Effect of Vegetable Oil Adjuvant on Wetting, Drift, and Deposition of Pesticide Droplets from UAV Sprayers on Litchi Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatments
2.2. Surface Tension
2.3. Wetting Time
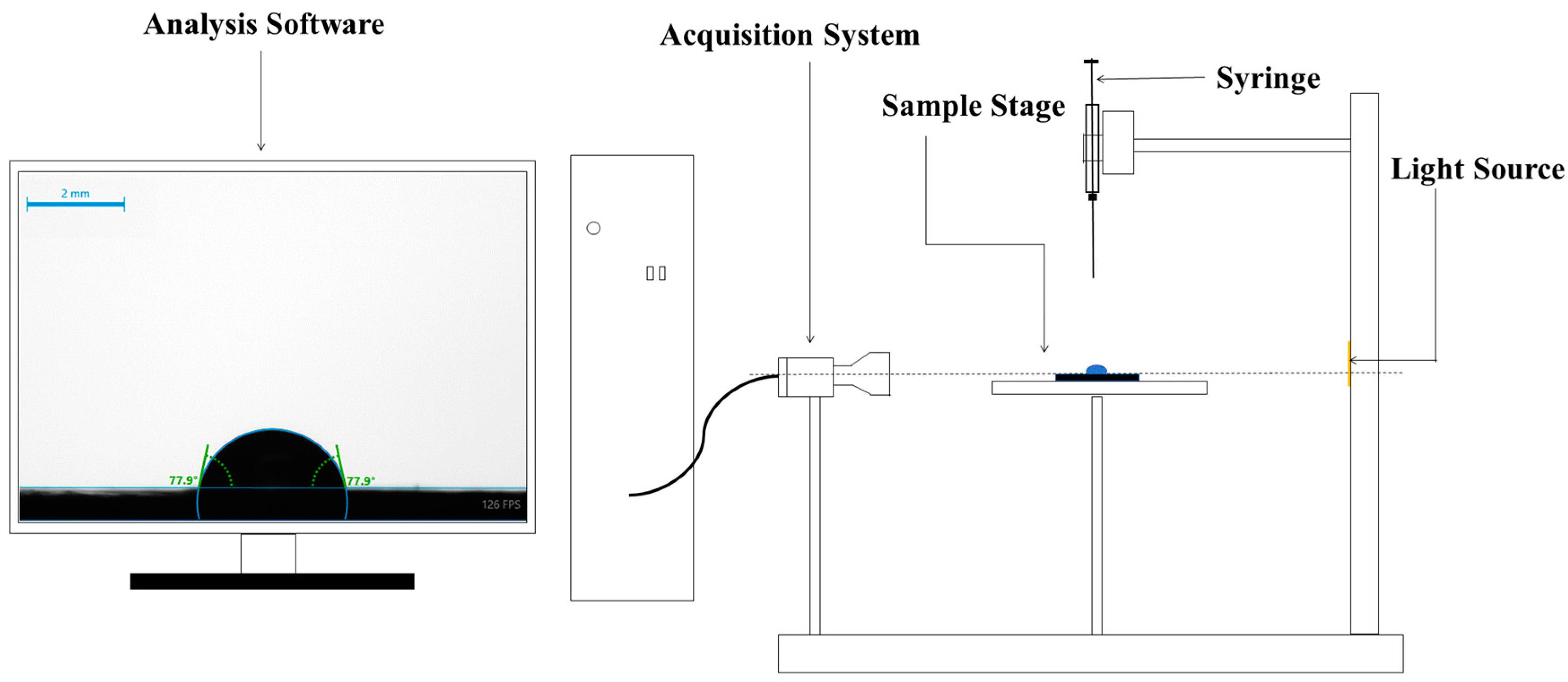
2.4. Contact Angle
2.5. Maximum Liquid Retention
2.6. Droplet Spread Areas on Litchi Leaves
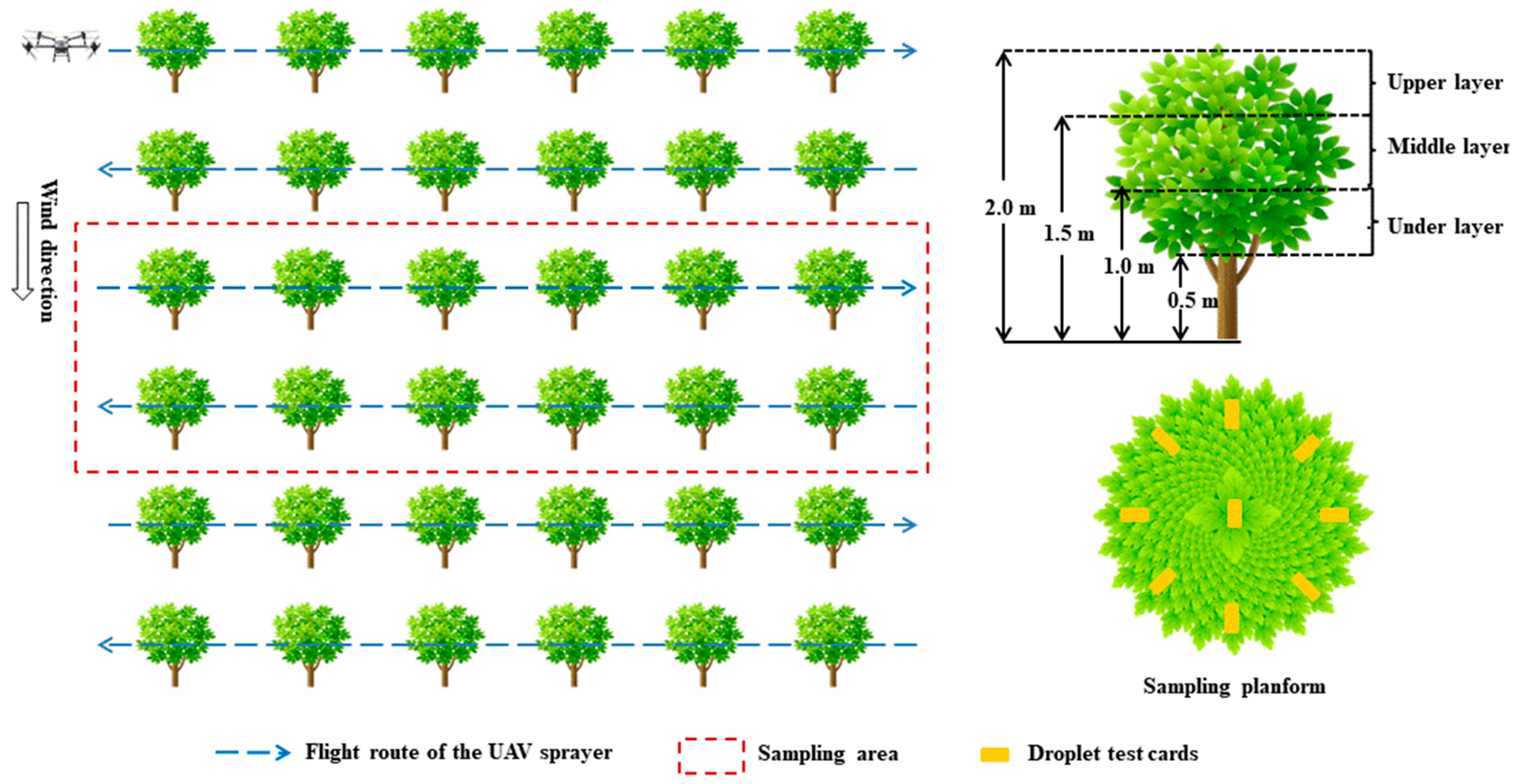
2.7. Spray Drift
2.8. Spray Deposited on the Litchi Leaves
2.9. Data Processing
3. Results
3.1. Physicochemical Properties of Spray Dilutions
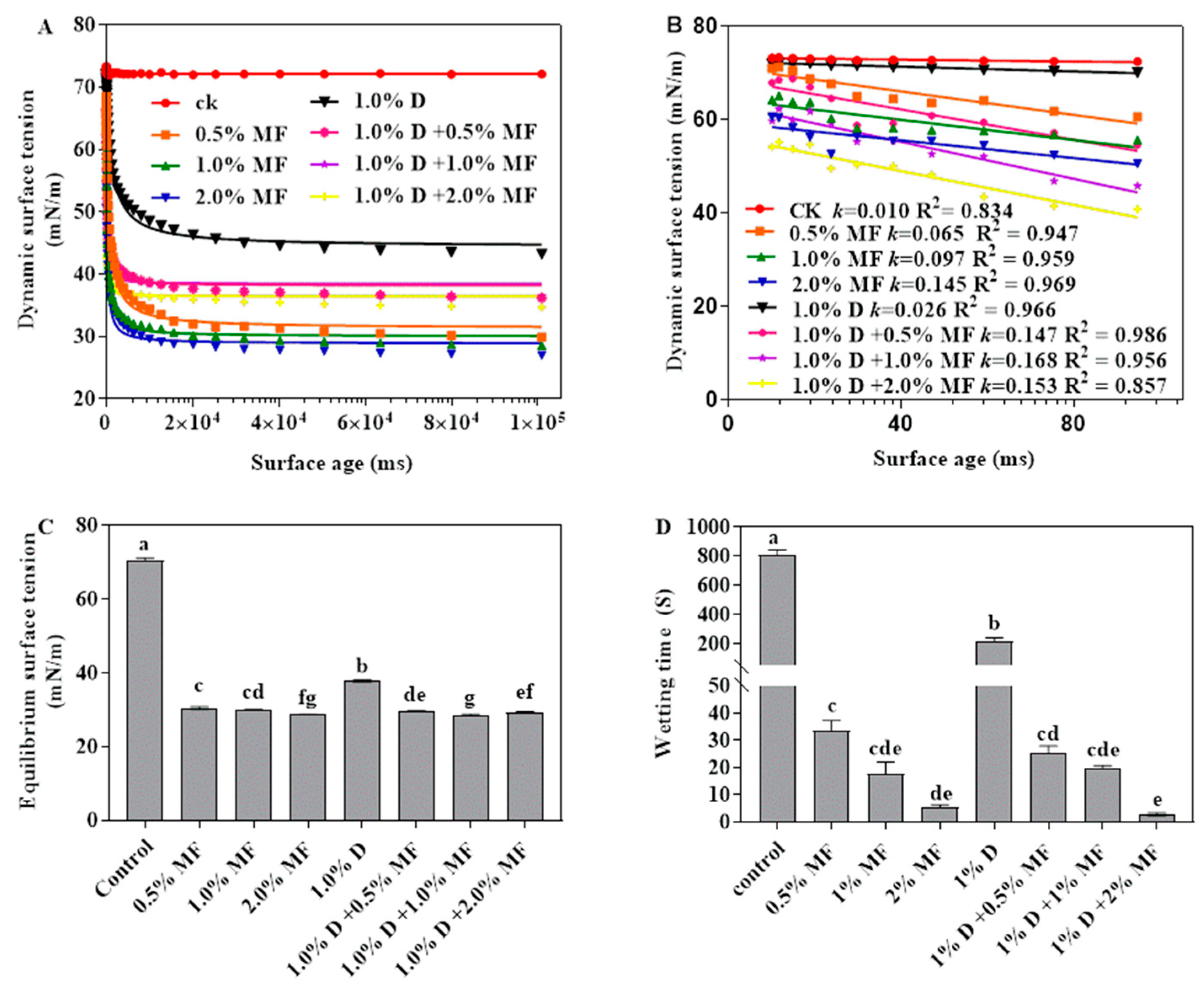
3.1.1. The Dynamic Surface Tension
3.1.2. The Equilibrium Surface Tension
3.1.3. The Wetting Time
3.2. Effects of MF on the Spreading and Retention of Pesticide Droplets on the Litchi Leaves
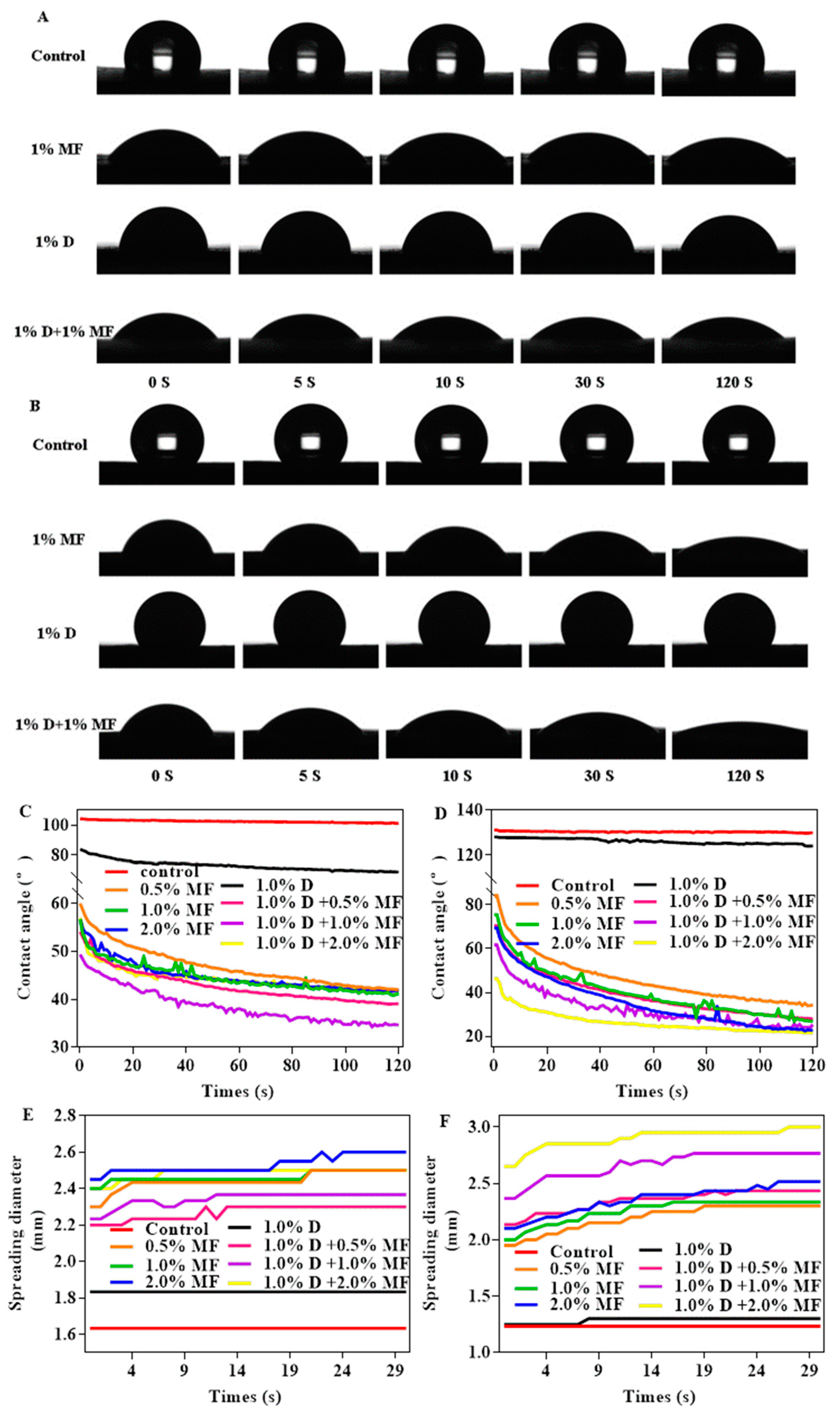
3.2.1. The Contact Angles and Spreading Diameter
3.2.2. The Maximum Liquid Retention
3.2.3. The Maximum Spreading Area and Evaporation Time
3.3. Effects of MF on Droplet Drift and Deposition
3.3.1. Effects of MF on the Spray Drift
3.3.2. Deposition of Spray Droplets on Litchi Canopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandhu, K.S.; Kaur, M.; Punia, S.; Ahmed, J. Rheological, thermal, and structural properties of high-pressure treated Litchi (Litchi chinensis) kernel starch. Int. J. Biol. Macromol. 