Abstract
This study examines the occurrence and distribution of root-knot nematodes (RKN), Meloidogyne spp., in Serbia through an official survey conducted from 2021 to 2023. A total of 241 plant and soil samples were collected from 25 districts across two regions: Northern Serbia (Vojvodina Province) and Central Serbia. RKN infestations were detected in 23.7% of the samples. Among the 57 identified populations, 5 Meloidogyne species were recorded: M. incognita, M. hapla, M. luci, M. arenaria and M. javanica. Meloidogyne luci was reported in Serbia for the first time, marking a significant finding for nematology in the region. This study highlights the importance of implementing effective pest management strategies to mitigate the agricultural impact of RKN in Serbia.
1. Introduction
Root-knot nematodes (RKNs) of the genus Meloidogyne (Göldi, 1892) are economically significant obligate polyphagous parasites that infect nearly all species of higher plants [1]. According to Blok et al. [2], annual vegetable crop losses attributed to RKN exceeds EUR 80 billion worldwide. These nematodes are distributed globally, attacking root systems and causing abnormal swelling known as galls, resulting in yield and quality reduction, particularly in vegetables and ornamental plants. Over 100 species have been described within this nematode genus [3]. Four species are considered major pests in agricultural systems: M. incognita (Kofoid and White, 1919) Chitwood, 1949; M. javanica (Treub, 1885) Chitwood, 1949; M. arenaria (Neal, 1889) Chitwood, 1949; and M. hapla Chitwood, 1949 [4]. Among the described RKN species, M. ethiopica Whitehead, 1968, is listed on the A1 quarantine list [5], while six species (M. chitwoodi Golden, O’Bannon, Santo & Finley 1980; M. enterolobii Yang & Eisenback, 1983; M. fallax Karssen,1996; M. graminicola Golden & Birchfield, 1965; M. luci Carneiro et al., 2014; and M. mali Itoh, Ohshima & Ichinoe,1969) are included in the A2 quarantine list of the European Plant Protection Organisation (EPPO) to prevent further spread of these harmful pests in Europe [6].
The agricultural and food sector is one of the most significant contributors to the Serbian economy. Agricultural land in Serbia spans approximately 5.1 million hectares (ha), accounting for over two-thirds of the country’s total area, with approximately 3.6 million ha classified as arable land [7], Aaccording to the Statistical Office of the Republic of Serbia [8].
Serbia is divided into two main regions—Northern Serbia (Vojvodina Province) and Central Serbia—comprising 30 districts in total based on political and administrative boundaries. The majority of arable land (67%) is allocated to cereal crops, 19% to industrial crops, 9% to fodder crops and 4% to other crops, including vegetables. Vegetable production is dominated by potato (Solanum tuberosum L.), followed by pepper (Capsicum annuum L.), onion (Allium cepa L.) and tomato (Solanum lycopersicum L.). Greenhouses and other protective structures account for 13% of the total area under vegetable cultivation
Only seven species of Meloidogyne have been reported in Serbia to date (Table 1). The first species initially identified as Heterodera marioni (nomen nudum) was first discovered in 1947 on cucumber (Cucumissativus L.) in a greenhouse located near Belgrade [9]. Meloidogyne incognita, M. arenaria, M. hapla, M. javanica and M. incognita acrita Chitwood, 1949 (a synonym for M. incognita) were detected on tomato, pepper, cucumber, lettuce (Lactuca sativa L.), celery (Apium graveolens L.), black nightshade (Solanum nigrum L.) and ornamental plants in greenhouses located in Central Serbia [10,11]. In the field, M. hapla, M. incognita, M. arenaria and M. naasi Franklin, 1965, were reported on sugar beet (Beta vulgaris L.), carrot (Daucus carota subsp. sativus Hoffm.), tobacco (Nicotiana tabacum L.), wheat (Triticum aestivum L.), barley (Hordeum vulgare L.) and sunflower (Helianthus annuus L.) in the central, western and southern regions of Serbia [11,12,13,14]. In the 1990s and 2000s, no systemic surveillance or new reports of RKN were recorded in the country. However, between 2014 and 2018, severe damage to potato crops (caused by M. incognita and M. arenaria) was recorded along the Serbian–Hungarian border [15,16]. M. incognita and M. hapla were also detected on tomato, carrot and parsnip (Pastinaca sativa L.) in greenhouses and fields near Leskovac, Veliko Gradište, Kruševac and Belgrade [17,18]. In addition, M. arenaria was identified on calla (Calla palustris L.) and the quarantine species M. luci was reported for the first time on tomato in a greenhouse in Northern Serbia [19,20].

Table 1.
Species of Meloidogyne spp. detected in Serbia.
Serbia was part of the former Yugoslavia until the 1990s. RKNs were also investigated in other countries of the former Yugoslavia (Croatia, Bosnia and Herzegovina, North Macedonia, Slovenia and Montenegro). By 2024, seven RNK species had been detected across these countries: M. arenaria, M. hapla and M. artiellia Franklin, 1961, in Croatia [21]; M. incognita, M. arenaria, M. hapla and M. javanica in Bosnia and Herzegovina [22,23]; M. incognita, M. arenaria, M. hapla and M. javanica in North Macedonia [24,25]; M. incognita, M. hapla, M. luci and M. arenaria in Slovenia [26,27]; and M. incognita, M. arenaria, M. javanica, M. hapla and M. ardenensis Santos, 1968, in Montenegro [28].
The identification of Meloidogyne species is crucial for developing effective management practises. Analysing the morphological characteristics of RKNs in combination with biochemical or molecular techniques has proven to be more dependable than relying on morphology alone, as morphological characteristics are often similar and may vary between individuals and species.
Currently, there is a lack of comprehensive data on the occurrence, geographical distribution and host plant records of RKN species in Serbia. Therefore, the aim of this study was to update the knowledge on the occurrence and distribution of Meloidogyne species in greenhouses and open fields in Serbia, as part of the official survey on quarantine RKNs conducted from 2021 to 2023. Accurate identification of RKNs using morphological (perineal pattern morphology) and molecular methods is a critical first step towards establishing an effective programme for the control of Meloidogyne species in the country.
