Abstract
The effects of hexanal supplementation in the storage atmosphere of ‘Fuji Kiku’ apples were investigated. The contents of volatile compounds (VOCs) in the headspace emitted by apple fruit during cold storage and in the headspace of apple fruit and juice during shelf life were determined. Hexanal treatment during storage significantly affected the VOC profile by stimulating the production or retention of key esters, including hexyl acetate, ethyl acetate, and butyl 2-methylbutanoate, during cold storage. Supplementation of hexanal also increased the production of linear esters, especially hexyl acetate, and promoted the formation of branched esters such as ethyl 2-methylbutanoate and hexyl 2-methylbutanoate during shelf life. Hexanal also increased the alcohol concentrations, with a significant increase in hexanol and 2-pentanol. Partial least squares discriminant analysis showed clear separation between control and hexanal-treated samples, with compounds like butyl hexanoate and 2-methyl-1-butanol being the most influential. Apple juice extracted from the flesh of hexanal-treated apples exhibited higher concentrations of key VOCs, including 2-methylbutyl acetate, hexyl acetate, and 2-methyl-1-butanol. No significant differences in firmness were observed; however, hexanal showed an inhibitory effect on the colour development of fruit. This study highlights the potential of hexanal in influencing aroma-related compounds and provides insight into strategies to improve postharvest aroma in apples.
1. Introduction
Apples are the third-most-produced fruit in the world, preceded by bananas and watermelons. However, a decreasing trend in apple consumption has been observed in Europe, possibly related to consumer expectations of the sensory properties of fruit, including aroma [1]. Volatile aroma compounds in fruits are known for their antimicrobial properties, but their main role is to significantly influence the sensory quality and thus the consumer’s perception of the fruit. Although apples contain more than 300 identified volatile compounds (VOCs), only a small proportion contribute significantly to their flavour. The most important of these are esters, alcohols, aldehydes, ketones, and ethers. The diversity and content of these compounds vary from variety to variety and are influenced by various factors before, during, and after harvest. As apples ripen, the VOCs convert from aldehydes to esters, to the extent that esters can account for over 80% of all aromatic compounds in ripe apples of certain varieties such as Golden Delicious and Golden Reinders [2,3,4,5]. The increasing consumer demand for larger quantities of out-of-season fruit and the complexity of distribution networks have led to the expansion of apple storage under controlled atmospheric conditions. A critical component of these storage systems is the ability to actively regulate and maintain a low oxygen environment to slow down the ripening process and preserve the quality of the fruit. However, this reduced oxygen content limits the availability of precursors required to synthesize the fruit esters, resulting in a reduction of aromatic compounds in the fruit [6].
Hexanal, a naturally occurring C6 aldehyde with high volatility, is released by plants via the lipoxygenase pathway when their tissues are disturbed. This compound is a primary VOC found in many fruits and vegetables, including apple fruit [2]. These volatiles are known for their characteristic odour and taste and are often used as flavours in the food industry. Hexanal is produced by the oxidative degradation of fatty acids, which contribute to the characteristic “green” descriptor in various fruits and vegetables [7]. As an antimicrobial aldehyde, hexanal is one of the VOCs that can be used for post-harvest treatment. The U.S. Food and Drug Administration has approved the use of hexanal as a food additive and allows a dosage of 3700 mg kg−1 based on its ORL-MAM LD50 classification [8,9]. The physical properties of hexanal include a melting point of 20 °C and a boiling point of 130 to 131 °C. Its heat capacity under constant pressure at 28.5 °C is measured at 210.4 J/mol·K [10].
In research, hexanal is applied using various methods, including pre-harvest spraying, post-harvest dipping, and vapour treatments, which have gained wide acceptance [11]. Pre-harvest spraying allows for uniform coverage of plants or fruits and may provide extended protection before harvest; however, weather conditions may affect efficacy. Post-harvest dipping also ensures direct contact with the entire surface of harvested produce and can be combined with other post-harvest treatments but requires additional handling of produce, potentially increasing the risk of damage. The advantages of vapor treatments are that they are a non-contact method, reducing the potential for mechanical damage, can penetrate into spaces between produce items, and are suitable for treating large volumes of produce simultaneously. However, they may require specialized equipment or facilities, and concentration control can be challenging.
The use of hexanal has been shown to have positive effects on extending the shelf life of both climacteric and non-climacteric fruit. These fruits also include apples [12,13,14], bananas [15], peaches [11], mangoes [16], papayas [17], and strawberries [18]. Research suggests that hexanal inhibits the breakdown of cell walls and membranes, as well as slows down the ethylene-mediated ripening process in fruits. Multiple investigations have indicated that hexanal could reduce the activity of phospholipase D, an enzyme that breaks down membrane phospholipids and initiates membrane degradation, leading to fruit softening [9]. Additionally, hexanal was also found to mildly suppress ethylene, a hormone which is responsible for ripening a climacteric fruit [15]. Fruits exposed to hexanal consistently showed delayed softening, decreased respiratory rates, and reduced ethylene production as well during storage when compared to untreated samples [15]. While hexanal application on fruits has demonstrated promising results in extending shelf life and preserving quality, there are potential disadvantages that warrant consideration. For instance, inappropriate application rates could result in undesirable effects on fruit quality. The efficacy of hexanal may vary depending on the fruit species and cultivar. Furthermore, high concentrations or prolonged exposure might induce tissue damage in certain fruits [2]. Additionally, hexanal may interfere with the production or metabolism of other significant volatile compounds.
The synthesis of aromatic VOCs is primarily influenced by the availability of the precursors. Therefore, the addition of hexanal during storage can potentially facilitate the regeneration of aromatic VOCs. While most of the research to date has focused on the effects of hexanal on the shelf life of apples, few studies have investigated its influence on aromatic compounds in apples. In particular, there has not yet been a study in which apples of the ‘Fuji Kiku’ variety were stored in a hexanal-enriched atmosphere for an extended period of time. Therefore, the aim of this study was to investigate the effects of hexanal addition to the storage atmosphere on the production of aroma compounds in ‘Fuji Kiku’ apples during extended cold storage and shelf life under different temperature conditions and time periods. In addition to evaluating the effects on aromatic compounds, this study also investigated VOCs in apple juice extracted from the apple flesh to determine whether hexanal penetrates and is metabolized in fruit. In addition, the effects of hexanal on the firmness and colour of apples, two important quality indicators, were investigated. By investigating these different aspects, the impact of hexanal on the quality of apples during and after storage should be clarified, contributing to the development of more efficient storage strategies and improving the overall quality of stored apples for the benefit of the food industry and consumers.
2. Materials and Methods
2.1. Plant Material, Treatments, and Storage
The apple fruit (Malus domestica Borkh.) cultivar ‘Fuji Kiku’ was obtained from a commercial orchard in Sadjarstvo Mirosan (46°13′46″ N, 15°11′05″ E; 248 m above sea level). The apples were harvested at the commercial maturity stage. A total of 52 kg of apples of uniform colour and size, free of disease and insect infestation, were selected for the study. Then, 26 kg of randomly selected apples were placed in two separate chambers with a volume of 125 L under 2 kPa O2 and 98 kPa N2 at 1 °C and 90–95% relative humidity (controlled atmosphere (CA)). The apples were then stored for 24 weeks. One chamber was an untreated control, whereas in the other chamber, consistent addition of hexanal gas was carried out to maintain a specific concentration of hexanal in the storage atmosphere, 70 µg/L within the chamber space. The concentration of hexanal applied was carefully chosen to exceed the endogenous levels naturally present in apples but remained below thresholds that could cause any toxic effects on apple tissue. Regarding the treatment time, while the duration in a controlled atmosphere (CA) typically varies depending on the apple variety, storage conditions, and market requirements, ‘Fuji’ apples are commonly stored in a CA for approximately 7 months. In our experiment, hexanal was continuously maintained in the storage chamber throughout the entire duration of cold storage. Therefore, the treatment duration was determined based on the storage period of the apples. The apples were stored for 24 weeks under these conditions.
2.2. Flesh Firmness
To determine the firmness of the apples, Fruit Texture Analyzer (Guss, Strand, Western Cape, South Africa), was used in this study. Firmness was measured at 24 weeks of cold storage and expressed as kg/cm2 after. Measurements were made at four locations (equatorial region, peel removal) on ten individual fruit samples using an 11 mm plunger.
2.3. Colour Measurements
The colour measurements were performed using a colorimeter (CR-400; Minolta, Kyoto, Japan). The Commission Internationale de l’Eclairage (CIE) parameters (L*, a*, b*) were determined on 15 fruits from each chamber after 24 weeks of cold storage. L* represents the brightness of the colour, a* represents the position between red (+) and green (−), and b* represents the scale between blue (−) and yellow (+). The total colour difference was calculated using Equation (1).
where ΔE is defined as ’very distinct’ for ΔE > 3, ‘distinct’ for 1.5 < ΔE < 3, and ‘nondistinct’ for ΔE < 1.5.
