Safety Evaluation of Herbicides in Maize and Soybean and Their Antioxidant Defense Responses to Thifensulfuron-Methyl and Flufenacet
Abstract
1. Introduction
2. Material and Methods
2.1. Planting Material for Greenhouse Experiment
2.2. Pre-Emergence Herbicides
2.3. Post-Emergence Herbicides
2.4. Measurement of Growth Parameters
2.5. Determination of Antioxidant Activity
2.6. Statistical Analysis
3. Results
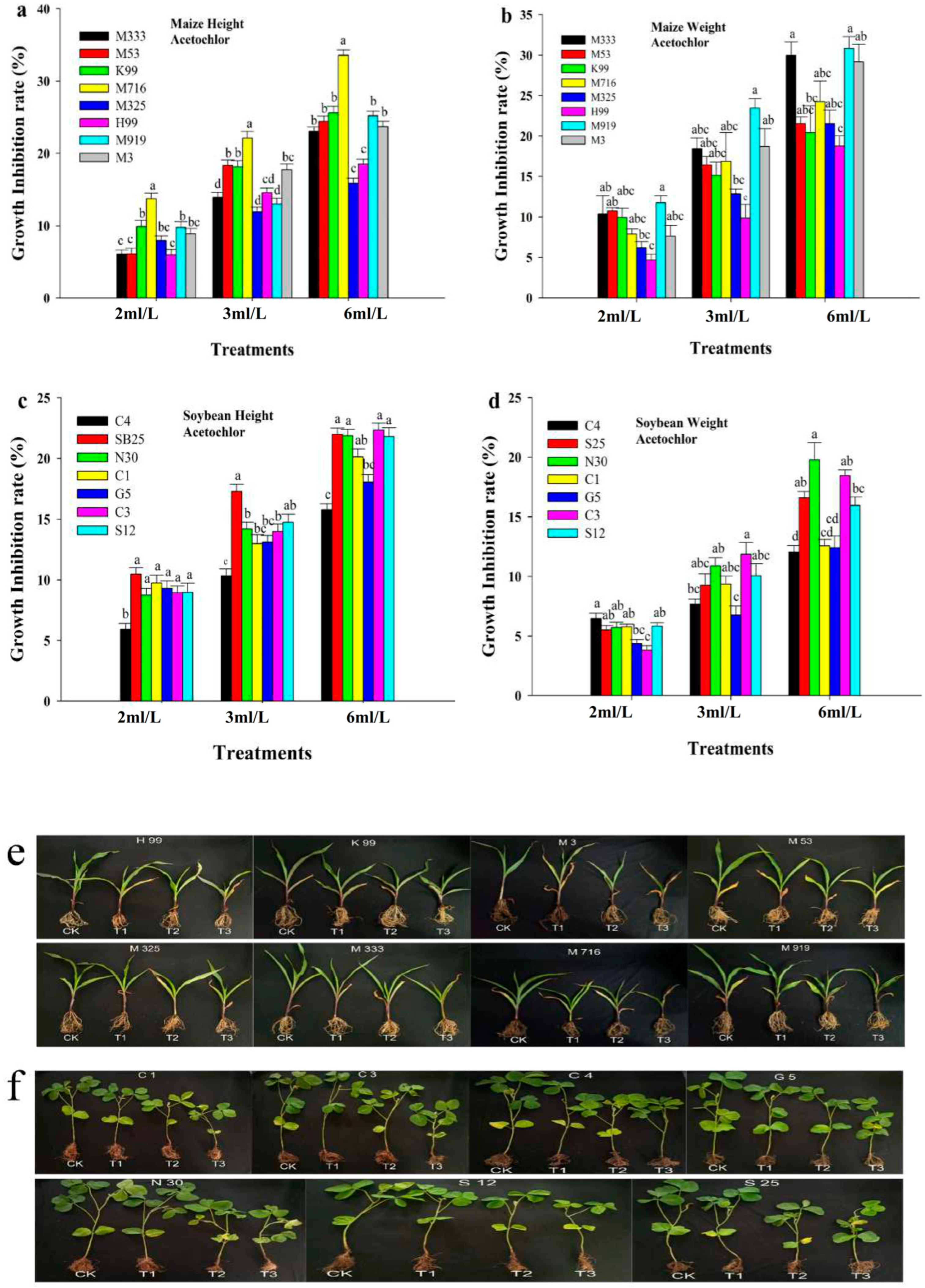
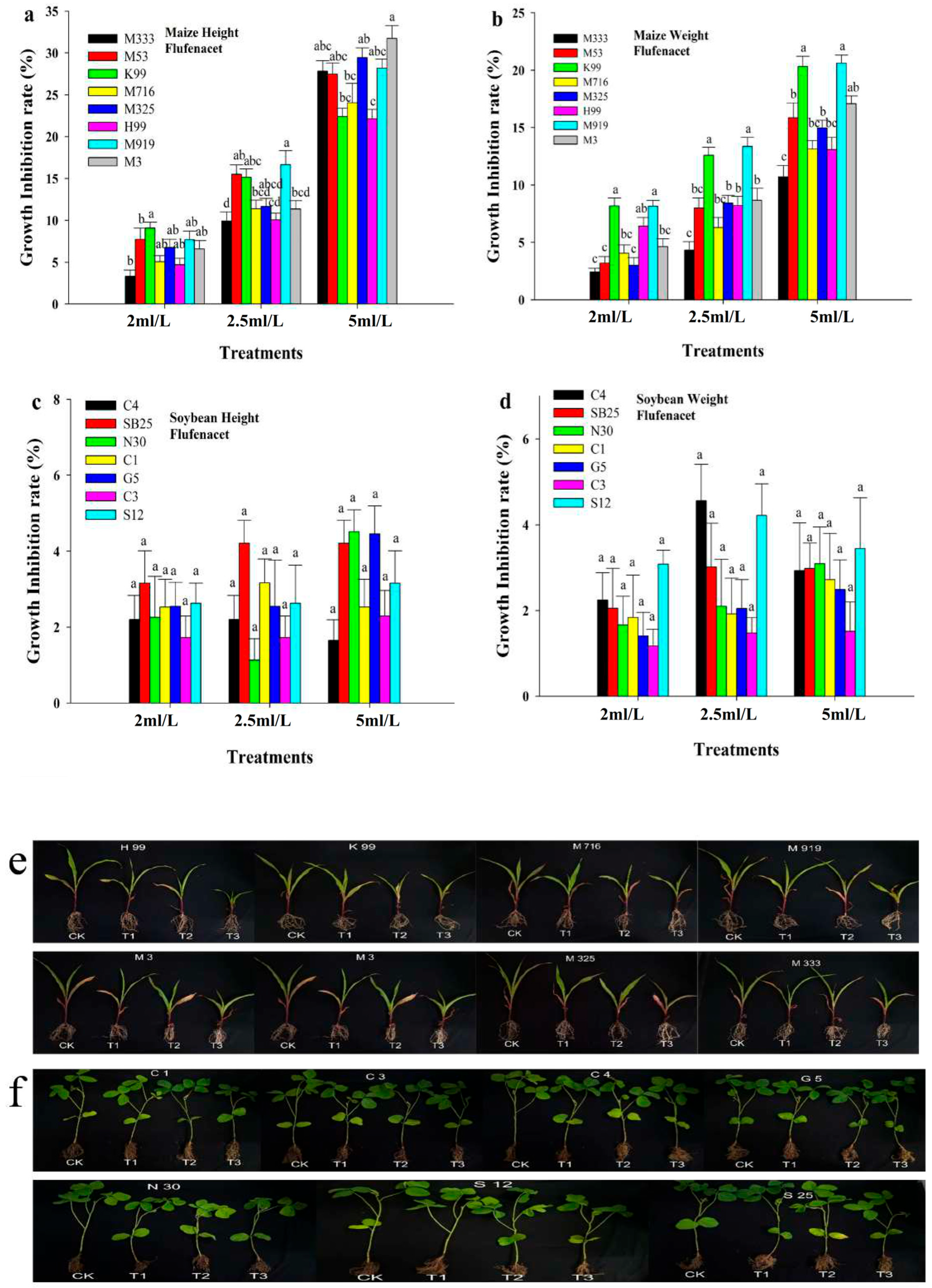
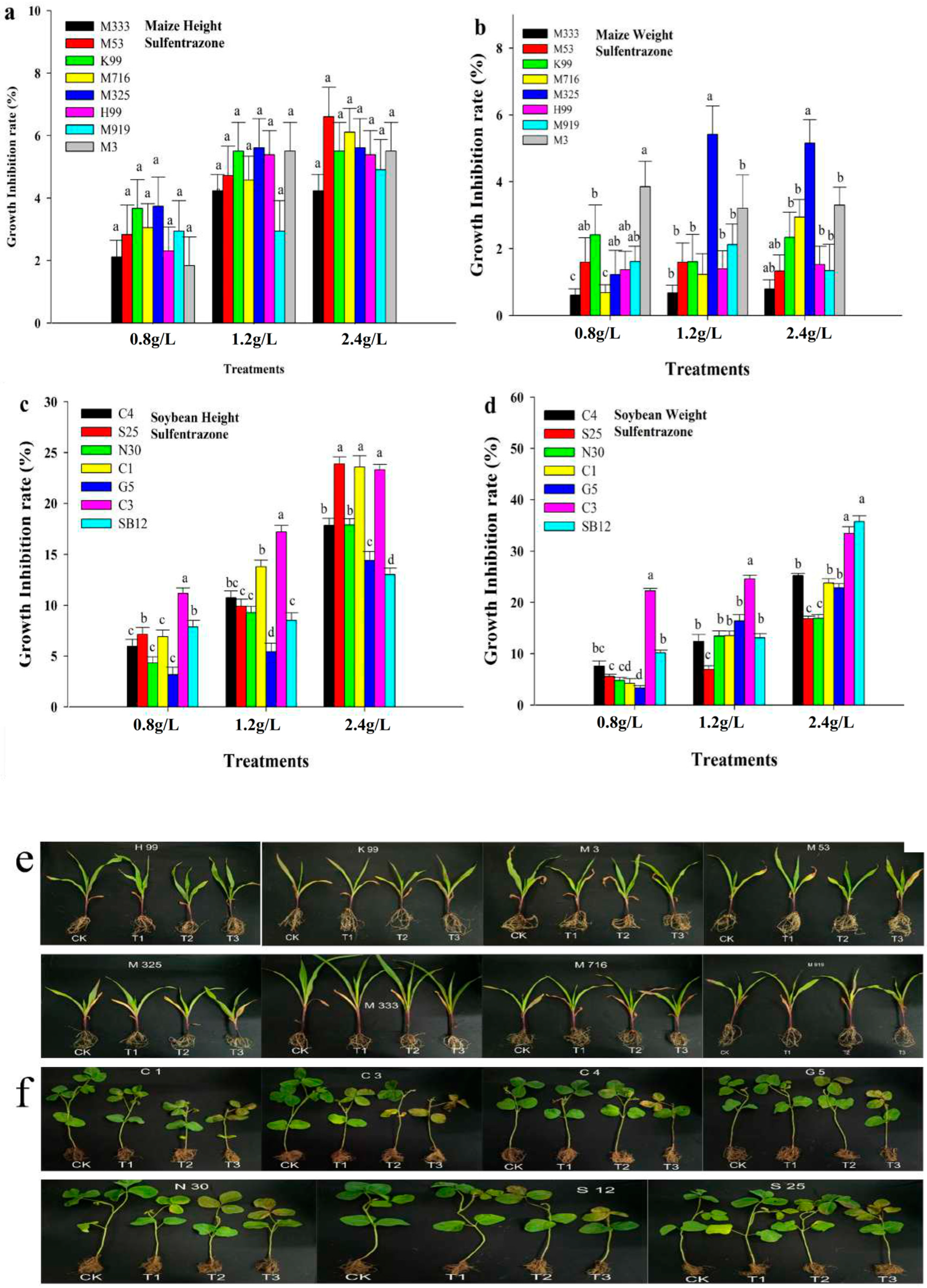
3.1. Effect of Pre-Emergence Herbicide on Maize and Soybean Growth Under Greenhouse Conditions
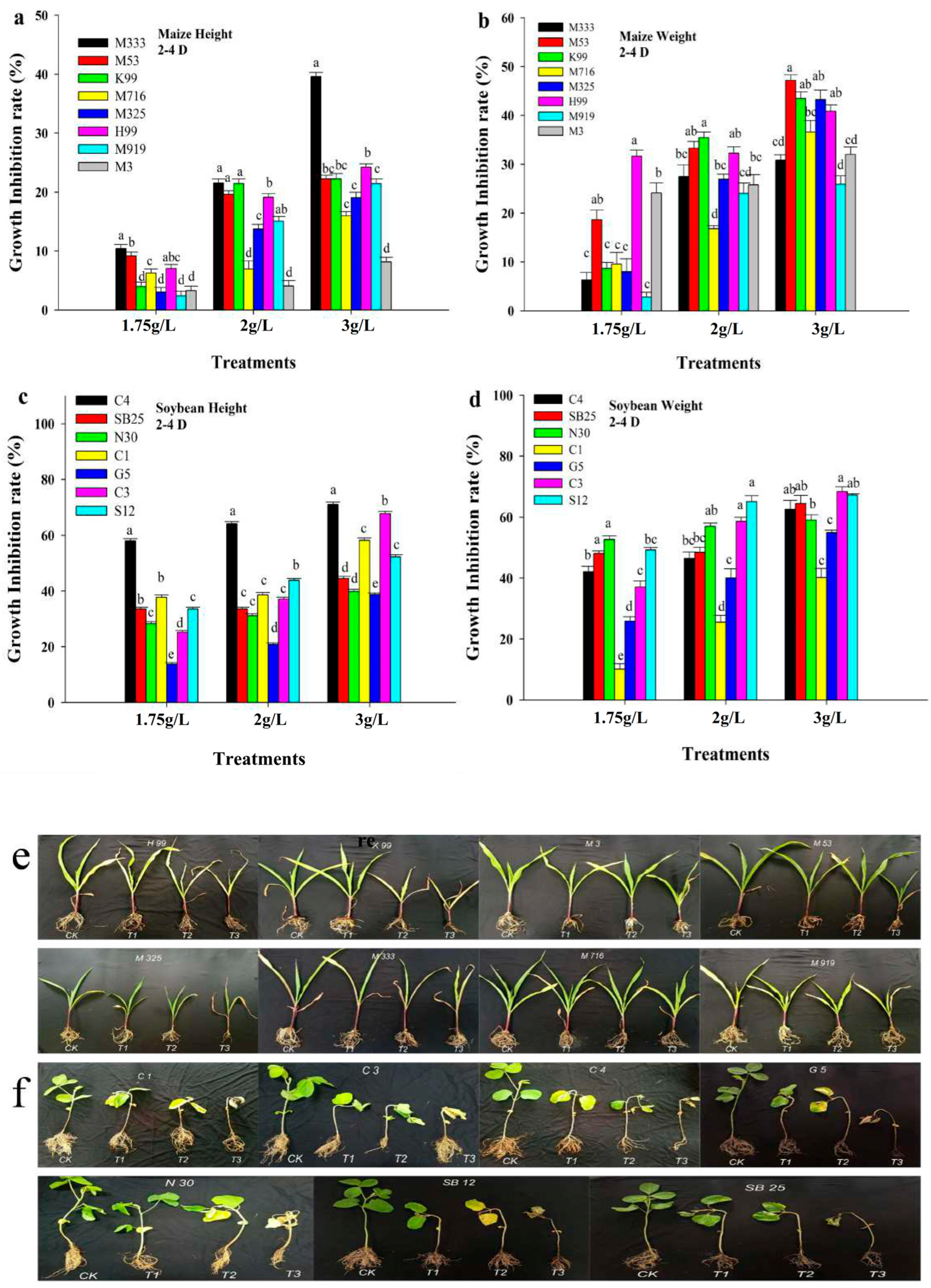
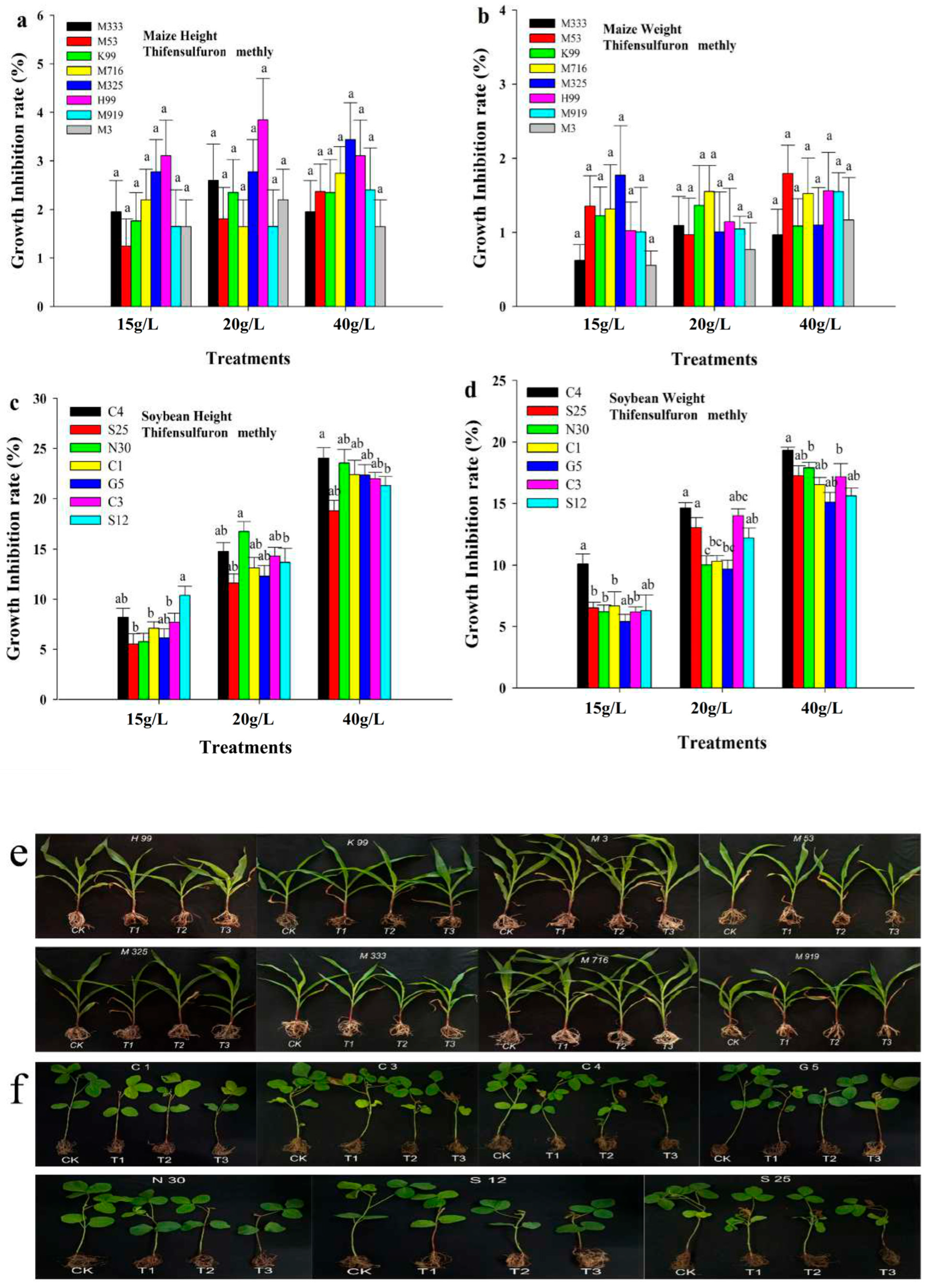
3.2. Effect of Post-Emergence Herbicide on Maize and Soybean Growth Under Greenhouse Conditions
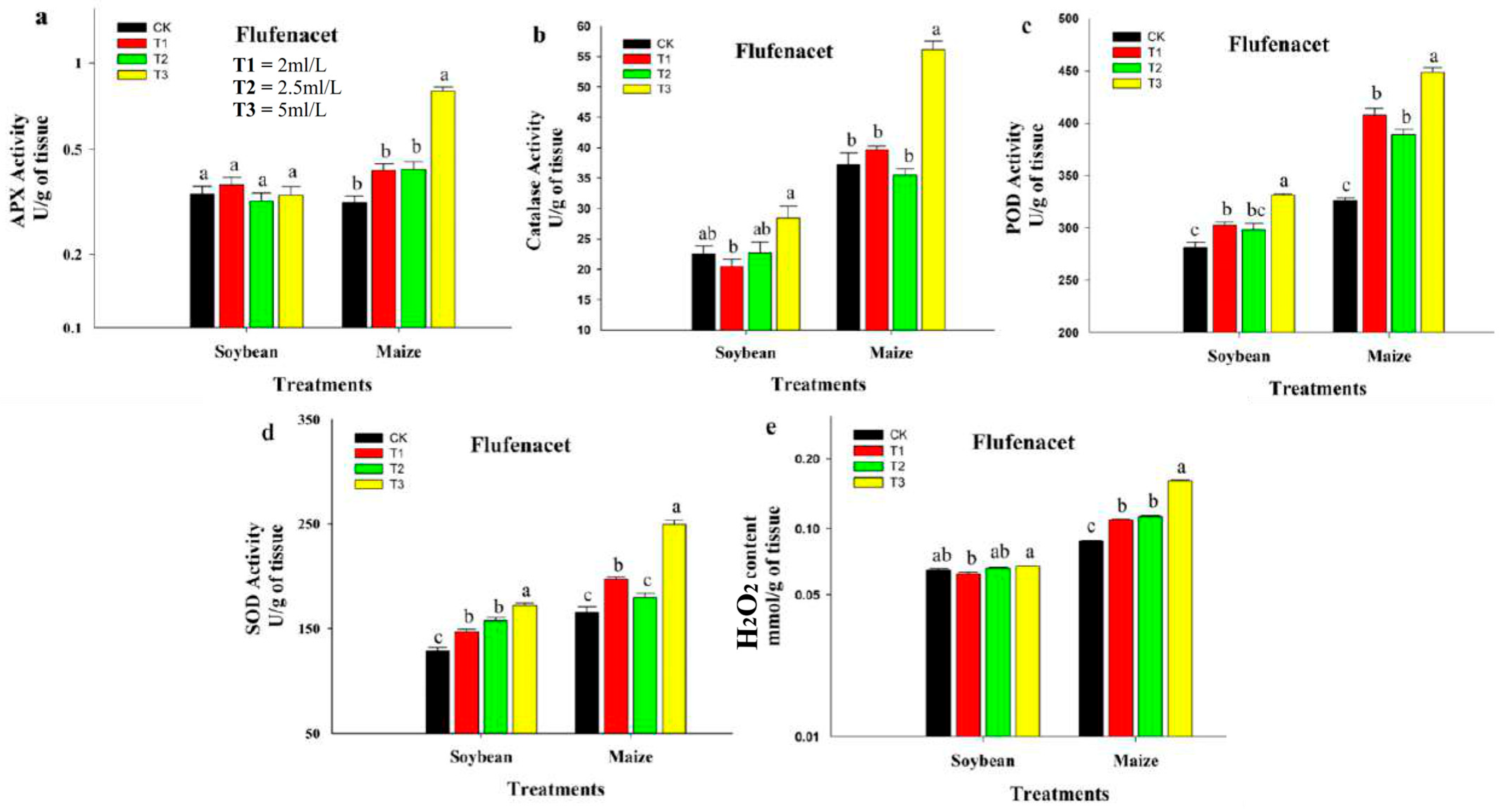
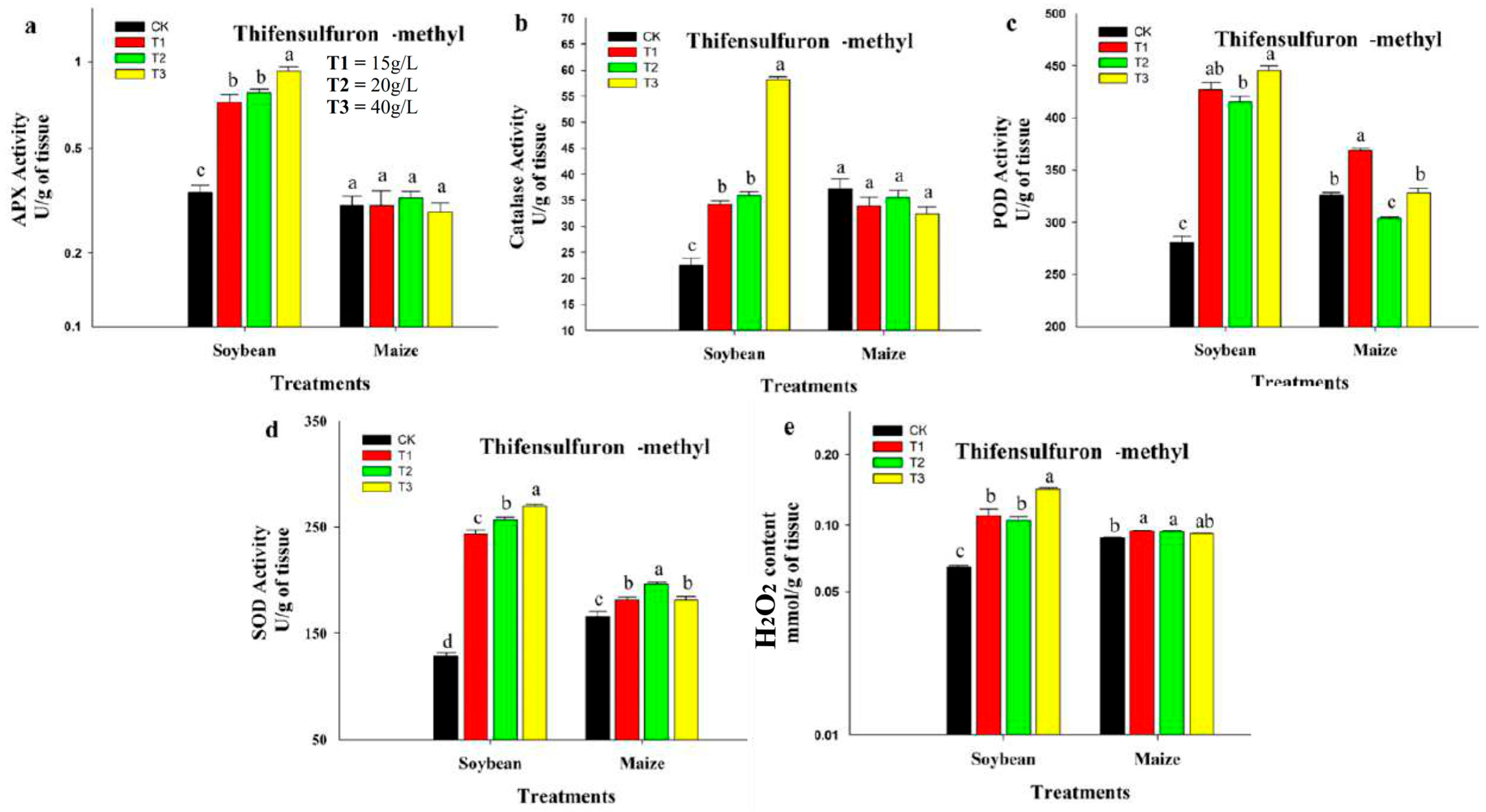
3.3. Effect of Flufenacet and Thifensulfuron-Methyl Herbicide on Antioxidant Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lithourgidis, A.S.; Vlachostergios, D.N.; Dordas, C.A.; Damalas, C.A. Dry matter yield, nitrogen content, and competition in pea–cereal intercropping systems. Eur. J. Agron. 2011, 34, 287–294. [Google Scholar] [CrossRef]
