The Effects of Reducing Nitrogen and Increasing Density in the Main Crop on Yield and Cadmium Accumulation of Ratoon Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Site and Materials
2.2. Experimental Design
2.3. Measurement Items and Methods
2.4. Statistical Analysis
3. Results
3.1. Effects of N Fertilizer and Density on the Yield of Ratoon Rice
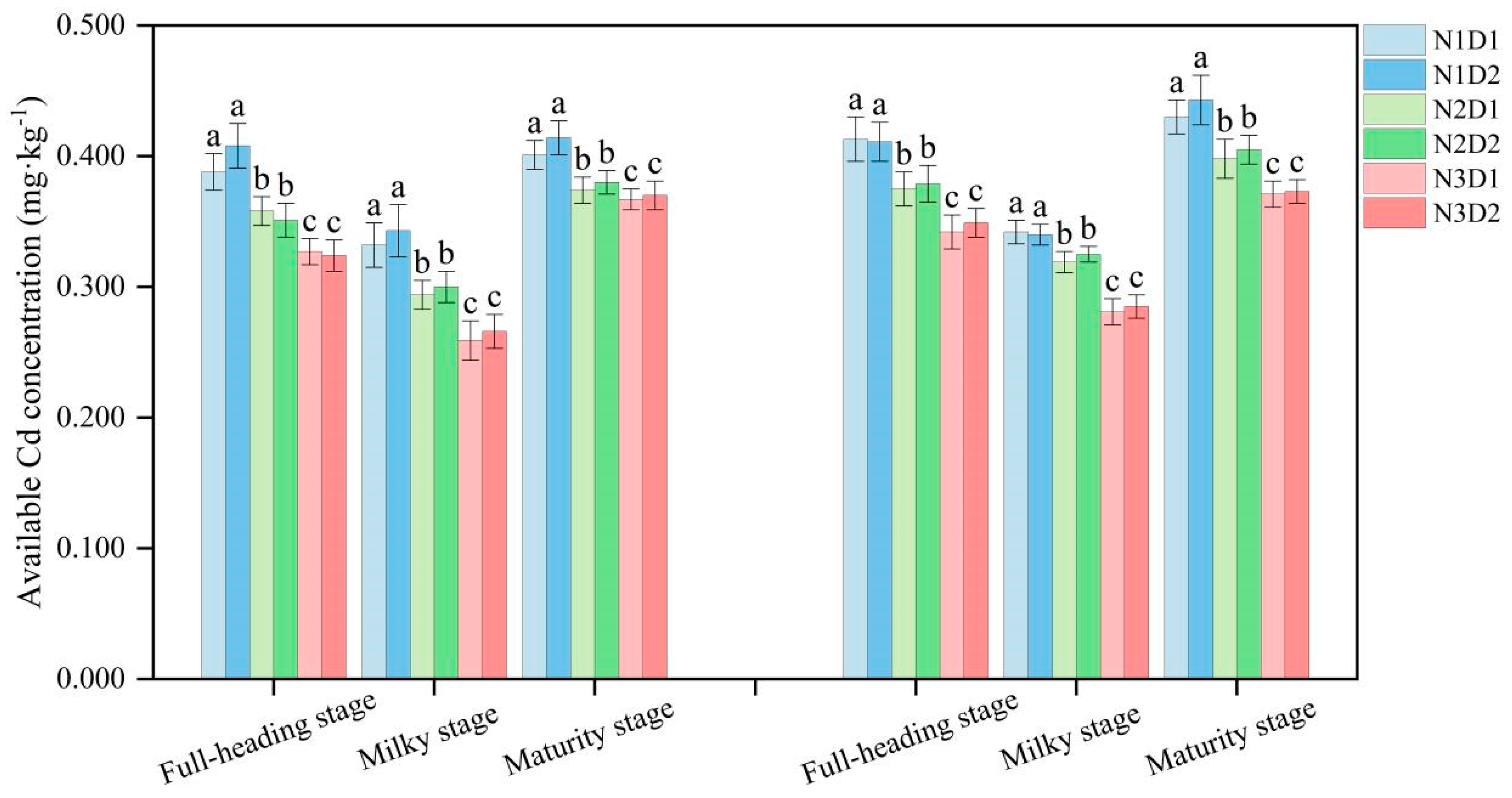
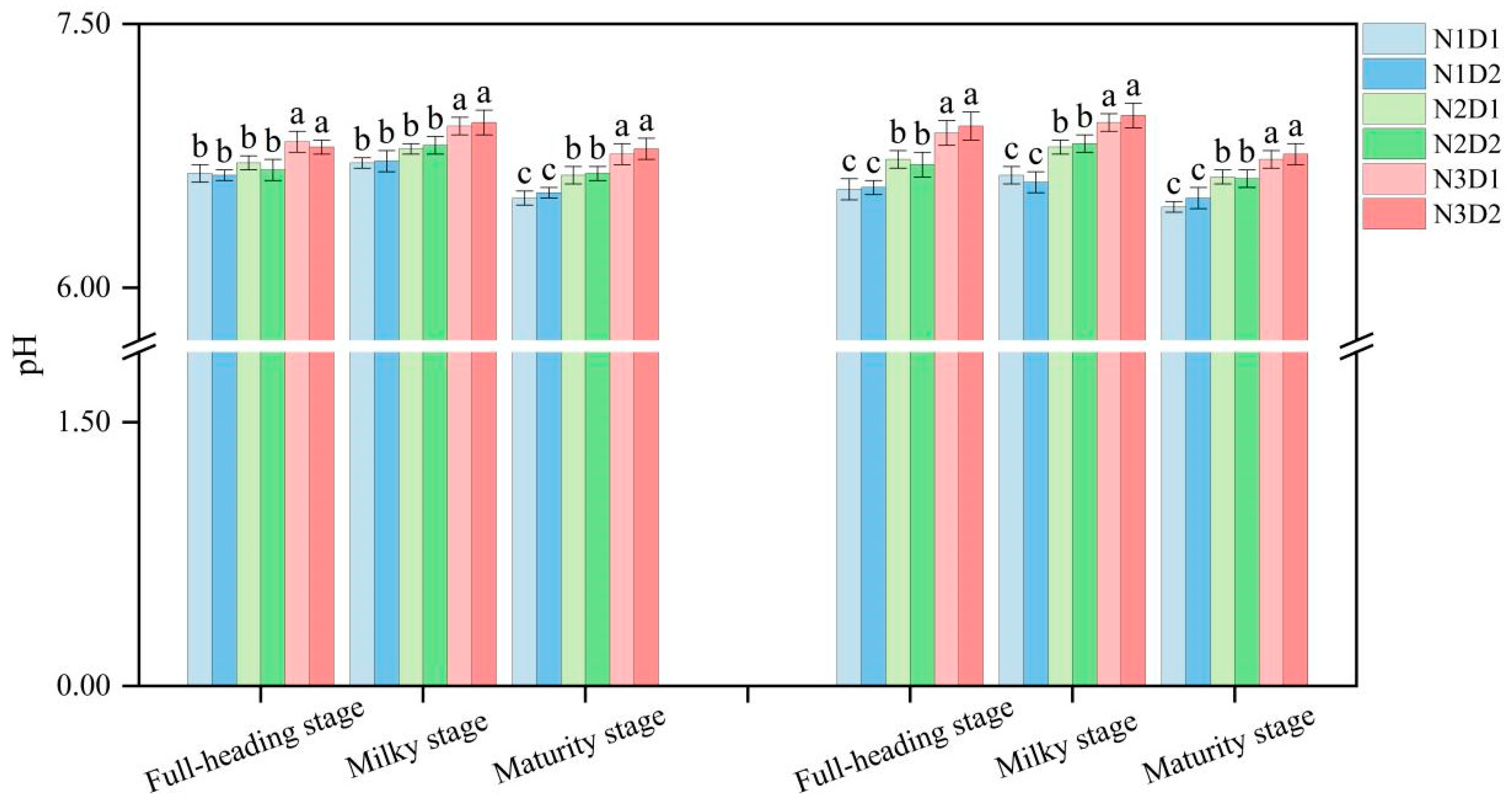
3.2. Effects of N Fertilizer and Density on the pH and Available Cd of Soil
3.3. Effects of N Fertilizer and Density on the Cd Accumulation Characteristics of Ratoon Rice
3.3.1. Effects of N Fertilizer and Density on the Cd Concentration of Ratoon Rice
3.3.2. Effects of N Fertilizer and Density on Cd Absorption and Translocation in Ratoon Rice
3.3.3. Effects of N Fertilizer and Density on Cd Accumulation of Ratoon Rice
4. Discussion
4.1. Effects of Reducing N Fertilizer and Increasing Density on Yield of Ratoon Rice
4.2. Effects of Reducing N Fertilizer and Increasing Density on Available Cd of Soil
4.3. Effects of Reducing N Fertilizer and Increasing Density on Cd Accumulation Characteristics of Ratoon Rice
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R.L. How does contamination of rice soils with Cd and Zn cause high incidence of human Cd disease in subsistence rice farmers. Curr. Pollut. Rep. 2015, 1, 13–22. [Google Scholar] [CrossRef]
- Singh, J.; Bhattu, M.; Verma, M.; Bechelany, M.; Brar, S.K.; Jadeja, R. Sustainable Valorization of Rice Straw into Biochar and Carbon Dots Using a Novel One-Pot Approach for Dual Applications in Detection and Removal of Lead Ions. Nanomaterials 2025, 15, 66. [Google Scholar] [CrossRef]
- Harrell, D.L.; Bond, J.A.; Blanche, S. Evaluation of main-crop stubble height on ratoon rice growth and development. Field Crops Res. 2009, 114, 396–403. [Google Scholar] [CrossRef]
- Yang, D.; Han, D.; Huang, J.; Peng, S. Reducing cadmium accumulation and increasing grain yield with proper timing of flood irrigation in the second season of ratoon rice. Field Crops Res. 2024, 306, 109222. [Google Scholar] [CrossRef]
- Yuan, S.; Jiang, Y.; Chen, P.; Tu, N.; Zhou, W.; Yi, Z. Effects of Cultivar Type and Node Position on Cadmium Accumulation Characteristics of Ratoon Rice. Agronomy 2024, 14, 1401. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Tao, L.; Cao, Z.; Tang, W.; Zhang, J.; Lu, Y. Regulatory mechanisms of nitrogen (N) on cadmium (Cd) uptake and accumulation in plants: A review. Sci. Total Environ. 2020, 708, 135186. [Google Scholar] [CrossRef]