2. Materials and Methods
2.1. Sampling
The official Meloidogyne survey was carried out by the Serbian Plant Protection Directorate of the Ministry of Agriculture, Forestry and Water Management (MAFWM) between June 2021 and October 2023 as part of the Plant Health Measures Programme. Below-ground plant material (roots and tubers) with soil was collected from greenhouses and fields by 27 agricultural advisory services in two regions across 25 districts (7 in Northern Serbia (Vojvodina Province) and 18 in Central Serbia). Samples of several symptomatic and asymptomatic root systems were collected from tomato, pepper, cucumber, melon (Cucumis melo L.), ornamental plants (calla, gladiolus (Gladiolus communis L.), potato, rose (Rosa cinnamomea L.), carrot, parsley (Petroselinum crispum (Mill.) Fuss), parsnip, horseradish (Armoracia rusticana G.Gaertn., B.Mey. & Scherb.), cabbage (Brassica oleracea L.) and black nightshade (Solanum nigrum L.) and were sampled equally across both regions (40 samples), totalling approximately 80 samples collected annually (Figure 1, Table 2). Sampling instructions were prepared by the laboratory for nematode diagnostics. Sample numbers were planned based on previous data on vegetable and ornamental production in 25 districts and were to be collected by the Serbian Plant Protection Directorate. Some samples were collected based on prior reports of infestation, while others were selected randomly. In addition to plant material, soil samples were collected from the same locations. Soil samples, with an approximate volume of 1500 mL per greenhouse or field, were taken using a shovel from a depth of 15–20 cm. This was intended to facilitate nematode reproduction in cases where unidentified RKN populations were present. The sampling tools were cleaned and disinfected with ethanol after each use. The samples were placed in plastic bags, individually coded and labelled with information on the farmer, field or greenhouse, plant host, cultivation conditions and location. The samples were stored in a refrigerator at approximately 4 °C and promptly transported to the laboratory for nematode analysis.

Figure 1.
(A) Samples were collected between 2021 and 2023 from the regions of Northern and Central Serbia, highlighted in blue with the border depicted by a bold black line between Northern Serbia (7 districts) and Central Serbia (18 districts). Samples were not collected in 5 districts, highlighted in red on this map. Map is from https://en.wikipedia.org/wiki/Administrative_districts_of_Serbia (accessed on 8 January 2025). (B) Symptoms caused by M. incognita (Kofoid and White, 1919) Chitwood, 1949, on potato (Solanum tuberosum L.) and M. arenaria (Neal, 1889) Chitwood, 1949, on carrot (Daucus carota subsp. sativus Hoffm.).

Table 2.
Number of samples collected according to growing conditions in Serbia from 2021 to 2023.
2.2. Nematode Extraction
Fresh plant material (roots and tubers) was analysed for the presence of galls. Galled roots were washed free of soil and the gall index (GI) was determined on a scale of 0–10 [29]. Females and egg masses were extracted from the roots by dissecting the tissue with needles with a stereomicroscope (Nikon SMZ 800N, Tokyo, Japan) at 40× magnification for morphological and molecular analysis. The remaining plant material and soil infected with RKN were heat-treated in an autoclave after nematode extraction.
2.3. Morphological Analysis
The morphological identification of the females was carried out by observing the perineal patterns. The perineal patterns were prepared following the procedures outlined by Hartman and Sasser [30]. The posterior part of the female was dissected using a surgical steel blade in 45% lactic acid, after which the body contents were removed and the cleaned cuticle was transferred to a drop of glycerol on a clean slide for permanent mounting. The morphology of the perineal patterns was examined with a microscope (Leica DM 1000 LED, Wetzlar, Germany) at 400× magnification and photographed using a digital camera (ICC50 W Camera—HDMI, Wetzlar, Germany). Species identification was performed based on the criteria established by Chitwood, 1949, according to Karssen [31].
2.4. Molecular Identification
The extracted egg masses or females were pooled in 150 µL of sterile water, and total DNA was isolated using the Quick-DNATM Fecal/Soil Microbe Miniprep kit, following the manufacturer’s instructions. Molecular identification was carried out according to PCR protocols and primers as described in [32,33,34,35,36]. Specific regions were amplified by the PCR method using 1 µL of 10 µmol forward and reverse primers, 1 U of Taq polymerase (GoTaq Flexi DNA polymerase by Promega, Madison, WI, USA) and 5 ng of genomic DNA in 25 µL of the total PCR reaction mixture volume. Depending on the primers (Table 3), the PCR reaction was performed according to the programmes and conditions described in Supplementary Material 1 (Tables S1–S4). Amplified PCR products were visualised on 1% agarose gel and the following bands were expected depending on the primers used. In the first step, based on the PCR with primers 194/195, the species of the genus Meloidogyne could be separated into three groups with the following expected bands: M. hapla (yielding a 700 bp fragment); M. incognita, M. arenaria and M. javanica (720 bp); M. enterolobii (780 bp); and M. chitwoodi and M. fallax (between 1600 and 1700 bp). The use of these primers resulted in consistent amplifications of DNA extracted from females and egg masses. The samples were further analysed by the PCR method, with primers MI-F and MI-R specific for the species M. incognita (999 bp); primers Fjav and Rjav specific for the species M. javanica (720 bp); primers Far and Rar specific for the species M. arenaria (420 bp); and primers JMV1, JMV2, JMV hapla and JMV tropical specific for M. chitwoodi (540 bp), M. fallax (670 bp), M. hapla (440 bp) and M. incognita (615 bp). Meloidogyne luci was identified using group-specific PCR, species-specific PCR and sequence analysis. The affiliation of the nematode to the tropical RKN group and the M. ethiopica group was determined using two PCR reactions. Identification was confirmed by a species-specific PCR of M. luci, which yielded a band of approximately 770 bp. In addition, identification was confirmed by sequence analyses. The region of the mtDNA was amplified with primers C2F3 (GGTCAATGTTCAGAAATTTGTGG) and 1108 (TACCTTTGACCAATCACGCT), cloned, sequenced (acc. no. OQ211107) and compared with other sequences of Meloidogyne spp. from Genbank. To identify the nematode M. luci belonging to the tropical root-knot nematode group (i.e., clade I of Meloidogyne spp.), the forward primer C2F3 and a group-specific reverse primer Mt575R yielded a 621 bp long amplicon specific for the tropical root-knot nematode group. The next step was to determine whether the nematode belongs to the M. ethiopica group (i.e., a group of three species: M. ethiopica, M. luci and M. inornata Lordello, 1956) by PCR reaction. The reaction with primers Me309F and Me549R resulted in a 241 bp long amplicon specific to the M. ethiopica group. Finally, M. luci was identified by the PCR reaction. The reaction with the Mlf and Mlr primers resulted in a band of approximately 770 bp.

Table 3.
Primers used for Meloidogyne spp. identification.
2.5. Culturing of Meloidogyne Populations
Infected plant material with soil from three unidentified samples collected in 2021 was used for a bioassay with tomato seedlings in 2022. Portions of the plant material with soil were transferred to one-litre pots in which a susceptible tomato variety S. lycopersicum L. “Marathon” (Superior Company, Serbia) was planted as a host plant. The plants were grown in a greenhouse over a period of 110 days and watered as necessary. Subsequently, the roots of the plant were analysed for the presence of galls. The roots were dissected with a stereomicroscope and females and egg masses were extracted and stored at −20 °C for detailed molecular analysis to identify the RKN species. Morphological analysis was performed on fresh material.