- Echarte, L.; Della, M.A.; Cerrudo, D.; Gonzalez, V.H.; Abbate, P. Yield response to plant density of maize and sunflower intercropped with soybean. Field Crops Res. 2011, 121, 423–429. [Google Scholar] [CrossRef]
- Inal, A.; Gunes, A.; Zhang, F.; Cakmak, I. Peanut/maize intercropping induced changes in rhizosphere and nutrient concentrations in shoots. Plant Physiol. Biochem. 2005, 45, 350–356. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S.; Laghari, G.M.; Laghari, A.H.; Soomro, A.A.; Jabeen, N. Effect of maize (Zea mays) and soybean (Glycine max) cropping systems on weed infestation and resource use efficiency. Agronomy 2024, 14, 2801. [Google Scholar] [CrossRef]
- Li, X.; Mu, Y.; Cheng, Y.; Liu, X.; Nian, H. Effects of intercropping sugarcane and soybean on growth; rhizosphere soil microbes; nitrogen and phosphorus availability. Acta Physiol. Plant. 2013, 35, 1113–1119. [Google Scholar] [CrossRef]
- Iqbal, N.; Hussain, S.; Ahmed, Z.; Yang, F.; Wang, X. Comparative analysis of maize-soybean strip intercropping systems: A review. Plant Prod. Sci. 2019, 22, 131–142. [Google Scholar] [CrossRef]
