A Global Meta-Analysis of Soil Carbon Stock in Agroforestry Coffee Cultivation
Abstract
1. Introduction
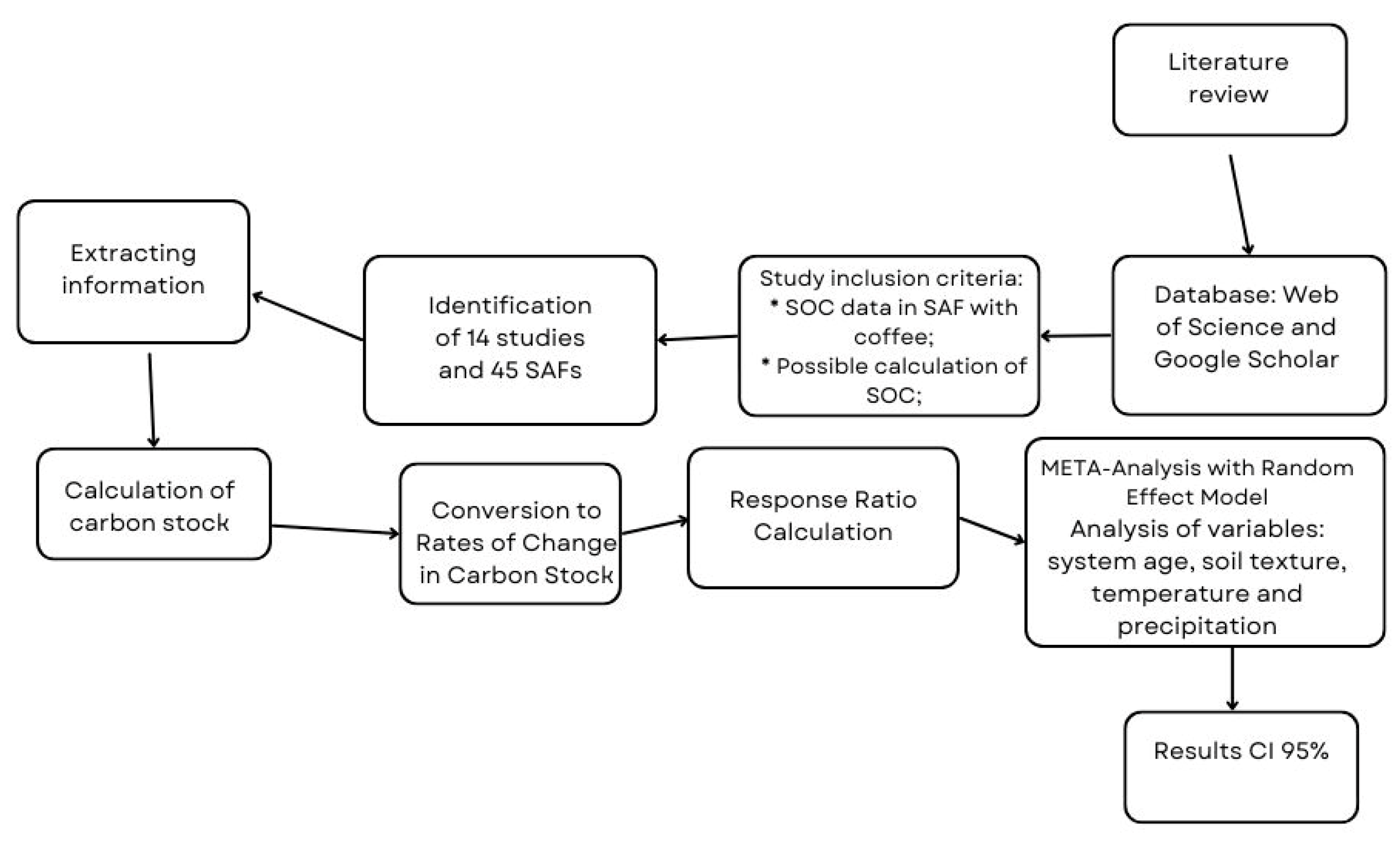
2. Materials and Methods
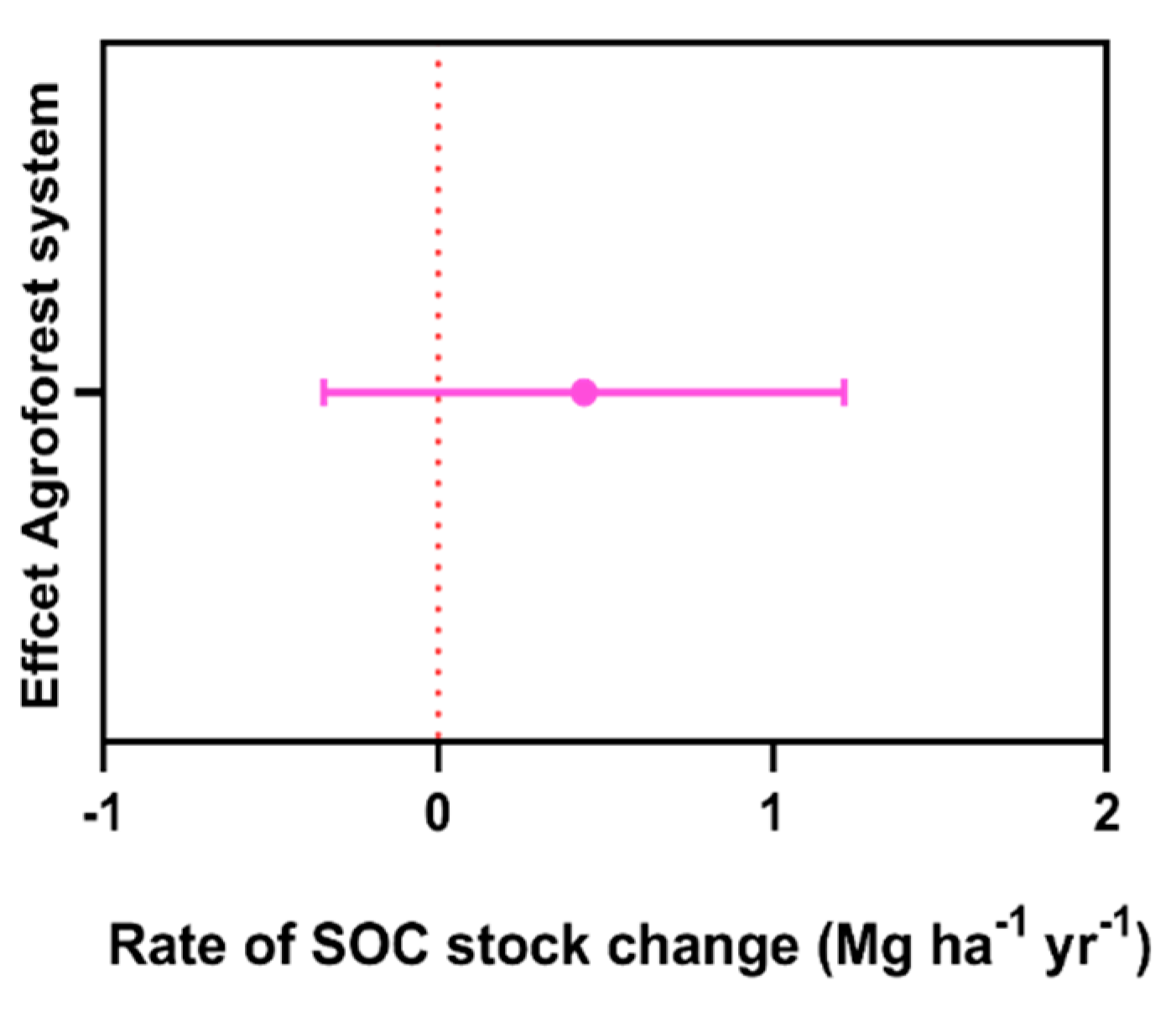
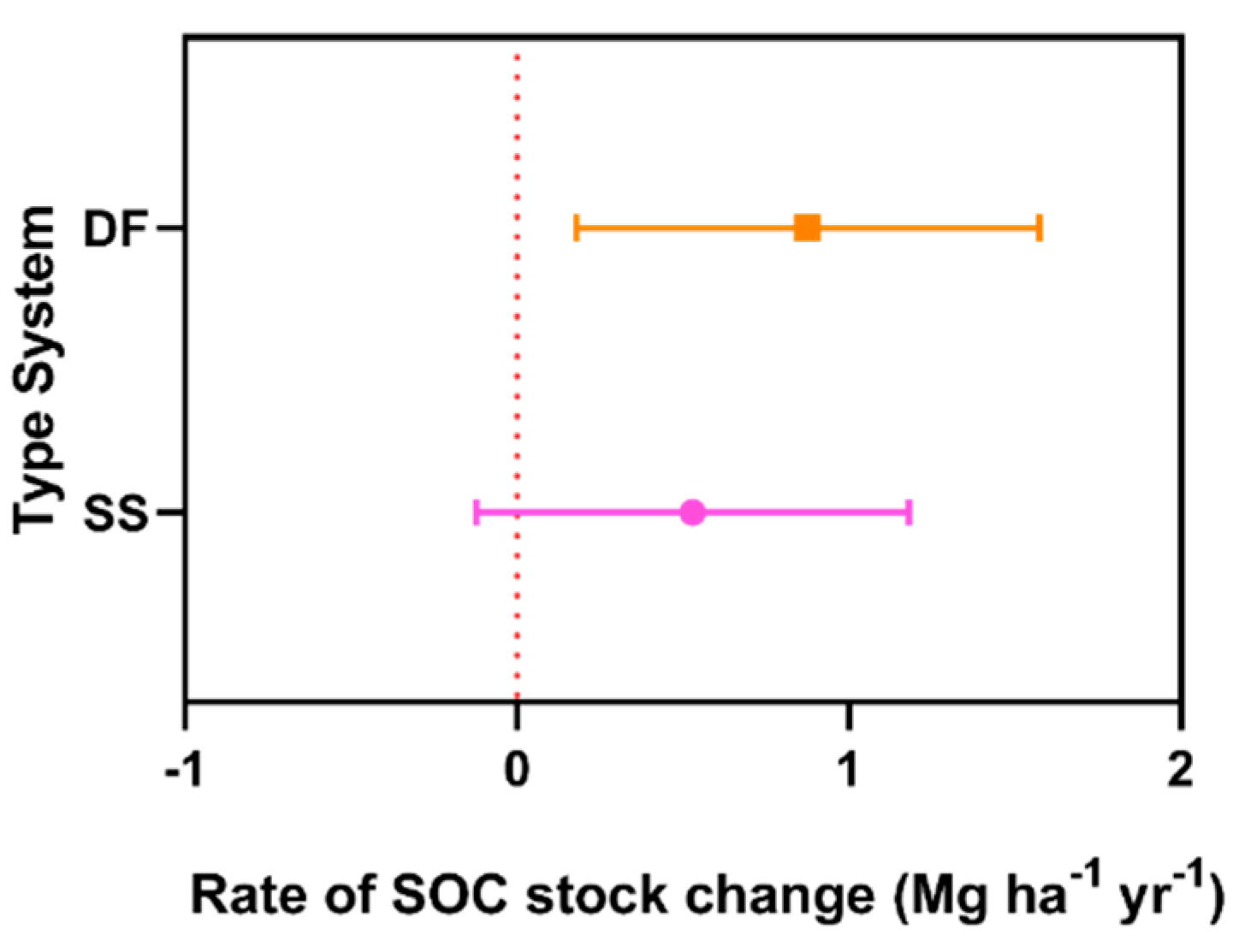
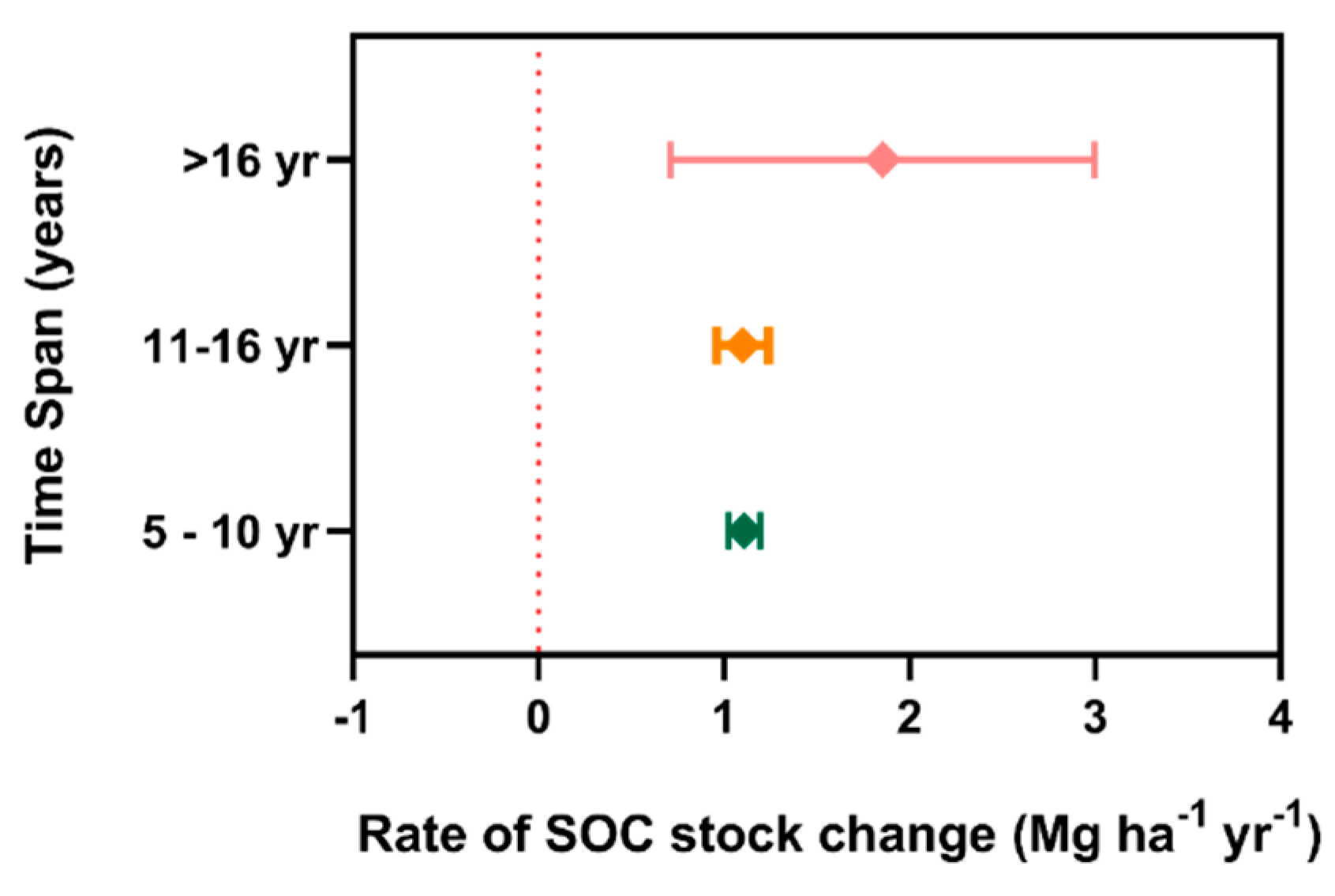
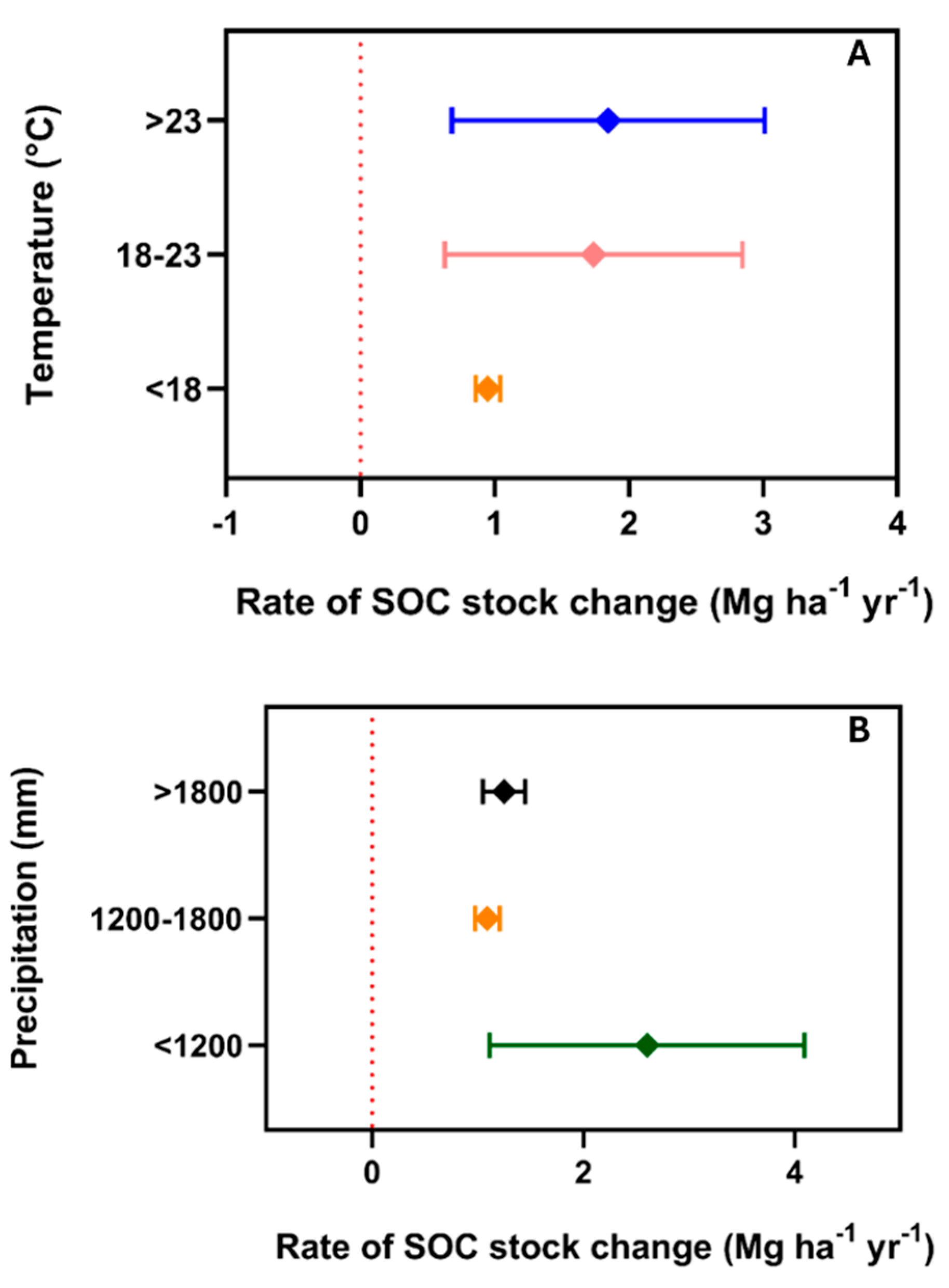
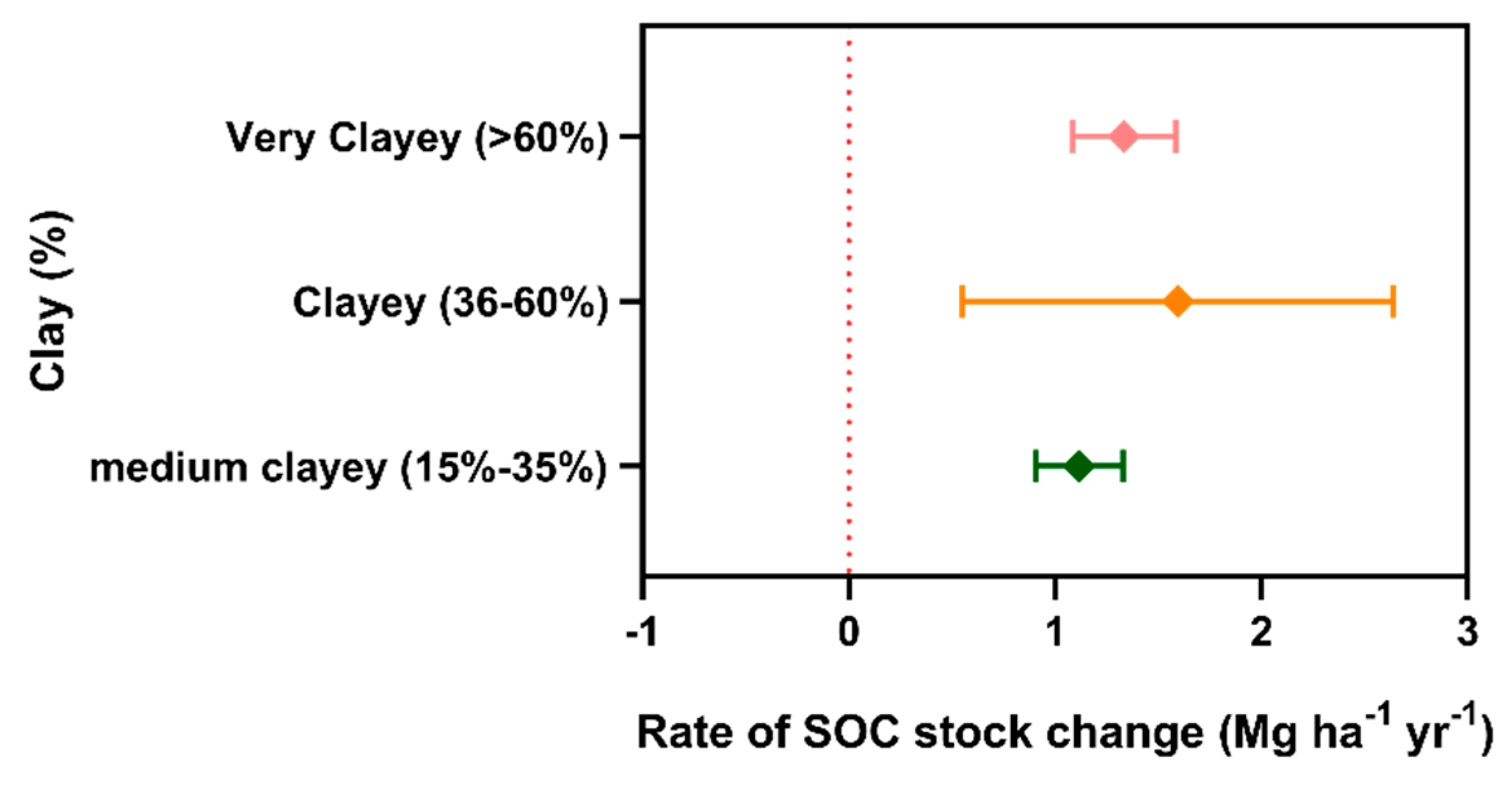
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mooney, H.A.; Ehleringer, J.R. Photosynthesis. In Plant Ecology, 2nd ed.; Crawley, M.J., Ed.; Blackwell Publishing: Oxford, UK, 2009; pp. 1–28. ISBN 978-0-632-03639-4. [Google Scholar]