2.6. Statistical and Meloidogyne spp. Community Analyses
To evaluate the differences in the frequency and abundance of Meloidogyne across regions and districts of Serbia, as well as the differences in the frequency of individual species of Meloidogyne, multiple proportion tests were performed using SPSS statistical software SPSS Statistics 30 (https://www.ibm.com/spss)/https://www.ibm.com/products/spss-statistics (accessed on 8 January 2025). For one of the tests, only samples identified at the species level were included. Hypothesis tests were performed with a significance level of α = 0.05. The occurrence and frequency of root-knot nematodes at both the genus and species levels were calculated using the following formulae [37].
Occurrence of the genus or species = Number of samples with RKN infection × 100/Total number of samples analysed
Absolute frequency = Number of samples with species × 100/Number of samples collected
Relative frequency = Frequency of occurrence of the species × 100/Sum of the frequency of all Meloidogyne spp.
3. Results
3.1. Meloidogyne Detection
A total of 241 plant material samples were collected from greenhouses and fields in two regions of Serbia and analysed between 2021 and 2023. Meloidogyne spp. were detected in 57 samples, which corresponds to 23.7% of the total. Supplementary Material 2 (Table S5) includes data for each positive sample, detailing the host plant, district, gall index, primer pairs and identified RKN species for the period from 2021 to 2023.
3.2. Identification, Distribution and New Host Plant Records for Meloidogyne Species
The identification of Meloidogyne species, based on the analysis of perineal pattern morphology in combination with molecular methods, indicated that the most common species among the 57 RKN populations was M. incognita, followed by M. hapla, M. luci and M. arenaria. In the first year of the survey, two mixed Meloidogyne populations were detected (M. hapla and M. incognita and M. hapla and M. javanica). The analysis of perineal pattern morphology included the examination of the vulva/anus area, lateral lines, surrounding cuticle striae, tail terminus and phasmids [38]. Variability in the perineal pattern was observed among the identified species of the tropical RKN group (M. arenaria, M. incognita and M. luci). The perineal shape ranged from rounded to ovoid in M. arenaria and M. incognita, whereas it was oval to squarish in M. luci. Characteristic lateral lines were observed in M. arenaria, whereas they were absent or only weakly developed in M. incognita and M. luci (Figure 2).
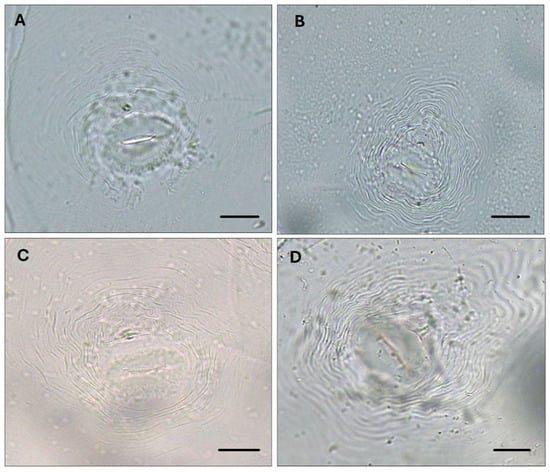
Figure 2.
Perineal patterns in Meloidogyne specimens: (A) M. hapla Chitwood, 1949; (B) M. incognita (Kofoid and White, 1919) Chitwood, 1949; (C) M. arenaria (Neal, 1889) Chitwood, 1949; (D) M. luci Carneiro et al., 2014. Bar = 20 μm.
Meloidogyne spp. were confirmed in 57 samples using PCR analysis. The primers Far/Rar produced a DNA fragment size of 420 bp specific to M. arenaria in 3 samples, while the primer set MI-F/MI-R yielded a 999 bp fragment specific to M. incognita in 39 samples. Multiplex PCR analysis with JMV1/JMV2/JMVhapla primers resulted in a 440 bp DNA fragment size specific to M. hapla in eight populations. In addition, M. luci was confirmed in five samples using the primer set Mlf/Mlr. In the first year of the survey (2021), three Meloidogyne populations could not be identified. In 2022, these populations were identified as M. luci following a bioassay conducted in a greenhouse, combined with additional morphological and molecular analyses. The first population of M. luci was collected in 2021 in a greenhouse in Northern Serbia (West Bačka district) on tomatoes, marking the first report of this species in Serbia. The second population, also identified as M. luci, was found on cucumbers at the same location. The third M. luci population was collected on cucumbers located in the Jablanica district. Morphological analysis of the perineal patterns initially suggested M. incognita but was later presumed to be M. luci based on a comparison with the originally described morphology of M. luci. The affiliation of the population to the tropical RKN group and the M. ethiopica group was determined using two PCR reactions. It was confirmed as M. luci through the detection of a species-specific 770 bp band and mtDNA sequence analyses. The sequence matched 100% with an unidentified Meloidogyne spp. from Serbia and 99.94% with sequences of M. luci populations from Slovenia, Greece and Iran. In the phylogenetic tree, all M. luci sequences, including the Meloidogyne sp. sequence (accession no. KM077449) from Serbia, are grouped into a single clade.
Meloidogyne spp. were detected in 11 out of 25 districts sampled in Serbia during the period between 2021 and 2023, with 5 districts located in Northern Serbia (Vojvodina Province) and 6 in Central Serbia. M. incognita was found in all districts where Meloidogyne spp. were detected: five in Northern Serbia (North Banat, South Banat, West Banat, South Banat, Srem) and six in Central Serbia (Mačva, Braničevo, Raška, Nišava, Jablanica, Pčinja). M. hapla was detected in six districts (North Banat, South Banat, South Bačka, Srem, Raška, Jablanica), M. arenaria in three districts (North Banat, South Banat, Braničevo) and M. luci in two districts (West Bačka, Jablanica). Two mixed Meloidogyne populations were detected: M. hapla and M. javanica in North Banat and Jablanica. Positive detections of Meloidogyne spp. were most frequent in Jablanica district and least frequent in Pčinja district. The districts with the highest species diversity were North Banat, Jablanica, West Bačka and South Banat, each with three species, followed by Srem, Braničevo and Raška, each with two species. Only one species was identified in South Bačka, Nišava, Mačva and Pčinja (Figure 3).
Statistical analysis confirmed that the abundance of Meloidogyne is not equal in all districts (p-value = 0.026 < 0.05). A more detailed statistical analysis revealed that the abundance of Meloidogyne in Pčinja district is significantly different (p-value < 0.05) from the West Bačka and South Bačka districts. Furthermore, Jablanica district significantly differs from the districts of Mačva, Braničevo and Raška. Statistically significant differences (p-value < 0.05) were observed in the abundance of Meloidogyne between the Jablanica and Pčinja districts.
