The Addition of Straw Affects the Response of Labile Soil Organic Carbon to the Freezing and Thawing Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Experimental Design
2.3. Soil Sample Analyses
2.4. Statistical Analysis
3. Results
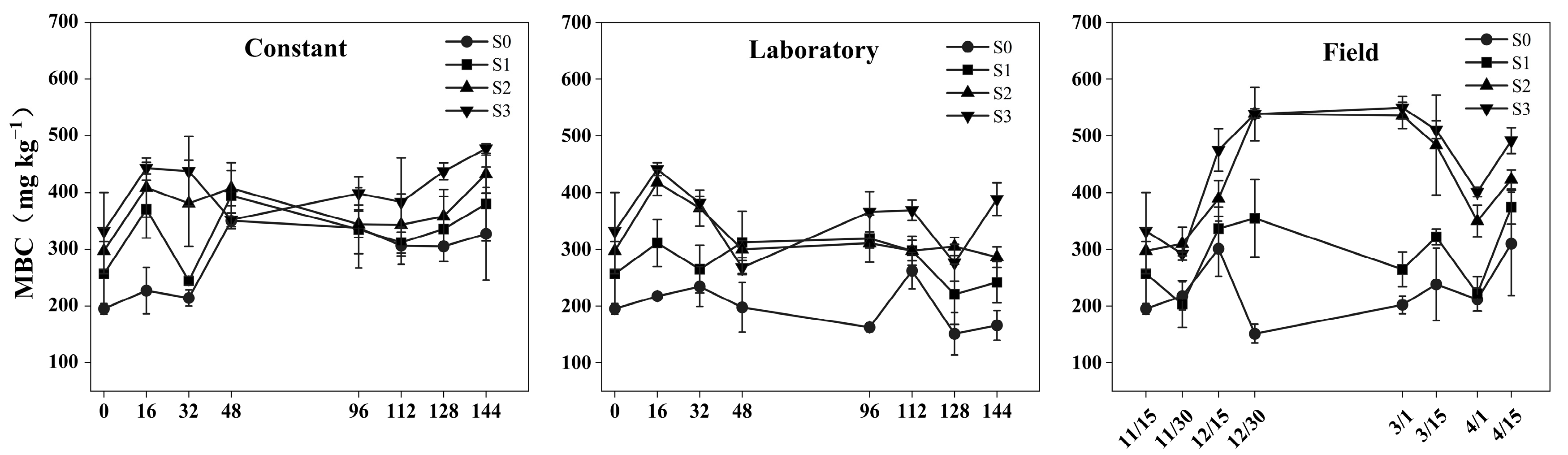
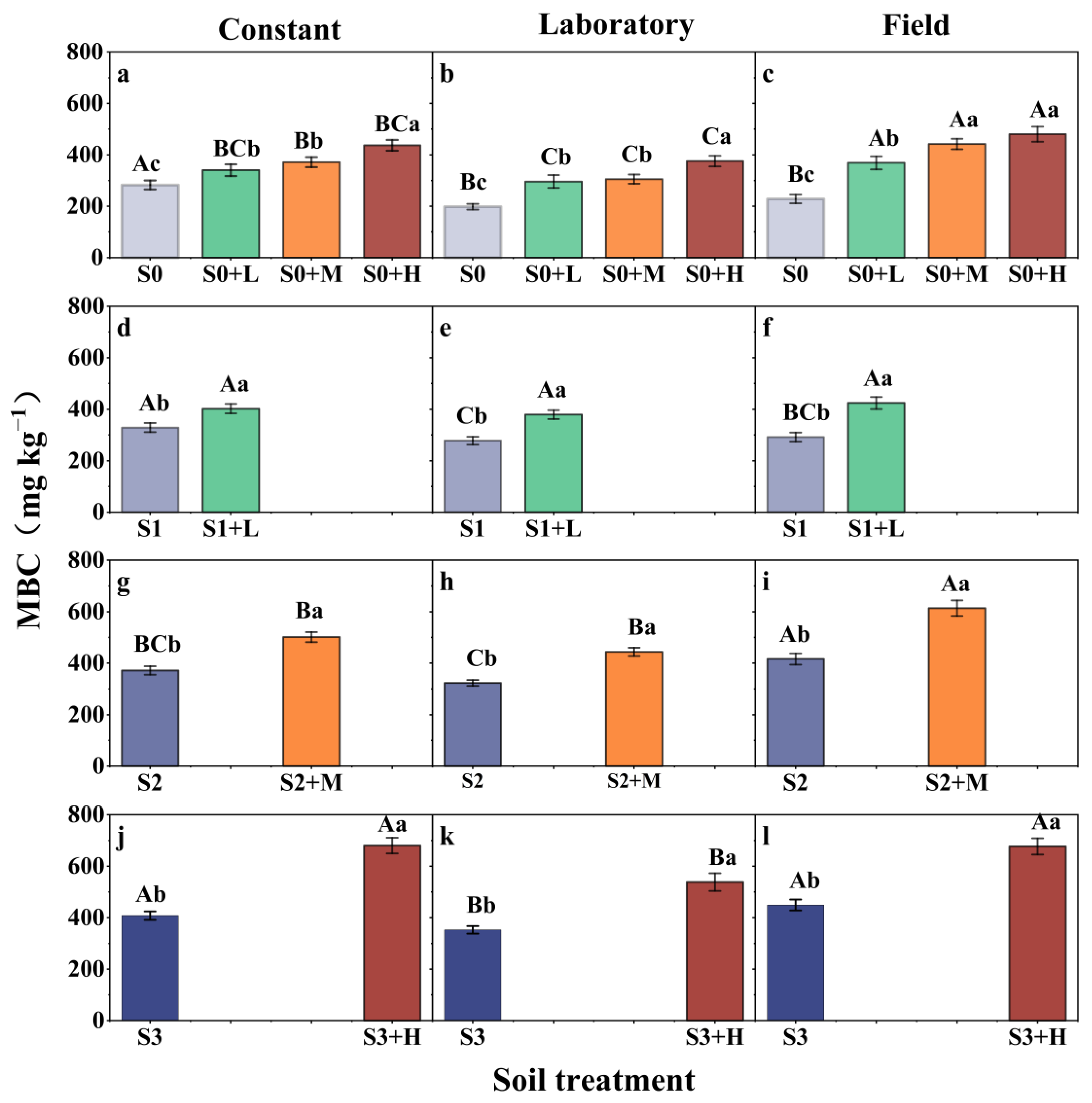
3.1. Changes in Soil MBC
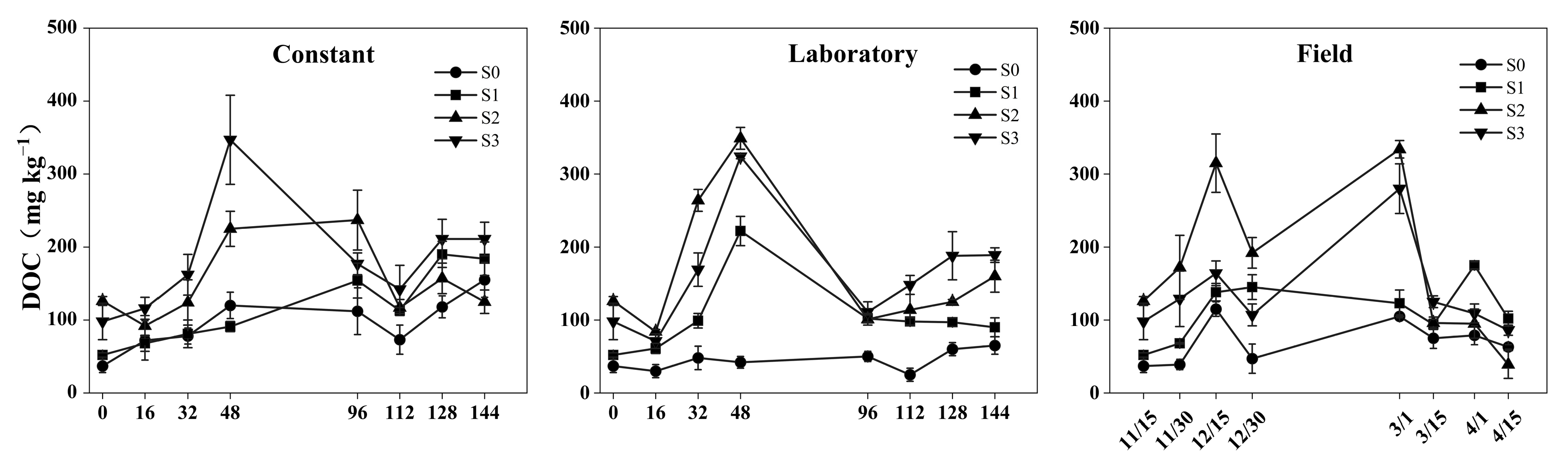
3.2. Changes in Soil DOC
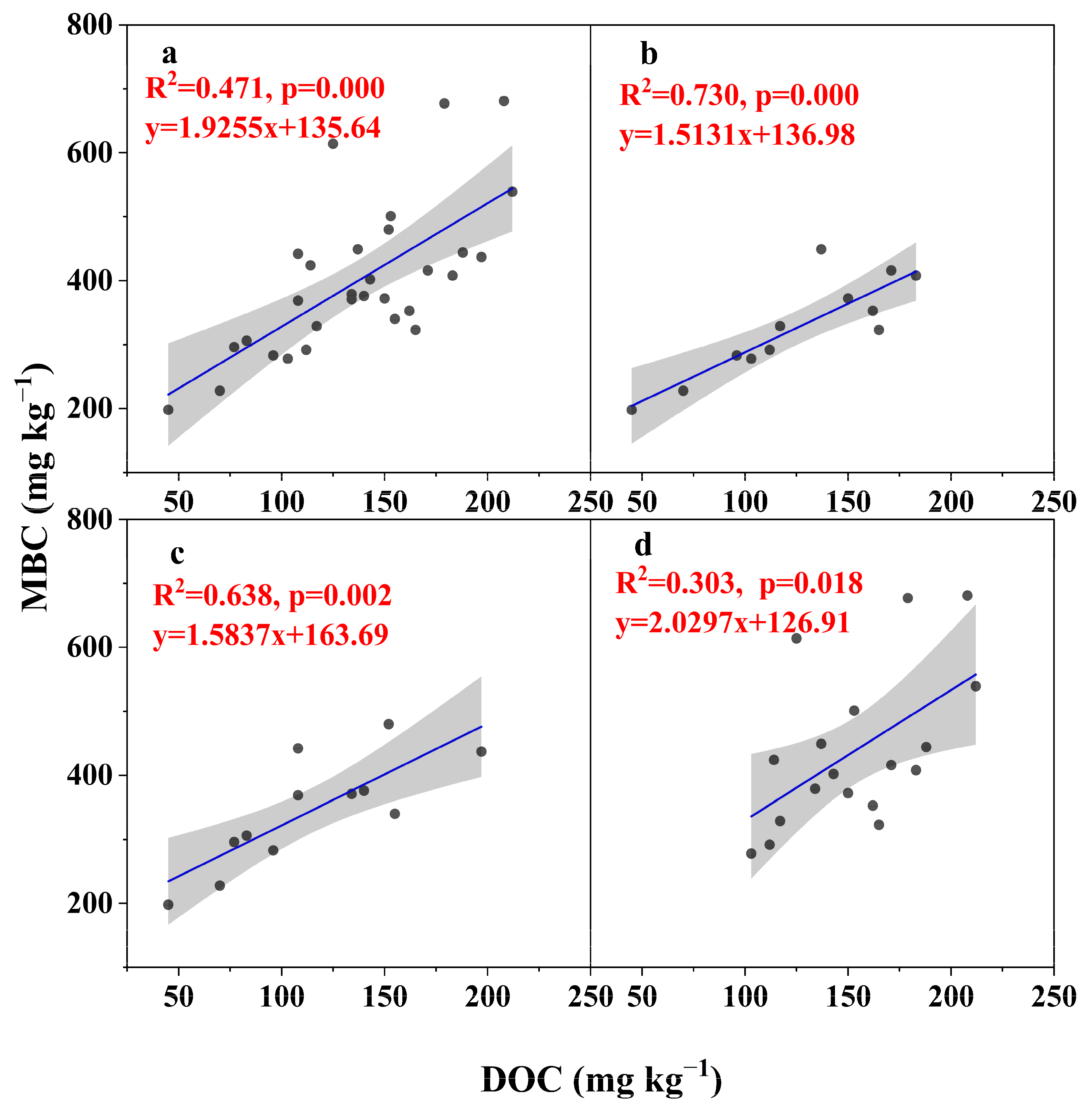
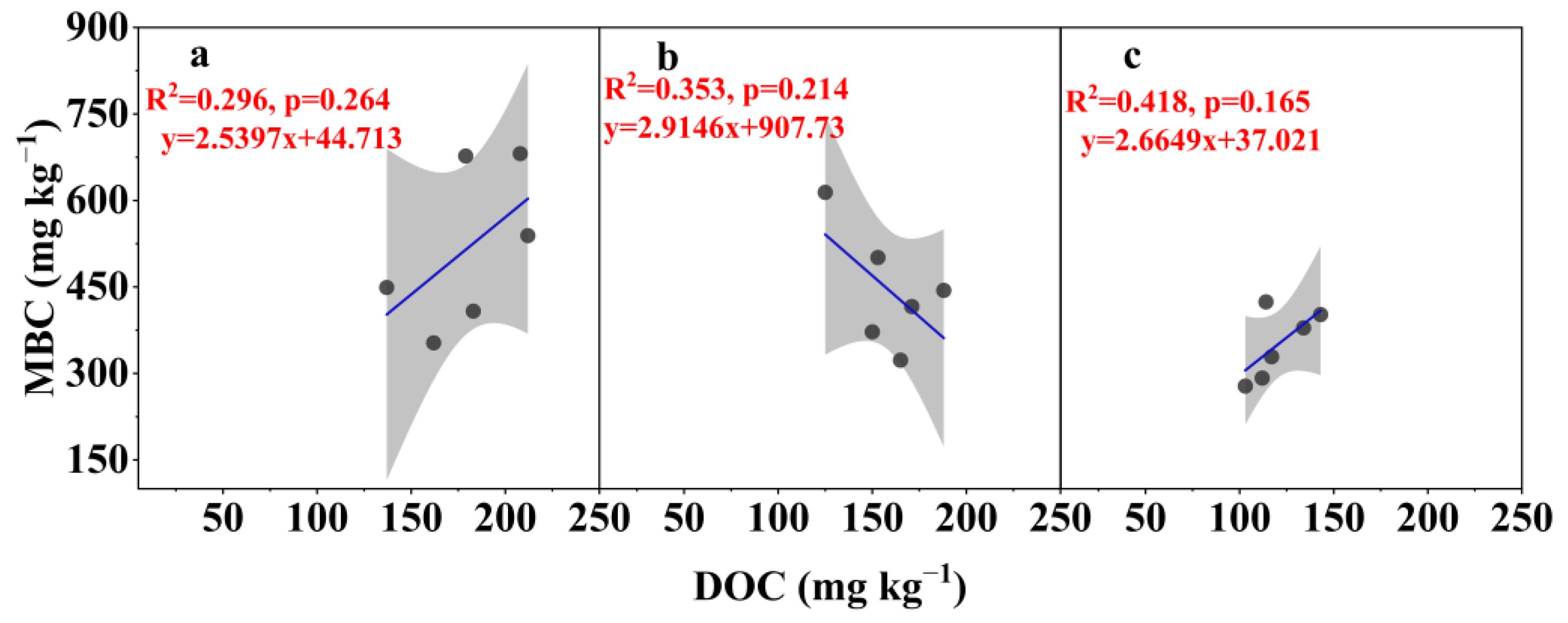
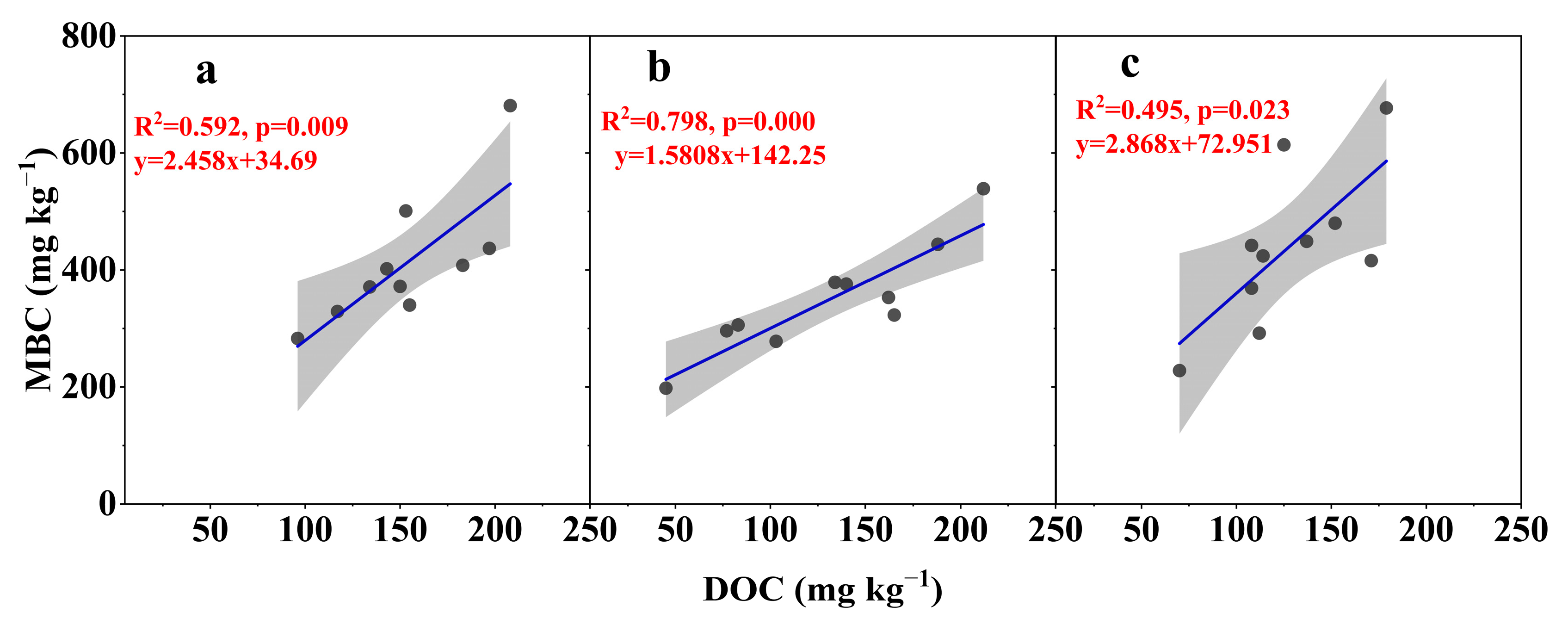
3.3. Relationship Between MBC and DOC
4. Discussion
4.1. Effects of the Freeze–Thaw Process on the MBC and DOC
4.2. Effects of Straw Addition on MBC and DOC Under Different Experimental Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larsen, K.S.; Jonasson, S.; Michelsen, A. Repeated Freeze–Thaw Cycles and Their Effects on Biological Processes in Two Arctic Ecosystem Types. Appl. Soil Ecol. 2002, 21, 187–195. [Google Scholar] [CrossRef]
- Watanabe, T.; Tateno, R.; Imada, S.; Fukuzawa, K.; Isobe, K.; Urakawa, R.; Oda, T.; Hosokawa, N.; Sasai, T.; Inagaki, Y.; et al. The Effect of a Freeze–Thaw Cycle on Dissolved Nitrogen Dynamics and Its Relation to Dissolved Organic Matter and Soil Microbial Biomass in the Soil of a Northern Hardwood Forest. Biogeochemistry 2019, 142, 319–338. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Pu, Y.; Li, T.; Xu, X.; Jia, Y.; Deng, O.; Gong, G. Dynamics of Soil Labile Organic Carbon Fractions and C-Cycle Enzyme Activities under Straw Mulch in Chengdu Plain. Soil Tillage Res. 2016, 155, 289–297. [Google Scholar] [CrossRef]
- Zhang, J.B.; Song, C.C.; Yang, W.Y. Land Use Effects on the Distribution of Labile Organic Carbon Fractions through Soil Profiles. Soil Sci. Soc. Am. J. 2006, 70, 660–667. [Google Scholar]
- Silveira, M.L.A. Dissolved Organic Carbon and Bioavailability of N and P as Indicators of Soil Quality. Sci. Agric. 2005, 62, 502–508. [Google Scholar] [CrossRef]
