Zero-Tillage Induces Reduced Bio-Efficacy Against Weed Species Amaranthus retroflexus L. Dependent on Atrazine Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Sampling
2.2. X-Ray CT Scanning and Image Processing
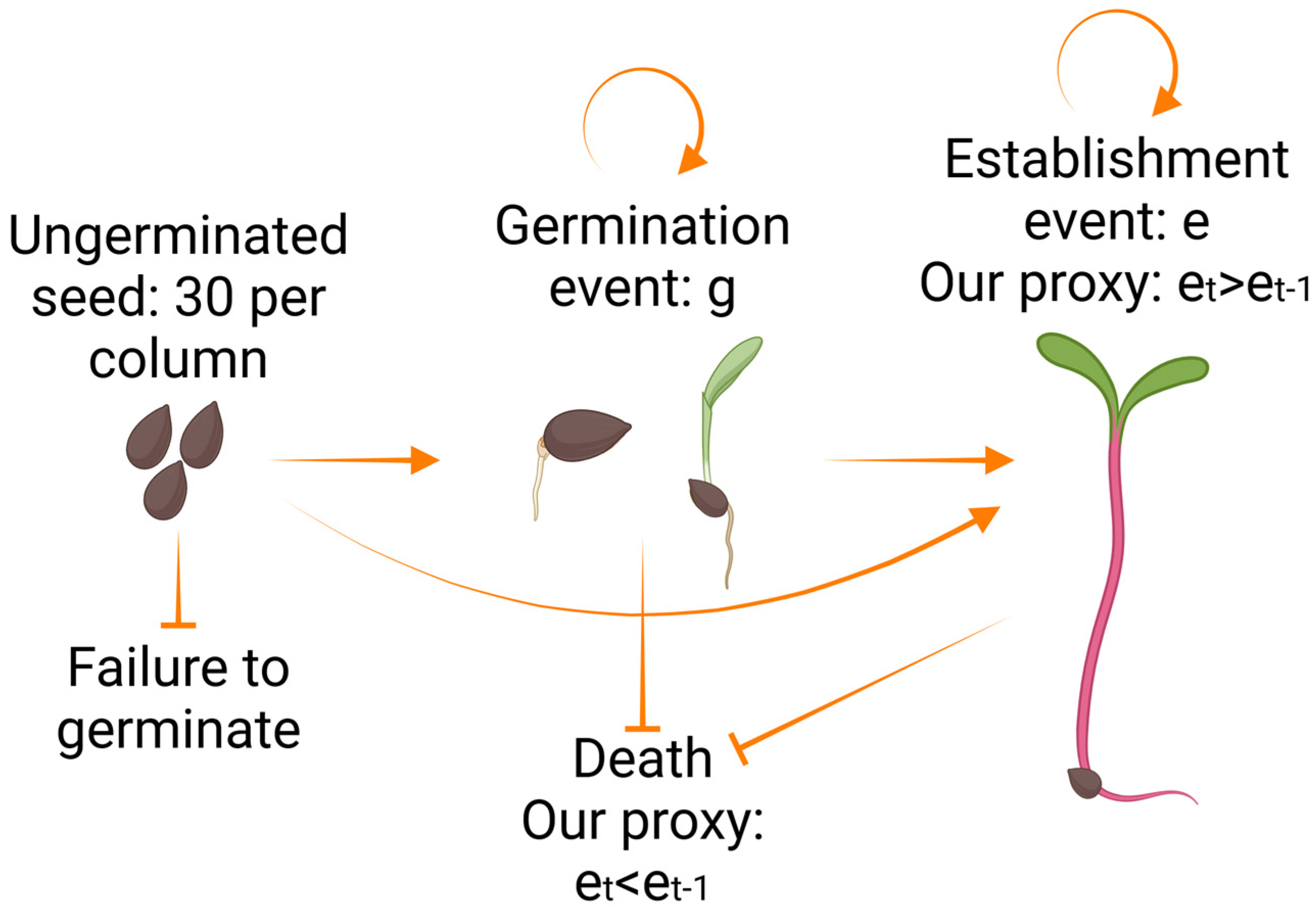
2.3. Bioefficacy Assessment
2.4. Basic Soil Properties
2.5. Gravimetric Water Content