- Young, G.B. Changes in herbicide use patterns and production practices resulting from glyphosate-resistant crops. Weed Technol. 2006, 20, 301–307. [Google Scholar] [CrossRef]
- Xu, Z.; Li, C.; Zhang, C.; Yu, Y.; van der Werf, W.; Zhang, F. Intercropping maize and soybean increases efficiency of land and fertilizer nitrogen use; A meta-analysis. Field Crops Res. 2020, 246, 107661. [Google Scholar] [CrossRef]
- Kocsy, G.; Tóth, B.; Berzy, T.; Szalai, G.; Jednákovits, A.; Galiba, G. Glutathione reductase activity and chilling tolerance are induced by a hydroxylamine derivative BRX-156 in maize and soybean. Plant Sci. J. 2001, 160, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, S.; Gao, R.; Liu, W.; Yong, T. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red: Far-red ratio. Field Crops Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Sondhia, S. Herbicides residues in soil, water, plants and non-targeted organisms and human health implications: An Indian perspective. Indian. J. Weed Sci. 2014, 46, 66–85. [Google Scholar]
- Riedo, J.; Wettstein, F.E.; Rösch, A.; Herzog, C.; Banerjee, S.; Büchi, L. Widespread occurrence of pesticides in organically managed agricultural soils-the ghost of a conventional agricultural past? Environ. Sci. Technol. 