- McGILL, W.B.; Cannon, K.R.; Robertson, J.A.; Cook, F.D. Dynamics of Soil Microbial Biomass and Water-Soluble Organic C in Breton L after 50 Years of Cropping to Two Rotations. Can. J. Soil Sci. 1986, 66, 1–19. [Google Scholar] [CrossRef]
- Marschner, B.; Bredow, A. Temperature Effects on Release and Ecologically Relevant Properties of Dissolved Organic Carbon in Sterilised and Biologically Active Soil Samples. Soil Biol. Biochem. 2002, 34, 459–466. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Shi, F.; Zhang, Y. Divergent Controlling Factors of Freeze–Thaw-Induced Changes in Dissolved Organic Carbon and Microbial Biomass Carbon between Topsoil and Subsoil of Cold Alpine Grasslands. CATENA 2024, 241, 108063. [Google Scholar] [CrossRef]
- Christensen, S.; Tiedje, J.M. Brief and Vigorous N2O Production by Soil at Spring Thaw. J. Soil Sci. 1990, 41, 1–4. [Google Scholar] [CrossRef]
- Suutari, M.; Laakso, S. Microbial Fatty Acids and Thermal Adaptation. Crit. Rev. Microbiol. 1994, 20, 285–328. [Google Scholar] [CrossRef]
- Herrmann, A.; Witter, E. Sources of C and N Contributing to the Flush in Mineralization upon Freeze–Thaw Cycles in Soils. Soil Biol. Biochem. 2002, 34, 1495–1505. [Google Scholar] [CrossRef]
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of Successive Soil Freeze-Thaw Cycles on Soil Microbial Biomass and Organic Matter Decomposition Potential of Soils. Soil Sci. Plant Nutr. 2004, 50, 821–829. [Google Scholar] [CrossRef]
- Song, Y.; Zou, Y.; Wang, G.; Yu, X. Altered Soil Carbon and Nitrogen Cycles Due to the Freeze-Thaw Effect: A Meta-Analysis. Soil Biol. Biochem. 2017, 109, 35–49. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, X.; Xie, Q.; Ma, X. Release of Carbon and Nitrogen from Alpine Soils During Thawing Periods in the Eastern Qinghai-Tibet Plateau. Water Air Soil Pollut. 2015, 226, 209. [Google Scholar] [CrossRef]