2021, 175, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zheng, J.; Chen, S.; Lan, Y.; Li, H.; Zeng, L.; Yue, X. The effect of Aceria litchii (Keifer) infestation on the surface properties of litchi leaf hosts. Pest. Manag. Sci. 2024, 80, 2647–2657. [Google Scholar] [CrossRef]
- Zhang, Y.; Huber, D.J.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Delay of postharvest browning in litchi fruit by melatonin via the enhancing of antioxidative processes and oxidation repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Voltz, M.; Andrieux, P.; Samouëlian, A.; Ponchant, L.; Grünberger, O.; Bajazet, T.; Comte, I.; Nanette, J.-B.; Onapin, G.; Bussière, F.; et al. Flow patterns and pathways of legacy and contemporary pesticides in surface waters in tropical volcanic catchments. Sci. Total Environ. 2023, 893, 164815. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, C.; Du, G.; Yu, F.; Yao, W.; Lan, Y. Evaluating the use of unmanned aerial vehicles for spray applications in mountain Nanguo pear orchards. Pest. Manag. Sci. 2024, 80, 3590–3602. [Google Scholar] [CrossRef]
- Wong, H.L.; Garthwaite, D.G.; Ramwell, C.T.; Brown, C.D. Assessment of exposure of professional agricultural operators to pesticides. Sic. Total Environ. 2018, 619, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Chen, S. Current status and trends of plant protection UAV and its spraying technology in China. Int. J. Precis. Agric. Aviat. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- García-Santos, G.; Scheiber, M.; Pilz, J. Spatial interpolation methods to predict airborne pesticide drift deposits on soils using knapsack sprayers. Chemosphere 2020, 258, 127231. [Google Scholar] [CrossRef] [PubMed]
- Katzman, D.; Bohbot-Raviv, Y.; Dubowski, Y. Does polyacrylamide-based adjuvant actually reduce primary drift of airborne pesticides? Sci. Total Environ. 2021, 775, 145816. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Wu, Y.; Song, R.; Gao, Y.; Zhang, S.; Zhao, K.; Wu, T.; Zhang, C.; Du, F. The simple strategy to improve pesticide bioavailability and minimize environmental risk by effective and ecofriendly surfactants. Sci. Total Environ. 2022, 851, 15816. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wu, Y.; Bao, Z.; Gao, Y.; Zhao, K.; Zhang, S.; Zhang, C.; Du, F. Machine Learning to predict the interfacial behavior of pesticide droplets on hydrophobic surfaces for minimizing environmental risk. ACS Sustain. Chem. Eng. 2022, 10, 14034–14044. [Google Scholar] [CrossRef]
- Hossard, L.; Schneider, C.; Voltz, M. A role-playing game to stimulate thinking about vineyard management practices to limit pesticide use and impacts. J. Clean. Prod. 2022, 380, 134913. [Google Scholar] [CrossRef]