- Iqbal, A.; Hussain, Q.; Mo, Z.; Hua, T.; Mustafa, A.E.-Z.M.A.; Tang, X. Vermicompost Supply Enhances Fragrant-Rice Yield by Improving Soil Fertility and Eukaryotic Microbial Community Composition under Environmental Stress Conditions. Microorganisms 2024, 12, 1252. [Google Scholar] [CrossRef]
- Huang, W.; Jia, Y.; Niu, C.; Zhang, H.; Wang, Y.; Feng, C. Effects of Carbon-Based Modified Materials on Soil Water and Fertilizer Retention and Pollution Control in Rice Root Zone. Sustainability 2024, 16, 6750. [Google Scholar] [CrossRef]
- Asmamaw, B.A. Effect of planting density on growth, yield and yield attributes of rice (Oryza sativa L.). Afr. J. Agric. Res. 2017, 12, 2713–2721. [Google Scholar] [CrossRef]
- Xu, F.; Wang, D.; Zhou, X.; Zhang, L.; Guo, X.; Liu, M.; Zhu, Y.; Xiong, H.; Guo, C.; Jiang, P. Integrating Nitrogen, Water, and Other Management Practices to Improve Grain and Ratoon Forage Yields in Perennial Rice. Plants 2024, 13, 3157. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gu, Y.; Lu, X.; Jiang, W.; Zhang, K.; Zhu, H.; Li, G.; Xu, F.; Zhu, Y.; Liu, G.; et al. Effects of One-Time Reduced Basal Application of Controlled-Release Nitrogen Fertilizer with Increased Planting Density on Yield and Nitrogen Utilization of Mechanically Transplanted Japonica Rice. Agronomy 2024, 14, 3072. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Geng, S.; Zhang, R.; Dou, M.; Wu, M.; Li, L.; Wang, D.; Wei, X.; Tian, P.; et al. Improvement of Transplanting Rice Yield and Nitrogen Use Efficiency by Increasing Planting Density in Northeast China Under the Optimal Nitrogen Split-Fertilizer Applications. Agriculture 2024, 14, 2015. [Google Scholar] [CrossRef]
- Xiang, H.; Ni, L.; Wang, F.; Zhao, B.; Wei, H.; Zhang, J. An effective planting model to decrease cadmium accumulation in rice grains and plants: Intercropping rice with wetland plants. Pedosphere 2023, 33, 355–364. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Lin, S.; Xie, Y. Soil pollution management in China: A brief introduction. Sustainability 2019, 11, 556. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Fang, B.; Fang, Y.; Chen, K.; Zhang, Y. Potential for high yield with increased seedling density and decreased N fertilizer application under seedling-throwing rice cultivation. Sci. Rep. 2019, 9, 731. [Google Scholar] [CrossRef]