- Henry, H.A.L. Soil Freeze–Thaw Cycle Experiments: Trends, Methodological Weaknesses and Suggested Improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- Perez-Mon, C.; Frey, B.; Frossard, A. Functional and Structural Responses of Arctic and Alpine Soil Prokaryotic and Fungal Communities under Freeze-Thaw Cycles of Different Frequencies. Front. Microbiol. 2020, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Grogan, P.; Michelsen, A.; Ambus, P.; Jonasson, S. Freeze–Thaw Regime Effects on Carbon and Nitrogen Dynamics in Sub-Arctic Heath Tundra Mesocosms. Soil Biol. Biochem. 2004, 36, 641–654. [Google Scholar] [CrossRef]
- Seitz, S.; Goebes, P.; Puerta, V.L.; Pereira, E.I.P.; Wittwer, R.; Six, J.; Van Der Heijden, M.G.A.; Scholten, T. Conservation Tillage and Organic Farming Reduce Soil Erosion. Agron. Sustain. Dev. 2019, 39, 4. [Google Scholar] [CrossRef]
- Liu, C.; Si, B.; Zhao, Y.; Wu, Z.; Lu, X.; Chen, X.; Han, X.; Zhu, Y.; Zou, W. Drivers of Soil Quality and Maize Yield under Long-Term Tillage and Straw Incorporation in Mollisols. Soil Tillage Res. 2025, 246, 106360. [Google Scholar] [CrossRef]
- Ekwunife, K.C.; Madramootoo, C.A.; Abbasi, N.A. Assessing the Impacts of Tillage, Cover Crops, Nitrification, and Urease Inhibitors on Nitrous Oxide Emissions over Winter and Early Spring. Biol. Fertil. Soils 2022, 58, 195–206. [Google Scholar] [CrossRef]
- Hao, X.; Han, X.; Wang, S.; Li, L.-J. Dynamics and Composition of Soil Organic Carbon in Response to 15 Years of Straw Return in a Mollisol. Soil Tillage Res. 2022, 215, 105221. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Xia, Z.; Zhang, X.; Zhu, M.; Gao, Y.; An, S.; Yu, W.; Ma, Q. The Mechanism of the Dose Effect of Straw on Soil Respiration: Evidence from Enzymatic Stoichiometry and Functional Genes. Soil Biol. Biochem. 2022, 168, 108636. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Cai, L. Effects of Different Straw Incorporation Amounts on Soil Organic Carbon, Microbial Biomass, and Enzyme Activities in Dry-Crop Farmland. Sustainability 2024, 16, 10588. [Google Scholar] [CrossRef]