2.6. Data Handling and Statistics
3. Results
3.1. Basic Soil Properties
3.2. Water Content over Time
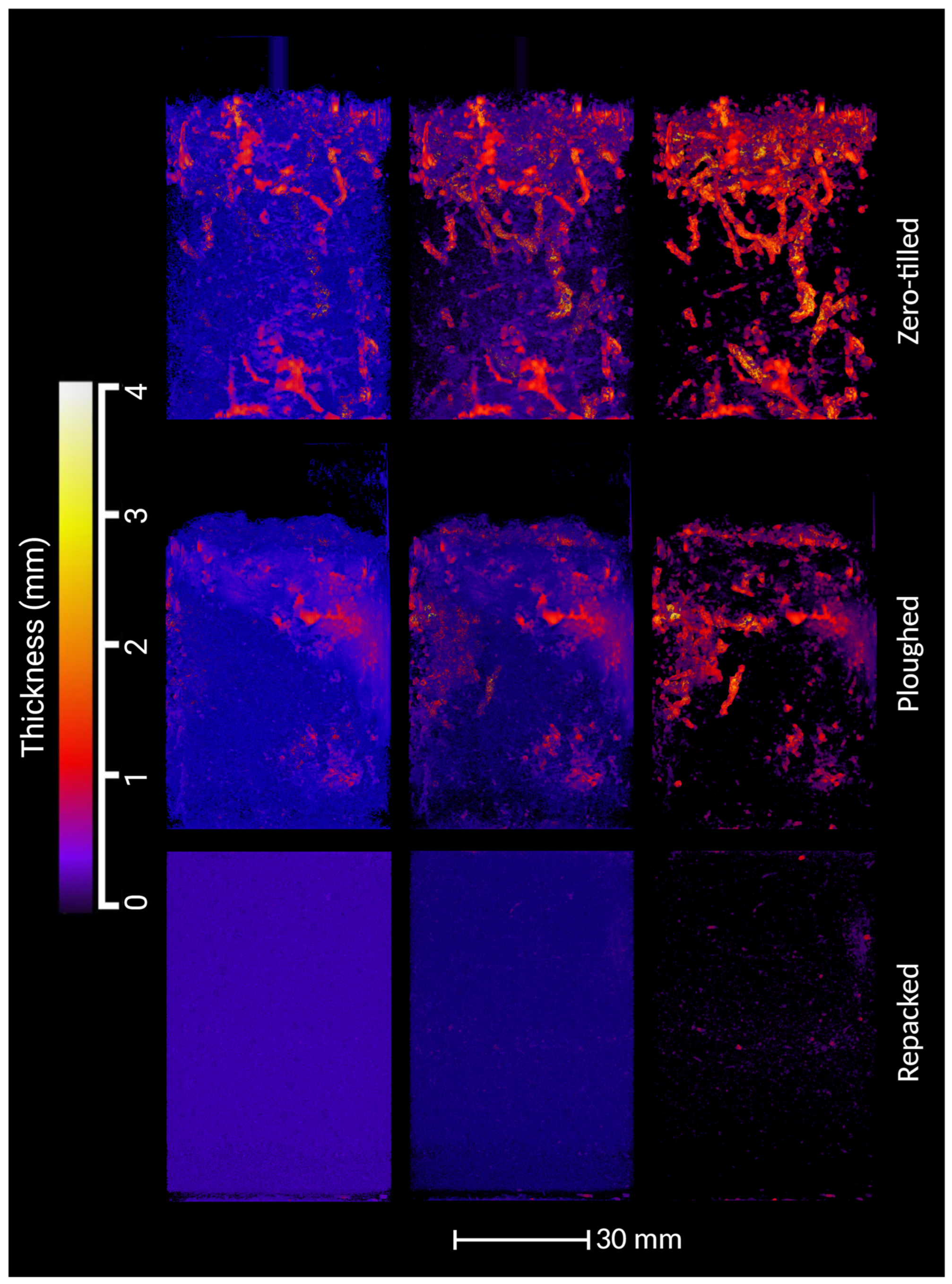
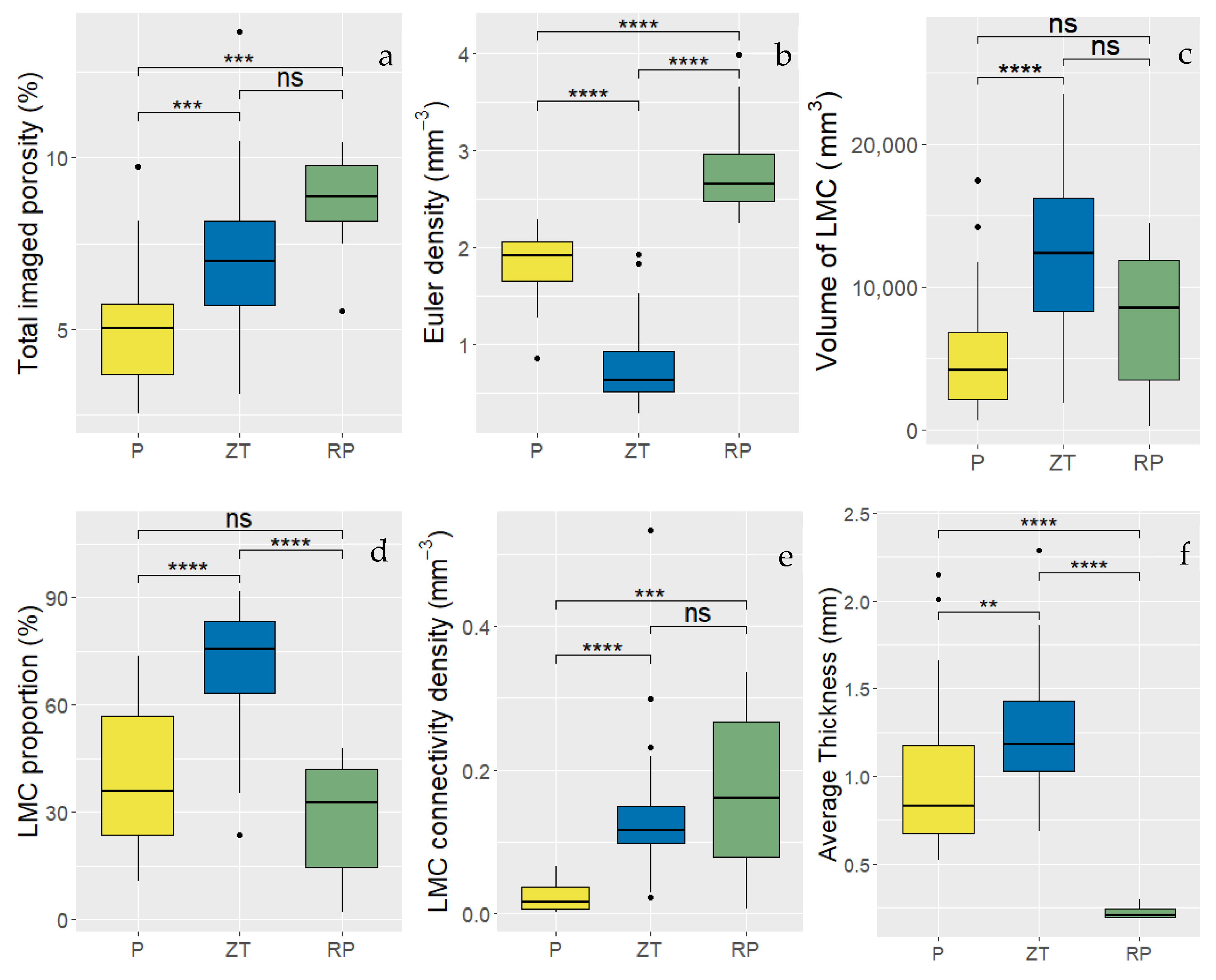
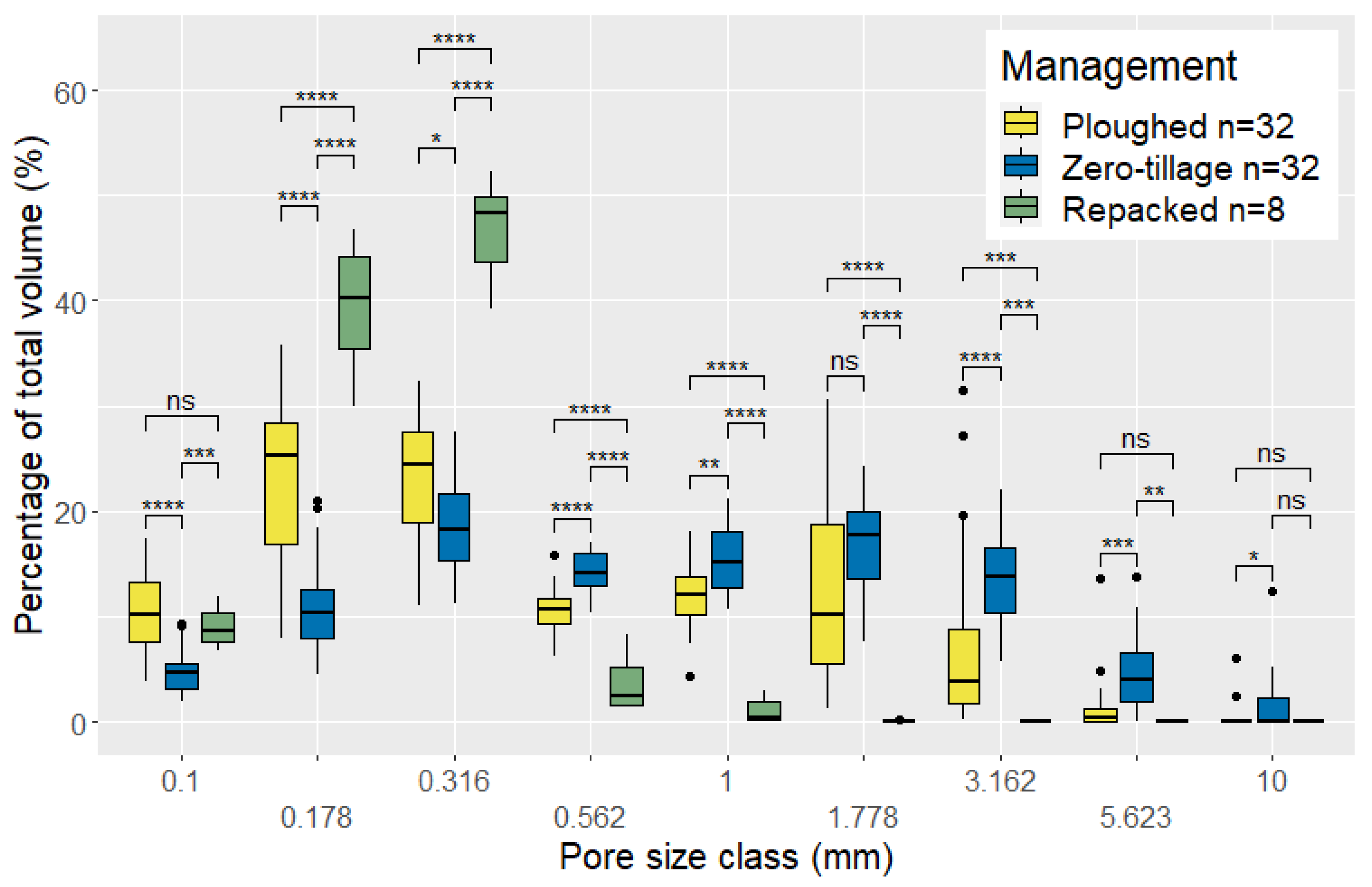
3.3. Structural Differences in Pore Networks Between Ploughed and ZT Soils
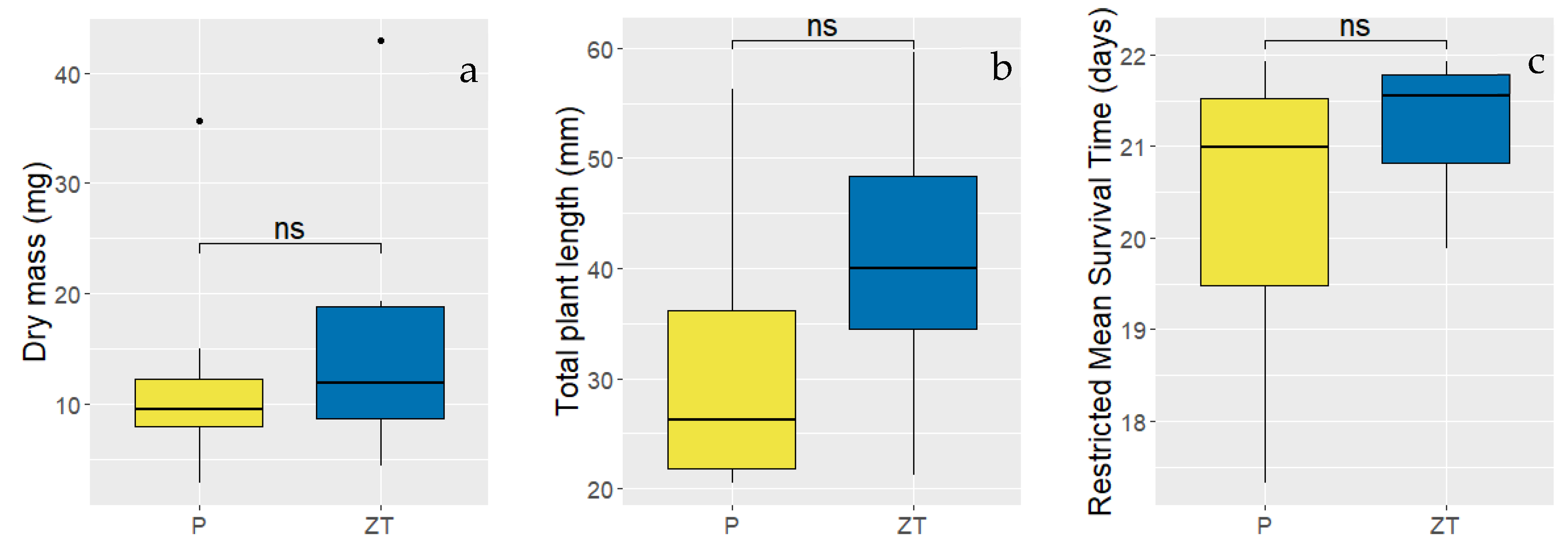
