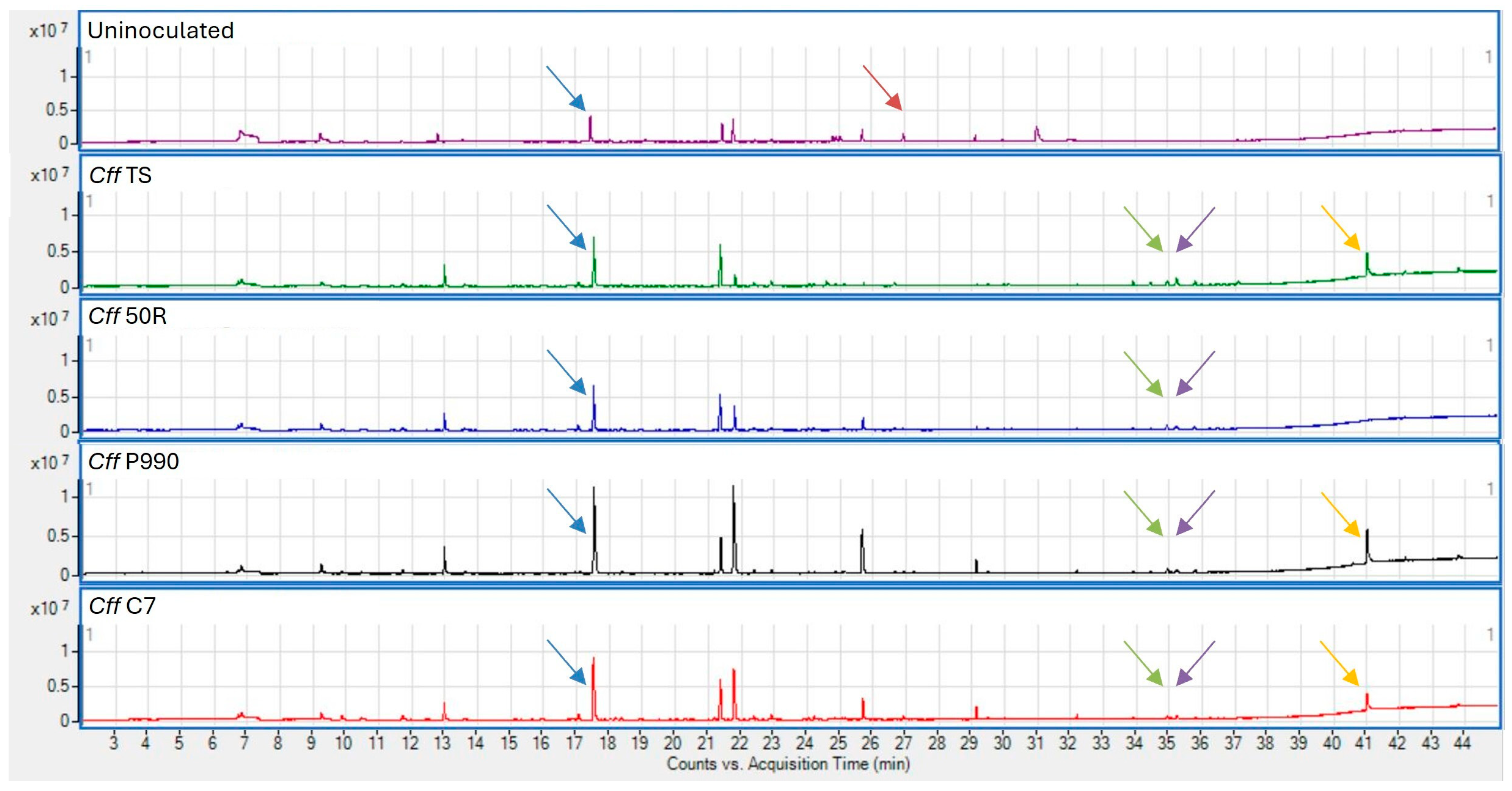
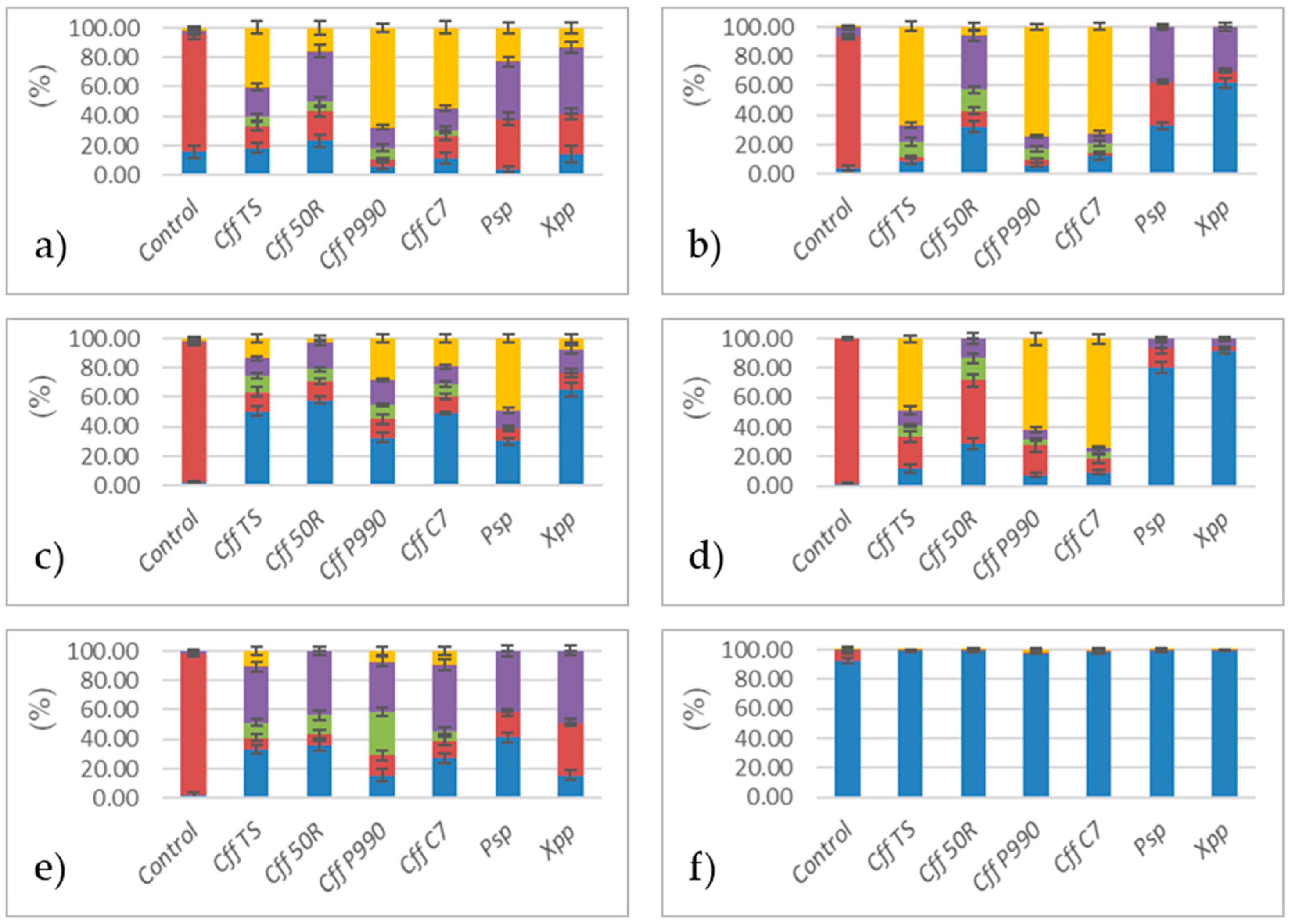
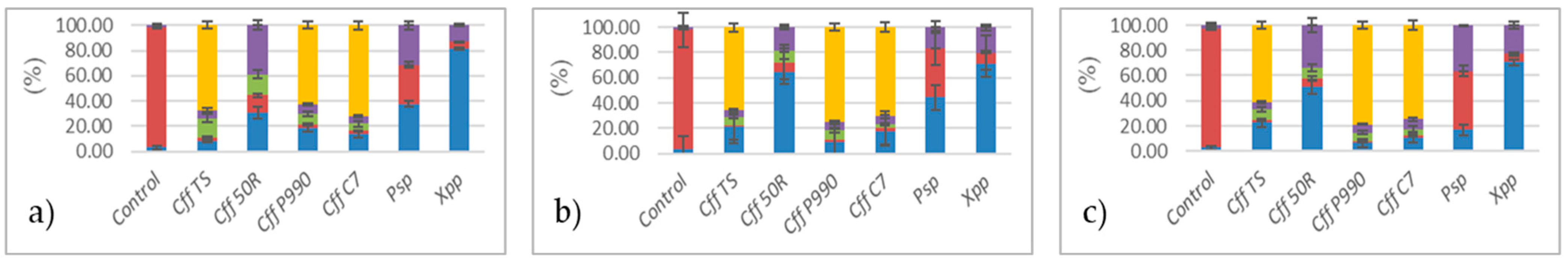
3.1. Analysis of Cff VOCs Produced During In Vitro Culture
3.2. Analysis of VOCs Produced by Artificially Cff-Infected Bean Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Rourke, J.A.; Bolon, Y.-T.; Bucciarelli, B.; Vance, C.P. Legume Genomics: Understanding Biology through DNA and RNA Sequencing. Ann. Bot. 2014, 113, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.E.; Thavarajah, D. Checking Agriculture’s Pulse: Field Pea (Pisum sativum L.), Sustainability, and Phosphorus Use Efficiency. Front. Plant Sci. 2019, 10, 1489. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 29 December 2024).