In this survey, some host plants were analysed for the presence of RKN species for the first time in Serbia. Meloidogyne species were found parasitising eight different host plants, including three in fields and five in greenhouses. In the fields, M. incognita was detected on potatoes, while M. arenaria was found on carrots and parsley in two districts with sandy soils (Northern Banat and Braničevo). In greenhouses, Meloidogyne spp. were most frequently found on peppers, followed by cucumbers, tomatoes, calla and melons. The species with the highest number of host plants identified during this study was M. incognita (five), followed by M. hapla (four), M. luci (two) and M. arenaria (two). To our knowledge, this is the first report of Meloidogyne spp. infestation of parsley in fields and infestation of melons in greenhouses in the country (Table 4). However, Meloidogyne species were not detected on roses, black nightshade, cabbage, horseradish or gladiolus in the fields.
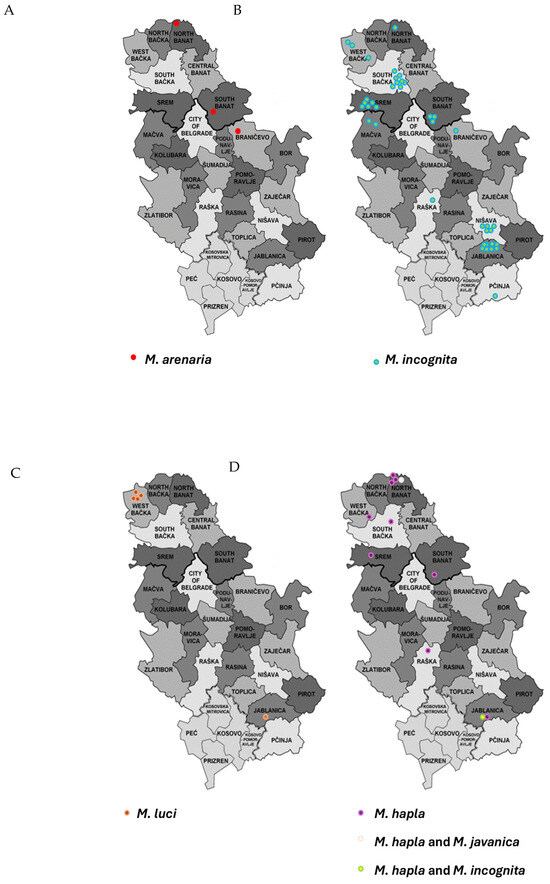
Figure 3.
Distribution of Meloidogyne sp. in Serbia. (A) M. arenaria, (B) M. incognita, (C) M. luci, (D) M. hapla and mixed populations. Each dot on the map represents a single population.
The map is from https://en.wikipedia.org/wiki/Administrative_districts_of_Serbia (accessed on 8 January 2025).

Table 4.
Districts and host plants associated with Meloidogyne species identified in Serbia in the period 2021–2023.
Table 4.
Districts and host plants associated with Meloidogyne species identified in Serbia in the period 2021–2023.
| Species | Region | District | Host Plant | Cultivation Conditions |
|---|---|---|---|---|
| M. arenaria | Northern Serbia | South Banat | Calla (C. palustris) | Greenhouse |
| North Banat | Carrot (D. carota subsp. sativus) | Field | ||
| Central Serbia | Braničevo | |||
| M. incognita | Northern Serbia | North Banat | Potato (S. tuberosum) | Field |
| South Banat | Calla (C. palustris) Pepper (C. annuum) Tomato (S. lycopersicum) Cucumber (C. sativus) | Greenhouse | ||
| West Bačka | ||||
| South Bačka | ||||
| Srem | ||||
| Central Serbia | Mačva | |||
| Braničevo | ||||
| Raška | ||||
| Nišava | ||||
| Jablanica | ||||
| Pčinja | ||||
| M. luci | Northern Serbia | West Bačka | Tomato (S. lycopersicum) Cucumber (C. sativus) | Greenhouse |
| Central Serbia | Jablanica | |||
| M. hapla | Northern Serbia | North Banat | Carrot (D. carota subsp. sativus) Parsley (P. crispum) | Field |
| South Banat | Pepper (C. annuum) Tomato (S. lycopersicum) Melon (C. melo) | Greenhouse | ||
| South Bačka | ||||
| Srem | ||||
| Central Serbia | Raška | |||
| Jablanica | ||||
| M. hapla and M. javanica | Northern Serbia | North Banat | Carrot (D. carota subsp. sativus) | Field |
| M. hapla and M. incognita | Central Serbia | Jablanica | Tomato (S. lycopersicum) | Greenhouse |
3.3. Meloidogyne spp. Community Analyses
Of the 57 samples infested with Meloidogyne spp. from the survey areas (Northern Serbia and Central Serbia), 3 samples were infested with M. arenaria, 39 with M. incognita, 5 with M. luci, 8 with M. hapla and 2 with Meloidogyne mixed populations (one with M. hapla and M. javanica and one with M. hapla and M. incognita) (Table 5). M. incognita was the most abundant species in both regions infested by Meloidogyne spp. The occurrence of M. incognita was 16.2%, followed by M. hapla (3.3%), M. luci (2.1%), M. arenaria (1.4%) and the two mixed populations (M. hapla and M. javanica and M. hapla and M. incognita), each with the same percentage (0.4%). M. incognita was the most prevalent species in both survey regions, with absolute and relative frequencies of 68.4% and 70.9%, respectively. This was followed by M. hapla (14.0% and 14.5%), M. luci (8.8% and 9.1%) and M. arenaria (5.3% and 5.5%), as observed in the absolute and relative frequency values, respectively. The occurrence of Meloidogyne spp. was higher in Northern Serbia (28.1%) compared to Central Serbia (19.2%). Additionally, the percentage incidence of M. arenaria, M. incognita, M. luci and M. hapla was higher in Northern Serbia than in Central Serbia. However, the observed differences were not substantial. Comparing the absolute and relative frequencies of all identified species in this survey, only the values for M. incognita were higher in Central Serbia (73.9% and 81.0%) compared to Northern Serbia (64.7% and 64.7%).

Table 5.
Community analyses of Meloidogyne spp. in surveyed areas of Serbia.
Statistical analysis of the different Meloidogyne species showed that the presence of M. incognita varied significantly (p-value = 0.000 < 0.05) compared to the rest of the species (M. arenaria, M. luci and M. hapla), indicating its dominance in Serbia. In contrast, the less frequent species M. arenaria did not differ significantly from M. luci or M. hapla (p-value > 0.05).
