Exploring Suitable Nitrification Inhibitor in an Intensively Cultivated Greenhouse Soil and Its Effect on the Abundance and Community of Soil Ammonia Oxidizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. Soil Mineral N Contents
2.3. Soil DNA Extraction and Real-Time Quantitative PCR
2.4. High-Throughput Amplicon Sequencing and Bioinformatics Analysis
2.5. Statistical Analysis
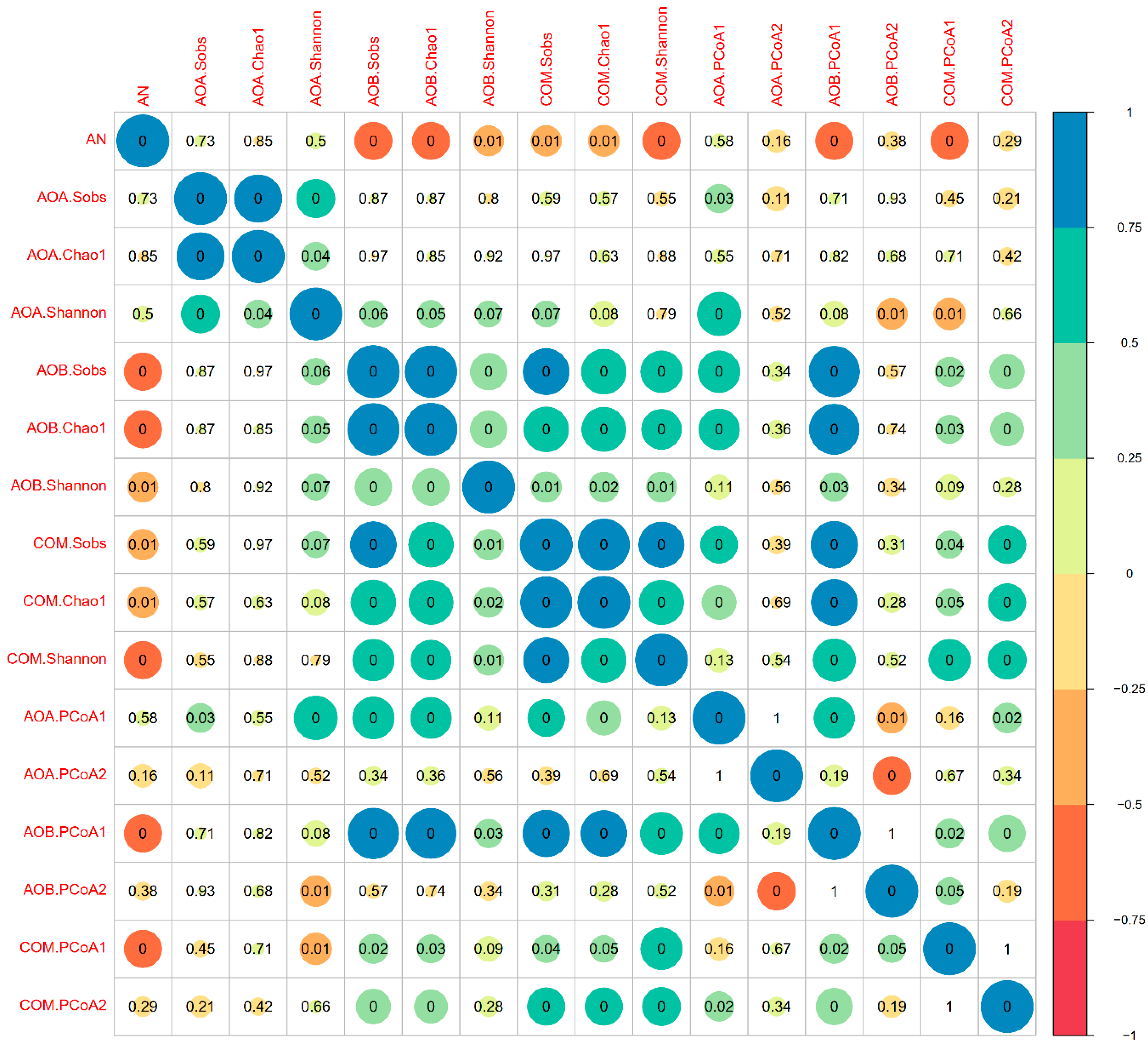
3. Results
3.1. The Change in Soil NH4+-N Content
3.2. The Change in the Abundance of Soil Ammonia Oxidizers After NP Incorporation
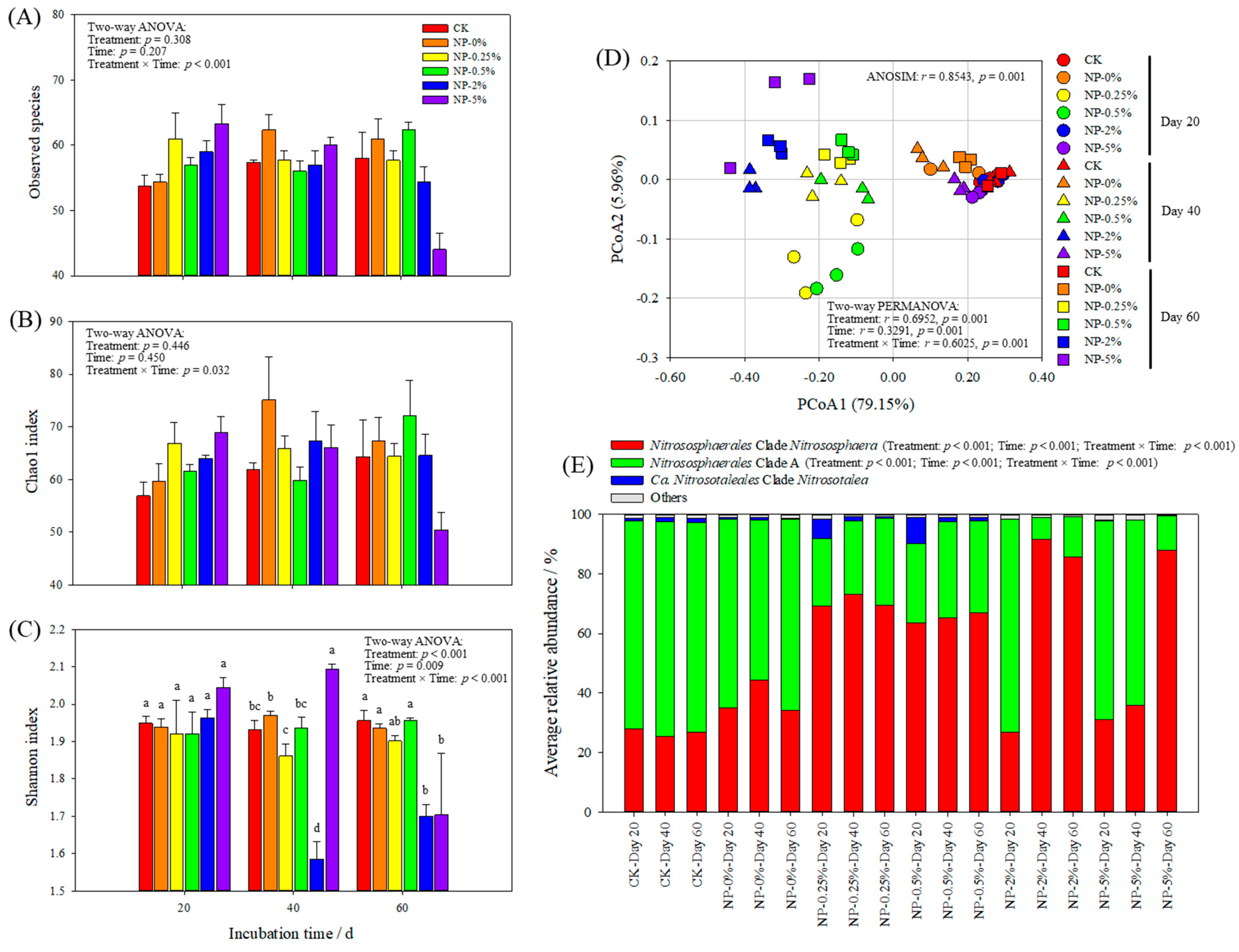
3.3. The Effect of NP Incorporation on AOA Community
3.4. The Effect of NP Incorporation on AOB Community
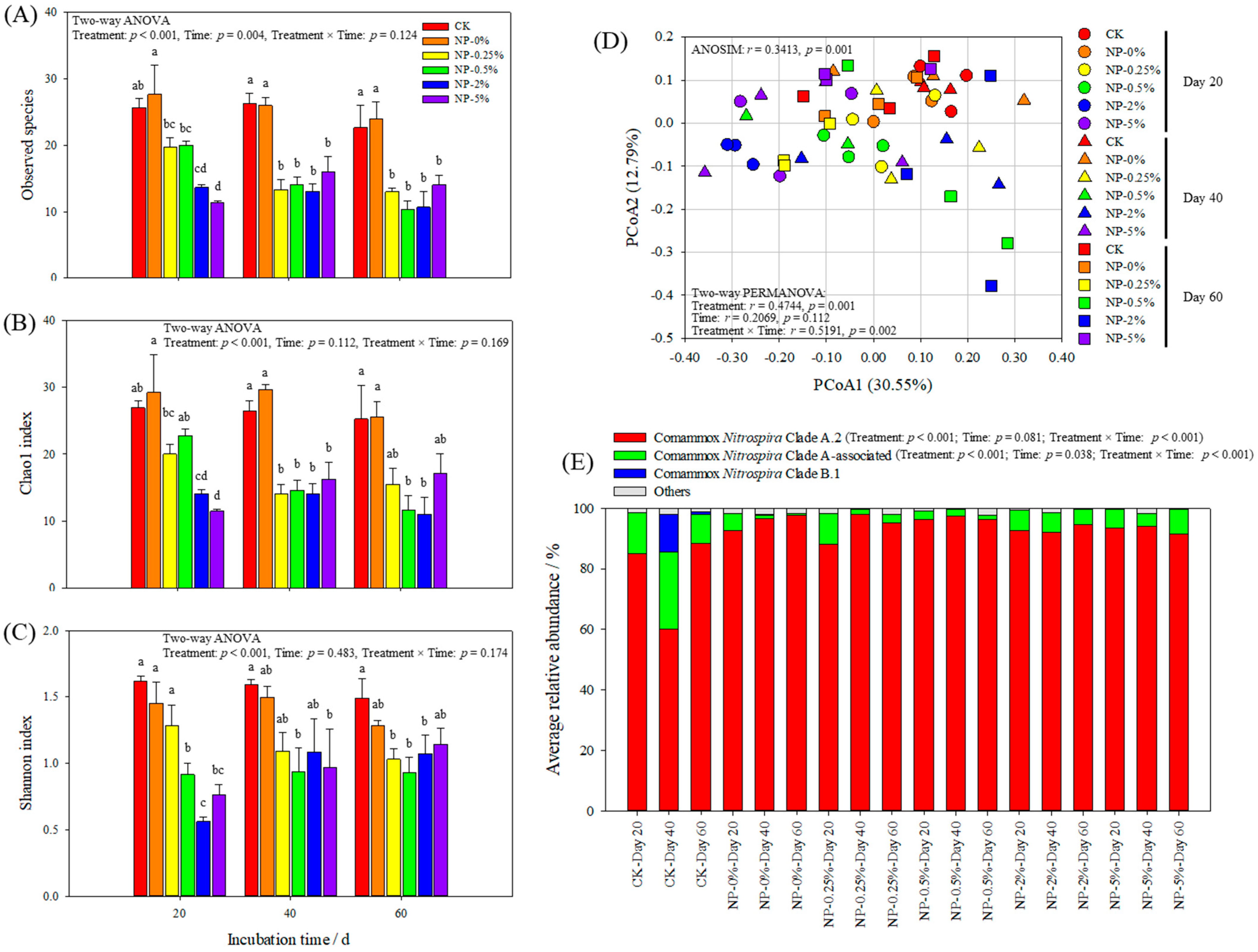
3.5. The Effect of NP Incorporation on Comammox Nitrospira Community
4. Discussion
4.1. NP as a Suitable NI in the Intensively Cultivated Greenhouse Soil Tested Here
4.2. NP Incorporation Suppressed AOB and Comammox Nitrospira Abundances Strongly
4.3. NP Incorporation Significantly Affected the Communities of Soil Ammonia Oxidizers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, W.; Hu, M.; Qian, D.; Xue, H.; Gao, N.; Lin, X. Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil 2021, 463, 1–18. [Google Scholar] [CrossRef]
- Motasim, A.M.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Abdul Sukor, A.S.; Adibah, A.M. Urea application in soil: Processes, losses, and alternatives—A review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.M.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv. Agron. 2009, 102, 267–322. [Google Scholar]
- Suman, J.; Rakshit, A.; Patra, A.; Dutta, A.; Tripathi, V.K.; Mohapatra, K.K.; Tiwari, R.; Krishnamoorthi, S. Enhanced efficiency N fertilizers: An effective strategy to improve use efficiency and ecological sustainability. J. Soil Sci. Plant Nutr. 2023, 23, 1472–1488. [Google Scholar] [CrossRef]
- Elrys, A.S.; Elnahal, A.S.; Abdo, A.I.; Desoky, E.M.; Selem, E.; Rady, M.M. Traditional, modern, and molecular strategies for improving the efficiency of nitrogen use in crops for sustainable agriculture: A fresh look at an old issue. J. Soil Sci. Plant Nutr. 2022, 22, 3130–3156. [Google Scholar] [CrossRef]
- Lam, S.K.; Wille, U.; Hu, H.; Caruso, F.; Mumford, K.; Liang, X.; Pan, B.; Malcolm, B.; Roessner, U.; Suter, H.; et al. Next-generation enhanced-efficiency fertilizers for sustained food security. Nat. Food 2022, 3, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Hallin, S. Geospatial variation in co-occurrence networks of nitrifying microbial guilds. Mol. Ecol. 2019, 28, 293–306. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y. Can phosphorus and nitrogen addition affect ammonia oxidizers in a high-phosphorus agricultural soil? Arch. Agron. Soil Sci. 2018, 64, 1728–1743. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Sakoula, D.; Koch, H.; Frank, J.; Jetten, M.S.M.; van Kessel, M.A.H.J.; Lücker, S. Enrichment and physiological characterization of a novel comammox Nitrospira indicates ammonium inhibition of complete nitrification. ISME J. 2021, 15, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Wagner, M. Nitrospira. Trends Microbiol. 2018, 26, 462–463. [Google Scholar] [CrossRef]
- Poghosyan, L.; Koch, H.; Lavy, A.; Frank, J.; van Kessel, M.A.H.J.; Jetten, M.S.M.; Banfield, J.F.; Lücker, S. Metagenomic recovery of two distinct comammox Nitrospira from the terrestrial subsurface. Environ. Microbiol. 2019, 21, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; van Kessel, M.A.H.J.; Lücker, S. Complete nitrification: Insights into the ecophysiology of comammox Nitrospira. Appl. Microbiol. Biotechnol. 2019, 103, 177–189. [Google Scholar] [CrossRef]
- Kits, K.D.; Sedlacek, C.J.; Lebedeva, E.V.; Han, P.; Bulaev, A.; Pjevac, P.; Daebeler, A.; Romano, S.; Albertsen, M.; Stein, L.Y.; et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 2017, 549, 269–272. [Google Scholar] [CrossRef]