- Lin, X.Q.; Zhu, D.F.; Chen, H.Z.; Zhang, Y.P. Effects of plant density and nitrogen application rate on grain yield and nitrogen uptake of super hybrid rice. Rice Sci. 2009, 16, 138–142. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, H.; Li, F.; Yang, T.; Shi, Y.; Gu, X.; Chen, M.; Jiang, S. Optimized Tillage Method Increased Rice Yield in Rice Ratooning System. Agriculture 2024, 14, 1768. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, S.; Peng, S. A Synthesis Analysis of the Relationship between Main and Ratoon Crop Grain Yields in Ratoon Rice. Agronomy 2024, 14, 2170. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Dai, S.; Wang, B. Surface Display of Multiple Metal-Binding Domains in Deinococcus radiodurans Alleviates Cadmium and Lead Toxicity in Rice. Int. J. Mol. Sci. 2024, 25, 12570. [Google Scholar] [CrossRef]
- Cecire, R.; Diana, A.; Giacomino, A.; Abollino, O.; Inaudi, P.; Favilli, L.; Bertinetti, S.; Cavalera, S.; Celi, L.; Malandrino, M. Rice Husk as a Sustainable Amendment for Heavy Metal Immobilization in Contaminated Soils: A Pathway to Environmental Remediation. Toxics 2024, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Alpha, J.M.; Chen, J.; Zhang, G. Effect of nitrogen fertilizer forms on growth, photosynthesis, and yield of rice under cadmium stress. J. Plant Nutr. 2009, 32, 306–317. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Lin, D.; Yao, Y.; Song, N.; Wang, F. Co-application of biochar and nitrogen fertilizer promotes rice performance, decreases cadmium availability, and shapes rhizosphere bacterial community in paddy soil. Environ. Pollut. 2022, 308, 119624. [Google Scholar] [CrossRef]
- Jalloh, M.A.; Chen, J.; Zhen, F.; Zhang, G. Effect of different N fertilizer forms on antioxidant capacity and grain yield of rice growing under Cd stress. J. Hazard. Mater. 2009, 162, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, L.; Tang, L.; Huang, F.; Xiao, N.; Zhang, L.; Song, B. Key Factors Controlling Cadmium and Lead Contents in Rice Grains of Plants Grown in Soil with Different Cadmium Levels from an Area with Typical Karst Geology. Agronomy 2024, 14, 2076. [Google Scholar] [CrossRef]
- Wu, W.; Ma, Q.; Zhao, Y.; Zhang, Q.; Tang, Y.; Luo, S.; Deng, X. Variation in Cd and As accumulation and health risk in rice-ratoon cropping system: Evidence from two-year field trials involving multiple cultivars in southern China. J. Cereal Sci. 2024, 120, 104046. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, P.; Gou, X.; Zhou, X.; Zhu, Y.; Liu, M.; Xu, F. Integrated water and nitrogen management practices to enhance yield and environmental goals in rice–ratoon rice systems. Agron. J. 2019, 111, 2821–2831. [Google Scholar] [CrossRef]
- Xu, C.; Wu, Z.; Zhu, Q.; Zhu, H.; Zhang, Y.; Huang, D. Effect of coated urea on cadmium accumulation in Oryza sativa L. grown in contaminated soil. Environ. Monit. Assess. 2015, 187, 716. [Google Scholar] [CrossRef]