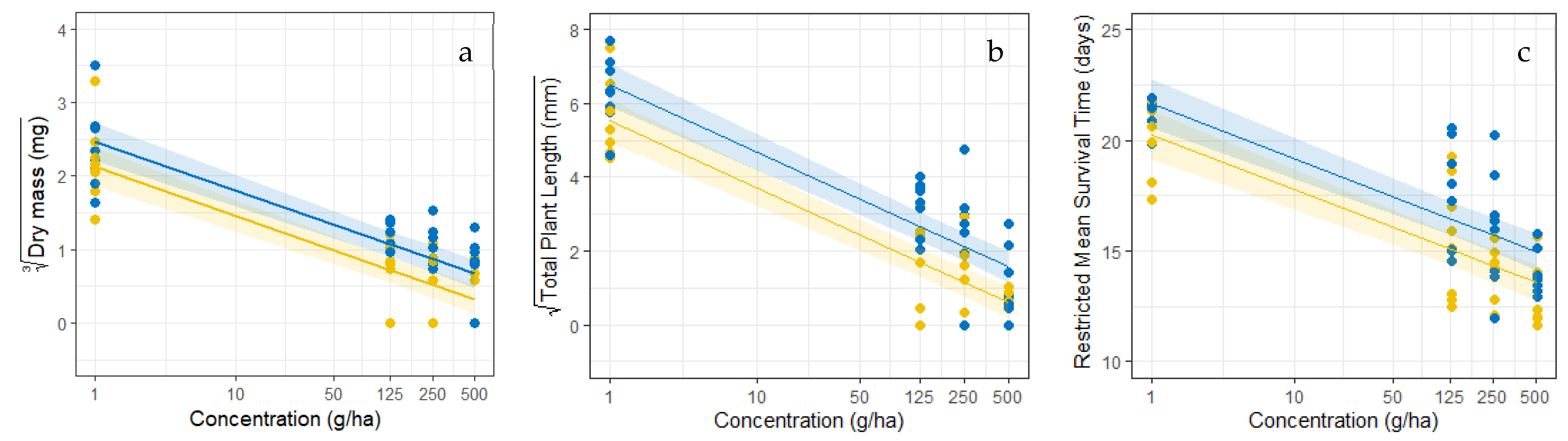
3.4. The Effect of Formulation Concentration on Bio-Efficacy
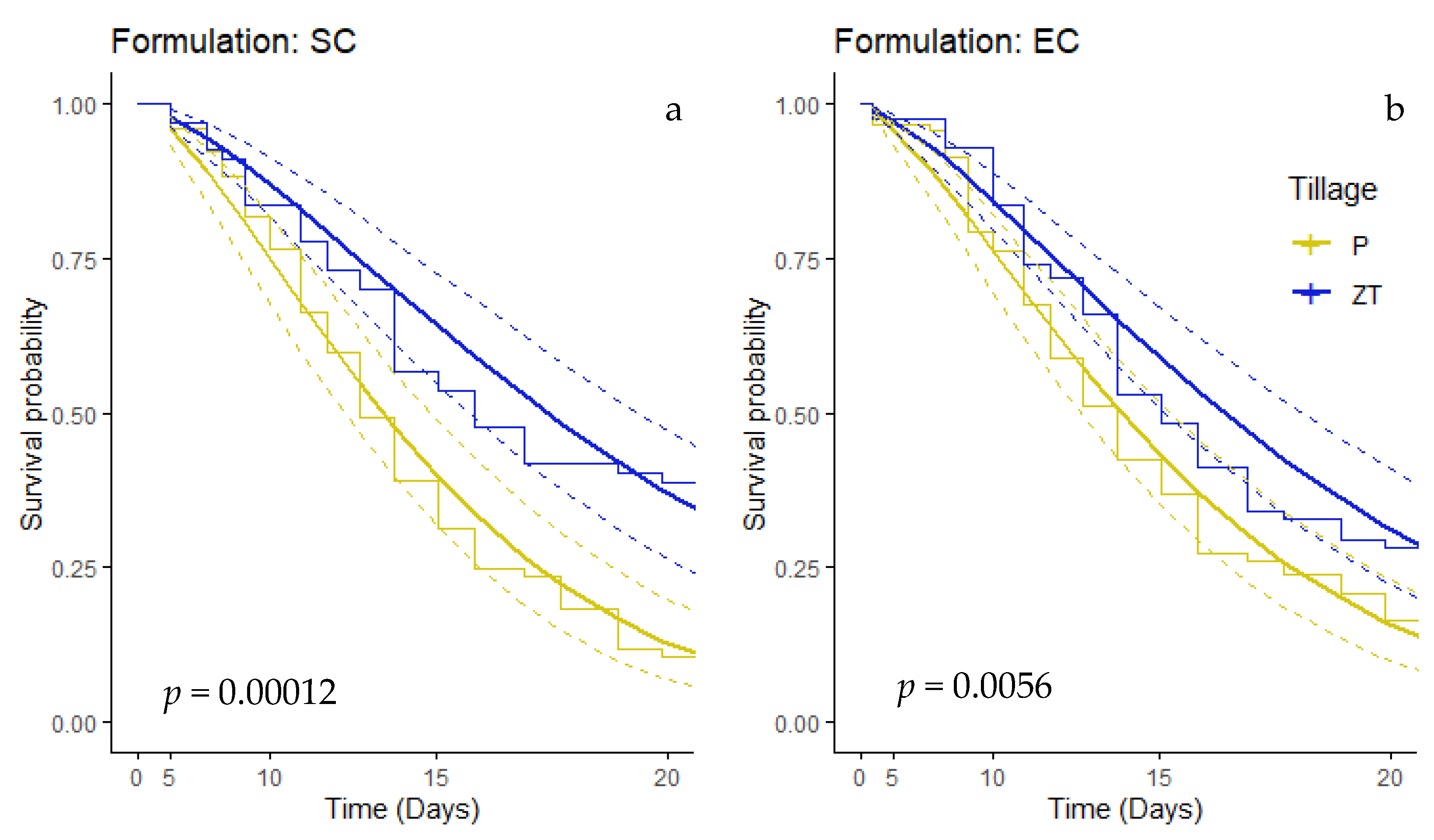
3.5. Effect of Tillage and Soil Structure on Formulation Bioefficacy
4. Discussion
4.1. Structural Differences Between Ploughed, ZT and Repacked Soil Columns
4.2. Herbicide Performance Under Experimental Conditions
4.3. Formulation Bioefficacy Dependent on Tillage
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DEFRA. Technical Annex: The Combined Environmental Land Management Offer, Agricultural Transition Plan 2021 to 2024. 2020. Available online: https://www.gov.uk/government/publications/agricultural-transition-plan-2021-to-2024 (accessed on 25 November 2024).