- Osdaghi, E.; Pakdaman Sardrood, B.; Bavi, M.; Akbari Oghaz, N.; Kimiaei, S.; Hadian, S. First Report of Curtobacterium flaccumfaciens pv. flaccumfaciens Causing Cowpea Bacterial Wilt in Iran. J. Phytopathol. 2015, 163, 653–656. [Google Scholar] [CrossRef]
- Osdaghi, E.; Young, A.J.; Harveson, R.M. Bacterial Wilt of Dry Beans Caused by Curtobacterium flaccumfaciens pv. flaccumfaciens: A New Threat from an Old Enemy. Mol. Plant Pathol. 2020, 21, 605–621. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (EFSA PLH Panel); Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Grégoire, J.-C.; Jaques Miret, J.A.; et al. Pest Categorisation of Curtobacterium flaccumfaciens pv. flaccumfaciens. EFSA J. 2018, 16, e05299. [Google Scholar] [CrossRef]
- Hedges, F. A Bacterial Wilt of the Bean Caused by Bacterium flaccumfaciens Nov. Sp. Science 1922, 55, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Hedges, F. Bacterial Wilt of Beans (Bacterium flaccumfaciens Hedges), Including Comparisons with Bacterium phaseoli. Phytopathology 1926, 16, 1–22. [Google Scholar]
- Zaumeter, W.J. Comparative Pathological Histology of Three Bacterial Diseases of Bean. J. Agric. Res. 1932, 44, 605–632. [Google Scholar]
- Harveson, R.M.; Schwartz, H.F.; Urrea, C.A.; Yonts, C.D. Bacterial Wilt of Dry-Edible Beans in the Central High Plains of the U.S.: Past, Present, and Future. Plant Dis. 2015, 99, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Erickson, R.S.; Balasubramanian, P.M.; Hsieh, T.F.; Conner, R.L. Resurgence of Bacterial Wilt of Common Bean in North America. Can. J. Plant Pathol. 2009, 31, 290–300. [Google Scholar] [CrossRef]
- Schuster, M.L.; Sayre, R.M. A Coryneform Bacterium Induces Purple-Colored Seed and Leaf Hypertrophy of Phaseolus vulgaris and Other Leguminosae. Phytopathology 1967, 57, 1064–1066. [Google Scholar]
- Harveson, R.M. The Multicolored Bacterium. Available online: https://www.apsnet.org/edcenter/apsnetfeatures/pages/bacterium.aspx (accessed on 29 December 2024).
- Osdaghi, E.; Taghavi, S.M.; Hamzehzarghani, H.; Fazliarab, A.; Harveson, R.M.; Lamichhane, J.R. Occurrence and Characterization of a New Red-Pigmented Variant of Curtobacterium flaccumfaciens, the Causal Agent of Bacterial Wilt of Edible Dry Beans in Iran. Eur. J. Plant Pathol. 2016, 146, 129–145. [Google Scholar] [CrossRef]
- Harveson, R.M.; Schwartz, H.F.; Vidaver, A.K.; Lambrecht, P.A.; Otto, K.L. New Outbreaks of Bacterial Wilt of Dry Bean in Nebraska Observed from Field Infections. Plant Dis. 2006, 90, 681. [Google Scholar] [CrossRef]
- Wood, B.A.; Easdown, W.J. A New Bacterial Disease of Mung Bean and Cowpea for Australia. Australas. Plant Pathol. 1990, 19, 16–21. [Google Scholar] [CrossRef]
- Sammer, U.F.; Reiher, K. Curtobacterium flaccumfaciens pv. flaccumfaciens on Soybean in Germany—A Threat for Farming. J. Phytopathol. 2012, 160, 314–316. [Google Scholar] [CrossRef]
- Pilik, R.; Tesic, S.; Ignatov, A.N.; Tarakanov, R.I.; Dorofeeva, L.V.; Lukyanova, A.A.; Evseev, P.V.; Dzhalilov, F.S.-U.; Miroshnikov, K.A. First Report of Curtobacterium flaccumfaciens pv. flaccumfaciens Causing Bacterial Wilt and Blight on Sunflower in Russia. Plant Dis. 2023, 107, 1621. [Google Scholar] [CrossRef]
- Curtobacterium flaccumfaciens pv. flaccumfaciens (CORBFL) [World Distribution]|EPPO Global Database. Available online: https://gd.eppo.int/taxon/CORBFL/distribution (accessed on 29 December 2024).