- Yu, H.Y.; Liu, C.; Zhu, J.; Li, F.; Deng, D.M.; Wang, Q.; Liu, C. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef]
- Li, A.; Fei, C.; Yang, H.; Zhu, M.; Wang, C.; Hu, H.; Ye, W. Enhanced Adsorption and Biomineralization of Cadmium and Arsenic in Irrigation Water by Biological Soil Crusts: The Key Roles of Iron/Manganese and Urea. Sustainability 2025, 17, 65. [Google Scholar] [CrossRef]
- Xu, X.; Mo, Q.; Cai, Z.; Jiang, Q.; Zhou, D.; Yi, J. Promoters, Key Cis-Regulatory Elements, and Their Potential Applications in Regulation of Cadmium (Cd) in Rice. Int. J. Mol. Sci. 2024, 25, 13237. [Google Scholar] [CrossRef]
- Liu, J.; Qian, M.; Cai, G.; Yang, J.; Zhu, Q. Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J. Hazard. Mater. 2007, 143, 443–447. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Cadmium minimization in rice. A review. Agron. Sustain. Dev. 2014, 34, 155–173. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef]
- Murakami, M.; Nakagawa, F.; Ae, N.; Ito, M.; Arao, T. Phytoextraction by rice capable of accumulating Cd at high levels: Reduction of Cd content of rice grain. Environ. Sci. Technol. 2009, 43, 5878–5883. [Google Scholar] [CrossRef]
- Yang, D.; Peng, S.; Qi, M.; Xiong, Z.; Deng, S.; Wang, F. Comparison of grain cadmium and arsenic concentration between main and ratoon crop in rice ratooning system. Food Chem. 2023, 399, 134017. [Google Scholar] [CrossRef]
| Season | Treatment | Effective Panicle (106 hm−2) | Grains Per Panicle | Seed Setting Rate (%) | Thousand-Grain Weight (g) | Theoretical Yield (t hm−2) | Actual Yield (t hm−2) |
|---|---|---|---|---|---|---|---|
| Main crop | N1 | 2.80 a | 169.04 a | 81.29 a | 22.42 a | 8.63 a | 8.29 a |
| N2 | 2.70 a | 167.51 a | 82.57 a | 22.47 a | 8.33 a | 7.99 a | |
| N3 | 2.55 b | 161.36 b | 82.22 a | 22.62 a | 7.62 b | 7.23 b | |
| D1 | 2.56 b | 165.89 a | 81.82 a | 22.54 a | 7.84 b | 7.46 b | |
| D2 | 2.80 a | 166.03 a | 82.22 a | 22.46 a | 8.58 a | 8.21 a | |
| N1D1 | 2.68 b | 167.67 a | 81.08 b | 22.53 ab | 8.21 b | 7.90 b | |
| N1D2 | 2.92 a | 170.41 a | 81.50 b | 22.30 b | 9.05 a | 8.68 a | |
| N2D1 | 2.59 c | 166.63 a | 82.63 a | 22.63 a | 8.05 b | 7.60 b | |
| N2D2 | 2.81 a | 168.39 a | 82.52 a | 22.30 b | 8.70 a | 8.38 a | |
| N3D1 | 2.42 c | 163.37 ab | 81.80 b | 22.45 ab | 7.25 c | 6.89 c | |
| N3D2 | 2.67 b | 159.35 b | 82.64 a | 22.78 a | 8.01 b | 7.57 b | |
| Ratoon crop | N1 | 3.24 a | 92.27 b | 75.44 a | 21.60 a | 4.94 a | 4.49 a |
| N2 | 3.06 a | 97.34 ab | 75.88 a | 20.70 a | 4.68 a | 4.29 a | |
| N3 | 2.80 b | 101.01 a | 75.11 a | 20.30 a | 4.29 b | 4.01 b | |
| D1 | 2.92 b | 95.69 a | 75.52 a | 21.07 a | 4.50 b | 4.13 b | |