- Ogieriakhi, M.O.; Woodward, R.T. Understanding Why Farmers Adopt Soil Conservation Tillage: A Systematic Review. Soil Secur. 2022, 9, 100077. [Google Scholar] [CrossRef]
- Soane, B.D.; Ball, B.C.; Arvidsson, J.; Basch, G.; Moreno, F.; Roger-Estrade, J. No-till in Northern, Western and South-Western Europe: A Review of Problems and Opportunities for Crop Production and the Environment. Soil Tillage Res. 2012, 118, 66–87. [Google Scholar] [CrossRef]
- Alskaf, K.; Sparkes, D.L.; Mooney, S.J.; Sjögersten, S.; Wilson, P. The Uptake of Different Tillage Practices in England. Soil Use Manag. 2020, 36, 27–44. [Google Scholar] [CrossRef]
- Ridley, L.; Parrish, G.; Chantry, T.; Richmond, A.; MacArthur, R.; Garthwaite, D. Arable Crops in the UK 2022; Fera: York, UK, 2024; Available online: https://pusstats.fera.co.uk/api/report-download/699 (accessed on 25 November 2024).
- European Commission. Joint Research Centre. Scientific Evidence Showing the Impacts of Nature Restoration Actions on Food Productivity; Publications Office: Luxembourg, 2022.
- Melander, B.; Munier-Jolain, N.; Charles, R.; Wirth, J.; Schwarz, J.; Van Der Weide, R.; Bonin, L.; Jensen, P.K.; Kudsk, P. European Perspectives on the Adoption of Nonchemical Weed Management in Reduced-Tillage Systems for Arable Crops. Weed Technol. 2013, 27, 231–240. [Google Scholar] [CrossRef]
- DEFRA. Code of Practice for Using Plant Protection Products. 2006. Available online: https://www.hse.gov.uk/pesticides/assets/docs/Code_of_Practice_for_using_Plant_Protection_Products_-_Complete20Code.pdf (accessed on 25 November 2024).
- DAERA. Code of Practice for Using Plant Protection Products; Department of Agriculture and Rural Development: Belfast, Northern Ireland, 2011; ISBN 978-1-84807-210-7. [Google Scholar]
- Flury, M. Experimental Evidence of Transport of Pesticides through Field Soils—A Review. J. Environ. Qual. 1996, 25, 25–45. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.-C.; García-Río, L. The Mobility and Degradation of Pesticides in Soils and the Pollution of Groundwater Resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Beltran, J.; Gerritse, R.G.; Hernandez, F. Effect of Flow Rate on the Adsorption and Desorption of Glyphosate, Simazine and Atrazine in Columns of Sandy Soils. Eur. J. Soil Sci. 1998, 49, 149–156. [Google Scholar] [CrossRef]
- Alletto, L.; Coquet, Y.; Benoit, P.; Heddadj, D.; Barriuso, E. Tillage Management Effects on Pesticide Fate in Soils. A Review. Agron. Sustain. Dev. 2010, 30, 367–400. [Google Scholar] [CrossRef]
- Silburn, D.M. Pesticide Retention, Degradation, and Transport Off-Farm. In No-Till Farming Systems for Sustainable Agriculture; Dang, Y.P., Dalal, R.C., Menzies, N.W., Eds.; Springer: Cham, Switzerland, 2020; pp. 281–297. ISBN 978-3-030-46408-0. [Google Scholar]
- Rasool, S.; Rasool, T.; Gani, K.M. A Review of Interactions of Pesticides within Various Interfaces of Intrinsic and Organic Residue Amended Soil Environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Holland, J.M. The Environmental Consequences of Adopting Conservation Tillage in Europe: Reviewing the Evidence. Agric. Ecosyst. Environ. 2004, 103, 1–25. [Google Scholar] [CrossRef]
- Elias, D.; Wang, L.; Jacinthe, P.-A. A Meta-Analysis of Pesticide Loss in Runoff under Conventional Tillage and No-till Management. Environ. Monit. Assess. 2018, 190, 79. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.K.; Mumma, R.O.; Watts, D.W. Leaching and Runoff Losses of Herbicides in a Tilled and Untilled Field. Agric. Ecosyst. Environ. 1991, 37, 303–314. [Google Scholar] [CrossRef]
- Elliott, J.A.; Cessna, A.J.; Nicholaichuk, W.; Tollefson, L.C. Leaching Rates and Preferential Flow of Selected Herbicides through Tilled and Untilled Soil. J. Environ. Qual. 2000, 29, 1650–1656. [Google Scholar] [CrossRef]
- Chen, X.; Bai, B. Experimental Investigation and Modeling of Particulate Transportation and Deposition in Vertical and Horizontal Flows. Hydrogeol. J. 2015, 23, 365–375. [Google Scholar] [CrossRef]
- Lucas, M.; Nguyen, L.T.T.; Guber, A.; Kravchenko, A.N. Cover Crop Influence on Pore Size Distribution and Biopore Dynamics: Enumerating Root and Soil Faunal Effects. Front. Plant Sci. 2022, 13, 928569. [Google Scholar] [CrossRef]
- Wardak, D.L.R.; Padia, F.N.; De Heer, M.I.; Sturrock, C.J.; Mooney, S.J. Zero-Tillage Induces Significant Changes to the Soil Pore Network and Hydraulic Function after 7 Years. Geoderma 2024, 447, 116934. [Google Scholar] [CrossRef]
- Taina, I.A.; Heck, R.J.; Elliot, T.R. Application of X-Ray Computed Tomography to Soil Science: A Literature Review. Can. J. Soil Sci. 2008, 88, 1–19. [Google Scholar] [CrossRef]