2021, 55, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, P.; Wang, X.; Jiang, M.; Wu, X.; Wu, C.; Tang, Q. Effect of soil sealing treatment on control of broadleaf weeds in soybean corn compound planting field. Plant Health Med. 2022, 1, 102–106, (In Chinese with English Abstract). [Google Scholar]
- Alptekin, H.; Ozkan, A.G.; Kulak, M. Management of weeds in maize by sequential or individual applications of pre-and post-emergence herbicides. Agriculture 2023, 13, 421. [Google Scholar] [CrossRef]
- Yang, L.; ZhiHong, M.; BingRu, L.; Hua, P.; Meng, Z. Registration status and existing problems of herbicides in the major crops in China. J. Food Qual. 2018, 9, 4483–4488. [Google Scholar]
- Priess, L.G.; Norsworthy, K.J.; Roberts, L.T.; Gbur, E.E. Impact of postemergence herbicides on soybean injury and canopy formation. Weed Technol. 2020, 34, 727–734. [Google Scholar] [CrossRef]
- Baghestani-Meybodi, M.A.; Eskandar, Z.; Soufizadeh, S.; Rahimian, M. Morphological and physiological characteristics which enhance competitiveness of winter wheat (Triticum aestivum) against Goldbachia laevigata. Iran. J. Weed Sci. 2005, 1, 111–126. [Google Scholar]