| D2 | 3.13 a | 98.05 a | 75.43 a | 20.66 a | 4.72 a | 4.44 a | |
| N1D1 | 3.13 b | 91.63 c | 74.89 b | 21.87 a | 4.84 a | 4.33 b | |
| N1D2 | 3.34 a | 92.91 c | 75.98 a | 21.33 a | 5.03 a | 4.64 a | |
| N2D1 | 2.95 b | 95.82 b | 76.24 a | 20.75 b | 4.50 b | 4.12 c | |
| N2D2 | 3.10 b | 98.85 ab | 75.51 ab | 20.65 b | 4.69 ab | 4.45 ab | |
| N3D1 | 2.69 c | 99.62 a | 75.42 ab | 20.59 b | 4.15 b | 3.85 c | |
| N3D2 | 2.94 b | 102.49 a | 74.79 b | 20.01 c | 4.43 b | 4.20 b |
| Treatment | Main Crop | Ratoon | ||||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Panicle | Root | Stem | Leaf | Panicle | |
| N1 | 1.63 a | 1.52 a | 0.53 a | 0.36 a | 2.00 a | 1.70 a | 0.94 a | 0.54 a |
| N2 | 1.49 b | 1.43 b | 0.44 b | 0.31 b | 1.83 b | 1.54 b | 0.81 b | 0.41 b |
| N3 | 1.38 c | 1.35 c | 0.40 c | 0.25 c | 1.73 c | 1.44 c | 0.71 c | 0.35 c |
| D1 | 1.49 a | 1.44 a | 0.46 a | 0.31 a | 1.86 a | 1.56 a | 0.81 a | 0.44 a |
| D2 | 1.51 a | 1.42 a | 0.45 a | 0.31 a | 1.85 a | 1.55 a | 0.83 a | 0.43 a |
| N1D1 | 1.57 b | 1.52 a | 0.53 a | 0.36 a | 2.00 a | 1.73 a | 0.92 a | 0.56 a |
| N1D2 | 1.72 a | 1.51 a | 0.53 a | 0.37 a | 2.01 a | 1.67 a | 0.96 a | 0.52 a |
| N2D1 | 1.47 c | 1.43 b | 0.45 b | 0.31 b | 1.82 b | 1.53 b | 0.84 b | 0.41 b |
| N2D2 | 1.51 bc | 1.43 b | 0.43 bc | 0.31 b | 1.84 b | 1.55 b | 0.77 b | 0.40 b |
| N3D1 | 1.39 d | 1.36 c | 0.40 c | 0.24 c | 1.75 c | 1.44 c | 0.69 c | 0.35 c |
| N3D2 | 1.36 d | 1.33 c | 0.39 c | 0.26 c | 1.71 c | 1.44 c | 0.73 bc | 0.35 c |
| Treatment | Main Crop | Ratoon Crop | ||||||
|---|---|---|---|---|---|---|---|---|
| Branch | Grain Husk | Empty Grain | Brown Rice | Branch | Grain Husk | Empty Grain | Brown Rice | |
| N1 | 0.33 a | 0.25 a | 0.15 a | 0.22 a | 1.09 a | 0.38 a | 0.29 a | 0.29 a |
| N2 | 0.30 b | 0.23 b | 0.12 b | 0.19 b | 1.02 b | 0.33 b | 0.27 b | 0.25 b |
| N3 | 0.24 c | 0.21 c | 0.11 c | 0.17 c | 0.95 c | 0.28 c | 0.21 c | 0.21 c |
| D1 | 0.29 a | 0.23 a | 0.13 a | 0.19 a | 1.03 a | 0.33 a | 0.26 a | 0.25 a |
| D2 | 0.29 a | 0.23 a | 0.12 a | 0.19 a | 1.01 a | 0.33 a | 0.25 a | 0.25 a |
| N1D1 | 0.33 a | 0.25 a | 0.15 a | 0.23 a | 1.11 a | 0.38 a | 0.29 a | 0.28 a |
| N1D2 | 0.33 a | 0.25 b | 0.15 ab | 0.21 a | 1.07 a | 0.37 a | 0.29 ab | 0.28 a |
| N2D1 | 0.31 b | 0.23 c | 0.13 b | 0.19 b | 1.03 a | 0.33 b | 0.27 b | 0.25 b |
| N2D2 | 0.30 c | 0.23 c | 0.11 c | 0.18 b | 1.02 a | 0.33 b | 0.27 b | 0.25 b |
| N3D1 | 0.24 d | 0.21 d | 0.11 c | 0.17 c | 0.95 b | 0.28 c | 0.22 c | 0.21 c |
| N3D2 | 0.24 d | 0.21 e | 0.10 d | 0.17 c | 0.95 b | 0.29 c | 0.21 c | 0.21 c |