- EPPO Global Database. Available online: https://gd.eppo.int/reporting/article-7341 (accessed on 29 December 2024).
- Curtobacterium flaccumfaciens pv. flaccumfaciens (CORBFL) [Netherlands]|EPPO Global Database. Available online: https://gd.eppo.int/taxon/CORBFL/distribution/NL (accessed on 29 December 2024).
- Curtobacterium flaccumfaciens pv. flaccumfaciens (CORBFL) [Switzerland]|EPPO Global Database. Available online: https://gd.eppo.int/taxon/CORBFL/distribution/CH (accessed on 29 December 2024).
- Kizheva, Y.; Pandova, M.; Dimitrova, M.; Gladicheva, Y.; Garkova, M.; Pirnareva, D.; Donchev, D.; Moncheva, P.; Hristova, P. First Report of Curtobacterium flaccumfaciens in Bulgaria. Pathogens 2024, 13, 483. [Google Scholar] [CrossRef]
- Implementing Regulation—2019/2072—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg_impl/2019/2072/oj/eng (accessed on 30 December 2024).
- Curtobacterium flaccumfaciens pv. flaccumfaciens (CORBFL) [Categorization]|EPPO Global Database. Available online: https://gd.eppo.int/taxon/CORBFL/categorization (accessed on 29 December 2024).
- Van Vuurde, J.W.L.; van den, B.G.W.; Birnbaum, Y. Immunofluorescence Microscopy and Enzyme-Linked Immunosorbent Assay as Potential Routine Tests for the Detection of Pseudomonas syringae phaseolicola and Xanthomonas campestris pv. phaseoli in Bean Seed. Seed Sci. Technol. 1983, 11, 547–559. [Google Scholar]
- Messenberg Guimaraés, P.; Palmano, S.; Smith, J.J.; Grossi de Sá, M.F.; Saddler, G.S. Development of a PCR Test for the Detection of Curtobacterium flaccumfaciens pv. flaccumfaciens. Antonie Van Leeuwenhoek 2001, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tegli, S.; Sereni, A.; Surico, G. PCR-Based Assay for the Detection of Curtobacterium flaccumfaciens pv. flaccumfaciens in Bean Seeds. Lett. Appl. Microbiol. 2002, 35, 331–337. [Google Scholar] [CrossRef]
- Tegli, S.; Biancalani, C.; Ignatov, A.N.; Osdaghi, E. A Powerful LAMP Weapon against the Threat of the Quarantine Plant Pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens. Microorganisms 2020, 8, 1705. [Google Scholar] [CrossRef]
- Tegli, S.; Gaudioso, D.; Stefanucci, D. Innovative Detection of the Quarantine Plant Pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens, Causal Agent of Bacterial Wilt of Leguminous Plants. In Plant Pathology: Method and Protocols; Luchi, N., Ed.; Springer: New York, NY, USA, 2022; pp. 251–261. ISBN 978-1-07-162517-0. [Google Scholar]
- Cavigli, L.; Gaudioso, D.; Faraloni, C.; Agati, G.; Tegli, S. Exploiting Bacterial Pigmentation for Non-Destructive Detection of Seed-Borne Pathogens by Using Photoacoustic Techniques. Sensors 2024, 24, 7616. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial Volatiles and Their Action Potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Ozawa, R.; Nishioka, T.; Boland, W.; Koch, T.; Kühnemann, F.; Takabayashi, J. Herbivore-Induced Volatiles Induce the Emission of Ethylene in Neighboring Lima Bean Plants. Plant J. 2002, 29, 87–98. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The Role of Volatiles in Plant Communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant Volatiles as Cues and Signals in Plant Communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef]
- Weise, T.; Kai, M.; Gummesson, A.; Troeger, A.; von Reuß, S.; Piepenborn, S.; Kosterka, F.; Sklorz, M.; Zimmermann, R.; Francke, W.; et al. Volatile Organic Compounds Produced by the Phytopathogenic Bacterium Xanthomonas campestris pv. vesicatoria 85-10. Beilstein J. Org. Chem. 2012, 8, 579–596. [Google Scholar] [CrossRef]