Total colour difference (ΔE) = ([Δa*]2 + [Δb*]2 + [ΔL*]2)1/2
2.4. Extraction of the VOCs from the Apple Fruit Headspace in the Storage Chambers During the Cold Storage
Apple volatiles were extracted using a solid-phase microextraction (SPME) fibre coated with a polydimethylsiloxane/divinylbenzene/carboxen/(PDMS/DVB/CAR) sorbent (1 cm long, 50/30 μm thick, StableFlex™, Supelco, Bellefonte, PA, USA) inserted into the chamber exit line sampling port for 5 min.
VOCs were sampled at the beginning of the storage period and after 2, 7, 12, 17, 20, and 24 weeks of cold storage.
2.5. Extraction of VOCs from the Apple Fruit Headspace During Shelf Life
For the analysis of volatile aroma compounds and to mimic retail conditions after storage, three apples from each cell were placed in a 2.5-L jar. Three jars from each chamber were stored at 1 °C and three jars were stored at 20 °C. VOCs from the apple fruit headspace were extracted using HS-SPME by exposing the fibre for 5 min after the needle was inserted into the jar. Subsequently, the fibre was desorbed using gas chromatography. The VOCs were analysed at 1, 7, and 14 days after jar closure.
2.6. Extraction of VOCs from the Apple Juice Headspace During Shelf Life
The extraction of juice was performed from apples stored for 24 h and 7 days at 20 °C. An amount of 0.5 mL of juice was squeezed from the apple flesh and placed in a 20 headspace vial. After 20 min, 1.5 mL of 5 M NaCl and IS 2-octanol 5 μL (332 mg/L) were added. The VOCs from the headspace of the apple juice were extracted using HS-SPME by exposing the fibre for 5 min after the needle was inserted through the silicone septum. The fibres were subsequently desorbed using a gas chromatograph. Each sample was analysed in five replicates. Analyses were carried out at 1 and 7 days of shelf life at 20 °C.
2.7. Determination of VOCs
Analysis by gas chromatography/mass spectrometry (GC/MS) was performed using an Agilent Technologies GC 7890 A gas chromatograph coupled to a Gerstel GmbH MPS2 multipurpose autosampler and an Agilent Technologies 5975C mass spectrometer (Agilent Technology, Santa Clara, CA, USA). The VOCs were desorbed in a GC injector port at 250 °C in splitless mode for 2 min. The gas chromatograph was equipped with a ZB-WAX capillary column (60 m × 0.32 mm i.d., 1 μm film thickness). Helium was used as the carrier gas, which flowed at 1.2 mL/min at 40 °C. The oven temperature program ran for 5 min at 40 °C and was then increased to 230 °C at 4 °C/min. A mass selective detector (5975C, Agilent Technologies) identified the VOCs in the range of 30 to 250 m/z, with the ion source and quadrupole temperatures maintained at 250 and 150 °C, respectively. VOC identification was performed by matching the mass spectra with the NIST 2.0 mass spectral database. When available, commercial standards were used for further confirmation. Calibration curves were generated for each compound to quantify VOCs. VOC concentrations were determined from the peak areas of selected ions and corresponding standards of known concentration. α-Farnesene was considered as a limonene equivalent, and for VOCs without available standards, quantification was performed as a butyl butanoate equivalent. The list of VOCs, the retention time, and the quantitative and qualitative ions of the VOCs used in the study are shown in Table A1.
2.8. Data Analysis
Statistical analyses were conducted using the SPSS software (version 23). Differences in maturity parameters and volatile production among various groups were evaluated using t-tests and one-way ANOVA with post-hoc Tukey’s tests. The results were considered statistically significant when the p-value was less than 0.05, corresponding to a 95% confidence interval. Visualization of the data as a heatmap and multivariate exploratory analysis were carried out using Partial Least Squares Discriminant Analysis (PLS-DA) via MetaboAnalyst (https://www.metaboanalyst.ca (accessed on 5 December 2024). The data matrix was normalized prior to multivariate analysis to ensure equal weighting of each variable during the study.
3. Results
3.1. Flesh Firmness and Colour
The analysis revealed no statistically significant differences in flesh firmness between the control and hexanal-treated fruit. The firmness of the fruit stored under hexanal conditions was measured at 6.59 ± 0.66 kg/cm2, while the control fruit exhibited a comparable value of 6.92 ± 0.71 kg/cm2. After 25 weeks of storage, no significant differences were observed in the L*, a*, and b* colour parameters between the control and treated samples. However, the total colour difference (ΔE) was calculated to be 5.62 ± 2.95, categorizing it as ‘very distinct’ (ΔE > 3), indicating an inhibitory effect of hexanal on colour development. These findings suggest that hexanal treatment had a minimal impact on the firmness and individual colour parameters of the fruit over the storage period. Nevertheless, the substantial total colour difference observed indicates that hexanal may have influenced the overall colour development of the treated fruit compared to the control (Table 1).

Table 1.
Flesh firmness, CIE (L*, a*, b*) parameters, and ΔE for apple fruit after 25 weeks of storage from control group and from the group treated with hexanal.
3.2. VOCs Profile from the Apple Fruit Headspace in the Storage Chambers During Cold Storage
In this study, 34 VOCs were identified, including esters, alcohols, aldehydes, terpenes, and ketones (Figure 1). Among the 25 esters identified, 2-methylbutyl acetate exhibited the highest concentration at the initiation of cold storage, followed by hexyl acetate, hexyl 2-methylbutanoate, and butyl acetate in both the control and hexanal-treated groups.
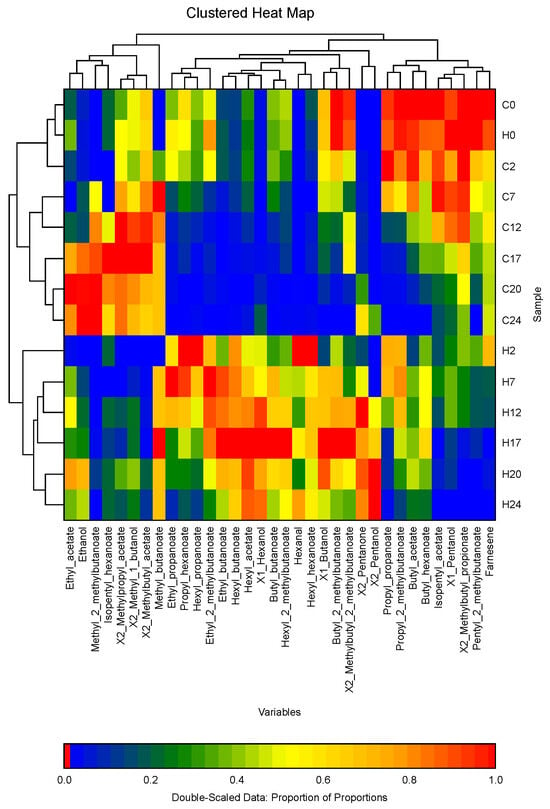
Figure 1.
Clustered heatmap of the VOCs of apple fruit headspace in the storage chambers during the 25 weeks of cold storage. C: control chamber, H: chamber with the addition of hexanal. Numbers (0, 2, 7, 12, 17, 20, 24) adjacent to the letters indicate the storage week.
The heatmap visualization, based on double-scaled data represented as the proportion of proportions, illustrates the volatile profiles of apple headspace samples stored under cold conditions. C represents the control chamber and H represents the chamber with the addition of hexanal. Numbers adjacent to the letters indicate the storage week. The heatmap clustered the VOCs into three major clusters. Cluster 1 includes compounds such as ethyl acetate, ethanol, methyl 2-methylbutanoate, isopentyl hexanoate, 2-methylpropyl acetate, 2-methyl-1-butanol, and methyl butanoate. Cluster 2 comprises hexyl and ethyl esters (except ethyl acetate), propyl hexanoate, 1-hexanol, butyl butanoate, hexanal, 1-butanol, butyl 2-methylbutanoate, 2-methylbutyl 2-methylbutanoate, 2-pentanone, and 2-pentanol. Cluster 3 comprises propyl propanoate, propyl 2-methylbutanoate, butyl acetate, butyl hexanoate, isopentyl acetate, 1-pentanol, 2-methylbutyl propionate, pentyl 2-methylbutanoate, and α-farnesene.
The volatile profiles of both groups were relatively similar during the initial stages of cold storage (C0 and H0), with the most prominent VOCs originating from Cluster 3. The concentration of these VOCs gradually decreased as storage progressed. However, as the storage period progresses, the profiles of the control and hexanal-treated groups began to diverge, as clearly illustrated in the heatmap. The heatmap separates the groups into two distinct clusters: one dominated by VOCs associated with the control group, and the other by those characteristic of the hexanal-treated group. In the later stages of storage, the hexanal-treated group was predominantly characterized by VOCs from Cluster 2, which were present in higher concentrations as compared to those in the control group. In contrast, the control group exhibited lower levels of these VOCs during the same period. Notably, in the later stages of storage, the control group showed an increase in VOCs from Cluster 1. In contrast, the hexanal-treated group contained lower concentrations of these VOCs during later weeks of storage.