- Ghosh, T.; Maity, P.P.; Rabbi, S.M.F.; Das, T.K.; Bhattacharyya, R. Application of X-Ray Computed Tomography in Soil and Plant -a Review. Front. Environ. Sci. 2023, 11, 1216630. [Google Scholar] [CrossRef]
- Wardak, D.L.R.; Padia, F.N.; De Heer, M.I.; Sturrock, C.J.; Mooney, S.J. Zero Tillage Has Important Consequences for Soil Pore Architecture and Hydraulic Transport: A Review. Geoderma 2022, 422, 115927. [Google Scholar] [CrossRef]
- Köhne, J.M.; Schlüter, S.; Vogel, H.-J. Predicting Solute Transport in Structured Soil Using Pore Network Models. Vadose Zone J. 2011, 10, 1082–1096. [Google Scholar] [CrossRef]
- Soto-Gómez, D.; Pérez-Rodríguez, P.; Vázquez-Juiz, L.; López-Periago, J.E.; Paradelo, M. Linking Pore Network Characteristics Extracted from CT Images to the Transport of Solute and Colloid Tracers in Soils under Different Tillage Managements. Soil Tillage Res. 2018, 177, 145–154. [Google Scholar] [CrossRef]
- Zhu, J.; Patzoldt, W.L.; Radwan, O.; Tranel, P.J.; Clough, S.J. Effects of Photosystem-II-Interfering Herbicides Atrazine and Bentazon on the Soybean Transcriptome. Plant Genome 2009, 2, 91–205. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Wirbisky, S.; Freeman, J. Atrazine Exposure and Reproductive Dysfunction through the Hypothalamus-Pituitary-Gonadal (HPG) Axis. Toxics 2015, 3, 414–450. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 10 March 2004 Concerning the Non-Inclusion of Atrazine in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing This Active Substance, 2004/248/EC. Off. J. Eur. Union 2004, 78, 53–55. [Google Scholar]
- USDA-NRCS. Effects of Conservation Practices on Water Erosion and Loss of Sediment at the Edge of the Field: A National Assessment Based on the 2003-06 CEAP Survey and APEX Modeling Databases; U.S. Department of Agriculture: Singapore; Natural Resources Conservation Service: Washington, DC, USA, 2017. Available online: https://www.nrcs.usda.gov/publications/ceap-crop-2017-sediment-loss.pdf (accessed on 25 November 2024).
- Ma, L.; Selim, H.M. Atrazine Retention and Transport in Soils. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Gunther, F.A., Eds.; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1996; Volume 145, pp. 129–173. ISBN 978-1-4612-7513-8. [Google Scholar]
- Sadeghi, A.M.; Isensee, A.R.; Shirmohammadi, A. Influence of Soil Texture and Tillage on Herbicide Transport. Chemosphere 2000, 41, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.C.; Costa, J.L.; Liedl, R.; Bedmar, F.; Daniel, P. Effects of Soil Type and Tillage Practice on Atrazine Transport through Intact Soil Cores. Geoderma 2006, 137, 161–173. [Google Scholar] [CrossRef]
- Khan, M.A.; Brown, C.D. Influence of Commercial Formulation on Leaching of Four Pesticides through Soil. Sci. Total Environ. 2016, 573, 1573–1579. [Google Scholar] [CrossRef]
- Gish, T.J.; Shirmohammadi, A.; Vyravipillai, R.; Wienhold, B.J. Herbicide Leaching under Tilled and No-Tillage Fields. Soil Sci. Soc. Am. J. 1995, 59, 895–901. [Google Scholar] [CrossRef]
- Hall, J.K.; Jones, G.A.; Hickman, M.V.; Amistadi, M.K.; Bogus, E.R.; Mumma, R.O.; Hartwig, N.L.; Hoffman, L.D. Formulation and Adjuvant Effects on Leaching of Atrazine and Metolachlor. J. Environ. Qual. 1998, 27, 1334–1347. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, D.; Matthew, C.; Wang, P.; Zheng, W. Photosynthetic Contribution of Cotyledons to Early Seedling Development in Cynoglossum divaricatum and Amaranthus retroflexus. N. Z. J. Bot. 2008, 46, 39–48. [Google Scholar] [CrossRef]
- Hamidzadeh Moghadam, S.; Alebrahim, M.T.; Tobeh, A.; Mohebodini, M.; Werck-Reichhart, D.; MacGregor, D.R.; Tseng, T.M. Redroot Pigweed (Amaranthus retroflexus L.) and Lamb’s Quarters (Chenopodium album L.) Populations Exhibit a High Degree of Morphological and Biochemical Diversity. Front. Plant Sci. 2021, 12, 593037. [Google Scholar] [CrossRef]
- Alskaf, K.; Mooney, S.J.; Sparkes, D.L.; Wilson, P.; Sjögersten, S. Short-Term Impacts of Different Tillage Practices and Plant Residue Retention on Soil Physical Properties and Greenhouse Gas Emissions. Soil Tillage Res. 2021, 206, 104803. [Google Scholar] [CrossRef]
- Alskaf, K. Conservation Agriculture for Sustainable Land Use: The Agronomic and Environmental Impacts of Different Tillage Practices and Plant Residue Retention: Farmer Uptake of Reduced Tillage in England. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2018. Available online: https://eprints.nottingham.ac.uk/51902/ (accessed on 25 November 2024).