- Martin, R.; Lim, E.J. Understanding the Unseen: CO2′s Connections in Life. Am. Biol. Teach. 2020, 82, 470–476. [Google Scholar] [CrossRef]
- Carroll, C.; Ray, J.C. Maximizing the effectiveness of national commitments to protected area expansion for conserving biodiversity and ecosystem carbon under climate change. Glob. Change Biol. 2021, 27, 3395–3414. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, Z.U.R.; Sabir, M.; Qadeer, A.; Naeem, A.; Murtaza, G.; Yousaf, H. Understanding the Causes of Climatic Change in the Environment. In Climate Change; Bandh, S.A., Ed.; Springer: Cham, Switzerland, 2022; pp. 37–47. [Google Scholar] [CrossRef]
- Lal, R.; Smith, P.; Jungkunst, H.F.; Mitsch, W.J.; Lehmann, J.; Nair, P.R.; McBratney, A.B.; Sá, J.C.d.M.; Schneider, J.; Zinn, Y.L.; et al. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018, 73, 145A–152A. [Google Scholar] [CrossRef]
- Lybbert, T.J.; Sumner, D.A. Agricultural technologies for climate change in developing countries: Policy options for innovation and technology diffusion. Food Policy 2012, 37, 114–123. [Google Scholar] [CrossRef]
- Cambou, A.; Cardinael, R.; Kouakoua, E.; Villeneuve, M.; Durand, C.; Barthès, B.G. Prediction of soil organic carbon stock using visible and near infrared reflectance spectroscopy (VNIRS) in the field. Geoderma 2016, 261, 151–159. [Google Scholar] [CrossRef]
- Davis, M.R.; Alves, B.J.R.; Karlen, D.L.; Kline, K.L.; Galdos, M.; Abulebdeh, D. Review of Soil Organic Carbon Measurement Protocols: A US and Brazil Comparison and Recommendation. Sustainability 2018, 10, 53. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 2015, 237, 149–158. [Google Scholar] [CrossRef]
- Lal, R. World cropland soils as a source or sink for atmospheric carbon. Adv. Agron. 2001, 71, 145–191. [Google Scholar] [CrossRef]
- Silva, E.R.A. Agenda 2030: ODS—Metas Nacionais dos Objetivos de Desenvolvimento Sustentável; IPEA: Brasília, DF, USA, 2018. Available online: https://repositorio.ipea.gov.br/bitstream/11058/8855/1/Agenda_2030_ods_metas_nac_dos_obj_de_desenv_susten_propos_de_adequa.pdf (accessed on 10 August 2024).