- Xiao, D. Alfisols and Closely Related Soils in China. Chin. Geogr. Sci. 1992, 2, 18–29. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, W.; Ma, Q.; Zhang, L. A modified Fumigation Extraction Method for the Determination of Soil Microbial Biomass Carbon. Chin. J. Soil Sci. 2009, 1, 0154-4. [Google Scholar]
- Ocio, J.A.; Brookes, P.C. An Evaluation of Methods for Measuring the Microbial Biomass in Soils Following Recent Additions of Wheat Straw and the Characterization of the Biomass That Develops. Soil Biol. Biochem. 1990, 22, 685–694. [Google Scholar] [CrossRef]
- Lin, Q.; Wu, Y.; Liu, H. Improvement on the fumigation method for determining soil microbial biomass carbon. Ecol. Mag. 1999, 2, 64–67. [Google Scholar]
- Guo, A.; Zhao, Z.; Zhang, P.; Yang, Q.; Li, Y.; Wang, G. Linkage between Soil Nutrient and Microbial Characteristic in an Opencast Mine, China. Sci. Total Environ. 2019, 671, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Chemical Analysis Method of Agricultural Soil; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Sparling, G. Ratio of Microbial Biomass Carbon to Soil Organic Carbon as a Sensitive Indicator of Changes in Soil Organic Matter. Soil Res. 1992, 30, 195. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Keeney, D.R.; McCarty, G.W. Effect of Freeze-Thaw Events on Mineralization of Soil Nitrogen. Biol. Fertil. Soils 1992, 14, 116–120. [Google Scholar] [CrossRef]
- Gao, D.; Bai, E.; Yang, Y.; Zong, S.; Hagedorn, F. A Global Meta-Analysis on Freeze-Thaw Effects on Soil Carbon and Phosphorus Cycling. Soil Biol. Biochem. 2021, 159, 108283. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Lipson, D.A. Microbial Growth under the Snow: Implications for Nutrient and Allelochemical Availability in Temperate Soils. Plant Soil 2004, 259, 1–7. [Google Scholar] [CrossRef]