- Shogaki, T. Mechanism of Sample Disturbance Caused by Tube Penetration: Model Tests on Toyoura Sand. Soils Found. 2017, 57, 527–542. [Google Scholar] [CrossRef]
- Williams, M.R.; McAfee, S.J.; Kent, B.E. Dye Tracers Reveal Potential Edge-Flow Effects in Undisturbed Lysimeters Sealed with Petrolatum. Vadose Zone J. 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive Machine Learning for (Bio)Image Analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Domander, R.; Felder, A.A.; Doube, M. BoneJ2–Refactoring Established Research Software. Wellcome Open Res. 2021, 6, 37. [Google Scholar] [CrossRef]
- Lindsey III, B.E.; Rivero, L.; Calhoun, C.S.; Grotewold, E.; Brkljacic, J. Standardized Method for High-Throughput Sterilization of Arabidopsis Seeds. JoVE 2017, 128, 56587. [Google Scholar] [CrossRef]
- Wang, X.; Yesbergenova-Cuny, Z.; Biniek, C.; Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. Revisiting the Role of Ethylene and N-End Rule Pathway on Chilling-Induced Dormancy Release in Arabidopsis Seeds. Int. J. Mol. Sci. 2018, 19, 3577. [Google Scholar] [CrossRef]
- Halbritter, A.H.; De Boeck, H.J.; Eycott, A.E.; Reinsch, S.; Robinson, D.A.; Vicca, S.; Berauer, B.; Christiansen, C.T.; Estiarte, M.; Grünzweig, J.M.; et al. The Handbook for Standardized Field and Laboratory Measurements in Terrestrial Climate Change Experiments and Observational Studies (ClimEx). Methods Ecol. Evol. 2020, 11, 22–37. [Google Scholar] [CrossRef]
- Brunes, A.P.; Araújo, Á.D.S.; Dias, L.W.; Villela, F.A.; Aumonde, T.Z. Seedling Length in Wheat Determined by Image Processing Using Mathematical Tools. Rev. Ciência Agronômica 2016, 47, 374–379. [Google Scholar] [CrossRef]
- Pribyl, D.W. A Critical Review of the Conventional SOC to SOM Conversion Factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Dettmann, U.; Kraft, N.N.; Rech, R.; Heidkamp, A.; Tiemeyer, B. Analysis of Peat Soil Organic Carbon, Total Nitrogen, Soil Water Content and Basal Respiration: Is There a ‘Best’ Drying Temperature? Geoderma 2021, 403, 115231. [Google Scholar] [CrossRef]
- O’Kelly, B.C. Oven-Drying Characteristics of Soils of Different Origins. Dry. Technol. 2005, 23, 1141–1149. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 25 November 2024).
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software; PBC: Boston, MA, USA, 2024; Available online: http://www.posit.co/ (accessed on 25 November 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using “Ggplot2”. 2024. Available online: https://CRAN.R-project.org/package=survminer (accessed on 25 November 2024).
- Jackson, C. Flexsurv: A Platform for Parametric Survival Modeling in R. J. Stat. Softw. 2016, 70, i08. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Dey, T.; Mukherjee, A.; Chakraborty, S. A Practical Overview and Reporting Strategies for Statistical Analysis of Survival Studies. Chest 2020, 158, S39–S48. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.J.; Nussenbaum, B.; Wang, E.W. A Practical Guide to Understanding Kaplan-Meier Curves. Otolaryngol.-Head Neck Surg. 2010, 143, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Damuzzo, V.; Agnoletto, L.; Leonardi, L.; Chiumente, M.; Mengato, D.; Messori, A. Analysis of Survival Curves: Statistical Methods Accounting for the Presence of Long-Term Survivors. Front. Oncol. 2019, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R.; Oakes, D. Analysis of Survival Data; Monographs on Statistics and Applied Probability; Chapman and Hall/CRC: New York, NY, USA, 1984; ISBN 0-412-224490-X. [Google Scholar]
- Pires, L.F.; Auler, A.C.; Roque, W.L.; Mooney, S.J. X-Ray Microtomography Analysis of Soil Pore Structure Dynamics under Wetting and Drying Cycles. Geoderma 2020, 362, 114103. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.A.T.; Cássaro, F.A.M.; Pires, L.F. Estimating Soil Porosity and Pore Size Distribution Changes Due to Wetting-Drying Cycles by Morphometric Image Analysis. Soil Tillage Res. 2021, 205, 104814. [Google Scholar] [CrossRef]
- Hao, H.; Hartmann, C.; Apichart, J.; Siwaporn, S.; Promsakha, S.; Richard, G.; Bruand, A.; Dexter, A.R. Slumping Dynamics in Tilled Sandy Soils under Natural Rainfall and Experimental Flooding. Soil Tillage Res. 2011, 114, 9–17. [Google Scholar] [CrossRef]
- Mitchell, J.; Johnston, I.G.; Bassel, G.W. Variability in Seeds: Biological, Ecological, and Agricultural Implications. J. Exp. Bot. 2017, 68, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Ahmadnia, F.; Alebrahim, M.T.; Nabati Souha, L.; MacGregor, D.R. Evaluation of Techniques to Break Seed Dormancy in Redroot Pigweed (Amaranthus Retroflexus). Food Sci. Nutr. 2024, 12, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L.; Williams, J.T.; Sagar, G.R. The Behaviour of Seeds in Soil: I. The Heterogeneity of Soil Surfaces and Its Role in Determining the Establishment of Plants from Seed. J. Ecol. 1965, 53, 273. [Google Scholar] [CrossRef]