- Boots, A.W.; Smolinska, A.; van Berkel, J.J.B.N.; Fijten, R.R.R.; Stobberingh, E.E.; Boumans, M.L.L.; Moonen, E.J.; Wouters, E.F.M.; Dallinga, J.W.; Schooten, F.J.V. Identification of Microorganisms Based on Headspace Analysis of Volatile Organic Compounds by Gas Chromatography–Mass Spectrometry. J. Breath. Res. 2014, 8, 027106. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Vidaver, A.K. Synthetic and Complex Media for the Rapid Detection of Fluorescence of Phytopathogenic Pseudomonads: Effect of the Carbon Source. Appl. Microbiol. 1967, 15, 1523–1524. [Google Scholar] [CrossRef]
- Dreiseikelmann, B.; Bunk, B.; Spröer, C.; Rohde, M.; Nimtz, M.; Wittmann, J. Characterization and Genome Comparisons of Three Achromobacter Phages of the Family Siphoviridae. Arch. Virol. 2017, 162, 2191–2201. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Škrlep, M.; Piasentier, E.; Calamai, L.; Franci, O.; Čandek-Potokar, M. The Effect of Ripening Time on the Chemical, Textural, Volatile and Sensorial Traits of Bicep Femoris and Semimembranosus Muscles of the Slovenian Dry-Cured Ham Kraški Pršut. Meat Sci. 2015, 100, 58–68. [Google Scholar] [CrossRef]
- Catola, S.; Kaidala Ganesha, S.D.; Calamai, L.; Loreto, F.; Ranieri, A.; Centritto, M. Headspace-Solid Phase Microextraction Approach for Dimethylsulfoniopropionate Quantification in Solanum lycopersicum Plants Subjected to Water Stress. Front. Plant Sci. 2016, 7, 1257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Informatics, N.O. of D. and NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 30 December 2024).
- Curtobacterium flaccumfaciens pv. flaccumfaciens (CORBFL), EPPO Global Database. Available online: https://gd.eppo.int/taxon/CORBFL/hosts (accessed on 29 December 2024).
- Blom, D.; Fabbri, C.; Connor, E.C.; Schiestl, F.P.; Klauser, D.R.; Boller, T.; Eberl, L.; Weisskopf, L. Production of Plant Growth Modulating Volatiles Is Widespread among Rhizosphere Bacteria and Strongly Depends on Culture Conditions. Environ. Microbiol. 2011, 13, 3047–3058. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M.T. Flavor Aspects of Pulse Ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Rajhi, I.; Baccouri, B.; Rajhi, F.; Mhadhbi, H.; Flamini, G. Monitoring the Volatile Compounds Status of Whole Seeds and Flours of Legume Cultivars. Food Biosci. 2021, 41, 101105. [Google Scholar] [CrossRef]
- Scott, H.R.; Scott, L.E. Process of Treating Nut Kernels to Produce Food Ingredients 1922. U.S. Patent 1,416,128, 16 May 1922. [Google Scholar]
- Nierop Groot, M.N.; de Bont, J.A.M. Conversion of Phenylalanine to Benzaldehyde Initiated by an Aminotransferase in Lactobacillus plantarum. Appl. Environ. Microbiol. 1998, 64, 3009–3013. [Google Scholar] [CrossRef]
- Yano, T.; Miyahara, Y.; Morii, N.; Okano, T.; Kubota, H. Pentanol and Benzyl Alcohol Attack Bacterial Surface Structures Differently. Appl. Environ. Microbiol. 2016, 82, 402–408. [Google Scholar] [CrossRef]
- Garrido, A.; Atencio, L.A.; Bethancourt, R.; Bethancourt, A.; Guzmán, H.; Gutiérrez, M.; Durant-Archibold, A.A. Antibacterial Activity of Volatile Organic Compounds Produced by the Octocoral-Associated Bacteria Bacillus sp. BO53 and Pseudoalteromonas Sp. GA327. Antibiotics 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Bonikowski, R.; Świtakowska, P.; Kula, J. Synthesis, Odour Evaluation and Antimicrobial Activity of Some Geranyl Acetone and Nerolidol Analogues. Flavour Fragr. J. 2015, 30, 238–244. [Google Scholar] [CrossRef]