- Mahoney, K.J.; Nurse, E.R.; Everman, J.W.; Sprague, L.C.; Sikkema, H.P. Tolerance of corn (Zea mays L.) to early and late glyphosate applications. Am. J. Plant Sci. 2014, 5, 4034–4042. [Google Scholar] [CrossRef]
- Nelson, A.K.; Renner, A.K. Soybean growth and development as affected by glyphosate and postemergence herbicide tank mixtures. J. Agron. 2001, 93, 428–434. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, S.; Lin, T.; Jiao, Y.; Cai, C.; Xue, C.; Ye, J.; Xue, X. The impact of fluroxypyr drift on soybean phytotoxicity and the safety drift thresholds. Agriculture 2024, 14, 2203. [Google Scholar] [CrossRef]
- Boulahia, K.; Carol, P.; Planchais, S.; Abrous, B.O. Phaseolus vulgaris seedlings exposed to prometryn herbicide contaminated soil trigger an oxidative stress response. J. Agric. Food Chem. 2016, 64, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lu, Y.; Zhang, J.; Tan, R.L.; Yang, H. Accumulation and toxicological response of atrazine in rice crops. Ecotoxicol. Environ. Saf. 2014, 102, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Neal Stewart, C.; Vargas, L. Defenses against ROS in crops and weeds: The effects of interference and herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, T.; Sen, G.; Mukherjee, J.; Das, R. Assessing the effect of herbicide diuron on river biofilm: A statistical model. Chemosphere 2021, 282, 131104. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhang, S.; Zhou, Q.; Xue, C.; Ye, J.; Ye, S.; Wu, C.; Han, H.; Mao, Z.; Ding, S.; et al. Experimental study of Quizalofop-p-Ethyl herbicide drift damage to corn and the safety amount of drift deposition. Agronomy 2023, 13, 2890. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H. Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol. Environ. Saf. 2009, 72, 1687–1693. [Google Scholar] [CrossRef]
- Vasilakoglou, B.I.; Eleftherohorinos, G.I. Persistence; efficacy; and selectivity of amide herbicides in corn. Weed Technol. 2003, 17, 381–388. [Google Scholar] [CrossRef]
- Hassan, M.N.; Nemat-Alla, M.M. Oxidative stress in herbicide-treated broad bean and maize plants. Acta Physiol. Plant. 2005, 27, 429–438. [Google Scholar] [CrossRef]
- Harre, T.N.; Nie, H.; Jiang, Y.; Young, G.B. Differential antioxidant enzyme activity in rapid-response glyphosate-resistant Ambrosia trifida. Pest Manag. Sci. 2018, 74, 2125–2132. [Google Scholar] [CrossRef]