- Nair, P.K.R. (Ed.) Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges; Springer: London, UK; New York, NY, USA, 2011; Volume 8, pp. 145–162. [Google Scholar]
- Lovison, J.; Gehrke, L.D.; Vieira, M. Proposta de implantação de um sistema silvipastoril no município de restinga seca. In Proceedings of the 13º SIEPE: Salão Internacional de Ensino, Pesquisa e Extensão, Online, 17–19 November 2021; Volume 13, p. 1. [Google Scholar]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Froufe, L.C.M.; Seoane, C.E.S. Levantamento fitossociológico comparativo entre sistema agroflorestal multiestrato e capoeiras como ferramenta para a execução da reserva legal. Pesqui. Florest. Bras. 2011, 31, 203–225. [Google Scholar] [CrossRef]
- De Stefano, A.; Jacobson, M.G. Soil carbon sequestration in agroforestry systems: A meta-analysis. Agrofor. Syst. 2018, 92, 285–299. [Google Scholar] [CrossRef]
- Sahoo, U.K.; Tripathi, O.P.; Nath, A.J.; Deb, S.; Das, D.J.; Gupta, A.; Devi, N.B.; Charturvedi, S.S.; Singh, S.L.; Kumar, A.; et al. Quantifying Tree Diversity, Carbon Stocks, and Sequestration Potential for Diverse Land Uses in Northeast India. Front. Environ. Sci. 2021, 9, 724950. [Google Scholar] [CrossRef]
- Manaye, A.; Tesfamariam, B.; Tesfaye, M.; Worku, A.; Gufi, Y. Tree diversity and carbon stocks in agroforestry systems in northern Ethiopia. Carbon Balance Manag. 2021, 16, 14. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Coffee Production 2020, by Country. Statista. 2024. Available online: https://www.statista.com/statistics/277137/world-coffee-production-by-leading-countries/ (accessed on 21 October 2024).
- EMBRAPA. Produção total de café no Mundo Deverá Atingir Volume Físico Equivalente a 1743 Milhões de Sacas na Safra 2023–2024. 2023. Available online: https://www.embrapa.br/busca-de-noticias/-/noticia/82856140/producao-total-de-cafe-no-mundo-devera-atingir-volume-fisico-equivalente-a-1743-milhoes-de-sacas-na-safra-2023-2024 (accessed on 10 August 2024).
- Jaramillo-Botero, C.; Santos, R.H.S.; Martinez, H.E.P.; Cecon, P.R.; Fardin, M.P. Production and vegetative growth of coffee trees under fertilization and shade levels. Sci. Agric. 2010, 67, 639–645. [Google Scholar] [CrossRef]
- Moreira, S.L.; Pires, C.V.; Marcatti, G.E.; Santos, R.H.; Imbuzeiro, H.M.; Fernandes, R.B. Intercropping of coffee with the palm tree, macauba, can mitigate climate change effects. Agric. For. Meteorol. 2018, 257, 379–390. [Google Scholar] [CrossRef]
- Dossa, E.L.; Fernandes, E.C.M.; Reid, W.S.; Ezui, K. Above- and belowground biomass, nutrient and carbon stocks contrasting an open-grown and a shaded coffee plantation. Agrofor. Syst. 2008, 72, 103–115. [Google Scholar] [CrossRef]
- Schmitt-Harsh, M.; Evans, T.P.; Castellanos, E.; Randolph, J.C. Carbon stocks in coffee agroforests and mixed dry tropical forests in the western highlands of Guatemala. Agrofor. Syst. 2012, 86, 141–157. [Google Scholar] [CrossRef]
- Negash, M.; Starr, M. Biomass and soil carbon stocks of indigenous agroforestry systems on the south-eastern Rift Valley escarpment, Ethiopia. Plant Soil 2015, 393, 95–107. [Google Scholar] [CrossRef]
- Zaro, G.C.; Caramori, P.H.; Yada Junior, G.M.; Sanquetta, C.R.; Filho, A.A.; Nunes, A.L.P.; Prete, C.E.C.; Voroney, P. Carbon sequestration in an agroforestry system of coffee with rubber trees compared to open-grown coffee in southern Brazil. Agrofor. Syst. 2020, 94, 799–809. [Google Scholar] [CrossRef]
- Ehrenbergerova, L.; Cienciala, E.; Kučera, A.; Guy, L.; Habrová, H. Carbon stock in agroforestry coffee plantations with different shade trees in Villa Rica, Peru. Agrofor. Syst. 2016, 90, 433–445. [Google Scholar] [CrossRef]