- İtmeç, M.; Bayat, A.; Bolat, A.; Toraman, M.C.; Soysal, A. Assessment of spray drift with various adjuvants in a wind tunnel. Agronomy 2022, 12, 2377. [Google Scholar] [CrossRef]
- Xiao, Q.; Xin, F.; Lou, Z.; Zhou, T.; Wang, G.; Han, X.; Lan, Y.; Fu, W. Effect of aviation spray adjuvants on defoliant droplet deposition and cotton defoliation efficacy sprayed by unmanned aerial vehicles. Agronomy 2019, 9, 217. [Google Scholar] [CrossRef]
- Meng, Y.; Zhong, W.; Liu, C.; Su, J.; Su, J.; Lan, Y.; Wang, Z.; Wang, M. UAV spraying on citrus crop: Impact of tank-mix adjuvant on the contact angle and droplet distribution. PeerJ 2022, 10, 13064. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Han, Y.; Li, X.; Andaloro, J.; Chen, P.; Hoffmann, W.C.; Han, X.; Chen, S.; Lan, Y. Field evaluation of spray drift and environmental impact using an agricultural unmanned aerial vehicle (UAV) sprayer. Sci. Total Environ. 2020, 737, 139793. [Google Scholar] [CrossRef]
- Zeeshan, M.; Li, H.; Yousaf, G.; Ren, H.; Liu, Y.; Arshad, M.; Dou, Z.; Han, X. Effect of formulations and adjuvants on the properties of acetamiprid solution and droplet deposition characteristics sprayed by UAV. Front. Plant Sci. 2024, 15, 1441193. [Google Scholar] [CrossRef]