- Papadopoulou, E.S.; Bachtsevani, E.; Katsoula, A.; Charami, C.; Lampronikou, E.; Vasileiadis, S.; Karpouzas, D.G. Nitrification inhibitors impose distinct effects on comammox bacteria and canonical ammonia oxidizers under high N fertilization regimes. Appl. Soil Ecol. 2024, 199, 105417. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, H.; Kang, Y.; Jiang, R.; Wang, Y.; Zhang, R.; Zhang, S.; Li, Y.; Li, P.; Yang, F.; et al. Comammox plays a functionally important role in the nitrification of rice paddy soil with different nitrogen fertilization levels. Appl. Soil Ecol. 2024, 193, 105120. [Google Scholar] [CrossRef]
- Li, C.; Hu, H.; Chen, Q.; Chen, D.; He, J. Comammox Nitrospira play an active role in nitrification of agricultural soils amended with nitrogen fertilizers. Soil Biol. Biochem. 2019, 138, 107609. [Google Scholar] [CrossRef]
- Feng, X.; Wang, M.; Li, Q.; Qin, Y.; Sun, B.; Tan, P.; Liu, H.; Li, C.; Zhang, J. Comammox dominate soil nitrification under different N fertilization regimes in semi-arid areas of Northeast China. Appl. Soil Ecol. 2024, 193, 105119. [Google Scholar] [CrossRef]
- Tang, J.; Su, L.; Fang, Y.; Wang, C.; Meng, L.; Wang, J.; Zhang, J.; Xu, W. Moderate nitrogen reduction increases nitrogen use efficiency and positively affects microbial communities in agricultural soils. Agriculture 2023, 13, 796. [Google Scholar] [CrossRef]
- Tao, R.; Ding, W.; Zhang, K.; Li, Y.; Li, J.; Hu, B.; Chu, G. Response of comammox Nitrospira clades A and B communities to long-term fertilization and rhizosphere effects and their relative contribution to nitrification in a subtropical paddy field of China. J. Environ. Manag. 2024, 367, 121939. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, S.; Ren, J.; Zhang, X.; Xie, W.; Meng, H.; He, H.; Zhang, L. Higher contribution by comammox bacteria than AOA and AOB to nitrification in the sediments of lake Taihu. Int. Biodeter. Biodegr. 2024, 187, 105709. [Google Scholar] [CrossRef]
- Ding, F.; He, T.; Qi, X.; Zhang, H.; An, L.; Xu, S.; Zhang, X. Comammox Nitrospira dominates the nitrification in artificial coniferous forest soils of the Qilian Mountains. Sci. Total Environ. 2024, 906, 167653. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, C.; Di, H.; Cameron, K.; Podolyan, A.; Shen, J.; Zhang, L.; Sirisena, K.; Godsoe, W. Contrasting response of comammox Nitrospira, ammonia oxidising bacteria, and archaea to soil pH and nitrogen inputs. Sci. Total Environ. 2024, 924, 171627. [Google Scholar] [CrossRef]
- Belyaeva, O.; Ward, G.; Wijesinghe, T.; Chen, D.; Suter, H. Pasture productivity benefits from strategic urease and nitrification inhibitor use are limited in rainfed temperate dairy pastures of southern Australia. Plant Soil 2024, 505, 317–334. [Google Scholar] [CrossRef]
- Padash, A.; Azarmi, R.; Toularoud, A.A.S.; Esmailpour, B.; Cruz, C. Use of symbiotic fungi to reduce the phytotoxic effect of DCD nitrification inhibitors in lettuce. Agriculture 2022, 12, 251. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Q.; Yang, J.; Wang, Y.; Yang, J.; Zhang, X.; Feng, G.; Cheng, Z.; Wang, S.; Su, H. Fabrication and release behavior of nitrapyrin microcapsules: Using modified melamine-formaldehyde resin as shell material. Sci. Total Envrion. 2020, 704, 135394. [Google Scholar] [CrossRef]
- Hsu, P.; Di, H.J.; Cameron, K.; Podolyan, A.; Chau, H.; Luo, J.; Miller, B.; Carrick, S.; Johnstone, P.; Ferguson, S.; et al. Comammox Nitrospira Clade B is the most abundant complete ammonia oxidizer in a dairy pasture soil and inhibited by dicyandiamide and high ammonium concentrations. Front. Microbiol. 2022, 13, 1048735. [Google Scholar] [CrossRef]
- Li, C.; Hu, H.; Chen, Q.; Chen, D.; He, J. Growth of comammox Nitrospira is inhibited by nitrification inhibitors in agricultural soils. J. Soils Sediments 2020, 20, 621–628. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Xu, Q.; Cao, G.; Guo, X.; Zhou, H.; Du, Y. Global analysis of nitrification inhibitors on grasslands nitrous oxide emission rates. Biochem. Syst. Ecol. 2021, 97, 104289. [Google Scholar] [CrossRef]
- Dawar, K.; Khan, A.; Sardar, K.; Fahad, S.; Saud, S.; Datta, R.; Danish, S. Effects of the nitrification inhibitor nitrapyrin and mulch on N2O emission and fertilizer use efficiency using 15N tracing techniques. Sci. Total Environ. 2021, 757, 1432739. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, J.J.; Wei, Z.; Dodla, S.K.; Fultz, L.M.; Gaston, L.A.; Xiao, R.; Park, J.; Scaglia, G. Nitrification inhibitors reduce nitrogen losses and improve soil health in a subtropical pastureland. Geoderma 2021, 388, 114947. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Liu, X.; Yao, P.; Ge, T.; Zhang, X. Spatiotemporal dynamics of the archaeal community in coastal sediments: Assembly process and co-occurrence relationship. ISME J. 2020, 14, 1463–1478. [Google Scholar] [CrossRef]