The heatmap underscores the significant role of hexanal treatment in modulating volatile profiles of apples during cold storage. Hexanal appears to stimulate the production or retention of esters such as hexyl acetate, ethyl acetate, and butyl 2-methylbutanoate, which are critical for preserving the characteristic aroma of apples.
3.3. VOCs Profile from the Apple Fruit Headspace During Shelf Life
The headspace concentrations of VOCs of apple fruits produced by apple fruits during 1, 7, and 14 days of shelf life at 1 and 20 °C are represented in Table 2. The incorporation of hexanal into the storage atmosphere significantly enhanced the production of linear esters during the shelf life of the ‘Kiku’ apples. This effect was most pronounced after 1 day of storage at 20 °C, with apples in the hexanal group yielding 7.5 times more linear esters than those in the control group.
Among the VOCs studied, hexanal exhibited the strongest effect on hexyl acetate production under all the conditions. The strongest increase occurred after 14 days of storage at 1 °C, with the apples from the hexanal group releasing more than 21 times the amount of hexyl acetate compared to the control group. It is noteworthy that hexanal consistently promoted the formation of all linear esters at least once during shelf life, with the exception of propyl esters, where no significant effect was observed.
Significant differences in the formation of branched esters were observed between the treatment groups. Apples stored in the hexanal-enriched atmosphere exhibited significantly higher concentrations of branched esters after 1 day of shelf life at 20 °C and after 7 days of shelf life at both 1 °C and 20 °C. Among these, 2-methylbutyl acetate was one of the most abundant esters in both groups. While the apples in the hexanal group had lower concentrations of 2-methylbutyl acetate than the control group after one day of shelf life at 1 °C, they produced almost double the amount of this ester after 14 days of shelf life at 20 °C. This ester generally demonstrated less sensitivity to storage temperature than other esters. Ethyl 2-methylbutanoate, an important aroma compound in apples due to its low odour threshold (0.13 μg/L [19]), was exclusively detected in apples from the hexanal group under all tested conditions, while it was absent in the control group. Notable changes were also observed for butyl 2-methylbutanoate, particularly after one day of storage. The apples in the hexanal group released five times more of this ester than the control group at 1 °C and 3.6 times more at 20 °C. Hexyl 2-methylbutanoate was the most dominant branched ester. Treatment with hexanal consistently led to significantly higher concentrations of hexyl 2-methylbutanoate compared to the control, with this effect being especially pronounced at higher temperatures and longer storage times. After 14 days of shelf life at 20 °C, the hexanal group exhibited seven times higher concentrations than the control group.
Although treatment with hexanal promoted the maintenance or increase of certain branched esters, inhibitory effects on the synthesis of other esters were also observed. In particular, apples in the hexanal group had lower concentrations of pentyl 2-methylbutanoate and isopentyl hexanoate than those in the control group. The influence of hexanal on ester production was dependent on both the duration and temperature during the shelf-life period. A similar trend was observed for 2-methylbutyl propionate in the presence of 2-methylbutyl acetate.
Alcohols serve as precursors for ester formation [2]. The group treated with hexanal exhibited significantly higher total alcohol concentrations after 1 day of shelf life at 1 °C and after 7 days of shelf life at 20 °C. The addition of hexanal to the storage atmosphere increased the concentrations of all alcohols during the shelf-life period, with the exception of ethanol, for which no significant differences were observed between the groups, and 2-methyl-1-butanol, which demonstrated an inhibitory effect of hexanal after 1 day of shelf life at 20 °C. The increased alcohol concentrations in the hexanal-treated group depended on shelf-life temperature and storage temperature. Apples from the hexanal group released 5–16 times more hexanol during the shelf-life period. This indicated that hexanal in the apples was effectively converted to hexanol by the action of alcohol dehydrogenase (ADH). Hexanal also increased the production of 2-pentanol under all shelf-life conditions. The most significant difference was observed after 14 days of shelf life at 20 °C, where apples from the hexanal group produced 10 times more 2-pentanol than the control group. Additionally, significant differences in 1-butanol concentrations were observed after 1 day of shelf life at 1 °C and 20 °C, with hexanal-treated apples producing approximately 4.5 to 7 times higher quantities of 1-butanol.
Hexanal itself was only detected in the hexanal-treated group, with the highest concentration detected after 1 day of shelf life at 1 °C. In the control group, hexanal levels were either absent or below the detection limit. After 7 and 14 days of shelf life at 20 °C, hexanal was no longer detected in the hexanal-treated group, suggesting that it was rapidly metabolized at higher temperatures or that enzymatic activity was limited at lower temperatures. Hexanal significantly affected the production of 2-pentanone under all storage conditions. During storage and ripening, oxidative conditions (presence of oxygen) likely promote the conversion of alcohols to ketones, such as 2-pentanone. Conversely, hexanal demonstrated an inhibitory effect on α-farnesene content at both 1 °C and 20 °C after 1 day of storage. However, after 14 days of shelf life at 1 °C, the hexanal-treated apples released higher quantities of α-farnesene than did the control group.
To better distinguish the volatile composition of apples between the control (C) and hexanal-treated (H) groups at different storage temperatures (1 °C and 20 °C) and shelf-life durations (1, 7, and 14 days), Partial Least Squares Discriminant Analysis (PLS-DA) was performed (Figure 2). The PLS-DA score plot revealed a clear separation between groups, with Component 1 explaining 31.1% of the variance and Component 2 accounting for 18.3%. The analysis demonstrated that hexanal-treated samples were distinctly separated from control samples, particularly at extended shelf-life durations and higher temperatures, reflecting the significant impact of hexanal on VOC production. The separation along component 1 captured most of the variance between the groups. The hexanal-treated groups were distinctly separated from the control groups, particularly at prolonged shelf-life durations and higher temperatures. Ellipses highlight the variance within each group. Hexanal-treated apples often exhibit larger ellipses, particularly at higher temperatures, and a prolonged shelf life, reflecting more dynamic changes in their metabolic profiles. Compounds such as butyl hexanoate (VIP score = 1.8), 2-methyl-1-butanol (1.7), 2-methylbutyl acetate (1.6), 2-methylpropanol (1.5), and 2-pentanol (1.4) were identified as the most discriminant VOCs influencing group separation, as indicated by their high Variable Importance in Projection (VIP) scores. These volatiles were predominantly associated with the control group, particularly after 14 days of shelf life at 1 °C. In contrast, 2-pentanol, 1-hexanol, ethyl-2-methylbutanoate, hexyl acetate, and 2-pentanone were characteristic of the hexanal-treated group. This analysis underscores the pronounced influence of hexanal treatment combined with storage temperature and duration on the volatile profiles of apples. These findings have significant implications for postharvest aroma enhancement strategies, demonstrating the potential of hexanal in modulating key aroma-related compounds.

Figure 2.
Partial Least Squares Discriminant Analysis (PLS-DA) of VOCs profile from the apple fruit headspace during shelf life. (a) PLS-DA score plot; (b) variable importance analysis of 15 important VOCs. C: Control group, H: hexanal-treated groups, 1d1: 1 day of shelf life at 1 °C, 7d1: 7 days of shelf life at 1 °C, 14d1: 14 days of shelf life at 1 °C; 1d20: 1 day of shelf life at 20 °C, 7d20: 7 days of shelf life at 20 °C, 14d20: 14 days of shelf life at 20 °C.

Table 2.
The headspace concentrations of VOCs released from the apple fruits during 1, 7, and 14 days of shelf life at 1 and 20 °C.
Table 2.
The headspace concentrations of VOCs released from the apple fruits during 1, 7, and 14 days of shelf life at 1 and 20 °C.