- Ren, L.; Wang, S.F.; Shi, X.J.; Cao, J.Y.; Zhou, J.B.; Zhao, X.J. Characterisation of sensitivity of Colletotrichum gloeosporioides and Colletotrichum capsici, causing pepper anthrac nose, to picoxystrobin. J. Plant Dis. Prot. 2020, 127, 657–666. [Google Scholar] [CrossRef]
- Hu, H.; Ma, Y.; Song, X.; Wang, D.; Ren, X.; Wu, C.; Liu, C.; Ma, X.; Shan, Y.; Meng, Y.; et al. Tank-mix adjuvants enhance pesticide efficacy by improving physicochemical properties and spraying characteristics for application to cotton with unmanned aerial vehicles. ACS Omega 2024, 9, 31011–31025. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Cang, T.; Zhao, X.; Wu, S.; Qi, P.; Wang, X.; Xu, X.; Wang, Q. Positive effects of an oil adjuvant on efficacy, dissipation and safety of pyrimethanil and boscalid on greenhouse strawberry. Ecotoxicol. Environ. Saf. 2018, 160, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wongsuk, S.; Qi, P.; Wang, C.; Zeng, A.; Sun, F.; Yu, F.; Zhao, X.; Xiongkui, H. Spray performance and control efficacy against pests in paddy rice by UAV-based pesticide application: Effects of atomization, UAV configuration and flight velocity. Pest. Manag. Sci. 2024, 80, 2072–2084. [Google Scholar] [CrossRef]
- ISO 8022; Surface Active Agents-Determination of Wetting Power by Immersion 1990. International Organization for Standardization: Geneva, Switzerland, 1990.