- Hargreaves, P.R.; Baker, K.L.; Graceson, A.; Bonnett, S.A.F.; Ball, B.C.; Cloy, J.M. Use of a nitrification inhibitor reduces nitrous oxide (N2O) emissions from compacted grassland with different soil textures and climatic conditions. Agric. Ecosyst. Environ. 2021, 310, 107307. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Ma, S.; Zheng, X.; Wang, Z.; Lu, C. Effects of commonly used nitrification inhibitors—Dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP), and nitrapyrin—On soil nitrogen dynamics and nitrifiers in three typical paddy soils. Geoderma 2020, 380, 114637. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, J.; He, J.; Zhang, L. Microbial mechanisms of nitrification inhibitors and their application. J. Agro-Environ. Sci. 2014, 33, 2077–2083. [Google Scholar]
- Hu, H.; He, J. Comammox—A newly discovered nitrification process in the terrestrial nitrogen cycle. J. Soils Sediments 2017, 17, 2709–2717. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.; Chu, G. Inhibition of DCD, DMPP and Nitrapyrin on soil nitrification and their appropriate use dosage. J. Plant Nutr. Fertil. 2017, 23, 54–61. [Google Scholar]
- O’Sullivan, C.A.; Duncan, E.G.; Whisson, K.; Treble, K.; Ward, P.R.; Roper, M.M. A colourimetric microplate assay for simple, high throughput assessment of synthetic and biological nitrification inhibitors. Plant Soil 2017, 413, 275–287. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Ren, X.; Chen, B.; Shen, C.; Wang, F. Long-term greenhouse vegetable cultivation alters the community structures of soil ammonia oxidizers. J. Soils Sediments 2019, 19, 883–902. [Google Scholar] [CrossRef]
- Li, L.; Zhao, C.; Wang, X.; Tan, Y.; Wang, X.; Liu, X.; Guo, B. Effects of nitrification and urease inhibitors on ammonia-oxidizing microorganisms, denitrifying bacteria, and greenhouse gas emissions in greenhouse vegetable fields. Environ. Res. 2023, 237, 116781. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Fang, X.; Zhou, W.; Alami, M.J.; Xu, S.; Huang, W.; Cui, S.; Gao, B. Regional N2O emission differences and the mitigation measurements in the vegetable production systems of China. J. Plant Nutr. Fertil. 2024, 30, 417–429. [Google Scholar]
- Cheng, X.; Tian, X.; Guo, Y.; Li, R.; Zhang, L.; Ji, Y.; Li, B. Effects of nitrification inhibitor/microbial inoculum on nitrogen fate in soil-vegetable system of greenhouse. J. Plant Nutr. Fertil. 2022, 28, 1466–1477. [Google Scholar]
- Liu, F.; Ma, X.; Zhang, F.; Liang, T.; Li, L.; Wang, J.; Chen, X.; Wang, X. Impact of nitrification inhibitors on vegetable production yield, nitrogen fertilizer use efficiency and nitrous oxide emission reduction in China: Meta analysis. Environ. Sci. 2022, 43, 5140–5148. [Google Scholar]
- Dorich, R.A.; Nelson, D.W. Direct colorimetric measurement of ammonium in potassium chloride extracts of soils. Soil Sci. Soc. Am. J. 1983, 47, 833–836. [Google Scholar] [CrossRef]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [PubMed]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, G.; Zhao, Z.; Dang, C.; Liu, W.; Zheng, M. Newly designed primer pair revealed dominant and diverse comammox amoA gene in full-scale wastewater treatment plants. Bioresour. Technol. 2018, 270, 580–587. [Google Scholar] [CrossRef]
- Xia, F.; Wang, J.; Zhu, T.; Zou, B.; Rhee, S.; Quan, Z. Ubiquity and diversity of complete ammonia oxidizers (Comammox). Appl. Environ. Microbiol. 2018, 84, e01390-18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Ren, X.; Shen, C.; Wang, F.; Wu, D. Influences of nitrogen forms on abundances and community structures of ammonia and nitrite oxidizers in a slightly alkaline upland soil. Arch. Agron. Soil Sci. 2021, 67, 152–165. [Google Scholar] [CrossRef]
- Lu, X.; Lu, P.; Yang, K. Restoration using Azolla imbricata increases nitrogen functional bacterial groups and genes in soil. Appl. Microbiol. Biotechnol. 2017, 101, 3849–3859. [Google Scholar] [CrossRef]