| Treatment | Main Crop | Ratoon Crop | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Panicle | Brown Rice | Root | Stem | Leaf | Panicle | Brown Rice | |
| N1 | 1.20 a | 1.12 a | 0.39 a | 0.27 a | 0.16 a | 1.47 a | 1.25 a | 0.69 a | 0.40 a | 0.20 a |
| N2 | 1.10 b | 1.05 b | 0.33 b | 0.23 b | 0.14 b | 1.35 b | 1.13 b | 0.59 b | 0.30 b | 0.18 b |
| N3 | 1.01 c | 0.99 c | 0.29 c | 0.19 c | 0.12 c | 1.27 c | 1.06 c | 0.52 c | 0.26 c | 0.16 c |
| D1 | 1.09 a | 1.06 a | 0.34 a | 0.22 a | 0.14 a | 1.36 a | 1.15 a | 0.60 a | 0.33 a | 0.19 a |
| D2 | 1.11 a | 1.05 a | 0.33 a | 0.23 a | 0.14 a | 1.36 a | 1.14 a | 0.61 a | 0.33 a | 0.19 a |
| N1D1 | 1.17 b | 1.12 a | 0.39 a | 0.26 a | 0.17 a | 1.47 a | 1.27 a | 0.67 a | 0.41 a | 0.21 a |
| N1D2 | 1.23 a | 1.11 a | 0.39 a | 0.27 a | 0.16 a | 1.48 a | 1.23 a | 0.72 a | 0.39 a | 0.20 a |
| N2D1 | 1.08 c | 1.05 b | 0.33 b | 0.23 b | 0.14 b | 1.34 b | 1.12 b | 0.62 b | 0.30 b | 0.18 b |
| N2D2 | 1.11 bc | 1.05 b | 0.32 bc | 0.22 b | 0.14 b | 1.35 b | 1.14 b | 0.57 b | 0.30 b | 0.18 b |
| N3D1 | 1.02 d | 1.00 c | 0.30 c | 0.18 c | 0.12 c | 1.28 c | 1.06 c | 0.50 c | 0.25 c | 0.15 c |
| N3D2 | 1.00 d | 0.98 c | 0.29 c | 0.19 c | 0.12 c | 1.26 c | 1.06 c | 0.54 bc | 0.26 c | 0.16 c |
| Treatment | Main Crop | Ratoon Crop | ||||||
|---|---|---|---|---|---|---|---|---|
| Root- Brown Rice | Stem- Brown Rice | Leaf- Brown Rice | Panicle- Brown Rice | Root- Brown Rice | Stem- Brown Rice | Leaf- Brown Rice | Panicle- Brown Rice | |
| N1 | 0.14 a | 0.15 a | 0.42 a | 0.61 a | 0.14 a | 0.16 a | 0.30 a | 0.52 a |
| N2 | 0.13 b | 0.13 b | 0.42 a | 0.60 a | 0.14 a | 0.16 a | 0.31 a | 0.61 a |
| N3 | 0.12 b | 0.13 b | 0.42 a | 0.66 a | 0.12 b | 0.15 b | 0.30 a | 0.61 a |
| D1 | 0.13 a | 0.14 a | 0.42 a | 0.64 a | 0.14 a | 0.16 a | 0.31 a | 0.57 a |
| D2 | 0.13 a | 0.13 a | 0.42 a | 0.61 a | 0.14 a | 0.16 a | 0.30 a | 0.59 a |
| N1D1 | 0.14 a | 0.15 a | 0.42 a | 0.63 ab | 0.14 a | 0.16 a | 0.31 a | 0.51 b |
| N1D2 | 0.13 b | 0.14 a | 0.40 a | 0.58 b | 0.14 a | 0.16 a | 0.28 a | 0.54 ab |
| N2D1 | 0.13 b | 0.13 b | 0.41 a | 0.60 ab | 0.14 a | 0.16 a | 0.30 a | 0.60 a |
| N2D2 | 0.12 b | 0.13 b | 0.42 a | 0.60 ab | 0.14 a | 0.16 a | 0.32 a | 0.62 a |
| N3D1 | 0.12 b | 0.13 b | 0.42 a | 0.70 a | 0.12 b | 0.15 b | 0.31 a | 0.60 a |
| N3D2 | 0.12 b | 0.12 b | 0.42 a | 0.64 ab | 0.12 b | 0.15 b | 0.29 a | 0.61 a |
| Tissues | Treatment | Transplanting Stage to Full-Heading Stage | Full-Heading Stage to Milky Stage | Milky Stage to Maturity Stage | Tissues | Treatment | Transplanting Stage to Full-Heading Stage | Full-Heading Stage to Milky Stage | Milky Stage to Maturity Stage |
|---|---|---|---|---|---|---|---|---|---|