4. Discussion
This study represents a detailed and continuous survey of the occurrence of RKNs in Serbia, conducted as part of the official survey of quarantine RKN species from 2021 to 2023. The survey covered 25 districts across two regions of Serbia (Northern and Central Serbia). The results revealed that out of a total of 241 samples of vegetable and ornamental plant materials collected from greenhouses and fields, 57 samples were infested with Meloidogyne spp. Five RKN species (M. arenaria, M. incognita, M. luci, M. javanica and M. hapla) were identified in this survey out of seven species previously detected in Serbia. To date, 25 species of root-knot nematodes have been recorded in Europe [39,40]. All species identified in this study have already been recorded in other countries in the region as well as in the countries of the former Yugoslavia. Our findings confirm previous reports on the occurrence of M. arenaria, M. incognita and M. hapla in six districts (North Banat, South Banat, Braničevo, Nišava, Jablanica and Pčinja) and their incidence in countries with a similar continental climate characterised by cold winters and hot summers, such as Bulgaria [41], Romania [42] and Hungary [43].
During this survey, the EPPO A2 quarantine species M. luci was detected for the first time in Serbia, in two districts (West Bačka and Jablanica), on tomato and cucumber. This species, belonging to the tropical RKN group, was first identified in Europe in 2003, in Slovenia, on tomato roots in a greenhouse in Dornberk [44], initially classified as M. ethiopica. Subsequently, M. ethiopica was later recorded in other countries within the EPPO region, including Greece [45], Italy [46] and Turkey [47]. In 2014, M. luci was described as a new species by Carneiro et al. [48], revealing its close relationship with M. ethiopica. The description of this sibling species prompted the re-evaluation of populations previously identified as M. ethiopica [49]. Research demonstrated that all examined populations of M. ethiopica from Slovenia, Italy, Greece and Turkey were, in fact, M. luci [50]. In addition, this species was later detected in Portugal, both on the mainland and in Azores, parasitising potato crops [51,52]. A comparison of the mtDNA sequence of M. luci obtained in this study with publicly available sequences revealed an identical match with a Meloidogyne population from Serbia that could not be identified at the species level in 2014. This suggests that M. luci may have been present in Serbia for at least a decade but remained unidentified due to limited biological material and the unavailability of species-specific PCR for M. luci at that time. It is also possible that M. luci is more widespread than currently recognised, as its detection is not part of regular phytosanitary surveys in many countries.
Species from the tropical RKN group cause significantly greater damage and yield losses in tropical than in temperate regions due to favourable environmental conditions. However, climate change seems to be influencing field distribution and the impact of these species in temperate regions of south-eastern Europe, including Serbia. Findings from a recent Euphresco MeloTrop project conducted between 2017 and 2020 on global warming and the distribution of tropical RKN species revealed 107 localities of RKN in France, Portugal, Serbia and Slovenia. The study showed that M. incognita was the most frequent species, followed by M. arenaria, M. javanica, M. hispanica, M. luci and M. enterolobii. [53]. In a separate RKN survey conducted in Portugal between 2017 and 2022, M. incognita and M. javanica were the most prevalent species, followed by M. arenaria and M. hapla. Rare species such as M. enterolobii, M. hispanica, M. luci and M. naasi were also detected. Climate change, with rising air temperatures and increased moisture, may lead to higher rates of RKN infection, development and reproduction, ultimately altering their geographic distribution and abundance [54]. Our results showed the presence of species from the tropical RKN group, including M. incognita, M. arenaria and one mixed population of M. hapla and M. javanica. The significant damage caused by these species in the field seems to be related to the sandy soil prevalent in the North Banat district. While these species are generally native to tropical and subtropical regions, they can also survive in fields with Mediterranean and continental European climates. Notably, their infectivity is maintained even after exposure to sub-freezing temperatures during winter, as observed with M. luci in Slovenia [55]. Global warming has resulted in the migration of tropical RKN species into new field environments and the emergence of previously undocumented RKN species in Europe. Meloidogyne infestations disrupt water and nutrient intake from the soil, making crops more vulnerable to increased temperatures and drought. Additionally, the increased occurrence of heat waves in southern Europe threatens the effectiveness of widely used host plant resistances, including the Mi-1 gene, which is the most important resistance gene against RKN in tomatoes. The prolonged use of a limited number of resistant plant varieties has facilitated the emergence of virulent RKN populations in fields across southern Europe. Current cultivars are insufficient to prevent the further spread of RKN, emphasising the urgent need for efficient and sustainable solutions to mitigate RKN-related crop losses. Addressing this issue will be a key focus of the NEM-EMERGE project, funded by the European Union [56]. Our findings confirmed the presence of tropical RKN species in the field and the occurrence of a newly identified species (M. luci) during this survey.
Meloidogyne spp. are among the most widespread nematodes and pose a threat to the production of a wide range of crops worldwide. These pathogens can be transmitted through infected plants, soil, running water and agricultural machinery. Moreover, controlling and eradicating RKN, particularly mixed species populations, is extremely challenging. Consequently, the primary aim is to minimise crop damage by keeping nematode densities as low as possible. Efficient detection and accurate identification are the first crucial steps in RKN management, but they present their own set of challenges. Morphological identification based on the characteristics of females and juveniles is unreliable due to interspecies variability and overlapping traits among different species. For instance, several RKN species could be, and likely have been, misidentified as M. incognita based on M. incognita-type perineal patterns, which are present in several RKN species. Biochemical methods, such as isozyme pattern analysis, offer a reliable alternative; however, they require bioassays to obtain females at a specific developmental stage, which can be time-consuming [57]. For this reason, PCR analysis is a more practical method for routine and rapid diagnosis of RKN species. Historically, analysis of perineal morphology was the only method applied to identify RKNs in Serbia. To our knowledge, this survey was the first application of the PCR method for RKN species identification in Serbia. Using species-specific primers, we identified M. incognita, M. arenaria and M. hapla as well as M. luci through DNA amplification from egg masses or females. Additionally, the identity of M. luci was confirmed through sequencing.
Various control strategies can be employed against RKNs, such as chemical nematicides, resistant cultivars and cultural practises. Among chemical nematicides, carbamates and organophosphates have been the most commonly used. However, some active ingredients have been banned or regulated due to their harmful effects on human health and the environment. These substances have been partially replaced by newer chemicals, such as fluopyram and biological nematicides based on fungal and bacterial agents [58]. Host resistance has proven effective against RKNs, but resistant cultivars are not available for all crops and markets. Furthermore, certain RKN species are not controlled by Mi gene-based resistance, and in some cases, populations of RKN species that were initially controlled by Mi gene-based resistance have evolved virulent populations capable of overcoming this resistance [59].