- Alves, R.J.E.; Wanek, W.; Zappe, A.; Richter, A.; Svenning, M.M.; Schleper, C.; Urich, T. Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J. 2013, 7, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Alves, R.J.E.; Zhang, D.; Han, L.; He, J.; Zhang, L. Time-dependent shifts in populations and activity of bacterial and archaeal ammonia oxidizers in response to liming in acidic soils. Soil Biol. Biochem. 2017, 112, 77–89. [Google Scholar] [CrossRef]
- Zou, W.; Liu, S.; Jiao, J.; Zhang, W.; Chen, Y.; Lakshmanan, P.; Lang, M.; Chen, X. Niche adaptation strategies of comammox Nitrospira in response to nitrogen addition in different types of soil. Appl. Soil. Ecol. 2024, 203, 105682. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Thapa, R.; Chatterjee, A.; Awale, R.; McGranahan, D.A.; Daigh, A. Effect of enhanced efficiency fertilizers on nitrous oxide emissions and crop yields: A Meta-analysis. Soil Sci. Soc. Am. J. 2016, 80, 1121–1134. [Google Scholar] [CrossRef]
- You, L.; Ros, G.H.; Chen, Y.; Shao, Q.; Young, M.D.; Zhang, F.; de Vries, W. Global mean nitrogen recovery efficiency in croplands can be enhanced by optimal nutrient, crop and soil management practices. Nat. Commun. 2023, 14, 5747. [Google Scholar] [CrossRef]
- Tufail, M.A.; Naeem, A.; Arif, M.S.; Farooq, T.H.; Shahzad, S.M.; Dar, A.A.; Albasher, G.; Shakoor, A. Unraveling the efficacy of nitrification inhibitors (DCD and DMPP) in reducing nitrogen gases emissions across agroecosystems: A three-decade global data synthesis (1993–2021). Fuel 2022, 324, 124725. [Google Scholar] [CrossRef]
- Verburg, K.; Thorburn, P.J.; Vilas, M.P.; Biggs, J.S.; Zhao, Z.; Bonnett, G.D. Why are the benefits of enhanced-efficiency fertilizers inconsistent in the field? Prerequisite conditions identified from simulation analyses. Agron. Sustain. Dev. 2022, 42, 73. [Google Scholar] [CrossRef]
- Chibuike, G.; Saggar, S.; Palmada, T.; Luo, J. The persistence and efficacy of nitrification inhibitors to mitigate nitrous oxide emissions from New Zealand pasture soils amended with urine. Geoderma Reg. 2022, 30, e00541. [Google Scholar] [CrossRef]
- Duan, P.; Zhang, Q.; Zhang, X.; Xiong, Z. Mechanisms of mitigating nitrous oxide emissions from vegetable soil varied with manure, biochar and nitrification inhibitors. Agric. Forest Meteorol. 2019, 278, 107672. [Google Scholar] [CrossRef]
- Fan, C.; Li, B.; Xiong, Z. Nitrification inhibitors mitigated reactive gaseous nitrogen intensity in intensive vegetable soils from China. Sci. Total Environ. 2018, 612, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Xi, R.; Long, X.; Huang, S.; Yao, H. pH rather than nitrification and urease inhibitors determines the community of ammonia oxidizers in a vegetable soil. AMB Express 2017, 7, 129. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, C.H.; Li, Q.L.; Li, B.; Zhu, Y.Y.; Xiong, Z.Q. A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agric. Ecosyst. Environ. 2015, 201, 43–50. [Google Scholar] [CrossRef]
- Gao, J.; Luo, J.; Lindsey, S.; Shi, Y.; Sun, Z.; Wei, Z.; Wang, L. Benefits and risks for the environment and crop production with application of nitrification inhibitors in China. J. Soil Sci. Plant Nutr. 2021, 21, 497–512. [Google Scholar] [CrossRef]
- Papadopoulou, E.S.; Bachtsevani, E.; Lampronikou, E.; Adamou, E.; Katsaouni, A.; Vasileiadis, S.; Thion, C.; Menkissoglu-Spiroudi, U.; Nicol, G.W.; Karpouzas, D.G. Comparison of novel and established nitrification inhibitors relevant to agriculture on soil ammonia- and nitrite-oxidizing isolates. Front. Microbiol. 2020, 11, 581283. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Chen, X.; Liu, S.; Zhou, M.; Gao, X. Biological and chemical nitrification inhibitors exhibited different effects on soil gross N nitrification rate and N2O production: A 15N microcosm study. Environ. Sci. Pollut. Res. 2023, 30, 116162–116174. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.S.; Uzair, M.; Maqbool, Z.; Fiaz, S.; Yousuf, M.; Yang, S.H.; Khan, M.R. Improving nitrogen use efficiency in aerobic rice based on insights into the ecophysiology of archaeal and bacterial ammonia oxidizers. Front. Plant Sci. 2022, 13, 913204. [Google Scholar] [CrossRef] [PubMed]