- Patel, K.F.; Tatariw, C.; MacRae, J.D.; Ohno, T.; Nelson, S.J.; Fernandez, I.J. Repeated Freeze–Thaw Cycles Increase Extractable, but Not Total, Carbon and Nitrogen in a Maine Coniferous Soil. Geoderma 2021, 402, 115353. [Google Scholar] [CrossRef]
- Yu, X.; Zou, Y.; Jiang, M.; Lu, X.; Wang, G. Response of Soil Constituents to Freeze–Thaw Cycles in Wetland Soil Solution. Soil Biol. Biochem. 2011, 43, 1308–1320. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Hu, X. Soil Open Pore Structure Regulates Soil Organic Carbon Fractions of Soil Aggregates under Simulated Freeze-Thaw Cycles as Determined by X-Ray Computed Tomography. J. Soil Sci. Plant Nutr. 2024, 24, 5235–5248. [Google Scholar] [CrossRef]
- Xue, P.; Fu, Q.; Li, T.; Liu, D.; Hou, R.; Li, Q.; Li, M.; Meng, F. Effects of Biochar and Straw Application on the Soil Structure and Water-Holding and Gas Transport Capacities in Seasonally Frozen Soil Areas. J. Environ. Manag. 2022, 301, 113943. [Google Scholar] [CrossRef]
- Schimel, P.; Clein, J.S. Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biol. Biochem. 1996, 28, 1061–1066. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.; Jung, J.Y. Responses of Dissolved Organic Carbon to Freeze–Thaw Cycles Associated with the Changes in Microbial Activity and Soil Structure. Cryosphere 2023, 17, 3101–3114. [Google Scholar] [CrossRef]
- Lei, K.; Dai, W.; Wang, J.; Li, Z.; Cheng, Y.; Jiang, Y.; Yin, W.; Wang, X.; Song, X.; Tang, Q. Biochar and Straw Amendments over a Decade Divergently Alter Soil Organic Carbon Accumulation Pathways. Agronomy 2024, 14, 2176. [Google Scholar] [CrossRef]
- Wang, F.; Bettany, J. Influence of Freeze-Thaw and Flooding on the Loss of Soluble Organic-Carbon and Carbon-Dioxide from Soil. J. Environ. Qual. 1993, 22, 709–714. [Google Scholar] [CrossRef]