Current control measures applied in Serbia against RKNs focus on agrotechnical measures in combination with the use of pesticides containing the active ingredient fluopyram, such as Velum® Prime, Bayer (registered for use from 2 August 2016 to 31 January 2025). This approach has proven effective in reducing Meloidogyne populations, as demonstrated in Slovenia [60]. In cases of infestation by quarantine RKN species, strict control measures must be implemented upon nematode detection. These measures include restrictions on plant material movement and interruption of susceptible host varieties until nematode densities fall below the detection threshold. These measures could also be helpful in the Western Bačka and Jablanica districts, where M. luci was detected, to prevent its further spread to other regions. The national surveillance programme for quarantine RKNs in Serbia continued in 2024 and will extend into 2025. The findings of this study on the occurrence and distribution of RKNs will assist decision-making bodies, phytosanitary inspectors, farmers, scientists, the breeding sector and plant protection companies in implementing effective plant protection, quarantine and monitoring measures to prevent the further spread of these pests.
5. Conclusions
This study shows that Meloidogyne species pose a significant threat to agricultural production and confirms their high occurrence and widespread distribution in Serbia. Meloidogyne infestations were identified on eight different plant hosts, and to our knowledge, this is the first report of Meloidogyne parasitising parsley in the fields and melons in the greenhouses of Serbia. This survey showed that M. incognita is currently the predominant RKN species in the country. Additionally, M. luci, an EPPO A2 quarantine species, was also discovered locally for the first time, with limited occurrences. The detection of new Meloidogyne species in various districts and new plant host records indicate that these infestations are likely linked to trade activity. Climate change and rising temperatures could significantly increase the spread and damage caused by tropical RKN species to various agricultural crops in temperate regions of south-eastern Europe, including Serbia, in future. This study implemented routine and rapid identification of Meloidogyne species, which is essential for developing and applying appropriate control strategies in Serbia. The findings presented here relative to the Meloidogyne species identified locally will assist in the development and establishment of efficient and sustainable measures and surveys aiming to prevent the introduction and spread of these pests across Europe.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15020372/s1: Supplementary Material 1. Supplementary Material Table S1: Amplification conditions with different primer pairs; Supplementary Material Table S2: Amplification conditions for identification of Meloidogyne luci with primers Mlf/Mlr; Supplementary Material Table S3: Amplification conditions for identification of Meloidogyne ethiopica group with primers Me309/Me549R; Supplementary Material Table S4: Amplification conditions for identification of tropical root-knot nematodes group with primers C2F3/Mt575. Supplementary Material 2. Supplementary Material Table S5: Sample data and PCR results for samples where Meloidogyne spp. was detected.
Author Contributions
Conceptualization, J.B and B.G.S.; methodology, J.B., I.L., S.Š., M.T., N.S. and B.G.S.; software, J.B.; validation, J.B. and B.G.S.; formal analysis, J.B. and B.G.S.; investigation, J.B., I.L., S.Š., M.T., N.S. and B.G.S.; resources, J.B.; data curation, J.B.; writing—original draft preparation, J.B and B.G.S.; writing—review and editing, J.B, I.L. and B.G.S.; visualization, J.B.; supervision, J.B. and B.G.S.; project administration, J.B.; funding acquisition, J.B. and S.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Serbian Plant Protection Directorate of MAFWM, as part of the Measures in Plant Health in the period 2021–2023 Programme and the Serbian Ministry of Education, Technological Development and Innovation, grant number 451-03-66/2024-03/200010, Slovenian Research And Innovation Agency—ARIS (P4-0072, P4-0431) and Republic of Slovenia, Ministry of Agriculture, Forestry and Food—MKGP (C2337).
Data Availability Statement
The data presented in this study are openly available in [https://plantarum.izbis.bg.ac.rs/browse?type=author&value=Ba%C4%8Di%C4%87%2C+Jasmina] (accessed on 20 January 2025).
Acknowledgments
The authors are grateful to colleagues Jelena Kušić-Tišma from the Institute of Molecular Genetics and Genetic Engineering and Ivica Dimkić and Tamara Janakiev from the Faculty of Biology, University of Belgrade, who performed part of the PCR testing presented in this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne Species—A Diverse Group of Novel and Important Plant Parasites. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; Oxon (CABI): Wallingford, UK, 2009; pp. 1–17. [Google Scholar]
- Blok, V.C.; Jones, J.T.; Phillips, M.S.; Trudgill, D.L. Parasitism genes and host range disparities in biotrophic nematodes: The conundrum of polyphagy versus specialisation. BioEssays 2008, 30, 249–259. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Palomares-Rius, J.E.; Castillo, P. Chapter 1 Taxonomic history. In Systematics of Root-Knot Nematodes (Nematoda: Meloidogynidae) Nematology Monographs and Perspectives; Hunt, D.J., Perry, R.N., Eds.; Brill: Leiden, The Netherlands, 2021; Volume 14, p. 857. [Google Scholar]
- Karssen, G. Chapter 5 Revision of the European Root-Knot Nematodes III. On Mono-and Dicotyledons. In The Plant-Parasitic Nematode Genus Meloidogyne Goldi, 1892 (Tylenchida) in Europe; Brill: Leiden, The Netherlands, 2002; p. 91. [Google Scholar]
- European and Mediterranean Plant Protection Organization. EPPO A1 List of Pests Recommended for Regulation as Quarantine Pests. Version 2023-09. 2023. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list (accessed on 8 January 2025).
- European and Mediterranean Plant Protection Organization. EPPO A2 List of Pests Recommended for Regulation as Quarantine Pests. Version 2023-09. 2023. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed on 8 January 2025).
- Anonymous. Study on the State of Agriculture in Five Applicant Country, Country Report: Serbia. ACOTRASS-Consortium. 2006. Available online: https://agriculture.ec.europa.eu/system/files/2020-02/ext-study-applicant-serbia_2006_en_0.pdf (accessed on 8 January 2025).
- Anonymous. Brochure: 2023 Census of Agriculture, Statistical Office of the Republic of Serbia 2024. Available online: https://popispoljoprivrede.stat.gov.rs/media/31091/brosura-engleski-sa-koricom.pdf (accessed on 8 January 2025).