- De Freitas-Silva, L.; Rodríguez-Ruiz, M.; Houmani, H.; da-Saliva Campos, L.; Corpas, F. Glyphosate-induced oxidative stress in Arabidopsis thaliana affecting peroxisomal metabolism and triggers activity in the oxidative phase of the pentose phosphate pathway (OxPPP) involved in NADPH generation. J. Plant Physiol. 2017, 218, 196–205. [Google Scholar] [CrossRef]
- dos-Santos Moura, C.; de-Almeida Silva, M. Physiological and biochemical responses of sugarcane to oxidative stress induced by water deficit and paraquat. Acta Physiol. Plant. 2015, 37, 172. [Google Scholar] [CrossRef]
- Zhu, J.; Patzoldt, L.W.; Radwan, O.; Tranel, P.; Clough, S. Effects of photosystem-II-interfering herbicides atrazine and bentazon on the soybean transcriptome. Plant Genome 2014, 2, 191–205. [Google Scholar] [CrossRef]
- Wang, Q.; Que, X.; Zheng, R.; Pang, Z.; Li, C. Phytotoxicity assessment of atrazine on growth and physiology of three emergent plants. Environ. Sci. Pollut. Res. 2015, 22, 9646–9657. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhong, G.; Zhou, J.; Liu, Y.; Pang, Y. Separate and combined effects of glyphosate and copper on growth and antioxidative enzymes in Salvinia natans (L.). Sci. Total Environ. 