- Zhang, J.; Zhou, T.; Zeng, J.; Yin, X.; Lan, Y.; Wen, S. Effects of temperature and humidity on the contact angle of pesticide droplets on rice leaf surfaces. J. Pestic. Sci. 2022, 47, 59–68. [Google Scholar] [CrossRef]
- Zhang, S. Effects of leaf surface roughness and contact angle on in vivo measurement of droplet retention. Agronomy 2022, 12, 2228. [Google Scholar] [CrossRef]
- Precipito, L.M.B.; Dario, G.; Oliveira, J.V.; Oliveira, R.B. Evaporation and wettability of fungicide spray, with or without adjuvant, on leaves of vegetables. Hortic. Bras. 2018, 36, 320–324. [Google Scholar] [CrossRef]
- ISO 22866; Equipment for Crop Protection-Methods for Field Measurement of Spray Drift. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 24253-1; Crop Protection Equipment-Spray Deposition Test for Field Crop. International Organization for Standardization: Geneva, Switzerland, 2015.
- Chen, S.; Lan, Y.; Zhou, Z.; Ouyang, F.; Wang, G.; Huang, X.; Deng, X.; Cheng, S. Effect of droplet size parameters on droplet deposition and drift of aerial spraying by using plant protection UAV. Agronomy 2020, 10, 195. [Google Scholar] [CrossRef]
- Zhu, H.; Salyani, M.; Fox, R.D. A portable scanning system for evaluation of spray deposit distribution. Comput. Electron. Agr. 2011, 76, 38–43. [Google Scholar] [CrossRef]
- Law, K.Y. Water-surface interactions and definitions for hydrophilicity, hydrophobicity and superhydrophobicity. JMS-PAC 2015, 87, 759–765. [Google Scholar] [CrossRef]
- Zheng, L.; Cheng, X.; Cao, L.; Chen, Z.; Huang, Q.; Song, B.J. Enhancing pesticide droplet deposition through O/W pickering emulsion: Synergistic stabilization by flower-like ZnO particles and polymer emulsifier. Chem. Eng. J. 2022, 434, 134761. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, M.; Sun, Z.; Li, L.; Shang, H.; Xi, W.; Li, B.; Li, Y.; Xu, Y.; Wu, X. Using tank-mix adjuvant improves the physicochemical properties and dosage delivery to reduce the use of pesticides in unmanned aerial vehicles for plant protection in wheat. Pest. Manag. Sci. 2022, 78, 2512–2522. [Google Scholar] [CrossRef]
- Dorr, G.J.; Forster, W.A.; Mayo, L.C.; McCue, S.W.; Kempthorne, D.M.; Hanan, J.; Turner, I.W.; Belward, J.A.; Young, J.; Zabkiewicz, J.A. Spray retention on whole plants: Modelling, simulations and experiments. Crop. Prot. 2016, 88, 118–130. [Google Scholar] [CrossRef]
- Meng, Y.; Wu, Q.; Zhou, H.; Hu, H. How tank-mix adjuvant type and concentration influence the contact angle on wheat leaf surface. PeerJ 2023, 11, 16464. [Google Scholar] [CrossRef] [PubMed]
- Eral, H.B.; ’t Mannetje, D.J.C.M.; Oh, J.M. Contact angle hysteresis: A review of fundamentals and applications. Colloid. Polym. Sci. 2012, 291, 247–260. [Google Scholar] [CrossRef]