- de Paulo, E.N.; Galindo, F.S.; Rabêlo, F.H.S.; Frazão, J.J.; Lavres, J. 3,4-Dimethylpyrazole Phosphate (DMPP) reduces nitrogen leaching in three tropical soils and improves the agronomic efficiency of nitrogen fertilizers applied to cotton. J. Soil Sci. Plant Nutr. 2022, 22, 2520–2533. [Google Scholar] [CrossRef]
- Shi, X.; Hu, H.; Müller, C.; He, J.; Chen, D.; Suter, H.C. Effects of the nitrification inhibitor 3,4-Dimethylpyrazole Phosphate on nitrification and nitrifiers in two contrasting agricultural soils. Appl. Environ. Microbiol. 2016, 82, 5236–5248. [Google Scholar] [CrossRef] [PubMed]
- Guardia, G.; Marsden, K.A.; Vallejo, A.; Jones, D.L.; Chadwick, D.R. Determining the influence of environmental and edaphic factors on the fate of the nitrification inhibitors DCD and DMPP in soil. Sci. Total Environ. 2018, 624, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Zhang, L.; Song, Y.; Li, Y.; Zheng, Y.; Zhang, J.; et al. Effects of nitrification inhibitors on soil nitrification and ammonia volatilization in three soils with different pH. Agronomy 2021, 11, 1674. [Google Scholar] [CrossRef]
- Liu, S.; Wu, D.; Ju, X.; Shen, J.; Cheng, Y.; Deng, N.; Song, X.; Di, H.; Li, P.; Han, L.; et al. Nitrification inhibitor induced microbial NH4+-N immobilization improves maize nitrogen use efficiency in strong ammonia oxidation soil. Soil Biol. Biochem. 2025, 202, 109687. [Google Scholar] [CrossRef]
- Hayden, H.L.; Phillips, L.A.; Marshall, A.J.; Condon, J.R.; Doran, G.S.; Wells, G.S.; Mele, P.M. Nitrapyrin reduced ammonia oxidation with different impacts on the abundance of bacterial and archaeal ammonia oxidisers in four agricultural soils. Appl. Soil Ecol. 2021, 157, 103759. [Google Scholar] [CrossRef]
- Papadopoulou, E.S.; Bachtsevani, E.; Papazlatani, C.V.; Rousidou, C.; Brouziotis, A.; Lampronikou, E.; Tsiknia, M.; Vasileiadis, S.; Ipsilantis, I.; Menkissoglu-Spiroudi, U.; et al. The effects of Quinone Imine, a new potent nitrification inhibitor, Dicyandiamide, and Nitrapyrin on target and off-target soil microbiota. Microbiol. Spectr. 2022, 10, e02403-21. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Fan, X.; Chen, H.; Ye, M.; Yan, G.; Li, T.; Peng, H.; Shengzhe, E.; Che, Z.; Wakelin, S.A.; et al. Inhibition of ammonia-oxidizing bacteria promotes the growth of ammonia-oxidizing archaea in ammonium-rich alkaline soils. Pedosphere 2022, 32, 532–542. [Google Scholar] [CrossRef]
- Lei, J.; Fan, Q.; Yu, J.; Ma, Y.; Yin, J.; Liu, R. A meta-analysis to examine whether nitrification inhibitors work through selectively inhibiting ammonia-oxidizing bacteria. Front. Microbiol. 2022, 13, 962146. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Tan, C.; Chen, H.; Ye, M.; Fan, X.; Zheng, W.; Gao, Z.; Peng, H.; Liang, Y. Insight into the role of competition in niche differentiation between ammonia-oxidizing archaea and bacteria in ammonium-rich alkaline soil: A network-based study. Soil Biol. Biochem. 2022, 168, 108638. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Chen, H.; Jiang, Y.; Ye, M.; Yan, G.; Peng, H.; Wakelin, S.A.; Liang, Y. 3, 4-Dimethylpyrazole phosphate is an effective and specific inhibitor of soil ammonia-oxidizing bacteria. Biol. Fertil. Soils 2021, 57, 753–766. [Google Scholar] [CrossRef]
- Yao, H.; Huang, S.; Qiu, Q.; Li, Y.; Wu, L.; Mi, W.; Dai, F. Effects of different fertilizers on the abundance and community structure of ammonia oxidizers in a yellow clay soil. Appl. Microbiol. Biotechnol. 2016, 100, 6815–6826. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, G.; Elsgaard, L.; Tzanakakis, V.A.; Franklin, R.B.; Brown, B.L.; Zanakis, G.; Monokrousos, N.; Anastopoulos, I.; Awad, M.; Ipsilantis, I.; et al. Impact of nitrapyrin on urea-based fertilizers in a Mediterranean calcareous soil: Nitrogen and microbial dynamics. Eur. J. Soil Sci. 2024, 75, e13553. [Google Scholar] [CrossRef]
- Gu, Y.; Mi, W.; Xie, Y.; Ma, Q.; Wu, L.; Hu, Z.; Dai, F. Nitrapyrin affects the abundance of ammonia oxidizers rather than community structure in a yellow clay paddy soil. J. Soils Sediments 2019, 19, 872–882. [Google Scholar] [CrossRef]