| Plus 1 Day of Shelf Life | Plus 7 Days of Shelf Life | Plus 14 Days of Shelf Life | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +1 °C | +20 °C | +1 °C | +20 °C | +1 °C | +20 °C | |||||||||||||||||||
| Control | Hexanal | Control | Hexanal | Control | Hexanal | Control | Hexanal | Control | Hexanal | Control | Hexanal | |||||||||||||
| VOC (OT (mg/L)) | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd |
| Ethyl acetate (3280 a) | /a | / | 0.09 b | 0.01 | /a | / | 0.18 b | 0.16 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| Ethyl butanoate (9 b) | /a | / | 0.11 b | 0.07 | /a | / | 0.08 b | 0.01 | / | / | 0.05 | 0.04 | / | / | 0.01 | 0.01 | / | / | 0.02 | 0.01 | / | / | 0.01 | 0.00 |
| Propyl propanoate (880 a) | 0.02 | 0.01 | 0.06 | 0.06 | 0.13 | 0.03 | 0.17 | 0.01 | / | / | 0.06 | 0.06 | 0.03 | 0.02 | 0.02 | 0.02 | / | / | 0.03 | 0.03 | 0.03 | 0.02 | 0.06 | 0.00 |
| Butyl acetate (66 a) | 0.26 a | 0.06 | 0.97 b | 0.03 | 0.46 a | 0.06 | 5.71 b | 0.23 | 0.15 a | 0.01 | 1.21 b | 0.62 | 0.08 | 0.03 | 0.13 | 0.07 | 0.07 | 0.01 | 0.81 | 0.52 | 0.06 | 0.03 | 0.18 | 0.01 |
| Butyl butanoate (100 c) | 0.02 a | 0.00 | 0.21 b | 0.11 | 0.20 a | 0.03 | 0.87 b | 0.07 | 0.02 | / | 0.21 | 0.14 | 0.03 | 0.01 | 0.04 | 0.03 | 0.01 | / | 0.11 | 0.09 | 0.03 | 0.01 | 0.08 | 0.01 |
| Hexyl acetate (2 c) | 0.93 a | 0.17 | 7.07 b | 2.06 | 2.25 a | 0.19 | 28.56 b | 1.24 | 0.38 a | 0.04 | 6.26 b | 1.34 | 0.40 a | 0.07 | 1.74 b | 0.44 | 0.14 a | 0.02 | 3.03 b | 1.58 | 0.12 a | 0.05 | 1.55 b | 0.29 |
| Propyl hexanoate (nf) | 0.01 | 0.00 | 0.01 | 0.01 | 0.04 | 0.01 | 0.04 | 0.01 | 0.01 | / | 0.01 | 0.01 | 0.03 | 0.01 | 0.02 | 0.03 | 0.01 | 0.01 | / | / | 0.03 | 0.02 | 0.07 | 0.01 |
| Hexyl propanoate (8 b) | 0.09 | 0.02 | 0.10 | 0.02 | 0.91 a | 0.21 | 2.11 b | 0.49 | 0.03 | 0.01 | 0.11 | 0.06 | 0.06 a | 0.02 | 0.22 b | 0.08 | 0.02 | / | 0.05 | 0.03 | 0.02 a | 0.01 | 0.25 b | 0.02 |
| Butyl hexanoate (700 b) | 0.05 | 0.01 | 0.04 | 0.01 | 0.30 | 0.07 | 0.33 | 0.03 | 0.07 a | 0.01 | 0.04 b | 0.01 | 0.10 | 0.04 | 0.10 | 0.10 | 0.05 | 0.02 | 0.03 | 0.01 | 0.08 | 0.04 | 0.31 | 0.37 |
| Hexyl butanoate (250 b) | 0.05 a | 0.01 | 0.41 b | 0.09 | 0.54 a | 0.24 | 2.28 b | 0.21 | 0.03 a | / | 0.23 b | 0.10 | 0.08 | 0.03 | 0.30 | 0.20 | 0.02 | / | 0.09 | 0.07 | 0.03 | 0.01 | 0.19 | 0.02 |
| Hexyl hexanoate (6400 a) | 0.14 | 0.03 | 0.22 | 0.10 | 0.76 a | 0.37 | 1.89 b | 0.12 | 0.10 a | 0.01 | 0.15 b | 0.01 | 0.19 | 0.08 | 0.58 | 0.38 | 0.07 | 0.01 | 0.08 | 0.01 | 0.07 | 0.03 | 0.27 | 0.02 |
| Total linear estres | 1.56 a | 0.15 | 9.29 b | 1.91 | 5.58 a | 1.08 | 42.23 b | 1.41 | 0.79 a | 0.06 | 8.33 b | 2.12 | 1.01 a | 0.32 | 3.16 b | 0.96 | 0.39 a | 0.03 | 4.25 b | 2.28 | 0.46 | 0.21 | 3.14 | 0.21 |
| Methyl 2-methylbutanoate (0.048 d) | / | / | / | / | 0.01 a | 0.00 | /b | / | / | / | / | / | 0.04 | 0.02 | 0.09 | 0.08 | / | / | / | / | 0.04 | 0.02 | 0.32 | 0.49 |
| 2-Methylpropyl acetate (25 a) | 0.06 a | 0.01 | 0.02 b | 0.01 | 0.09 a | 0.01 | 0.05 b | 0.01 | 0.04 | 0.00 | 0.04 | 0.01 | 0.03 | 0.01 | 0.04 | 0.01 | 0.03 | 0.00 | 0.03 | 0.01 | 0.01 | 0.00 | 0.07 | 0.00 |
| Ethyl 2-methylbutanoate (0.13 b) | /a | / | 0.19 b | 0.08 | /a | / | 0.12 b | 0.03 | / | / | 0.08 | 0.07 | / | / | 0.21 | 0.36 | / | / | 0.03 | 0.03 | / | / | 0.14 | 0.03 |
| 2-Methylbutyl acetate (11 c) | 9.30 a | 1.67 | 4.57 b | 1.59 | 19.20 | 0.66 | 18.64 | 0.99 | 7.26 | 1.34 | 7.91 | 0.53 | 7.77 | 1.80 | 11.84 | 2.47 | 5.23 | 0.94 | 6.90 | 0.98 | 3.35 a | 0.99 | 12.65 b | 1.16 |
| Propyl 2-methylbutanoate (0.02 d) | 0.02 | 0.00 | 0.17 | 0.11 | 0.07 a | 0.01 | 0.30 b | 0.05 | 0.01 | 0.00 | 0.18 | 0.15 | 0.07 | 0.03 | 0.05 | 0.05 | 0.01 | 0.01 | 0.10 | 0.09 | 0.14 | 0.09 | 0.26 | 0.04 |
| Isopentyl acetate (7.2 d) | 0.04 a | 0.00 | 0.03 b | 0.00 | 0.13 a | 0.01 | 0.21 b | 0.02 | 0.02 a | 0.00 | 0.05 b | 0.01 | 0.03 | 0.01 | 0.04 | 0.01 | 0.01 | 0.00 | 0.03 | 0.02 | 0.01 | 0.01 | 0.05 | 0.00 |
| 2-Methylbutyl propionate (nf) | 0.03 a | 0.02 | /b | / | 0.65 a | 0.12 | 0.13 b | 0.03 | 0.04 a | 0.01 | 0.01 b | 0.01 | 0.20 | 0.05 | 0.23 | 0.09 | 0.02 a | 0.01 | /b | / | 0.08 a | 0.03 | 0.32 b | 0.02 |
| Butyl 2-methylbutanoate (17 b) | 0.05 a | 0.01 | 0.26 b | 0.09 | 0.26 a | 0.06 | 0.94 b | 0.14 | 0.07 | 0.01 | 0.32 | 0.19 | 0.19 | 0.09 | 0.29 | 0.23 | 0.05 | 0.01 | 0.22 | 0.15 | 0.25 | 0.14 | 0.73 | 0.16 |
| 2-Methylbutyl 2-methylbutanoate (nf) | 0.07 | 0.03 | 0.12 | 0.03 | 0.45 | 0.08 | 0.54 | 0.07 | 0.12 | 0.03 | 0.17 | 0.08 | 0.89 a | 0.30 | 2.20 b | 0.76 | 0.13 | 0.03 | 0.14 | 0.06 | 0.72 | 0.29 | 3.42 | 0.70 |
| Pentyl 2-methylbutanoate (nf) | 0.01 a | 0.00 | /b | / | 0.06 | 0.01 | 0.02 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.07 | 0.04 | 0.10 | 0.06 | 0.01 a | 0.00 | /b | 0.00 | 0.06 | 0.03 | 0.21 | 0.05 |
| Hexyl 2-methylbutanoate (22 c) | 0.73 a | 0.03 | 1.98 b | 0.08 | 3.66 a | 0.58 | 8.97 b | 0.78 | 0.70 a | 0.11 | 2.34 b | 0.28 | 1.94 a | 0.84 | 8.81 b | 1.69 | 0.56 a | 0.07 | 1.73 b | 0.36 | 1.00 a | 0.47 | 11.58 b | 0.91 |
| Isopentyl hexanoate (nf) | 0.04 a | 0.02 | 0.02 b | 0.00 | 0.44 a | 0.10 | 0.14 b | 0.02 | 0.08 a | 0.02 | 0.02 b | 0.00 | 0.30 | 0.10 | 0.43 | 0.21 | 0.10 a | 0.02 | 0.02 b | 0.00 | 0.12 | 0.04 | 0.48 | 0.09 |
| Total branched estres | 10.35 | 1.76 | 7.36 | 1.27 | 25.03 a | 1.22 | 30.06 b | 0.52 | 8.36 a | 1.49 | 11.12 b | 0.24 | 11.53 a | 3.17 | 24.32 b | 2.89 | 6.15 | 1.07 | 9.21 | 1.65 | 5.78 | 1.87 | 29.95 | 0.79 |
| Ethanol (10,000 a) | 0.22 | 0.10 | 0.52 | 0.26 | 0.76 | 1.09 | 0.91 | 0.56 | 0.23 | 0.16 | 0.13 | 0.03 | 0.30 | 0.40 | 0.37 | 0.57 | 0.11 | 0.09 | 0.09 | 0.02 | 0.03 | 0.01 | 0.22 | 0.08 |
| 2-Pentanol (nf) | 0.01 a | 0.00 | 0.07 b | 0.00 | 0.03 a | 0.01 | 0.15 b | 0.03 | 0.01 a | 0.00 | 0.09 b | 0.01 | 0.03 a | 0.01 | 0.28 b | 0.05 | 0.01 a | 0.00 | 0.07 b | 0.00 | 0.02 a | 0.00 | 0.42 b | 0.02 |
| 1-Butanol (492 a) | 0.25 a | 0.06 | 1.82 b | 0.85 | 0.71 a | 0.13 | 3.33 b | 0.27 | 0.15 | 0.03 | 1.65 | 0.95 | 0.14 | 0.02 | 0.20 | 0.11 | 0.05 | 0.02 | 0.94 | 0.67 | 0.08 | 0.04 | 0.35 | 0.03 |
| 2-Methyl-1-butanol (1200 a) | 7.76 | 0.40 | 5.35 | 0.86 | 10.28 a | 2.47 | 8.97 b | 0.52 | 8.08 | 1.91 | 7.89 | 1.24 | 9.56 | 1.57 | 11.22 | 1.24 | 6.89 | 0.62 | 6.97 | 1.61 | 7.66 | 1.58 | 17.60 | 1.70 |
| 1-Pentanol (150 a) | 0.05 | 0.01 | 0.05 | 0.02 | 0.10 | 0.02 | 0.12 | 0.02 | 0.03 a | 0.01 | 0.07 b | 0.02 | 0.04 | 0.01 | 0.07 | 0.02 | 0.02 a | 0.00 | 0.05 b | 0.02 | 0.03 | 0.01 | 0.12 | 0.03 |
| 1-Hexanol (2500 a) | 0.74 a | 0.16 | 7.31 b | 0.44 | 1.01 a | 0.17 | 12.84 b | 0.69 | 0.29 a | 0.10 | 4.65 b | 0.83 | 0.35 a | 0.09 | 1.85 b | 0.02 | 0.14 | 0.03 | 2.25 | 0.89 | 0.13 a | 0.04 | 2.59 b | 0.00 |
| Total alcohols | 9.03 a | 0.35 | 15.12 b | 1.99 | 12.89 | 3.64 | 26.31 | 0.83 | 8.78 | 2.00 | 14.47 | 2.94 | 10.42 a | 1.87 | 13.99 b | 0.49 | 7.22 | 0.73 | 10.37 | 3.20 | 7.94 | 1.51 | 20.81 | 0.64 |