- Batista, É.R.; Franco, A.D.J.; Silva, A.P.V.D.; Silva, J.A.G.F.D.; Tavares, D.S.; Souza, J.K.D.; Silva, A.O.; Barbosa, M.V.; Santos, J.V.D.; Carneiro, M.A.C. Organic Substrate Availability and Enzyme Activity Affect Microbial-Controlled Carbon Dynamics in Areas Disturbed by a Mining Dam Failure. Appl. Soil Ecol. 2022, 169, 104169. [Google Scholar] [CrossRef]
- Liu, M.; Lin, H.; Li, J. Are There Links between Nutrient Inputs and the Response of Microbial Carbon Use Efficiency or Soil Organic Carbon? A Meta-Analysis. Soil Biol. Biochem. 2025, 201, 109656. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, J.; Liang, S.; Li, Z.; Wang, J.; Wang, S. Early Thawing after Snow Removal and No Straw Mulching Accelerates Organic Carbon Cycling in a Paddy Soil in Northeast China. J. Environ. Manag. 2018, 209, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, R.; Shibata, H.; Kuroiwa, M.; Inagaki, Y.; Tateno, R.; Hishi, T.; Fukuzawa, K.; Hirai, K.; Toda, H.; Oyanagi, N.; et al. Effects of Freeze–Thaw Cycles Resulting from Winter Climate Change on Soil Nitrogen Cycling in Ten Temperate Forest Ecosystems throughout the Japanese Archipelago. Soil Biol. Biochem. 2014, 74, 82–94. [Google Scholar] [CrossRef]
- Hou, R.; Li, T.; Fu, Q.; Liu, D.; Li, M.; Zhou, Z.; Li, Q.; Zhao, H.; Yu, P.; Yan, J. Effects of Biochar and Straw on Greenhouse Gas Emission and Its Response Mechanism in Seasonally Frozen Farmland Ecosystems. CATENA 2020, 194, 104735. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal—A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- He, Z.; Hou, R.; Fu, Q.; Li, T.; Zhang, S.; Su, A. Evolution Mechanism of Soil Hydrothermal Parameters under Freeze-Thaw Cycles: Regulatory Significance of Straw and Biochar. J. Clean Prod. 2023, 385, 135787. [Google Scholar] [CrossRef]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of Long-term Straw Return on Soil Organic Carbon Storage and Sequestration Rate in North China Upland Crops: A Meta-analysis. Glob. Change Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, N.; Zhang, Z.; Xu, J.; Tao, B.; Meng, Y. Short-Term Responses of Soil Organic Carbon and Carbon Pool Management Index to Different Annual Straw Return Rates in a Rice–Wheat Cropping System. CATENA 2015, 135, 283–289. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; He, P.; Zhang, Y.; Li, L.-J. Changes in Soil Microbial Community and Carbon Use Efficiency in Freeze-Thaw Period Restored after Growth Season under Warming and Straw Return. Appl. Soil Ecol. 2025, 205, 105779. [Google Scholar] [CrossRef]
- Puissant, J.; Cécillon, L.; Mills, R.T.E.; Robroek, B.J.M.; Gavazov, K.; De Danieli, S.; Spiegelberger, T.; Buttler, A.; Brun, J.-J. Seasonal Influence of Climate Manipulation on Microbial Community Structure and Function in Mountain Soils. Soil Biol. Biochem. 2015, 80, 296–305. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Collins, D.; Li, B.; Zhu, J.; Tavakkoli, E. Nutrient Supply Enhanced Wheat Residue-Carbon Mineralization, Microbial Growth, and Microbial Carbon-Use Efficiency When Residues Were Supplied at High Rate in Contrasting Soils. Soil Biol. Biochem. 2018, 126, 168–178. [Google Scholar] [CrossRef]

| Soil | Total C (g kg−1) | Total N (g kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | Available N (mg kg−1) | pH |
|---|---|---|---|---|---|---|
| S0 | 11.72 | 1.03 | 7.47 | 98.0 | 99.7 | 6.78 |
| S1 | 14.18 | 1.19 | 5.37 | 105 | 104 | 6.81 |
| S2 | 15.94 | 1.33 | 5.05 | 120 | 114 | 6.84 |
| S3 | 17.72 | 1.46 | 5.00 | 127 | 126 | 6.90 |
| Freeze–Thaw Treatment | Induction Days | S0 | S1 | S2 | S3 |
|---|---|---|---|---|---|
| Constant | 0 | 1.67 a | 1.81 a | 1.87 a | 1.88 a |
| 16 | 1.91 a | 2.70 a | 2.61 a | 2.52 a | |
| 32 | 1.81 a | 1.77 a | 2.44 a | 2.49 a | |
| 48 | 2.97 a | 2.83 a | 2.60 a | 1.97 a | |
| 96 | 2.78 a | 2.44 a | 2.19 a | 2.27 a | |
| 112 | 2.56 a | 2.22 a | 2.23 a | 2.18 a | |
| 128 | 2.57 a | 2.37 a | 2.33 a | 2.53 a | |
| 144 | 2.79 a | 2.76 a | 2.76 a | 2.71 a | |