| Total accumulation | N1 | 2811.09 a | 1395.86 a | 6977.46 a | Stem | N1 | 1384.33 a | −149.97 a | 5283.43 a |
| N2 | 2259.05 b | 1008.09 b | 6369.55 b | N2 | 1046.93 b | −249.24 c | 4831.97 b | ||
| N3 | 1714.29 c | 1020.15 b | 5789.36 c | N3 | 698.41 c | −171.98 b | 4693.49 b | ||
| D1 | 2148.92 b | 1086.24 b | 6038.60 b | D1 | 1001.18 a | −179.13 a | 4714.56 b | ||
| D2 | 2374.04 a | 1196.50 a | 6718.97 a | D2 | 1085.26 a | −201.66 a | 5158.03 a | ||
| N1D1 | 2806.60 a | 1304.16 a | 6702.93 b | N1D1 | 1462.14 a | −217.50 bc | 5143.92 b | ||
| N1D2 | 2815.58 a | 1487.57 a | 7251.99 a | N1D2 | 1306.52 b | −82.45 a | 5422.94 a | ||
| N2D1 | 2103.81 c | 1003.26 b | 5988.31 c | N2D1 | 1018.76 c | −220.82 bc | 4612.98 c | ||
| N2D2 | 2414.30 b | 1012.93 b | 6750.79 ab | N2D2 | 1075.10 c | −277.67 c | 5050.95 b | ||
| N3D1 | 1536.34 e | 951.28 b | 5424.58 d | N3D1 | 522.64 e | −99.09 ab | 4386.78 c | ||
| N3D2 | 1892.23 d | 1089.02 b | 6154.13 c | N3D2 | 874.17 d | −244.87 c | 5000.20 b | ||
| Leaf | N1 | 880.58 a | 303.68 a | 74.00 a | Panicle | N1 | 546.19 a | 1242.16 a | 1620.02 a |
| N2 | 743.55 b | 257.75 b | 0.03 b | N2 | 468.58 b | 999.59 b | 1537.55 b | ||
| N3 | 600.42 c | 229.11 b | −33.30 b | N3 | 328.64 c | 963.02 b | 1129.17 c | ||
| D1 | 685.35 b | 248.22 a | 24.51 a | D1 | 404.51 b | 1017.15 b | 1299.53 b | ||
| D2 | 797.68 a | 278.81 a | 2.64 a | D2 | 491.09 a | 1119.36 a | 1558.30 a | ||
| N1D1 | 845.26 ab | 273.35 ab | 80.49 a | N1D1 | 499.20 b | 1248.31 a | 1478.51 abc | ||
| N1D2 | 915.89 a | 334.01 a | 67.52 a | N1D2 | 593.17 a | 1236.00 a | 1761.53 a | ||
| N2D1 | 673.18 c | 252.84 ab | 3.51 b | N2D1 | 411.87 c | 971.24 b | 1371.81 bc | ||
| N2D2 | 813.92 b | 262.67 ab | −3.45 b | N2D2 | 525.28 b | 1027.93 b | 1703.29 ab | ||
| N3D1 | 537.60 d | 218.48 b | −10.46 b | N3D1 | 302.46 d | 831.89 c | 1048.26 d | ||
| N3D2 | 663.24 c | 239.74 b | −56.15 c | N3D2 | 354.81 d | 1094.15 b | 1210.08 cd |
| Tissues | Treatment | Transplanting Stage to Full-Heading Stage | Full-Heading Stage to Milky Stage | Milky Stage to Maturity Stage | Tissues | Treatment | Transplanting Stage to Full-Heading Stage | Full-Heading Stage to Milky Stage | Milky Stage to Maturity Stage |
|---|---|---|---|---|---|---|---|---|---|
| Total accumulation | N1 | 3074.57 a | 6777.85 a | 599.50 a | Stem | N1 | 2243.58 a | 3837.76 a | −1051.56 b |
| N2 | 2634.92 b | 5768.32 b | 286.93 c | N2 | 1916.22 b | 3356.68 b | −1023.17 b | ||
| N3 | 2270.63 c | 4995.05 c | 507.07 b | N3 | 1681.92 c | 2836.31 c | −811.98 a | ||
| D1 | 2519.63 b | 5596.33 b | 538.85 a | D1 | 1849.77 b | 3263.07 b | −983.42 a | ||