- Grujičić, G. Efikasnost nekih nematocida kod suzbijanja korenove nematode u staklarama. Zašt. Bilja 1959, 55, 63–68. (In Serbian) [Google Scholar]
- Grujičić, G. Prilog proučavanju ekologije Meloidogyne spp. Zašt. Bilja 1959, 54, 69–75. (In Serbian) [Google Scholar]
- Jovičić, D.; Grujičić, G. Root-knot nematodes (Meloidogyne spp.) in the SR Serbia [Yugoslavia]. Zašt. Bilja 1986, 37, 31–40, (In Serbian with English abstract). [Google Scholar]
- Grujičić, G. Korenova nematoda (Meloidogyne naasi Franklin) u Srbiji. Prethodno saopštenje. Zašt. Bilja 1967, 18, 193–197. (In Serbian) [Google Scholar]
- Grujičić, G.; Borić, B. Korenova nematoda (Meloidogyne incognita Chitwood) na suncokretu u Vojvodini. Zašt. Bilja 1971, 112, 143–145. (In Serbian) [Google Scholar]
- Grujičić, G.; Jovičić, D. Harmful nematofauna in tobacco fields in Serbia. Zašt. Bilja 1988, 39, 149–157, (In Serbian with English abstract). [Google Scholar]
- Bačić, J.; Gerič Stare, B.; Strajnar, P.; Širca, S.; Urek, G. First report of a highly damaged potato crop from Serbia caused by Meloidogyne incognita. Plant Dis. 2016, 100, 1021–1022. [Google Scholar] [CrossRef]
- Bačić, J.; Bosnić, D.; Samardžić, J.; Avdalović, R.; Mickovski Stefanović, V.; Tišma Kušić, J. Occurrence of root-knot nematode Meloidogyne arenaria in the potato field in Serbia. Ratar. Povrt. 2022, 59, 51–55. [Google Scholar] [CrossRef]
- Milovanović, N.; Petrović, V.; Oro, V. Status of Root-Knot Nematodes in Serbia. Bilj. Lek. 2017, 45, 415–424, (In Serbian with English abstract). [Google Scholar]
- Oro, V. The Root-Knot Nematodes on Root Vegetables. Bilj. Lek. 2020, 48, 636–645. [Google Scholar] [CrossRef]
- Bačić, J.; Kušić, J.; Strajnar, P.; Gerič Stare, B.; Širca, S. First Report of Meloidogyne arenaria on Calla (Zantedeschia aethiopica) in Serbia. In Proceedings of the Abstract Book of 7th International Congress of Nematology, Antibes Juan Les Pins, France, 1–6 May 2022; p. 527. [Google Scholar]
- Bačić, J.; Pavlović, M.; Kušić-Tišma, J.; Širca, S.; Theuerschuh, M.; Gerič Stare, B. First Report of the Root-Knot Nematode Meloidogyne luci on Tomato in Serbia. Plant Dis. 2023, 107, 2554. [Google Scholar] [CrossRef] [PubMed]
- Rehak Biondić, T.; Puškarić, J.; Gerič Stare, B.; Brmež, M. The status of root-knot nematodes of the Meloidogyne Genus in Croatia, with a special reference to the quarantine species. Poljoprivreda 2023, 29, 27–34. [Google Scholar] [CrossRef]
- Klindić, O. Vrste nematoda prouzrokovača korenovih guka–Meloidogyne spp. na području Južne Hercegovine. Zašt. Bilja 1968, 102, 411–430. (In Serbian) [Google Scholar]
- Nježić, B.; Mitrović, B.; Macanović, I.; Grujić, N.; Waeyenberge, L. Occurrence of the Northern Root-Knot Nematode in Republic of Srpska, Bosnia and Herzegovina. In Proceedings of the 31st International Symposium of the European Society of Nematologists, Adana, Turkey, 23–27 September 2012; p. 199. [Google Scholar]
- Krnjaić, D.J. Fitopatogene nematode u staklarama SR Makedonije. Zašt. Bilja 1977, 142, 429–433. (In Serbian) [Google Scholar]
- Dautova, M.; Gommers, F.J. Meloidogyne spp. In Macedonia: Distribution and virulence for Mi resistance in tomato. Nem. Medit. 2000, 28, 121–128. [Google Scholar]
- Širca, S.; Urek, G.; Karssen, G. The incidence of the root-knot nematode Meloidogyne incognita and Meloidogyne hapla in Slovenia. Acta Agric. Slov. 2004, 83, 15–22. [Google Scholar] [CrossRef]
- Širca, S.; Urek, G. Dissemination of the root knot nematodes, Meloidogyne spp. in Slovenia. Dissertationes 2004, 46, 161–170, ISNN 0352-5090. Available online: https://plus.cobiss.net/cobiss/ul/sl/bib/ul/22863917 (accessed on 26 January 2025).
- Pajović, I.; Bird, G.W.; Širca, S.; Urek, G.; Rajković, D.; Barši, L.; Radivojević, M. Meloidogyne (Nematoda: Heteroderidae) detected in greenhouses in Zeta-Bjelopavlići valley. Nat. Mont. 2010, 9, 913–925. [Google Scholar]
- Zeck, W.M. A rating scheme for field evaluation of root-knot infestations. Pflanzenschutz-Nachrichten Bayer 1971, 24, 141–144. [Google Scholar]
- Hartman, K.M.; Sasser, J.N. Identification of Meloidogyne Species on the Basis of Different Host Test and Perineal Pattern Morphology. In An Advanced Treatise on Meloidogyne: Methodology; Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; Department of Plant Pathology, North Carolina State University: Raleigh, NC, USA, 1985; Volume 2, pp. 69–77. [Google Scholar]
- Karssen, G. The Plant-Parasitic Nematode Genus Meloidogyne Goldi, 1892 (Tylenchida) in Europe; Brill: Leiden, The Netherlands, 2002; p. 160. [Google Scholar]
- Adam, M.A.M.; Phillips, M.S.; Blok, V.C. Molecular diagnostic key for identification for single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.). Plant Pathol. 2007, 56, 190–197. [Google Scholar] [CrossRef]
- Wishart, J.; Phillips, M.S.; Blok, V.C. Ribosomal intergenic spacer: A polymerase chain reaction diagnostic for Meloidogyne chitwoodi, M. fallax, and M. hapla. Phytopathology 2002, 92, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Powers, T.O.; Harris, T.S. A polymerase chain reaction method for identification of five mayor Meloidogyne species. J. Nematol. 1993, 25, 1–6. [Google Scholar] [PubMed]
- Gerič Stare, B.; Aydinli, G.; Devran, Z.; Mennan, S.; Strajnar, P.; Širca, S. Recognition of species belonging to Meloidogyne ethiopica group and development of a diagnostic method for its detection. Eur. J. Plant Pathol. 2019, 154, 621–633. [Google Scholar] [CrossRef]
- Maleita, C.; Cardoso, M.S.J.; Rusinque, L.; Estevez, I.; Abrantes, I. Species-Specific Molecular Detection of the Root-Knot Nematode Meloidogyne luci. Biology 2021, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Norton, D.C. Ecology of Plant-Parasitic Nematodes; Wiley-Interscience: New York, NY, USA, 1978; p. 268. [Google Scholar]