- Fisk, L.M.; Maccarone, L.D.; Barton, L.; Murphy, D.V. Nitrapyrin decreased nitrification of nitrogen released from soil organic matter but not amoA gene abundance at high soil temperature. Soil Biol. Biochem. 2015, 88, 214–223. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Yao, H.; Wang, J.; Dai, F.; Wu, Y.; Chapman, S. Nitrification and urease inhibitors improve rice nitrogen uptake and prevent denitrification in alkaline paddy soil. Appl. Soil Ecol. 2020, 154, 103665. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Gao, Q.; Zhao, H.; Wang, G.; Tian, G.; Liu, J.; Yang, J. Study on the mechanism of nitrapyrin microcapsule suspension effectively improving nitrification inhibition rate in black soil. Ecotox. Environ. Saf. 2023, 265, 115539. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.G.; O’Sullivan, C.A.; Simonsen, A.K.; Roper, M.M.; Peoples, M.B.; Treble, K.; Whisson, K. The nitrification inhibitor 3,4,-dimethylpyrazole phosphate strongly inhibits nitrification in coarse-grained soils containing a low abundance of nitrifying microbiota. Soil Res. 2017, 55, 28–37. [Google Scholar] [CrossRef]
- Cui, P.; Fan, F.; Yin, C.; Li, Z.; Song, A.; Wan, Y.; Liang, Y. Urea- and nitrapyrin-affected N2O emission is coupled mainly with ammonia oxidizing bacteria growth in microcosms of three typical Chinese arable soils. Soil Biol. Biochem. 2013, 66, 214–221. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.; Fan, H.; Luo, D.; Chapman, S.J.; Yao, H. 15N-DNA stable isotope probing reveals niche differentiation of ammonia oxidizers in paddy soils. Appl. Microbiol. Biotechnol. 2024, 108, 342. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Zhou, X.; Alam, M.S.; Fan, J.; Guo, Z.; Zhang, H.; Gubry-Rangin, C.; Jia, Z. Long-term adaptation of acidophilic archaeal ammonia oxidisers following different soil fertilisation histories. Microb. Ecol. 2022, 83, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Bei, Q.; Reitz, T.; Schädler, M.; Hodgskiss, L.H.; Peng, J.; Schnabel, B.; Buscot, F.; Eisenhauer, N.; Schleper, C.; Heintz-Buschart, A. Metabolic potential of Nitrososphaera-associated clades. ISME J. 2024, 18, wrae086. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Stieglmeier, M.; Dai, J.; Urich, T.; Schleper, C. Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol. Lett. 2013, 344, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Issifu, S.; Acharya, P.; Kaur-Bhambra, J.; Gubry-Rangin, C.; Rasche, F. Biological nitrification inhibitors with antagonistic and synergistic effects on growth of ammonia oxidisers and soil nitrification. Microb. Ecol. 2024, 87, 143. [Google Scholar] [CrossRef]
- Oton, E.V.; Quince, C.; Nicol, G.W.; Prosser, J.I.; Gubry-Rangin, C. Phylogenetic congruence and ecological coherence in terrestrial Thaumarchaeota. ISME J. 2016, 10, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hink, L.; Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef]
- Fan, X.; Yin, C.; Chen, H.; Ye, M.; Zhao, Y.; Li, T.; Wakelin, S.A.; Liang, Y. The efficacy of 3,4-dimethylpyrazole phosphate on N2O emissions is linked to niche differentiation of ammonia oxidizing archaea and bacteria across four arable soils. Soil Biol. Biochem. 2019, 130, 82–93. [Google Scholar] [CrossRef]
- Deng, N.; Gubry-Rangin, C.; Song, X.; Ju, X.; Liu, S.; Shen, J.; Di, H.; Han, L.; Zhang, L. AOB Nitrosospira cluster 3a.2 (D11) dominates N2O emissions in fertilised agricultural soils. J. Environ. Manag. 2024, 355, 120504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Huang, G.; He, S.; Zhou, N.; Wang, M.; Dang, C.; Wang, J.; Zheng, M. Abundance and community composition of comammox bacteria in different ecosystems by a universal primer set. Sci. Total Environ. 2019, 691, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jiang, Y.; Wang, S.; Wang, X.; Zhu, G. Biogeographic distribution of comammox bacteria in diverse terrestrial habitats. Sci. Total Environ. 2020, 717, 137257. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, B.; Li, Y.; Jiang, D.; Zhou, Y.; Ding, A.; Zong, Y.; Ling, X.; Zhang, S.; Lu, H. Ubiquity, diversity, and activity of comammox Nitrospira in agricultural soils. Sci. Total Environ. 2020, 706, 135684. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wu, D.; Chen, D.; Xu, P.; Tang, Y. Abundance, diversity, and community composition of comammox Nitrospira in tea (Camellia sinensis) plantation soils. J. Soil Sci. Plant Nutr. 2024, 24, 7901–7918. [Google Scholar] [CrossRef]