- Rubab, M.; Chelliah, R.; Saravanakumar, K.; Barathikannan, K.; Wei, S.; Kim, J.-R.; Yoo, D.; Wang, M.-H.; Oh, D.-H. Bioactive Potential of 2-Methoxy-4-Vinylphenol and Benzofuran from Brassica oleracea L. Var. Capitate f, Rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat. Foods 2020, 9, 568. [Google Scholar] [CrossRef]
- Lone, A.S.; Ravindran, K.C.; Jeandet, P. Evaluation of Antimicrobial Activity and Bioactive Compound Analysis of Verbascum thapsus L. A Folklore Medicinal Plant. Phytomed. Plus 2024, 4, 100560. [Google Scholar] [CrossRef]
- Huang, Z.; Dostal, L.; Rosazza, J.P. Microbial Transformations of Ferulic Acid by Saccharomyces cerevisiae and Pseudomonas fluorescens. Appl. Environ. Microbiol. 1993, 59, 2244–2250. [Google Scholar] [CrossRef]
- Mishra, S.; Sachan, A.; Vidyarthi, A.S.; Sachan, S.G. Transformation of Ferulic Acid to 4-Vinyl Guaiacol as a Major Metabolite: A Microbial Approach. Rev. Environ. Sci. Biotechnol. 2014, 13, 377–385. [Google Scholar] [CrossRef]

| Strain | International Code 1 | Colony Color and Type | Origin | Year | |
|---|---|---|---|---|---|
| Host | Country | ||||
| Cff TS | ICMP 2584 CFBP 3418 | yellow–fluidal | Phaseolus vulgaris | Hungary | 1957 |
| Cff 50R | ICMP 22071 CFBP 8819 | red–fluidal | Phaseolus vulgaris | Iran | 2014 |
| Cff P990 | ICMP 22053 CFBP 8820 | yellow–fluidal | Capsicum annum | Iran | 2015 |
| Cff C7 | J24 | orange–fluidal | Phaseolus vulgaris | USA | 2004 |
| Psp | IPV-BO 2325 | creamy dark | Phaseolus vulgaris | Italy | not available |
| Xpp | 95-61 | yellow–fluidal | Phaseolus vulgaris | USA | not available |
| Plant Grains | Variety | Origin | Latitude | Longitude |
|---|---|---|---|---|
| Phaseolus vulgaris | Cannellino | Argentina | −31.4200 | −64.1887 |
| Phaseolus vulgaris | Borlotto | Argentina | −31.6406 | −60.6917 |
| Glycine max | Sandokan | Italy | 44.7271 | 11.2892 |
| Vigna unguiculata | Occhio del Valdarno | Italy | 43.5166 | 11.5666 |
| Pisum sativum | Primavera | Italy | 41.5055 | 15.3391 |
| Phaseolus coccineus | Spagna Bianco | Italy | 40.9151 | 14.7954 |
| Lens culinaris | Delle Crete | Italy | 43.3351 | 11.3158 |
| Triticum durum | Senatore Cappelli | Italy | 43.4676 | 11.0434 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudioso, D.; Calamai, L.; Tegli, S. Fly High: Volatile Organic Compounds for the Early Detection of the Seed-Borne Pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens. Agronomy 2025, 15, 497. https://doi.org/10.3390/agronomy15020497
Gaudioso D, Calamai L, Tegli S. Fly High: Volatile Organic Compounds for the Early Detection of the Seed-Borne Pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens. Agronomy. 2025; 15(2):497. https://doi.org/10.3390/agronomy15020497
Chicago/Turabian StyleGaudioso, Dario, Luca Calamai, and Stefania Tegli. 2025. "Fly High: Volatile Organic Compounds for the Early Detection of the Seed-Borne Pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens" Agronomy 15, no. 2: 497. https://doi.org/10.3390/agronomy15020497
APA StyleGaudioso, D., Calamai, L., & Tegli, S. (2025). Fly High: Volatile Organic Compounds for the Early Detection of the Seed-Borne Pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens. Agronomy, 15(2), 497. https://doi.org/10.3390/agronomy15020497