| 2-Pentanone (2300 d) | 0.03 a | 0.01 | 0.19 b | 0.02 | 0.03 a | 0.01 | 0.30 b | 0.13 | 0.02 a | 0.00 | 0.17 b | 0.02 | 0.01 a | 0.01 | 0.13 b | 0.05 | 0.01 a | / | 0.13 b | 0.02 | /a | / | 0.17 b | 0.01 |
| Hexanal (5 a) | /a | / | 11.72 b | 2.34 | /a | / | 0.67 b | 0.10 | /a | / | 0.55 b | 0.06 | / | / | / | / | /a | / | 0.14 b | 0.01 | / | / | / | / |
| α -Farnesene (87 a) | 1.33 a | 0.02 | 0.21 b | 0.00 | 9.63 a | 0.32 | 1.92 b | 0.26 | 1.21 | 0.20 | 0.24 | 0.01 | 6.79 | 1.32 | 4.30 | 1.91 | 1.00 a | 0.05 | 0.18 b | 0.03 | 4.20 | 1.51 | 7.01 | 4.95 |
| Total other | 1.36 a | 0.01 | 12.13 b | 2.36 | 9.66 a | 0.33 | 2.89 b | 0.15 | 1.22 | 0.20 | 0.96 | 0.08 | 6.80 | 1.32 | 4.43 | 1.95 | 1.01 a | 0.05 | 0.44 b | 0.05 | 4.21 | 1.51 | 6.45 | 4.96 |
Data are means ± SD (n = 3). VOC: Volatile compound; OT: odour threshold; nf: not found. Statistically significant differences are indicated by different letters in the same row under identical shelf-life conditions. Conversely, the mean values without accompanying letters did not exhibit significant differences. OT for VOC in water from reference: a [20],b [19],c [2],d [21].
3.4. VOCs from the Apple Juice Headspace During Shelf Life
To investigate whether hexanal metabolism occurred in the flesh of the apples, juice was extracted from the flesh of intact fruits for VOCs analysis using HS-SPME. This was performed after 24 h and 7 days of shelf life at 20 °C. A total of nine alcohols, seven esters, two aldehydes, and one ketone were identified. Notably, compounds such as 3-hexanol, trans-2-hexenal, 2-Methyl-2-butenol, heptanol, 6-methyl-5-hepten-2-ol, and ethylhexanol were detected exclusively in the juice and not in the intact fruit (Table 3).

Table 3.
The headspace concentrations of VOC from the apple juice headspace during shelf life after 1 and 7 days of shelf life at 20 °C. Data are means ± SD (n = 5).
No significant differences in hexanal content were observed between the control and hexanal-treated groups in the flesh juice. However, in the hexanal-treated group, there was a noticeable increase in several VOCs after one day of shelf life at 20 °C. In both groups, the most abundant compound was 2-methylbutyl acetate, with the hexanal-treated group exhibiting more than twice the quantity of this compound compared to the control. The second most abundant compound was 2-methyl-1-butanol, which was found in greater quantities in intact apples from the control group. Interestingly, in flesh juice, the hexanal-treated group contained higher levels of 2-methyl-1-butanol compared to the control group.
In addition, compounds such as 2-pentanone, isopentyl acetate, 3-hexanol and 2-methylbutyl 2-methylbutanoate were only detected in the hexanal-treated group after one day of shelf life, while 2-pentanone and 3-hexanol remained even after 7 days of shelf life. After 7 days of shelf life at 20 °C, the differences between the two groups diminished, with only 2-pentanone, 2-pentanol, and 3-hexanol present in higher concentrations in the hexanal-treated group. These results indicate that the influence of hexanal on the profile of VOC in fruit juice is primarily limited to the early stages of shelf life.
4. Discussion
The aim of this study was to investigate the impact of hexanal supplementation on the storage atmosphere of ‘Fuji Kiku’ apples, specifically focusing on the concentration of VOCs in the apple fruit headspace within the storage chambers during cold storage, as well as in the headspace of apple fruit and apple juice after 1 and 7 days of shelf life. Furthermore, this research assessed the effect of hexanal on flesh firmness and colour following extended cold storage.
Hexanal treatment has been demonstrated to significantly enhance fruit firmness across various species, contributing to improved shelf life and quality retention during storage [22,23,24]. Furthermore, studies have shown that hexanal can decrease the expression of genes responsible for producing enzymes associated with ethylene production [25], which is associated with improving the firmness [26]. In our investigation, no significant differences in firmness were observed between the control group and ‘Fuji Kiku’ apples exposed to a hexanal-enriched atmosphere during cold storage.
Sulaimankhil et al. [14] performed research on applying 0.03% hexanal as a dip treatment (3 min immersion) to freshly harvested ‘Royal Delicious’ apples. The authors came to the conclusion that the treated fruits maintained better flesh firmness after 3 months of storage at 1–2 °C. The application of low-concentration hexanal as a dip treatment has been shown to be the most effective in preserving firmness in the above-mentioned study. This positive outcome is primarily due to the fact that higher concentrations of hexanal can potentially induce detrimental effects on fruit texture. Consistent with our results, hexanal applied to ’Honeycrisp’ apples as a preharvest spray did not exhibit statistically significant differences in firmness between hexanal-treated and control fruits. However, the fruits treated with hexanal only retained a greater firmness compared to the control only after removal from 30 days of cold storage and subsequent storage for 14 days at room temperature [27].
Our results indicated an inhibitory effect of hexanal on colour development. This inhibitory effect on colour could potentially be attributed to hexanal’s influence on ripening processes or pigment formation during storage. Similarly, Cheema at al. observed that tomatoes treated with hexanal exhibited a less intense red colour compared to control fruit, suggesting an inhibition of the ripening processes [23]. The authors assert that the reduced intensity of the red colour in tomatoes treated with the hexanal formulation provides clear evidence of inhibited ripening processes. The inhibitory effect on colour development and delay of fruit ripening was also observed on bananas [28] and cherries [29]. Hexanal applied as a preharvest spray did not demonstrate significant differences in colour parameters between treatments and controls in ’Honeycrisp’ apples [27]. Although our results contradict the findings of previous studies on the efficacy of hexanal in enhancing fruit firmness and colour development, it is essential to consider that the impact of hexanal treatment may vary depending on the apple cultivar, storage conditions, and hexanal concentration. The absence of significant differences in firmness between the treated and control ‘Fuji Kiku’ suggests that this particular variety might be less sensitive to hexanal or that other factors may influence the results.