- Ruiz-García, P.; Monterroso-Rivas, A.I.; Valdés-Velarde, E.; Escamilla-Prado, E.; Gómez-Díaz, J.D. Carbon stocks in coffee (C. arabica L.) agroforestry systems in the face of climate change: México case. Agron. Mesoam. 2022, 33, 1–21. [Google Scholar] [CrossRef]
- Espinoza-Domínguez, W.; Krishnamurthy, L.; Vázquez-Alarcón, A.; Torres-Rivera, A. Almacén de carbono en sistemas agroforestales con café. Rev. Chapingo Ser. Cienc. For. Ambiente 2012, 18, 57–70. [Google Scholar] [CrossRef]
- Solis, R.; Vallejos-Torres, G.; Arévalo, L.; Marín-Díaz, J.; Ñique-Alvarez, M.; Engedal, T.; Bruun, T.B. Carbon stocks and the use of shade trees in different coffee growing systems in the Peruvian Amazon. J. Agric. Sci. 2020, 158, 450–460. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nair, P.K.R.; Nair, V.D.; Viswanath, S.; Bhattacharjee, A. Depth-wise distribution of soil-carbon stock in aggregate-sized fractions under shaded-perennial agroforestry systems in the Western Ghats of Karnataka, India. Agrofor. Syst. 2019, 94, 341–358. [Google Scholar] [CrossRef]
- Niguse, G.; Iticha, B.; Kebede, G.; Chimdi, A. Contribution of coffee plants to carbon sequestration in agroforestry systems of Southwestern Ethiopia. J. Agric. Sci. 2022, 160, 440–447. [Google Scholar] [CrossRef]
- Tumwebaze, S.B.; Byakagaba, P. Soil organic carbon stocks under coffee agroforestry systems and coffee monoculture in Uganda. Agric. Ecosyst. Environ. 2016, 216, 188–193. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nair, P.R.; Nair, V.D.; Bhattacharjee, A.; Filho, E.D.M.V.; Muschler, R.G.; Noponen, M.R. Do coffee agroforestry systems always improve soil carbon stocks deeper in the soil?—A case study from Turrialba, Costa Rica. Forests 2019, 11, 49. [Google Scholar] [CrossRef]
- Segnini, A.; Posadas, A.; da Silva, W.T.; Milori, D.M.; Gavilan, C.; Claessens, L.; Quiroz, R. Quantifying soil carbon stocks and humification through spectroscopic methods: A scoping assessment in EMBU-Kenya. J. Environ. Manag. 2019, 234, 476–483. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Blanchart, E.; Skiba, U.; Hénault, C.; Harmand, J.-M. Changes in carbon stock and greenhouse gas balance in a coffee (Coffea arabica) monoculture versus an agroforestry system with Inga densiflora, in Costa Rica. Agric. Ecosyst. Environ. 2012, 148, 102–110. [Google Scholar] [CrossRef]
- Álvarez-Arteaga, G.; Calderón, N.E.; Krasilnikov, P.; García-Oliva, F. Almacenes de carbono en bosques montaños de niebla de la Sierra Norte de Oaxaca, México. Agrociencia 2013, 47, 171–180. [Google Scholar]
- Oliveira, D.M.S.; Tavares, R.L.M.; Loss, A.; Madari, B.E.; Cerri, C.E.P.; Alves, B.J.R.; Pereira, M.G.; Cherubin, M.R. Climate-smart ag-riculture and soil C sequestration in Brazilian Cerrado: A systematic review. Rev. Bras. Ciência Solo 2023, 47, e0220055. [Google Scholar]
- Silva, L.J.; Oliveira, D.M.; Santos, R.S.; Oliveira, P.A.; Freitas, D.A.; Cherubin, M.R.; Cerri, C.E. Soil carbon dynamics in integrated agricultural systems in Minas Gerais state, Brazil: A meta-analysis. Geoderma Reg. 2024, 36, e00761. [Google Scholar] [CrossRef]
- Adams, D.C.; Gurevitch, J.; Rosenberg, M.S. Resampling tests for meta-analysis of ecological data. Ecology 1997, 78, 1277–1283. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Gattinger, A.; Gimeno, B.S. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: A meta-analysis. Agric. Ecosyst. Environ. 2013, 168, 25–36. [Google Scholar] [CrossRef]
- Vieira, M.V.M.; Giunti, O.D.; Gris, C.F.; Silva, A.V. Indicadores de Sustentabilidade e Influência de Sistemas Agroflorestal e Convencional Sobre a Qualidade do solo e do café Arábica em Piumhi-MG. Rev. Verde Agroecol. E Desenvolv. Sustentável 2015, 10, 224–238. Available online: https://www.gvaa.com.br/revista/index.php/RVADS/article/view/3329/3499 (accessed on 20 November 2024). [CrossRef][Green Version]