| D2 | 2800.44 a | 6097.81 a | 390.15 b | D2 | 2044.71 a | 3424.09 a | −941.05 a | ||
| N1D1 | 2965.44 ab | 6485.85 b | 751.36 a | N1D1 | 2158.40 b | 3742.96 a | −1031.22 ab | ||
| N1D2 | 3183.69 a | 7069.85 a | 447.64 ab | N1D2 | 2328.75 a | 3932.55 a | −1071.9 ab | ||
| N2D1 | 2485.14 c | 5609.59 cd | 255.29 b | N2D1 | 1834.80 d | 3305.6 b | −1111.76 b | ||
| N2D2 | 2784.70 b | 5927.04 c | 318.56 b | N2D2 | 1997.64 c | 3407.76 b | −934.57 ab | ||
| N3D1 | 2108.32 d | 4693.54 e | 609.90 ab | N3D1 | 1556.10 e | 2740.65 c | −807.27 a | ||
| N3D2 | 2432.94 c | 5296.55 de | 404.24 ab | N3D2 | 1807.74 d | 2931.96 c | −816.69 a | ||
| Leaf | N1 | 459.26 a | 928.05 a | −228.47 a | Panicle | N1 | 371.73 a | 2012.05 a | 1879.53 a |
| N2 | 418.45 b | 788.02 b | −251.09 a | N2 | 300.26 b | 1623.62 b | 1561.18 b | ||
| N3 | 338.50 c | 680.59 c | −117.74 a | N3 | 250.21 c | 1478.15 c | 1436.79 c | ||
| D1 | 389.13 b | 752.51 b | −189.48 a | D1 | 280.73 b | 1580.75 b | 1711.75 a | ||
| D2 | 421.67 a | 845.26 a | −208.72 a | D2 | 334.06 a | 1828.46 a | 1539.92 b | ||
| N1D1 | 450.36 a | 815.22 b | −191.35 a | N1D1 | 356.68 b | 1927.67 a | 1973.93 a | ||
| N1D2 | 468.16 a | 1040.88 a | −265.58 a | N1D2 | 386.78 a | 2096.42 a | 1785.12 b | ||
| N2D1 | 389.22 bc | 747.87 c | −228.63 a | N2D1 | 261.12 c | 1556.12 c | 1595.68 c | ||
| N2D2 | 447.67 ab | 828.17 bc | −273.55 a | N2D2 | 339.39 b | 1691.11 b | 1526.68 c | ||
| N3D1 | 327.82 c | 694.44 c | −148.46 a | N3D1 | 224.40 c | 1258.45 d | 1565.63 c | ||
| N3D2 | 349.18 c | 666.74 c | −87.02 a | N3D2 | 276.02 c | 1697.85 b | 1307.95 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Zheng, D.; Chen, P.; Yuan, S.; Yi, Z. The Effects of Reducing Nitrogen and Increasing Density in the Main Crop on Yield and Cadmium Accumulation of Ratoon Rice. Agronomy 2025, 15, 485. https://doi.org/10.3390/agronomy15020485
Tian Q, Zheng D, Chen P, Yuan S, Yi Z. The Effects of Reducing Nitrogen and Increasing Density in the Main Crop on Yield and Cadmium Accumulation of Ratoon Rice. Agronomy. 2025; 15(2):485. https://doi.org/10.3390/agronomy15020485
Chicago/Turabian StyleTian, Qinqin, Dechao Zheng, Pingping Chen, Shuai Yuan, and Zhenxie Yi. 2025. "The Effects of Reducing Nitrogen and Increasing Density in the Main Crop on Yield and Cadmium Accumulation of Ratoon Rice" Agronomy 15, no. 2: 485. https://doi.org/10.3390/agronomy15020485
APA StyleTian, Q., Zheng, D., Chen, P., Yuan, S., & Yi, Z. (2025). The Effects of Reducing Nitrogen and Increasing Density in the Main Crop on Yield and Cadmium Accumulation of Ratoon Rice. Agronomy, 15(2), 485. https://doi.org/10.3390/agronomy15020485