- Semenov, S.; Trybala, A.; Agogo, H.; Kovalchuk, N.; Ortega, F.; Rubio, R.G.; Starov, V.M.; Velarde, M.G. Evaporation of droplets of surfactant solutions. Langmuir 2013, 10, 1021. [Google Scholar] [CrossRef]
- Gao, X.; Wang, D.; Jiang, Z.; Li, X.; Chen, G. Effect of adjuvants on the wetting behaviors of bifenthrin droplets on tea leaves. Appl. Sci. 2022, 12, 4217. [Google Scholar] [CrossRef]
- Dorr, G.J.; Wang, S.; Mayo, L.C.; McCue, S.W.; Forster, W.A.; Hanan, J.; He, X. Impaction of spray droplets on leaves: Influence of formulation and leaf character on shatter, bounce and adhesion. Exp. Fluids 2015, 56, 143. [Google Scholar] [CrossRef]
- Appah, S.; Jia, W.; Ou, M.; Wang, P.; Amoah, A.E. Analysis of potential impaction and phytotoxicity of surfactant-plant surface interaction in pesticide application. Corp. Prot. 2020, 127, 104961. [Google Scholar] [CrossRef]
- Massinon, M.; Lebesu, F. Experimental method for the assessment of agricultural spray retention based on high-speed imaging of drop impact on a synthetic superhydrophobic surface. Biosyst. Eng. 2012, 112, 56–64. [Google Scholar] [CrossRef]
- Cao, C.; Song, Y.; Zhou, Z.; Cao, L.; Li, F.; Huang, Q. Effect of adhesion force on the height pesticide droplets bounce on impaction with cabbage leaf surfaces. Soft Matter 2018, 14, 8030–8035. [Google Scholar] [CrossRef]
- Katzman, D.; Zivan, O.; Dubowski, Y. Assessing the influence of polymer-based anti-drift adjuvants on the photolysis, volatilization, and secondary drift of pesticides after application. Atmosphere 2023, 14, 1627. [Google Scholar] [CrossRef]
- Wang, L.; Xia, S.; Zhang, H.; Li, Y.; Huang, Z.; Qiao, B.; Zhong, L.; Cao, M.; He, X.; Wang, C.; et al. Tank-mix adjuvants improved spray performance and biological efficacy in rice insecticide application with unmanned aerial vehicle sprayer. Pest. Manag. Sci. 2024, 80, 4371–4385. [Google Scholar] [CrossRef]
- Chen, P.; Lan, Y.; Huang, X.; Qi, H.; Wang, G.; Wang, J.; Wang, L.; Xiao, H. Droplet deposition and control of planthoppers of different nozzles in two-stage rice with a quadrotor unmanned aerial vehicle. Agronomy 2020, 10, 303. [Google Scholar] [CrossRef]
- Zhao, H.; Song, J.L.; Zeng, A.J.; He, X.H. Correlations between dynamic surface tension and droplet diameter. Trans. Chin. Soc. Agric. Mach. 2009, 40, 74–79. (In Chinese) [Google Scholar]


| Treatments | Humidity/% | Temperature/°C | Wind Speed/m·s−1 | Wind Direction/° |
|---|---|---|---|---|
| Control | 77.25 ± 0.37 | 27.07 ± 0.14 | 1.53 ± 0.60 | 87.05 ± 17.08 |
| 1.0% MF | 70.30 ± 0.25 | 30.18 ± 0.13 | 1.15 ± 0.37 | 105.67 ± 6.00 |
| Treatments | Humidity/% | Temperature/°C | Wind Speed/m·s−1 | Wind Direction/° |
|---|---|---|---|---|
| Control | 57.24 ± 0.29 | 30.85 ± 0.81 | 1.97 ± 0.49 | 25.50 ± 1.20 |