- Bastos, T.R.d.S.; Barreto-Garcia, P.A.B.; Mendes, I.d.C.; Monroe, P.H.M.; de Carvalho, F.F. Response of soil microbial biomass and enzyme activity in coffee-based agroforestry systems in a high-altitude tropical climate region of Brazil. Catena 2023, 230, 107–270. [Google Scholar] [CrossRef]
- Islam, M.; Dey, A.; Rahman, M. Effect of Tree Diversity on Soil Organic Carbon Content in the Homegarden Agroforestry System of North-Eastern Bangladesh. Small Scale For. 2015, 14, 91–101. [Google Scholar] [CrossRef]
- Cardozo, E.G.; Rousseau, G.X.; Celentano, D.; Salazar, H.F.; Gehring, C. Effect of species richness and vegetation structure on carbon storage in agroforestry systems in the Southern Amazon of Bo-livia. Rev. Biol. Trop. 2018, 66, 1481–1495. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, H.Y.H.; Bork, E.W.; Carlyle, C.N.; Chang, S.X. Carbon accumulation in agroforestry systems is affected by tree species diversity, age and regional climate: A global me-ta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1817–1828. [Google Scholar] [CrossRef]
- Rocha, G.P.; Fernandes, L.A.; Cabacinha, C.D.; Lopes, I.D.P.; Ribeiro, J.M.; Frazão, L.A.; Sampaio, R.A. Caracterização e estoques de carbono de sistemas agroflorestaisno Cerrado de Minas Gerais. Cienc. Rural. 2014, 44, 1197–1203. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Frazão, L.A.; Fernandes, L.A.; Sampaio, R.A.; Cardoso, P.H.S.; Oliveira, A.L.G. Fertilidade do solo e estoques de carbono e nitrogênio sob sistemas agroflorestais no Cerrado mineiro. Cienc. Florest. 2019, 29, 913–923. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Gama-Rodrigues, E.F.; Nair, P.K.R.; Nair, V.D.; Gama-Rodrigues, A.C.; Baligar, V.C.; Machado, R.C.R. Carbon Storage in Soil Size Fractions Under Two Cacao Agroforestry Systems in Bahia, Brazil. Environ. Manag. 2010, 45, 274–283. [Google Scholar] [CrossRef]
- Iwata, B.D.F.; Leite, L.F.C.; Araujo, A.S.F.; Nunes, L.A.P.L.; Gehring, C.; Campos, L.P. Sistemas agroflorestais e seus efeitos sobre os atributos químicos em Argissolo Vermelho-Amarelo do Cerrado piauiense. Rev. Bras. Eng. Agríc. Ambient. 2012, 16, 730–738. [Google Scholar] [CrossRef]
- Zeng, X.; Feng, J.; Yu, D.; Wen, S.; Zhang, Q.; Huang, Q.; Delgado-Baquerizo, M.; Liu, Y. Local temperature increases reduce soil microbial residues and carbon stocks. Glob. Change Biol. 2022, 28, 6433–6445. [Google Scholar] [CrossRef]
- Wanzeler, R.T.S.; Costa, J.d.P.R.d.; dos Santos, C.A. Variabilidade horária do perfil de temperatura do solo em um pomar de mangueiras (Mangifera indica l.), na localidade de Cuiarana, Salinópolis—PA. Estac. Cient. 2016, 6, 117–124. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Y.; Bi, Q.; Chen, Z.; Zhang, B. Response of leaf and soil C, N and P stoichiometry in different Pinus massoniana forest types to slope aspect in the Dabie mountains region of North subtropical, China. Front. Environ. Sci. 2023, 11, 1148986. [Google Scholar] [CrossRef]
- He, N.; Chen, Q.; Han, X.; Yu, G.; Li, L. Warming and increased precipitation individually influence soil carbon sequestration of Inner Mongolian grasslands, China. Agric. Ecosyst. Environ. 2012, 158, 184–191. [Google Scholar] [CrossRef]
- Becker, A.E.; Horowitz, L.S.; Ruark, M.D.; Jackson, R.D. Surface-soil carbon stocks greater under well-managed grazed pasture than row crops. Soil Sci. Soc. Am. J. 2022, 86, 758–768. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Haddix, M.L.; Gregorich, E.G.; Helgason, B.L.; Janzen, H.; Ellert, B.H.; Cotrufo, M.F. Climate, carbon content, and soil texture control the independent formation and persistence of particulate and mineral-associated organic matter in soil. Geoderma 2020, 363, 114–160. [Google Scholar] [CrossRef]