- Shah, A.S.; Hsu, P.; Chisholm, C.; Podolyan, A.; Cameron, K.; Luo, J.; Stenger, R.; Carrick, S.; Hu, W.; Ferguson, S.A.; et al. Nitrification inhibitor chlorate and nitrogen substrates differentially affect comammox Nitrospira in a grassland soil. Front. Microbiol. 2024, 15, 1392090. [Google Scholar] [CrossRef] [PubMed]



| Target Gene | Primer Name | Sequence | Amplification Condition | Reference |
|---|---|---|---|---|
| AOA amoA | Arch-amoAF Arch-amoAR | STAATGGTCTGGCTTAGACG/GCGGCCATCCATCTGTATGT | 95 °C for 3 min, 40 cycles of 95 °C for 5 s, 58 °C for 30 s and 72 °C for 1 min | [47] |
| AOB amoA | BamoA-1F BamoA-2R | GGGGTTTCTACTGGTGGT/CCCCTCKGSAAAGCCTTCTTC | [48] | |
| Comammox Nitrospira amoA | ComamoA-AF ComamoA-SR | AGGNGAYTGGGAYTTCTGG/CCGVACATACATRAAGCCCAT | [49] |
| Target Gene | Primer Name | Sequence | Amplification Condition | Reference |
|---|---|---|---|---|
| AOA amoA | Arch-amoAF Arch-amoAR | STAATGGTCTGGCTTAGACG/GCGGCCATCCATCTGTATGT | 95 °C for 3 min, 40 cycles of 95 °C for 30 s, 60 °C for 30 s, 72 °C for 45 s and 72 °C for 10 min | [47] |
| AOB amoA | BamoA-1F BamoA-2R | GGGGTTTCTACTGGTGGT/CCCCTCKGSAAAGCCTTCTTC | 95 °C for 3 min, 40 cycles of 95 °C for 30 s, 60 °C for 30 s, 72 °C for 45 s and 72 °C for 10 min | [48] |
| Comammox Nitrospira amoA | A189F C576R CA209F C576R | GGNGACTGGGAYTTYTGG/GAAGCCCATRTARTCNGCC GAYTGGAARGAYCGNCA/GAAGCCCATRTARTCNGCC | 94 °C for 5 min, 20 cycles of 94 °C for 60 s, 52 °C for 50 s, 72 °C for 50 s and 72 °C for 10 min 94 °C for 5 min, 30 cycles of 94 °C for 60 s, 50 °C for 50 s, 72 °C for 50 s and 72 °C for 10 min | [50] |
| Experimental Type | Soil Type | Dosage Applied | Change in Microbial Abundance | Reference | ||
|---|---|---|---|---|---|---|
| AOA | AOB | COM | ||||
| Laboratory incubation | Calcareous soil | 1% | Not sig. | Declined | — | [69] |
| Laboratory incubation | Black soil | 0.1% | Increased | Declined | Increased | [36] |
| Laboratory incubation | Red soil | 0.1% | Increased | Declined | — | [36] |
| Laboratory incubation | Purple soil | 0.1% | Increased | Declined | — | [36] |
| Field experiment | Yellow clay soil | Not mentioned | Decreased | Not sig. | — | [82] |
| Laboratory incubation | Pasture soil | Not mentioned | Not sig. | Declined | Declined | [30] |
| Laboratory incubation | Arable soil | Not mentioned | Not sig. | Declined | Declined | [30] |
| Laboratory incubation | Sandy loam soil | At an equivalent rate of 2.5 L hm−2 | Not sig. | Declined | — | [83] |
| Field experiment | Fimi-Orthic Anthrosols | Not mentioned | Decreased | Declined | — | [62] |
| Field experiment | Oxisols | 0.25% | Decreased | Not sig. | — | [84] |
| Laboratory incubation | Haplic Arenosol | 9 µg active ingredient g−1 of dry soil | — | Not sig. | — | [85] |
| Laboratory incubation | Vertosol | At an equivalent rate of 2.5 L hm−2 | Increased | Declined | — | [76] |
| Laboratory incubation | Tenosol | At an equivalent rate of 2.5 L hm−2 | Increased | Declined | — | [76] |
| Laboratory incubation | Sodosol | At an equivalent rate of 2.5 L hm−2 | Increased | Declined | — | [76] |
| Laboratory incubation | Calcarosol | At an equivalent rate of 2.5 L hm−2 | Increased | Not sig. | — | [76] |
| Field experiment | Paddy soil | 0.2% | Declined | Not sig. | — | [86] |
| Laboratory incubation | Loamy soil | 50 mg kg−1 of dry soil | Not sig. | Declined | Not sig. | [17] |
| Laboratory incubation | Red soil | 0.1% | Not sig. | Declined | — | [64] |
| Laboratory incubation | Black soil | 0.2% | — | Declined | — | [87] |
| Laboratory incubation | Cambisol—acidic | 0.86 mg kg−1 of dry soil | Declined | Not sig. | — | [77] |
| Laboratory incubation | Cambisol—acidic | 5 mg kg−1 of dry soil | Declined | Declined | — | [77] |
| Laboratory incubation | Cambisol—alkaline | 0.86 mg kg−1 of dry soil | Not sig. | Declined | — | [77] |
| Laboratory incubation | Cambisol—alkaline | 5 mg kg−1 of dry soil | Declined | Declined | — | [77] |
| Laboratory incubation | Sandy soil—Tenosol | 5 mg kg−1 of dry soil | Not sig. | Declined | — | [88] |
| Laboratory incubation | Sandy soil—Hydrosol | 5 mg kg−1 of dry soil | — | Declined | — | [88] |