- Karssen, G.; Wesemael, W.; Moens, M. Root-knot nematodes. In Plant Nematology, 2nd ed.; Perry, R.N., Moens, M., Eds.; Oxon (CABI): Wallingford, UK, 2013; pp. 73–108. [Google Scholar]
- Wesemael, W.M.L.; Viaene, N.; Moens, M. Root-knot nematodes (Meloidogyne spp.) in Europe. Nematology 2011, 13, 3–16. [Google Scholar] [CrossRef]
- Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; Liébanas, G.; Rapoport, H.F.; Castillo, P.; Palomares-Rius, J. Diversity of root-knot nematodes of the genus Meloidogyne Göeldi, 1892 (Nematoda: Meloidogynidae) associated with olive plants and environmental cues regarding their distribution in southern Spain. PLoS ONE 2018, 13, e0198236. [Google Scholar] [CrossRef] [PubMed]
- Samaliev, H.Y.; Salkova, D.S.; Baycheva, O.T.; Zinovieva, S.V.; Udalova, Z.V. Investigations of the Root-Knot Nematodes of the Genus Meloidogyne (Göldi, 1887) on the Territories of Bulgaria and Russian Federation. Russ. J. Parasitol. 2018, 12, 94–98. [Google Scholar] [CrossRef]
- Boros, L.; Fanelli, E.; Sesan, E.T.; Dobrin, I.; Iacomi, B.; De Luka, F. Detection and characterization of root-knot nematodes (Meloidogyne spp.) associated with three host plants in Romania. Rom. Biotechnol. Lett. 2018, 23, 14097–14106. [Google Scholar] [CrossRef]
- Amin, A.W. Root-knot nematodes (Meloidogyne spp.) in Hungary. OEPP EPPO Bull. 1994, 24, 417–422. [Google Scholar] [CrossRef]
- Širca, S.; Urek, G.; Karssen, G. First report of the root-knot nematode Meloidogyne ethiopica on tomato in Slovenia. Plant Dis. 2004, 88, 680. [Google Scholar] [CrossRef] [PubMed]
- Conceição, I.L.; Tzortzakakis, E.A.; Gomes, P.; Abrantes, I.; Da Cunha, M.J. Detection of the root-knot nematode Meloidogyne ethiopica in Greece. Eur. J. Plant Pathol. 2012, 134, 451–457. [Google Scholar] [CrossRef]
- Maleita, C.M.; Simões, M.J.; Egas, C.; Curtis, R.H.C.; Abrantes, I.M.d.O. Biometrical, biochemical, and molecular diagnosis of Portuguese Meloidogyne hispanica isolates. Plant Dis. 2012, 96, 865–874. [Google Scholar] [CrossRef]
- Aydınlı, G.; Mennan, S.; Devran, Z.; Širca, S.; Urek, G. First report of the root-knot nematode Meloidogyne ethiopica on tomato and cucumber in Turkey. Plant Dis. 2013, 97, 1262. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.M.; Correa, V.R.; Almeida, M.R.A.; Gomes, A.C.M.; Demi, A.M.; Castagnone-Sereno, P.; Karssen, G. Meloidogyne luci n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitising different crops in Brazil, Chile and Iran. Nematology 2014, 16, 289–301. [Google Scholar] [CrossRef]
- Janssen, T.; Karssen, G.; Verhaeven, M.; Coyne, D.; Bert, W. Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype-based diagnostics and reveals evidence of recent reticulate evolution. Sci. Rep. 2016, 6, 22591. [Google Scholar] [CrossRef] [PubMed]
- Gerič Stare, B.; Strajnar, P.; Sušić, N.; Urek, G.; Širca, S. Reported populations of Meloidogyne ethiopica in Europe identified as Meloidogyne luci. Plant Dis. 2017, 101, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Maleita, C.; Esteves, I.; Cardoso, J.M.S.; Cunha, M.J.; Carneiro, M.D.G.; Abrantes, I. Meloidogyne luci, a new root-knot nematode parasitizing potato in Portugal. Plant Pathol. 2018, 67, 366–376. [Google Scholar] [CrossRef]
- Rusinque, L.; Nobrega, F.; Cordeiro, L.; Serra, C.; Inácio, M.L. First detection of Meloidogyne luci (Nematoda: Meloidogynidae) parasitizing potato in the Azores, Portugal. Plants 2021, 10, 99. [Google Scholar] [CrossRef]
- Širca, S.; Gerič Stare, B.; Strajnar, P.; Knapič, M.; Žibrat, U.; Folcher, L.; Ollivier, F.; Buisson, A.; Chappe, A.M.; Inacio, M.L.; et al. Global Warming and Distribution of Root-Knot Nematode Species of the Tropical Group (MeloTrop). Available online: https://drop.euphresco.net/data/bdc46db0-344b-4b5e-bcd4-e25888588ec3 (accessed on 8 January 2025).
- Rusinque, L.; Camacho, M.J.; Serra, C.; Nobrega, F.; Inacio, M.L. Root-knot nematode assessment: Species identification, distribution, and new host records in Portugal. Front Plant Sci. 2023, 14, 1230968. [Google Scholar] [CrossRef]
- Strajnar, P.; Širca, S.; Knapič, M.; Urek, G. Effect of Slovenian climatic conditions on the development and survival of the root-knot nematode Meloidogyne ethiopica. Eur. J. Plant. Pathol. 2010, 129, 81–88. [Google Scholar] [CrossRef]
- Anonymous; NEM-EMERGE. An Integrated Set of Novel Approaches to Counter the Emergence and Proliferation of Invasive and Virulent Soil Borne Nematodes. Available online: https://nem-emerge.eu/project/work-packages/ (accessed on 8 January 2025).
- Hunt, D.J.; Handoo, Z.A. Taxonomy Identification and Principal Species. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; Oxon (CABI): Wallingford, UK, 2009; pp. 55–97. [Google Scholar] [CrossRef]
- Sušič, N.; Žibrat, V.; Sinkovič, L.; Vončina, A.; Razinger, J.; Knapič, M.; Sedlar, A.; Širca, S.; Gerič Stare, B. From Genome to Field-Observation of the Multimodal Nematicidal and Plant Growth-Promoting Effects of Bacillus firmus I-1582 on Tomatoes using Hyperspectral Remote Sensing. Plants 2020, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.M. Understanding molecular plant-nematode interactions to develop alternative approaches for nematode control. Plants 2022, 11, 2141. [Google Scholar] [CrossRef]
- Širca, S.; Sušič, N.; Theuerechuh, M.; Gerič Stare, B. Successful Eradication the Root-Knot Nematode Meloidogyne luci. In Proceedings of the 16th Slovenian Conference on Plant Protection with International Participation, Bohinjska Bistrica, Slovenia, 5–6 March 2024; pp. 127–128. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).