Fruit aromatic compounds are secondary metabolites synthesized through a series of enzymatic reactions during the fruit’s growth and postharvest stages. Numerous studies have identified primary metabolites such as fatty acids, amino acids, and carbohydrates as key precursors for the production of these VOCs [30]. The aromatic profile of the fruit is determined by a combination of factors, including the composition, concentration, and odour threshold of individual VOCs. The volatile esters, which exhibit in high proportion and have low odour thresholds, primarily contribute to fruit aroma [31]. These esters typically comprise fruity aromas and play an important role in the flavour profile of apples [32]. VOCs in fruits can be categorized into two main groups: primary aromatic compounds, which are naturally produced by the fruit during its growth and ripening (referred to as primary aroma), and secondary aromatic compounds, which are formed as the results of tissue damage, such as peeling, cutting, or pressing (secondary aroma). The formation of secondary aromatic compounds occurs through the release of enzymes from damaged cells, catalysing reactions involving VOCs [33]. Consequently, the volatile aromatic profile of apple juice differs from that of intact apples [34]. Numerous VOCs that are important for fruit aroma are produced by cell disruption, which occurs during cutting or crushing. Among these, aldehydes and six-carbon alcohols, such as cis-3-hexenal, its structural isomer trans-2-hexenal, hexanal, and the corresponding alcohols, are synthesised when the lipoxygenase (LOX) pathway becomes active after tissue damage [35]. In our study, esters were the predominant VOCs in intact apples, while alcohols and aldehydes were more prevalent in apple juice, such as 3-hexenol, trans-2-hexenal, 2-methyl-2-buten-1-ol, heptanol, 6-methyl-5-hepten-2-ol, and ethylhexanol, which were detected exclusively in the juice and not in the intact fruit.
Esters can be classified into linear-chain and branched-chain esters based on their carbon skeleton structures, with precursors typically originating from fatty acid metabolism for linear-chain esters and branched-chain amino acid metabolism for branched-chain esters [2,36]. In this study, butyl acetate, hexyl acetate, 2-methylbutyl acetate, and ethyl 2-methylbutyrate were identified as the most important esters in ripe apple fruits due to their high concentrations. Among the 25 esters identified, 2-methylbutyl acetate exhibited the highest concentration at the onset of cold storage, followed by hexyl acetate, hexyl 2-methylbutanoate, and butyl acetate in both the control and hexanal-treated groups. These findings are consistent with those of previous studies, which also reported high concentrations of 2-methylbutyl acetate, hexyl acetate, and butyl acetate in ‘Redchief’ apples [37]. Furthermore, esters such as butyl acetate, 2-methylbutyl acetate, butyl 2-methylbutanoate, hexyl acetate, and hexyl 2-methylbutyrate have been reported to be the most abundant volatiles in ‘Fuji’ apples [38], In the later stages of storage, the hexanal-treated group was predominantly characterized by esters, including hexyl and ethyl esters (with the exception of ethyl acetate), propyl hexanoate, butyl butanoate, butyl 2-methylbutanoate, 2-methylbutyl 2-methylbutanoate, and 2-methylpropyl acetate. These compounds were present in higher concentrations in the hexanal-treated group compared to the control group. Conversely, the control group exhibited higher levels of ethyl acetate, methyl 2-methylbutanoate, isopentyl hexanoate, 2-methylpropyl acetate, and methyl butanoate, which were more prominent in the control group. It is worth noting that apples are usually consumed a week or more after storage chambers are unsealed. Consequently, the observed decrease in metabolite compounds and other esters, which contribute to the characteristic flavour of the fruit, is particularly significant [39]. The influence of hexanal on ester production was evident throughout the storage period, shelf life, and even in the secondary aroma as detected in apple juice. The impact varies depending on time and temperature. Hexanal in particular had the strongest effect after one day of shelf life at 20 °C, with increased levels of hexyl acetate, hexyl propionate, 2-methylbutyl acetate, 2-methylbutyl propionate, and hexyl 2-methylbutanoate. Hexyl acetate has been identified as the most abundant linear ester, characterised by an odour threshold of 2 µg/L and sweet and fruity descriptors [2]. Its high concentration significantly contributed to the aroma of ‘Fuji’ apples. The apple fruit headspace in the storage chambers from the hexanal group contained higher levels of this ester compared to the control group. This trend continued throughout the shelf life, with hexanal-treated apples showing increased formation of hexyl acetate, particularly after one day of shelf life at 20 °C. Furthermore, analysis of VOCs in apple juice revealed elevated levels of hexyl acetate in the hexanal-treated group after one day of shelf life at 20 °C, but no such increase was observed after seven days of shelf life. Hexyl 2-methylbutanoate, another prominent ester, was more abundant in the hexanal-treated group during cold storage and throughout shelf life under all conditions. However, this ester was no longer detected in the apple juice. Hexyl 2-methylbutanoate is typically associated with descriptors such as “apple” and “grape,” and has an odour threshold of 22 µg/L [2]. In the hexanal-treated group, there was also a notable increase in other esters that were not directly associated with hexanal or its aldehyde. For instance, ethyl 2-methylbutanoate, an important aroma compound in apples with a very low odour threshold (0.13 µg/L [19]) and described with fruity and strawberry notes [2], was found exclusively in the hexanal-treated apples under all shelf life conditions, but was absent in the control group. This compound was not detected in the juices of either group. Alcohols serve as precursors for ester formation, with alcohol acetyltransferases (AATs) being crucial enzymes in this biosynthetic pathway, catalysing the final step using coenzyme A donors and alcohol acceptors [40]. The type and concentration of the alcohol substrates significantly influenced ester production. For example, MpAAT1 preferentially forms hexyl esters with C3, C6, and C8 CoAs, and its preference for acetate esters is dependent on substrate concentration. The metabolism of alcohols in apple fruit tissue demonstrates that higher alcohols are esterified more rapidly, and both cortex and peel tissues can acetylate butanol and 2-methylpropanol at all stages of maturity [40].
In our study, 2-methyl-1-butanol was found to be the predominant alcohol, which is consistent with the ‘Fuji’ apples from the study by Qi et al. [41]. In the later stages of storage, the hexanal-treated group exhibited higher levels of 1-hexanol, 1-butanol, and 1-propanol compared to the control group. This trend continued during shelf life, with apples from the hexanal group producing consistently higher concentrations of these alcohols, and their elevated levels were also observed in apple juice. Conversely, hexanal inhibits the production of 2-methyl-1-butanol in intact apples. However, the apple juice of the hexanal-treated group contained higher concentrations of this alcohol after 1 d of storage at 20 °C. During shelf life, apples rapidly metabolize hexanal, with this process occurring more rapidly at higher temperatures. Apples from the hexanal group exhibited a 5–16-fold increase in hexanol release during the shelf-life period. Following 14 days of shelf life at 20 °C, hexanal was no longer detectable in the headspace of the intact apples. This observation suggests that the enzymes responsible for hexanal conversion, such as alcohol dehydrogenase (ADH), which catalyses the conversion of hexanal to hexanol, are regenerated following long-term cold storage. Similar results were reported by Song et al. [42], who observed that hexanal was rapidly converted into aroma volatiles in ’Jonagold’ and ’Golden Delicious’ apple slices. After 20–30 h of treatment, a significant increase in the production of hexanol and hexyl acetate was noted. It is noteworthy that hexanal was no longer detectable in the headspace of the treated fruit within 16 h of treatment. Similarly, Fan et al. found that the treatment of ’Golden Delicious’ apples with hexanal at 22 °C for 2 days resulted in the formation of volatiles such as hexyl acetate, hexyl butanoate, butyl acetate, and hexyl-2-methylbutanoate. However, after 7 days, the concentrations of these compounds returned to their original levels [12].
The addition of hexanal to the storage atmosphere exhibited a significant effect on the concentration and profile of VOCs throughout the storage period and during shelf life. These findings suggest that the addition of hexanal to the storage atmosphere could be a valuable strategy for maintaining the sensory quality of apples during prolonged storage.
Hexanal can act as a precursor for various esters and other VOCs. Its presence in the storage environment appears to stimulate the production of certain volatiles, particularly esters and alcohols. Our results suggest that hexanal not only enhanced the production of specific aroma compounds but also played a role in preserving or promoting the synthesis of particular volatiles over time. This contributed to the overall aroma profile of ‘Fuji Kiku’ apples during storage and after shelf life. These results may indicate the involvement of hexanal in modulating the enzymatic pathways related to the formation of VOCs, especially by stimulating alcohol and ester synthesis. For example, Rowan et al., using deuterated hexanal, demonstrated in ‘Granny Smith’ and ‘Red Delicious’ apples that hexanal can be metabolized to hexanoic acid [43]. This compound can then undergo β-oxidation to form butanoic acid, which serves as a precursor for butyl and butanoate esters, or α-oxidation to form pentanoic acid, which is the basis for pentyl and pentanoate ester formation.