| Laboratory incubation | Upland alluvial soil | 0.3 mg kg−1 of dry soil | Declined | Declined | — | [89] |
| Laboratory incubation | Paddy soil | 0.3 mg kg−1 of dry soil | Declined | Declined | — | [89] |
| Laboratory incubation | Upland black soil | 0.3 mg kg−1 of dry soil | Not sig. | Not sig. | — | [89] |
| Experimental Type | Soil Type | Dosage Applied | Main Findings | Reference | ||
|---|---|---|---|---|---|---|
| AOA | AOB | COM | ||||
| Laboratory incubation | Calcareous soil | 1% | Richness: not sig. Diversity: not sig. Structure: not sig. | Richness: not sig. Diversity: not sig. Structure: significantly changed; average relative abundance of the Nitrosospira sp. Nsp17 Clade declined, whereas that of the Nitrosospira sp. 9SS1 and Nitrosospira briensis Clades increased | — | [69] |
| Field experiment | Oxisols | 0.25% | Richness: not mentioned Diversity: not mentioned Structure: not sig. | Richness: not mentioned Diversity: not mentioned Structure: not sig. | — | [84] |
| Laboratory incubation | Loamy soil | 50 mg kg−1 of dry soil | Richness: not sig. Diversity: not sig. Structure: not sig. | Richness: not sig. Diversity: not sig. Structure: significantly changed; reduced the average relative abundance of the Nitrosospira sp. NI5 Clade | Richness: declined Diversity: declined Structure: significantly changed; slightly increased the average relative abundance of Comammox Nitrospira Clade A without statistical significance | [17] |
| Laboratory incubation | Cambisol—Acidic | 0.86 mg kg−1 of dry soil | Richness: not sig. Diversity: not sig. Structure: not sig. | Richness: not sig. Diversity: not sig. Structure: significantly changed; increased the average relative abundance of some ASVs belonging to the Nitrosospira sp. Nsp5 Clade | — | [77] |
| Laboratory incubation | Cambisol—Acidic | 5 mg kg−1 of dry soil | Richness: not sig. Diversity: not sig. Structure: significantly changed; decreased the average relative abundance of an unclassified ASV | Richness: not sig. Diversity: not sig. Structure: significantly changed; lowered the average relative abundance of some ASVs belonging to the Nitrosospira briensis and Nitrosospira sp. Nsp65 Clades | — | [77] |
| Laboratory incubation | Cambisol—Alkaline | 0.86 mg kg−1 of dry soil | Richness: not sig. Diversity: not sig. Structure: not sig. | Richness: not sig. Diversity: declined Structure: significantly changed; the majority of the ASVs were significantly affected by the NP belonging to the Nitrosospira briensis group | — | [77] |
| Laboratory incubation | Cambisol—Alkaline | 5 mg kg−1 of dry soil | Richness: not sig. Diversity: not sig. Structure: significantly changed; suppressed the average relative abundance of the ASVs belonging to the Nitrososphaerales ε-2.2 Clade, but favored the ASVs belonging to the Nitrososphaerales γ Clade | Richness: declined Diversity: declined Structure: significantly changed; the majority of the ASVs were significantly affected by the NP belonging to the Nitrosospira briensis group | — | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cheng, Y.; Zhang, Y.; Wang, F.; Li, Y.; Shen, C.; Chen, B. Exploring Suitable Nitrification Inhibitor in an Intensively Cultivated Greenhouse Soil and Its Effect on the Abundance and Community of Soil Ammonia Oxidizers. Agronomy 2025, 15, 255. https://doi.org/10.3390/agronomy15020255
Liu X, Cheng Y, Zhang Y, Wang F, Li Y, Shen C, Chen B. Exploring Suitable Nitrification Inhibitor in an Intensively Cultivated Greenhouse Soil and Its Effect on the Abundance and Community of Soil Ammonia Oxidizers. Agronomy. 2025; 15(2):255. https://doi.org/10.3390/agronomy15020255
Chicago/Turabian StyleLiu, Xing, Yanan Cheng, Ying Zhang, Fei Wang, Yonggang Li, Changwei Shen, and Bihua Chen. 2025. "Exploring Suitable Nitrification Inhibitor in an Intensively Cultivated Greenhouse Soil and Its Effect on the Abundance and Community of Soil Ammonia Oxidizers" Agronomy 15, no. 2: 255. https://doi.org/10.3390/agronomy15020255
APA StyleLiu, X., Cheng, Y., Zhang, Y., Wang, F., Li, Y., Shen, C., & Chen, B. (2025). Exploring Suitable Nitrification Inhibitor in an Intensively Cultivated Greenhouse Soil and Its Effect on the Abundance and Community of Soil Ammonia Oxidizers. Agronomy, 15(2), 255. https://doi.org/10.3390/agronomy15020255