- Hassink, J.; Whitmore, A.P. A Model of the Physical Protection of Organic Matter in Soils. Soil Sci. Soc. Am. J. 1997, 61, 131–139. [Google Scholar] [CrossRef]
- McLaren, R.G.; Cameron, K.C. Soil Science, 2nd ed.; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
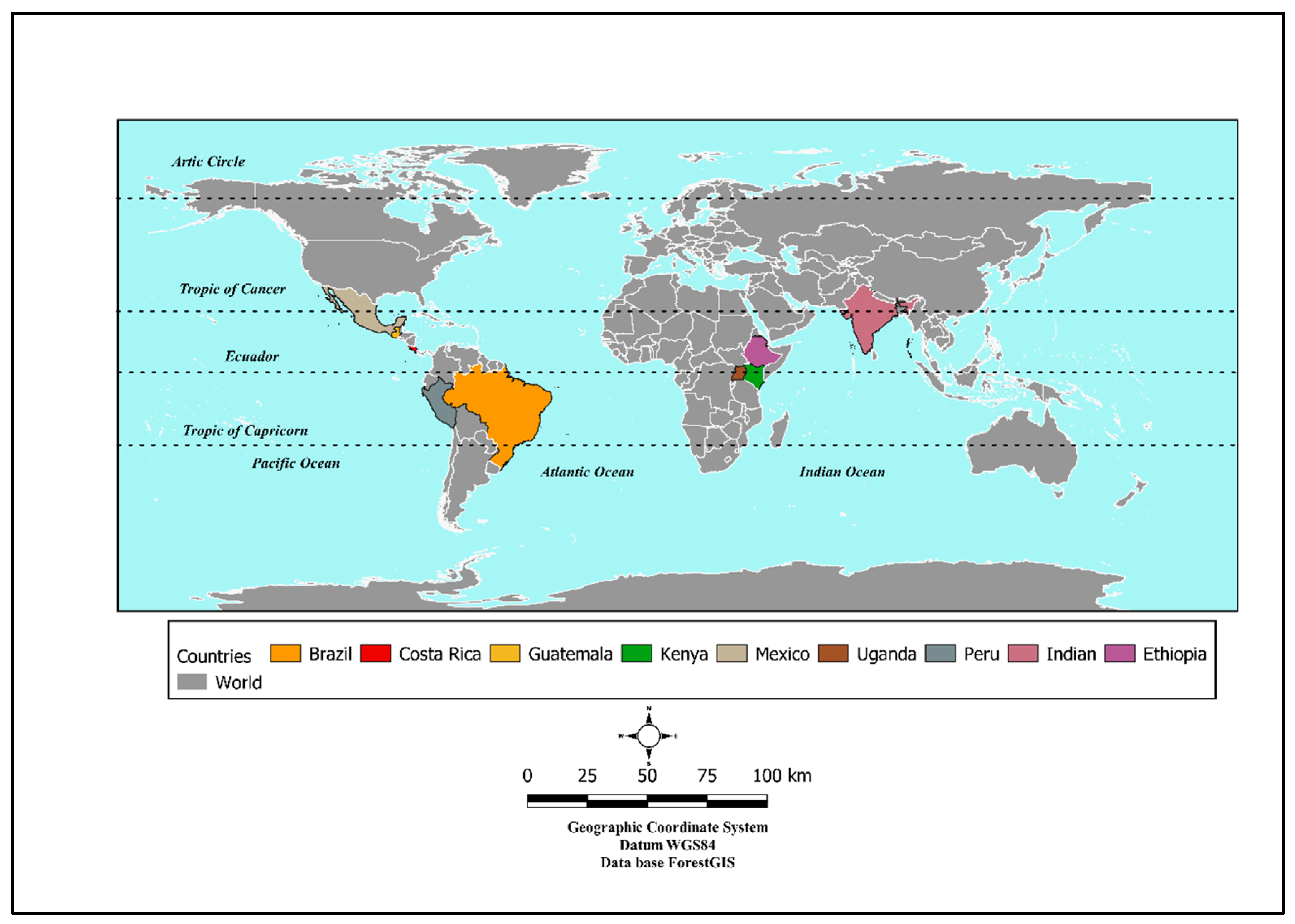
| Reference | Country | AFS Type | Depth (cm) | Time Span (yrs) | Clay Content | P (mm) | T (°C) |
|---|---|---|---|---|---|---|---|
| Negash and Starr [25] | Ethiopia | Diversified | 0–60 | 100 | Clayey | 1000 | 20.5 |
| Diversified | 0–60 | 100 | Clayey | 1000 | 20.5 | ||
| Diversified | 0–60 | 100 | Clayey | 1000 | 26.5 | ||
| Zaro et al. [26] | Brazil | Simple | 0–70 | 16 | Clayey | 1641 | 21.1 |
| Ehrenbergerova et al. [27] | Peru | Simple | 0–30 | 15 | Medium | 1550 | 17.8 |
| Simple | 0–30 | 15 | Medium | 1540 | 17.8 | ||
| Simple | 0–30 | 7 | Medium | 1660 | 17.8 | ||
| Ruiz Garcia et al. [28] | Mexico | Diversified | 0–30 | 50 | Very Clayey | 1900 | 18.8 |
| Simple | 0–30 | 50 | Very Clayey | 1900 | 18.8 | ||
| Simple | 0–30 | 50 | Very Clayey | 1900 | 18.8 | ||
| Espinoza-Dominguez [29] | Mexico | Simple | 0–30 | 20 | Very Clayey | 1844 | 19 |
| Simple | 0–30 | 20 | Very Clayey | 1844 | 19 | ||
| Simple | 0–30 | 20 | Very Clayey | 1844 | 19 | ||
| Simple | 0–30 | 20 | Very Clayey | 1844 | 19 | ||
| Solis et al. [30] | Peru | Diversified | 0–60 | 10 | Clayey | 1800 | 25 |
| Simple | 0–60 | 12 | Clayey | 1800 | 25 | ||
| Schmitt-Harsh et al. [24] | Guatemala | Diversified | 0–10 | 42 | Medium | 2504 | 21 |
| Chatterjee et al. [31] | India | Simple | 0–100 | 75 | Clayey | 2400 | 26 |
| Diversified | 0–100 | 65 | Medium | 2400 | 26 | ||
| Niguse et al. [32] | Ethiopia | Diversified | 0–60 | 20 | Medium | 1950 | 18.5 |
| Simple | 0–60 | 20 | Medium | 1950 | 18.5 | ||
| Tumwebaze and Byakagaba [33] | Uganda | Diversified | 0–30 | 20 | Very Clayey | 987.3 | 21.7 |
| Diversified | 0–30 | 20 | Very Clayey | 875 | 22.7 | ||
| Chatterjee, et al. [34] | Costa Rica | Simple | 0–100 | 15 | Clayey | 2600 | 22 |
| Simple | 0–100 | 15 | Clayey | 2600 | 22 | ||
| Simple | 0–100 | 15 | Clayey | 2600 | 22 | ||
| Simple | 0–100 | 15 | Clayey | 2600 | 22 | ||
| Segnini et al. [35] | Kenya | Simple | 0–30 | 20 | Clayey | 2905 | 19.3 |
| Hergoualc’h et al. [36] | Costa Rica | Simple | 0–40 | 9 | Clayey | 2300 | 21 |
| Arteaga et al. [37] | Mexico | Diversified | 0–40 | 30 | Medium | 990 | 21.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, V.M.; Miranda Júnior, M.S.; Silva, L.J.; Teixeira, M.V.; Teixeira, G.; Schossler, K.; Freitas, D.A.F.d.; Oliveira, D.M.d.S. A Global Meta-Analysis of Soil Carbon Stock in Agroforestry Coffee Cultivation. Agronomy 2025, 15, 480. https://doi.org/10.3390/agronomy15020480
Gomes VM, Miranda Júnior MS, Silva LJ, Teixeira MV, Teixeira G, Schossler K, Freitas DAFd, Oliveira DMdS. A Global Meta-Analysis of Soil Carbon Stock in Agroforestry Coffee Cultivation. Agronomy. 2025; 15(2):480. https://doi.org/10.3390/agronomy15020480
Chicago/Turabian StyleGomes, Vanessa Matos, Marcos Santana Miranda Júnior, Libério J. Silva, Marcus Vinícius Teixeira, Guilherme Teixeira, Karina Schossler, Diego Antônio França de Freitas, and Dener Márcio da Silva Oliveira. 2025. "A Global Meta-Analysis of Soil Carbon Stock in Agroforestry Coffee Cultivation" Agronomy 15, no. 2: 480. https://doi.org/10.3390/agronomy15020480
APA StyleGomes, V. M., Miranda Júnior, M. S., Silva, L. J., Teixeira, M. V., Teixeira, G., Schossler, K., Freitas, D. A. F. d., & Oliveira, D. M. d. S. (2025). A Global Meta-Analysis of Soil Carbon Stock in Agroforestry Coffee Cultivation. Agronomy, 15(2), 480. https://doi.org/10.3390/agronomy15020480