- Ramezanpoor, M.; Salehian, H.; Babanezhad, E.; Rezvani, M. The Leaching of Atrazine and Plant Species Sensitivity to Atrazine Using Bioassays and Chemical Analyses. Soil Sediment Contam. Int. J. 2022, 31, 456–467. [Google Scholar] [CrossRef]
- Kolekar, P.D.; Phugare, S.S.; Jadhav, J.P. Biodegradation of Atrazine by Rhodococcus Sp. BCH2 to N-Isopropylammelide with Subsequent Assessment of Toxicity of Biodegraded Metabolites. Environ. Sci. Pollut. Res. 2014, 21, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Barrios, R.E.; Gaonkar, O.; Snow, D.; Li, Y.; Li, X.; Bartelt-Hunt, S.L. Enhanced Biodegradation of Atrazine at High Infiltration Rates in Agricultural Soils. Environ. Sci. Process. Impacts 2019, 21, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, Q.; Hao, L.; Zhang, L.; Li, X.; Zheng, M. The Metabolism of a Novel Cytochrome P450 (CYP77B34) in Tribenuron-Methyl-Resistant Descurainia Sophia L. to Herbicides with Different Mode of Actions. Int. J. Mol. Sci. 2022, 23, 5812. [Google Scholar] [CrossRef]
- Grayling, K.M.; Young, S.D.; Roberts, C.J.; de Heer, M.I.; Shirley, I.M.; Sturrock, C.J.; Mooney, S.J. The Application of X-Ray Micro Computed Tomography Imaging for Tracing Particle Movement in Soil. Geoderma 2018, 321, 8–14. [Google Scholar] [CrossRef]
- Koestel, J.; Larsbo, M. Imaging and Quantification of Preferential Solute Transport in Soil Macropores. Water Resour. Res. 2014, 50, 4357–4378. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, M.; Tomar, I.S.; Morya, J. Evaluation of Atrazine 50% WP Herbicide for Weed Control in Maize (Zea mays L.) of Jhabua Hills Zone of M.P. JAS 2021, 8, 311–317. [Google Scholar] [CrossRef]
- Kumar, S.; Chakraborty, P.; Anderson, S. X-Ray Computed Tomography for Studying Solute Transport in Soils. In X-Ray Imaging of the Soil Porous Architecture; Jon Mooney, S., Young, I.M., Heck, R.J., Peth, S., Eds.; Springer: Cham, Switzerland, 2022; pp. 99–112. ISBN 978-3-031-12175-3. [Google Scholar]
- Larsbo, M.; Stenström, J.; Etana, A.; Börjesson, E.; Jarvis, N.J. Herbicide Sorption, Degradation, and Leaching in Three Swedish Soils under Long-Term Conventional and Reduced Tillage. Soil Tillage Res. 2009, 105, 200–208. [Google Scholar] [CrossRef]
- Zablotowicz, R.M.; Locke, M.A.; Gaston, L.A. Tillage and Cover Effects on Soil Microbial Properties and Fluometuron Degradation. Biol. Fertil. Soils 2007, 44, 27–35. [Google Scholar] [CrossRef]
- Chowdhury, I.F.; Rohan, M.; Stodart, B.J.; Chen, C.; Wu, H.; Doran, G.S. Persistence of Atrazine and Trifluralin in a Clay Loam Soil Undergoing Different Temperature and Moisture Conditions. Environ. Pollut. 2021, 276, 116687. [Google Scholar] [CrossRef]
- Johnson, B.; Zimmer, M. Soil Applied Herbicides and Rainfall for Activation. In Pest&Crop Newsletter; Purdue University: West Lafayette, IN, USA, 2023. [Google Scholar]
- Nimmo, J.R. Porosity and Pore Size Distribution. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-409548-9. [Google Scholar] [CrossRef]
| n | BD | SD | n | OM | SD | |
|---|---|---|---|---|---|---|
| ZT | 4 | 0.953 a | 0.017 | 3 | 6.31 a | 0.60 |
| P | 4 | 1.016 b | 0.031 | 4 | 5.52 a | 0.39 |
| Total Imaged Porosity (%) | Euler Density (mm−3) | |||||
|---|---|---|---|---|---|---|
| Tillage | Mean | SD | Mean | SD | ||
| P | 4.94 | a | 1.65 | 1.83 | a | 0.33 |
| ZT | 6.98 | b | 2.26 | 0.769 | b | 0.404 |
| RP | 8.67 | b | 1.60 | 2.85 | c | 0.63 |
| Volume of LMC (mm3) | Proportion of LMC (%) | |||||
| Tillage | Mean | SD | Mean | SD | ||
| P | 5160 | a | 4213 | 38.8 | a | 19.1 |
| ZT | 12571 | b | 6004 | 70.2 | ab | 17.1 |
| RP | 7825 | ab | 5171 | 28.7 | b | 17.0 |
| LMC connectivity density (mm−3) | LMC average thickness (mm) | |||||
| Tillage | Mean | SD | Mean | SD | ||
| P | 0.0244 | a | 0.0212 | 0.970 | a | 0.402 |
| ZT | 0.136 | b | 0.095 | 1.24 | b | 0.33 |
| RP | 0.174 | b | 0.125 | 0.224 | c | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardak, D.L.R.; Padia, F.N.; de Heer, M.I.; Sturrock, C.J.; Mooney, S.J. Zero-Tillage Induces Reduced Bio-Efficacy Against Weed Species Amaranthus retroflexus L. Dependent on Atrazine Formulation. Agronomy 2025, 15, 360. https://doi.org/10.3390/agronomy15020360
Wardak DLR, Padia FN, de Heer MI, Sturrock CJ, Mooney SJ. Zero-Tillage Induces Reduced Bio-Efficacy Against Weed Species Amaranthus retroflexus L. Dependent on Atrazine Formulation. Agronomy. 2025; 15(2):360. https://doi.org/10.3390/agronomy15020360
Chicago/Turabian StyleWardak, D. Luke R., Faheem N. Padia, Martine I. de Heer, Craig J. Sturrock, and Sacha J. Mooney. 2025. "Zero-Tillage Induces Reduced Bio-Efficacy Against Weed Species Amaranthus retroflexus L. Dependent on Atrazine Formulation" Agronomy 15, no. 2: 360. https://doi.org/10.3390/agronomy15020360
APA StyleWardak, D. L. R., Padia, F. N., de Heer, M. I., Sturrock, C. J., & Mooney, S. J. (2025). Zero-Tillage Induces Reduced Bio-Efficacy Against Weed Species Amaranthus retroflexus L. Dependent on Atrazine Formulation. Agronomy, 15(2), 360. https://doi.org/10.3390/agronomy15020360