2019, 655, 1448–1456. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, S.; Yang, J.; Yu, L.; Shang, J.; Yang, W.; Wang, X. Safety Evaluation of Herbicides in Maize and Soybean and Their Antioxidant Defense Responses to Thifensulfuron-Methyl and Flufenacet. Agronomy 2025, 15, 2833. https://doi.org/10.3390/agronomy15122833
Hamza S, Yang J, Yu L, Shang J, Yang W, Wang X. Safety Evaluation of Herbicides in Maize and Soybean and Their Antioxidant Defense Responses to Thifensulfuron-Methyl and Flufenacet. Agronomy. 2025; 15(12):2833. https://doi.org/10.3390/agronomy15122833
Chicago/Turabian StyleHamza, Sohail, Jizhi Yang, Liping Yu, Jing Shang, Wenyu Yang, and Xuegui Wang. 2025. "Safety Evaluation of Herbicides in Maize and Soybean and Their Antioxidant Defense Responses to Thifensulfuron-Methyl and Flufenacet" Agronomy 15, no. 12: 2833. https://doi.org/10.3390/agronomy15122833
APA StyleHamza, S., Yang, J., Yu, L., Shang, J., Yang, W., & Wang, X. (2025). Safety Evaluation of Herbicides in Maize and Soybean and Their Antioxidant Defense Responses to Thifensulfuron-Methyl and Flufenacet. Agronomy, 15(12), 2833. https://doi.org/10.3390/agronomy15122833