While previous studies have explored the use of hexanal for extending the shelf life of apples and other fruits, our study provides a novel focus on its effects on the VOCs profiles, specifically in the ’Fuji Kiku’ apple variety. This variety has not been extensively studied in the context of hexanal supplementation, which addresses a significant gap in the literature. Our research is distinguished by its emphasis on the influence of hexanal on aroma-related compounds during both cold storage and shelf life under different conditions. Unlike most studies that primarily investigate firmness and general quality parameters, we employed advanced analytical techniques, such as GC/MS and PLS-DA, to elucidate specific changes in the aromatic profiles of apples and apple juice. These findings provide deeper insights into the metabolic pathways influenced by hexanal and its potential to enhance consumer-perceived quality by modulating key esters, alcohols, and other volatiles critical for apple aroma. While the study successfully demonstrates the influence of hexanal on VC profiles during cold storage and short-term shelf life, its effects diminish over an extended shelf life, particularly at higher temperatures. This temporal limitation could pose challenges for commercial applications, where storage durations and conditions vary. Moreover, the absence of sensory evaluations leaves a gap in understanding the practical implications of VC changes for consumer experience. To enhance the study’s impact, future research could include comparative analyses across multiple apple varieties to generalize the findings. Sensory evaluations would provide a direct link between chemical changes and consumer preferences, strengthening the practical relevance of the results. Dose-response studies could optimize hexanal concentrations for specific storage conditions, and mechanistic investigations into the enzymatic pathways influenced by hexanal would offer deeper insights. Additionally, exploring combinations of hexanal with other postharvest technologies, such as modified atmosphere packaging or ethylene inhibitors, could extend its efficacy. While the study demonstrates hexanal’s potential to modulate aroma-related compounds, further research is needed to refine its application for broader adoption in the fruit industry. Furthermore, our study extends beyond storage effects by examining the impact of hexanal treatment on apple juice aroma, which has practical implications for the juice industry. The inclusion of both intact fruit and juice headspace analyses offers a broader understanding of hexanal’s application. These unique aspects of our research contribute to the body of knowledge on postharvest treatments, with potential applications for improving both the sensory and nutritional quality of apples, particularly under varying storage and distribution conditions.
5. Conclusions
In conclusion, this study showed that the addition of hexanal to the storage atmosphere significantly affected the VOC profiles of ‘Fuji Kiku’ apples during long-term cold storage as well as during shelf life. Hexanal stimulated the production and retention of important VOCs in ‘Kiku Fuji’ apples during storage and shelf life. This study highlights the potential of hexanal in enhancing aroma-related compounds and offers new strategies to improve the aroma of apples after harvest. These findings could lead to improved storage techniques that maintain or improve the quality and aroma of apples, benefiting both the fruit industry and consumers. The potential of hexanal to modulate aroma-related compounds in apples should be further investigated by studying its effects on different apple varieties and storage conditions.
Author Contributions
E.J.: Performed the experiments, data curation, visualization, writing—original draft preparation; R.V.: writing—review and editing; E.Z.: conceptualization, methodology, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Slovenian Research and Innovation Agency, through research program ≫Integrated food technology and nutrition≪ (P4-0234) and by the Department of Food Science and Technology, Biotechnical Faculty, University of Ljubljana, Jamnikarjeva 101, SI-1000 Ljubljana.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank the Department of Food Science and Technology, Biotechnical Faculty, University of Ljubljana, Jamnikarjeva 101, SI-1000 Ljubljana.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
VOCs, retention time, qualitative (first), quantitative, and ions used in the study.
Table A1.
VOCs, retention time, qualitative (first), quantitative, and ions used in the study.
| RT | ION | |
|---|---|---|
| Ethyl acetate | 10.04 | 61.0, 70.1, 88.1 |
| Ethanol | 11.81 | 31.1. 45.1, 43.0 |
| Ethyl propanoate | 12.78 | 102.1. 75.00, 87.1 |
| Propyl acetate | 13.49 | 61.10, 73.10 |
| 2-Pentanone | 13.682 | 86.1, 71.1, 58.1 |
| Methyl butanoate | 13.97 | 74.0, 87.10, 58.90 |
| Methyl 2-methylbutanoate | 14.92 | 88.1, 101.10, 69.00 |
| 2-Methylpropyl acetate | 15.04 | 73.00, 61.10, 101.10 |
| Ethyl butanoate | 16.04 | 71.10, 88.10, 101.00 |
| Propyl propanoate | 16.37 | 75.00, 86.90, 59.10 |
| Ethyl 2-methylbutanoate | 16.66 | 102.10, 85.10, 115.10 |
| Butyl acetate | 17.6 | 73.10, 61.10, 87.10 |
| Hexanal | 18.12 | 72.10, 82.00, 67.10 |
| 2-Pentanol | 19.29 | 73.10, 87.00, 40.90 |
| 2-Methylbutyl acetate | 19.64 | 70.10, 61.10, 85.10 |
| Propyl 2-methylbutanoate | 20.26 | 85.10, 103.10, 74.10 |
| 1-Butanol | 20.4 | 56.10, 41.10, 75.10 |
| Isopentyl acetate | 21.76 | 70.10, 61.00, 73.10 |
| 2-Methylbutyl propionate | 22.32 | 70.1, 87.00, 75.10 |
| 3-Hexanol | 22.36 | 59.10, 73.10, 55.10 |
| 2-Methyl-1-butanol | 22.88 | 70.10, 57.10, 53.10 |
| Butyl butanoate | 23.49 | 71.10, 89.10, 101.1 |
| Trans-2-hexenal | 23.94 | 69.10, 83.10, 98.20 |
| Butyl 2-methylbutanoate | 23.99 | 103.1, 85.10, 130.00 |
| 1-Pentanol | 24.57 | 55.10, 70.10, 31.10 |
| Hexyl acetate | 25.6 | 84.10, 69.10, 101.10 |
| 2-Methylbutyl 2-methylbutanoate | 25.84 | 70.10, 85.00, 103.10 |
| 2-Heptanol | 27.05 | 83.10, 70.10, 98.10 |
| Propyl hexanoate | 27.3 | 99.10, 117,310, 61.00 |
| 2-Methyl-2-butenol | 27.34 | 71.10, 86.10, 53.10 |
| Pentyl 2-methylbutanoate | 27.61 | 103.00, 85.10, 70.10 |
| Hexyl propanoate | 28.06 | 75.10, 84.20, 69.10 |
| 1-Hexanol | 28.41 | 56.10, 69.10, 84.10 |
| Butyl hexanoate | 30.67 | 117.10, 99.10, 71.10 |
| Hexyl butanoate | 30.76 | 89.10, 84.10, 71.10 |
| Hexyl 2-methylbutanoate | 31.11 | 103.10, 85.10, 74.10 |
| Heptanol | 31.96 | 70.1, 56.20, 83.10 |
| Isopentyl hexanoate | 32.01 | 70.10, 99.10, 117.10 |
| 6-Methyl-5-hepten-2-ol | 32.2 | 95.10, 69.10, 111.10 |
| Ethylhexanol | 33.09 | 57.2, 83.20, 112.20 |
| Hexyl hexanoate | 37.11 | 117.10, 99.10, 84.10 |
| α-Farnesene | 41.33 | 93.10, 107.10, 119.10 |
References
- Fedrigotti, V.B.; Fischer, C. Why per capita apple consumption is falling: Insights from the literature and case evidence from south tyrol. Horticulturae 2020, 6, 79. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Sepúlveda, D.R.; González-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A review. Food Technol. Biotechnol. 2016, 54, 375. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral Scents and Fruit Aromas: Functions, Compositions, Biosynthesis, and Regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef]
- Roberts, G.; Spadafora, N.D. Analysis of Apple Flavours: The Use of Volatile Organic Compounds to Address Cultivar Differences and the Correlation between Consumer Appreciation and Aroma Profiling. J. Food Qual. 2020, 2020. [Google Scholar] [CrossRef]
- Yang, S.; Hao, N.; Meng, Z.; Li, Y.; Zhao, Z. Identification, comparison and classification of volatile compounds in peels of 40 apple cultivars by hs–spme with gc–ms. Foods 2021, 10, 1051. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Mi, H.; Pristijono, P.; Ge, Y.; Lv, J.; Li, Y.; Liu, B. Tissue-Specific Recovery Capability of Aroma Biosynthesis in ‘Golden Delicious’ Apple Fruit after Low Oxygen Storage. Agronomy 2022, 12, 2794. [Google Scholar] [CrossRef]
- Ashitha, G.N.; Sunny, A.C.; Nisha, R. Effect of Pre-harvest and Post-harvest Hexanal Treatments on Fruits and Vegetables: A Review. Agric. Rev. 2020, 41, 124–131. [Google Scholar] [CrossRef]