| Laboratory | 0 | 1.67 ab | 1.81 a | 1.87 b | 1.88 bc |
| 16 | 1.81 ab | 2.15 a | 2.58 a | 2.49 a | |
| 32 | 1.96 ab | 1.83 a | 2.28 ab | 2.14 ab | |
| 48 | 1.64 ab | 2.22 a | 1.85 b | 1.50 c | |
| 96 | 1.36 b | 2.26 a | 1.92 b | 2.04 ab | |
| 112 | 2.25 a | 2.10 a | 1.80 b | 2.02 ab | |
| 128 | 1.28 b | 1.56 a | 1.85 b | 1.53 c | |
| 144 | 1.41 b | 1.73 a | 1.80 b | 2.15 ab | |
| Field | 0 | 1.67 ab | 1.81 bc | 1.87 c | 1.88 c |
| 16 | 1.79 ab | 1.44 c | 1.93 c | 1.65 c | |
| 32 | 2.48 ab | 2.36 ab | 2.40 bc | 2.72 ab | |
| 48 | 1.28 b | 2.51 ab | 3.33 a | 3.04 a | |
| 96 | 1.66 ab | 1.91 bc | 3.35 a | 3.06 a | |
| 112 | 2.03 ab | 2.34 ab | 3.06 ab | 2.90 ab | |
| 128 | 1.81 ab | 1.63 c | 2.23 bc | 2.28 bc | |
| 144 | 2.63 a | 2.73 a | 2.67 bc | 2.86 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Ma, Q.; Li, S.; Xia, Z.; Zhou, C.; Gao, Y.; Zhang, X.; An, S.; Jiang, X.; Yu, W. The Addition of Straw Affects the Response of Labile Soil Organic Carbon to the Freezing and Thawing Process. Agronomy 2025, 15, 479. https://doi.org/10.3390/agronomy15020479
Zhu M, Ma Q, Li S, Xia Z, Zhou C, Gao Y, Zhang X, An S, Jiang X, Yu W. The Addition of Straw Affects the Response of Labile Soil Organic Carbon to the Freezing and Thawing Process. Agronomy. 2025; 15(2):479. https://doi.org/10.3390/agronomy15020479
Chicago/Turabian StyleZhu, Mengmeng, Qiang Ma, Shuailin Li, Zhuqing Xia, Changrui Zhou, Yun Gao, Xinhui Zhang, Siyu An, Xiao Jiang, and Wantai Yu. 2025. "The Addition of Straw Affects the Response of Labile Soil Organic Carbon to the Freezing and Thawing Process" Agronomy 15, no. 2: 479. https://doi.org/10.3390/agronomy15020479
APA StyleZhu, M., Ma, Q., Li, S., Xia, Z., Zhou, C., Gao, Y., Zhang, X., An, S., Jiang, X., & Yu, W. (2025). The Addition of Straw Affects the Response of Labile Soil Organic Carbon to the Freezing and Thawing Process. Agronomy, 15(2), 479. https://doi.org/10.3390/agronomy15020479