| 1.0% MF | 54.02 ± 0.88 | 32.19 ± 0.25 | 2.03 ± 0.67 | 47.06 ± 3.08 |
| Treatments | Adaxial | Abaxial | ||
|---|---|---|---|---|
| Maximum Spreading Area (mm2) | Evaporation Time (s) | Maximum Spreading Area (mm2) | Evaporation Time (s) | |
| Control | 0.57 ± 0.04 d | 336.33 ± 20.55 a | 0.43 ± 0.01 d | 722.67 ± 11.02 a |
| 0.5% MF | 2.18 ± 0.32 ab | 302.67 ± 23.12 a | 1.95 ± 0.44 ab | 255.67 ± 32.72 de |
| 1.0% MF | 2.40 ± 0.11 a | 283.33 ± 8.08 bc | 2.07 ± 0.01 ab | 231.33 ± 12.22 e |
| 2.0% MF | 2.16 ± 0.24 ab | 295.33 ± 43.32 abc | 2.19 ± 0.04 a | 227.00 ± 10.82 e |
| 1.0% D | 1.06 ± 0.06 c | 313.00 ± 6.08 a | 0.46 ± 0.05 d | 314.33 ± 12.50 b |
| 1.0% D + 0.5% MF | 1.82 ± 0.14 b | 322.37 ± 29.94 a | 1.87 ± 0.10 b | 299.67 ± 10.79 bc |
| 1.0% D + 1.0% MF | 2.04 ± 0.05 b | 296.33 ± 3.22 abc | 1.65 ± 0.12 bc | 278.00 ± 11.36 cd |
| 1.0% D + 2.0% MF | 2.20 ± 0.31 ab | 280.67 ± 39.31 bc | 1.85 ± 0.06 b | 287.00 ± 27.62 bcd |
| Treatments | CDR (%) | Spray Drift Buffer Zone Distance (90% CDR) (m) |
|---|---|---|
| Water (Control) | 28.75 ± 2.34 a | 8.05 ± 0.70 a |
| 1% MF | 14.92 ± 2.16 b | 5.68 ± 0.49 b |
| Treatments | Droplet Deposition Density/(Drops/cm2) | Droplet Coverage Ratio/% | DV50/μm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Upper Layer | Middle Layer | Lower Layer | Upper Layer | Middle Layer | Lower Layer | Upper Layer | Middle Layer | Lower Layer | |
| Water (Control) | 50.52 ± 0.94 a | 35.87 ± 4.59 b | 30.10 ± 1.08 b | 4.50 ± 0.24 a | 3.11 ± 0.83 b | 2.01 ± 0.12 b | 165.50 ± 5.52 a | 159.73 ± 10.38 a | 148.05 ± 5.04 b |
| 1% MF | 63.24 ± 10.25 a | 49.56 ± 2.65 a | 34.86 ± 0.63 a | 6.38 ± 1.95 a | 4.96 ± 0.35 a | 2.87 ± 0.05 a | 169.13 ± 5.76 a | 167.36 ± 4.38 a | 161.63 ± 5.60 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Geng, Z.; Pan, B.; Jiang, L.; Lin, Y. Effect of Vegetable Oil Adjuvant on Wetting, Drift, and Deposition of Pesticide Droplets from UAV Sprayers on Litchi Leaves. Agronomy 2025, 15, 293. https://doi.org/10.3390/agronomy15020293
Wang B, Geng Z, Pan B, Jiang L, Lin Y. Effect of Vegetable Oil Adjuvant on Wetting, Drift, and Deposition of Pesticide Droplets from UAV Sprayers on Litchi Leaves. Agronomy. 2025; 15(2):293. https://doi.org/10.3390/agronomy15020293
Chicago/Turabian StyleWang, Bingjie, Ziqiong Geng, Bo Pan, Lei Jiang, and Yong Lin. 2025. "Effect of Vegetable Oil Adjuvant on Wetting, Drift, and Deposition of Pesticide Droplets from UAV Sprayers on Litchi Leaves" Agronomy 15, no. 2: 293. https://doi.org/10.3390/agronomy15020293
APA StyleWang, B., Geng, Z., Pan, B., Jiang, L., & Lin, Y. (2025). Effect of Vegetable Oil Adjuvant on Wetting, Drift, and Deposition of Pesticide Droplets from UAV Sprayers on Litchi Leaves. Agronomy, 15(2), 293. https://doi.org/10.3390/agronomy15020293