- FDA. NOF/\LAB Laboratories Voe (Volatile Organic Compounds); FDA: Silver Spring, MD, USA, 2019. [Google Scholar]
- Sulaimankhil, Z.; Sethi, S.; Sharma, R.R.; Verma, M.K.; Bhowmik, A. Influence of hexanal concentration and exposure time on quality of cold stored apples (Malus domestica). Indian J. Agric. Sci. 2021, 91, 713–717. [Google Scholar] [CrossRef]
- Vasil’ev, V.G.; Bykova, T.A.; Lebedev, B.V. Thermodynamics of hexanal at 0–330 K. Russ. J. Phys. Chem. 1991, 65, 51–54. [Google Scholar]
- Ranjan, S.; Chandrasekaran, R.; Paliyath, G.; Lim, L.T.; Subramanian, J. Effect of hexanal loaded electrospun fiber in fruit packaging to enhance the post harvest quality of peach. Food Packag. Shelf Life 2020, 23, 100447. [Google Scholar] [CrossRef]
- Fan, L.; Song, J.; Beaudry, R.M.; Hildebrand, P.D. Effect of hexanal vapor on spore viability of Penicillin expansum, lesion development on whole apples and fruit volatile biosynthesis. J. Food Sci. 2006, 71, M105–M109. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biol. Technol. 2003, 30, 113–121. [Google Scholar] [CrossRef]
- Sulaimankhil, Z.; Sethi, S.; Sharma, R.R.; Verma, M.K.; Dahuja, A.; Bhowmik, A. Influence of aqueous hexanal on quality of ‘Royal Delicious’ apple during cold storage. Acta Physiol. Plant. 2021, 43, 134. [Google Scholar] [CrossRef]
- Yumbya, P.; Ambuko, J.; Hutchinson, M.; Owino, W.; Juma, J.; Machuka, E.; Mutuku, J.M. Transcriptome analysis to elucidate hexanal’s mode of action in preserving the post-harvest shelf life and quality of banana fruits (Musa acuminata). J. Agric. Food Res. 2021, 3, 100114. [Google Scholar] [CrossRef]
- Nagarajan, V.; Kizhaeral, S.S.; Subramanian, M.; Rajendran, S.; Ranjan, J. Encapsulation of a Volatile Biomolecule (Hexanal) in Cyclodextrin Metal-Organic Frameworks for Slow Release and Its Effect on Preservation of Mangoes. ACS Food Sci. Technol. 2021, 1, 1936–1944. [Google Scholar] [CrossRef]
- Hutchinson, M.J.; Ouko, J.R.; Yumbya, P.M.; Ambuko, J.L.; Owino, W.O.; Subramanian, J. Efficacy of Hexanal Field Spray on the Postharvest Life and Quality of Papaya Fruit (Carica papaya L.) in Kenya. Adv. Agric. 2022, 2022. [Google Scholar] [CrossRef]
- Öz, A.T.; Kafkas, E. Volatile compositions of strawberry fruit during shelf life using pre and postharvest hexanal treatment. J. Food Process. Preserv. 2022, 46, e16464. [Google Scholar] [CrossRef]
- Wu, X.; Bi, J.; Fauconnier, M.L. Characteristic Volatiles and Cultivar Classification in 35 Apple Varieties: A Case Study of Two Harvest Years. Foods 2022, 11, 690. [Google Scholar] [CrossRef]
- Ma, N.; Zhu, J.; Wang, H.; Qian, M.C.; Xiao, Z. Comparative Investigation of Aroma-Active Volatiles in (“Ruixue”, “Liangzhi”, “Crystal Fuji,” and “Guifei”) Apples by Application of Gas Chromatography-Mass Spectrometry-Olfactometry (GC-MS-O) and Two-Dimensional Gas Chromatography-Quadrupole Mass Spectrometry (GC × GC-qMS) Coupled with Sensory Molecular Science. J. Agric. Food Chem. 2024, 72, 25229–25250. [Google Scholar]
- Kreissl, J.; Mall, V.; Steinhaus, P.; Steinhaus, M. Leibniz-LSB@TUM Odorant Database, Version 1.2; Leibniz Institute for Food Systems Biology at the Technical University of Munich: Freising, Germany, 2022. [Google Scholar]
- Cheema, A.; Padmanabhan, P.; Amer, A.; Parry, M.J.; Lim, L.-T.; Subramanian, J.; Paliyath, G. Postharvest hexanal vapor treatment delays ripening and enhances shelf life of greenhouse grown sweet bell pepper (Capsicum annum L.). Postharvest Biol. Technol. 2017, 136, 80–89. [Google Scholar] [CrossRef]
- Cheema, A.; Padmanabhan, P.; Subramanian, J.; Blom, T.; Paliyath, G. Improving quality of greenhouse tomato (Solanum lycopersicum L.) by pre- and postharvest applications of hexanal-containing formulations. Postharvest Biol. Technol. 2014, 95, 13–19. [Google Scholar] [CrossRef]
- Öz, A.T.; Eryol, B.; Ali, M.A. Postharvest hexanal application delays senescence and maintains quality in persimmon fruit during low temperature storage. J. Sci. Food Agric. 2023, 103, 7653–7663. [Google Scholar] [CrossRef]
- Tiwari, K.; Paliyath, G. Microarray analysis of ripening-regulated gene expression and its modulation by 1-MCP and hexanal. Plant Physiol. Biochem. 2011, 49, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.G.; Do Prado, S.B.R.; Andrade, S.C.S.; Fabi, J.P. Systems biology applied to the study of papaya fruit ripening: The influence of ethylene on pulp softening. Cells 2021, 10, 2339. [Google Scholar] [CrossRef]
- Sriskantharajah, K.; El Kayal, W.; Ayyanath, M.M.; Saxena, P.K.; Sullivan, A.J.; Paliyath, G.; Subramanian, J. Preharvest spray hexanal formulation enhances postharvest quality in ‘Honeycrisp’ apples by regulating phospholipase d and calcium sensor proteins genes. Plants 2021, 10, 2332. [Google Scholar] [CrossRef]
- Ashwini, T.; Ganapathy, S.; Subramanian, K.S.; Indu Rani, C.; Guru Meenakshi, G. Effect of Hexanal Vapour on Postharvest Quality and Shelf Life of Banana var. Grand Naine. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2441–2450. [Google Scholar] [CrossRef]
- Sharma, M.; Jacob, J.K.; Subramanian, J.; Paliyath, G. Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 239–247. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dong, L.; Zhou, Q.; Wang, J.W.; Chang, N.; Liu, Z.Y.; Ji, S.J. Effects of intermittent warming on aroma-related esters of 1-methylcyclopropene-treated “Nanguo” pears during ripening at room temperature. Sci. Hortic. 2015, 185, 82–89. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Pontesegger, N.; Rühmer, T.; Siegmund, B. Physicochemical Attributes, Aroma Profile, and Sensory Quality of Organic Crimson Crisp Apples after Storage. Foods 2023, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. New Zealand J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Contreras, C.; Beaudry, R. Lipoxygenase-associated apple volatiles and their relationship with aroma perception during ripening. Postharvest Biol. Technol. 2013, 82, 28–38. [Google Scholar] [CrossRef]
- Li, D.; Guo, J.; Ma, H.; Pei, L.; Liu, X.; Wang, H.; Chen, R.; Zhao, Z.; Gao, H. Changes in the VOC of Fruits at Different Refrigeration Stages of ‘Ruixue’ and the Participation of Carboxylesterase MdCXE20 in the Catabolism of Volatile Esters. Foods 2023, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Ferenczi, A.; Sugimoto, N.; Beaudry, R.M. Emission patterns of esters and their precursors throughout ripening and senescence in ‘Redchief Delicious’ apple fruit and implications regarding biosynthesis and aroma perception. J. Am. Soc. Hortic. Sci. 2021, 146, 297–328. [Google Scholar] [CrossRef]
- Yang, S.; Yu, J.; Yang, H.; Zhao, Z. Genetic analysis and QTL mapping of aroma volatile compounds in the apple progeny ‘Fuji’ × ‘Cripps Pink’. Front. Plant Sci. 2023, 14, 1048846. [Google Scholar] [CrossRef] [PubMed]
- Donadel, J.Z.; Thewes, F.R.; de Oliveira Anese, R.; Schultz, E.E.; Berghetti, M.R.P.; Ludwig, V.; Klein, B.; Cichoski, A.J.; Barin, J.S.; Both, V.; et al. Key volatile compounds of ‘Fuji Kiku’ apples as affected by the storage conditions and shelf life: Correlation between volatile emission by intact fruit and juice extracted from the fruit. Food Res. Int. 2019, 125, 108625. [Google Scholar] [CrossRef]
- Souleyre EJ, F.; Greenwood, D.R.; Friel, E.N.; Karunairetnam, S.; Newcomb, R.D. An alcohol acyl transferase from apple (cv. Royal Gala), MpAAT1, produces esters involved in apple fruit flavor. FEBS J. 2005, 272, 3132–3144. [Google Scholar] [CrossRef]
- Qi, W.; Wang, H.; Zhou, Z.; Yang, P.; Wu, W.; Li, Z.; Li, X. Ethylene Emission as a Potential Indicator of Fuji Apple Flavor Quality Evaluation Under Low Temperature. Hortic. Plant J. 2020, 6, 231–239. [Google Scholar] [CrossRef]
- Song, J.; Leepipattanawit, R.; Deng, W.; Beaudry, R.M. Hexanal Vapor Is a Natural, Metabolizable Fungicide: Inhibition of Fungal Activity and Enhancement of Aroma Biosynthesis in Apple Slices. J. Am. Soc. Hort. Sci. 1996, 121, 937–942. [Google Scholar] [CrossRef]
- Rowan, D.D.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of straight-chain ester volatiles in Red Delicious and Granny Smith apples using deuterium-labeled precursors. J. Agric. Food Chem. 1999, 47, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).