Optimizing Water–Carbon Coupling Through a Trait-Based Framework Integrating WCCI and Dual-Filter CATS Model
Abstract
1. Introduction
- (1)
- To quantify and compare the existing WCCI across the five stratified microhabitat zones (A-E).
- (2)
- To evaluate how key environmental filters (soil moisture, nutrients, and erosion) shape existing community trait distributions and functional performance (WCCI).
- (3)
- To develop and apply a dual-filter CATS optimization model to identify zone-specific species assemblages that maximize the CWM-WCCI.
- (4)
- To compare the functional composition of these optimized assemblages, thereby providing practical, tailored restoration prescriptions.
2. Materials and Methods
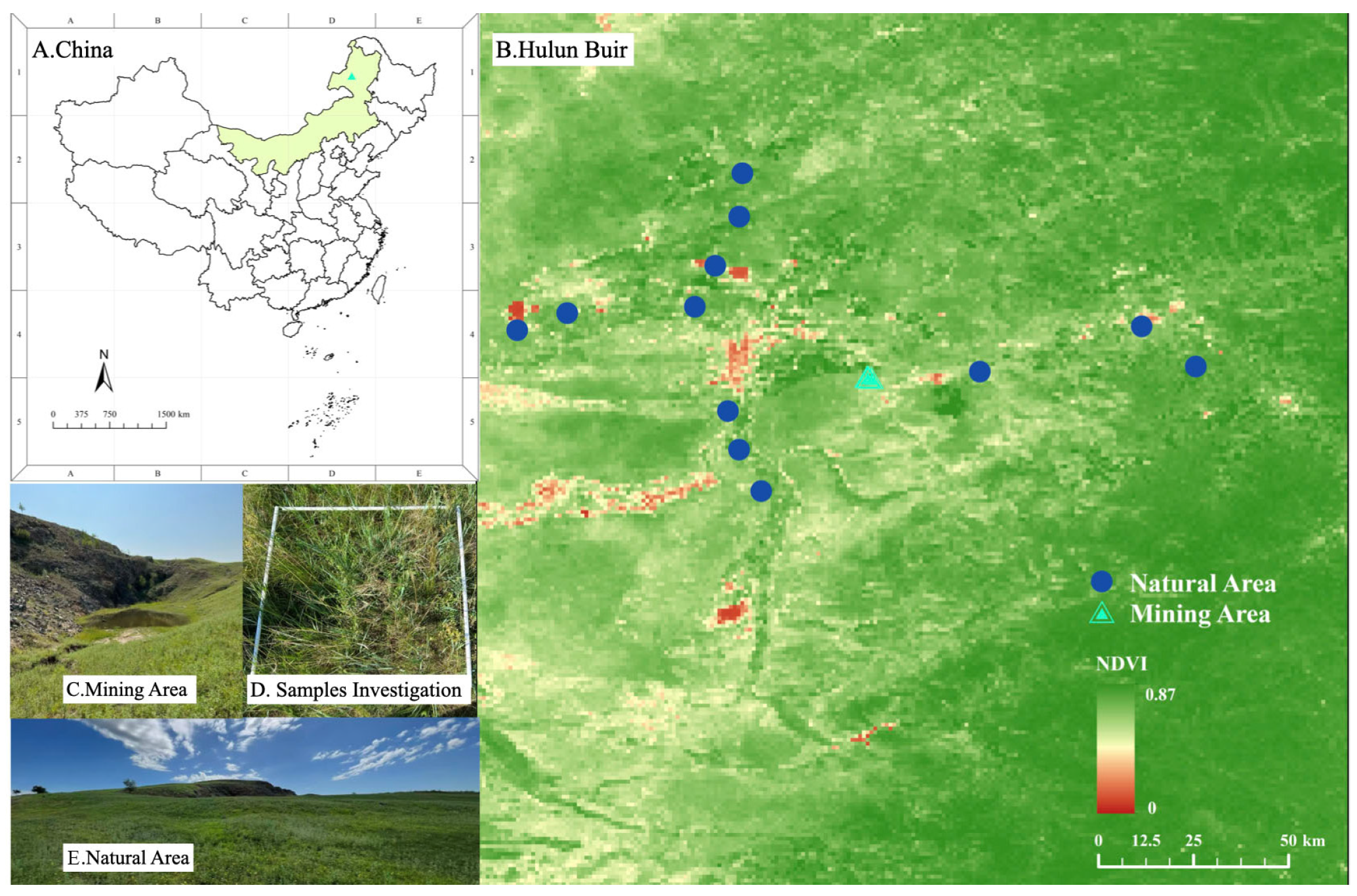
2.1. Study Area
2.2. Sampling Design and Field Data Collection
2.3. Objective 1 and 2: Quantifying Environmental Filters and WCCI
2.3.1. Microhabitat Stratification
2.3.2. Calculation of the Water-Carbon Coupling Index (WCCI)
- (1)
- Water Conservation Score (): This represents the community’s strategy for efficient resource use. We selected the inverse of Specific Leaf Area (1/SLA) as the sole proxy. 1/SLA (equivalent to Leaf Mass per Area, LMA) is a cornerstone trait of the Leaf Economics Spectrum (LES). Higher 1/SLA values strongly indicate a “conservative” resource strategy, characterized by denser leaf tissues, enhanced drought tolerance, and higher intrinsic water-use efficiency (WUE) under water-limited conditions.
- (2)
- Carbon Accumulation Score (): This represents the community’s potential for biomass storage (“carbon stock”). It is calculated as the aggregate of Plant Height (H) and Foliage Cover (FC). Taller stature (H) indicates greater vertical biomass potential, while higher foliage cover (FC) represents horizontal community-level carbon stocks. Together, they provide a robust proxy for the standing carbon capacity of the vegetation.
2.3.3. Trait-Environment Analysis [66]
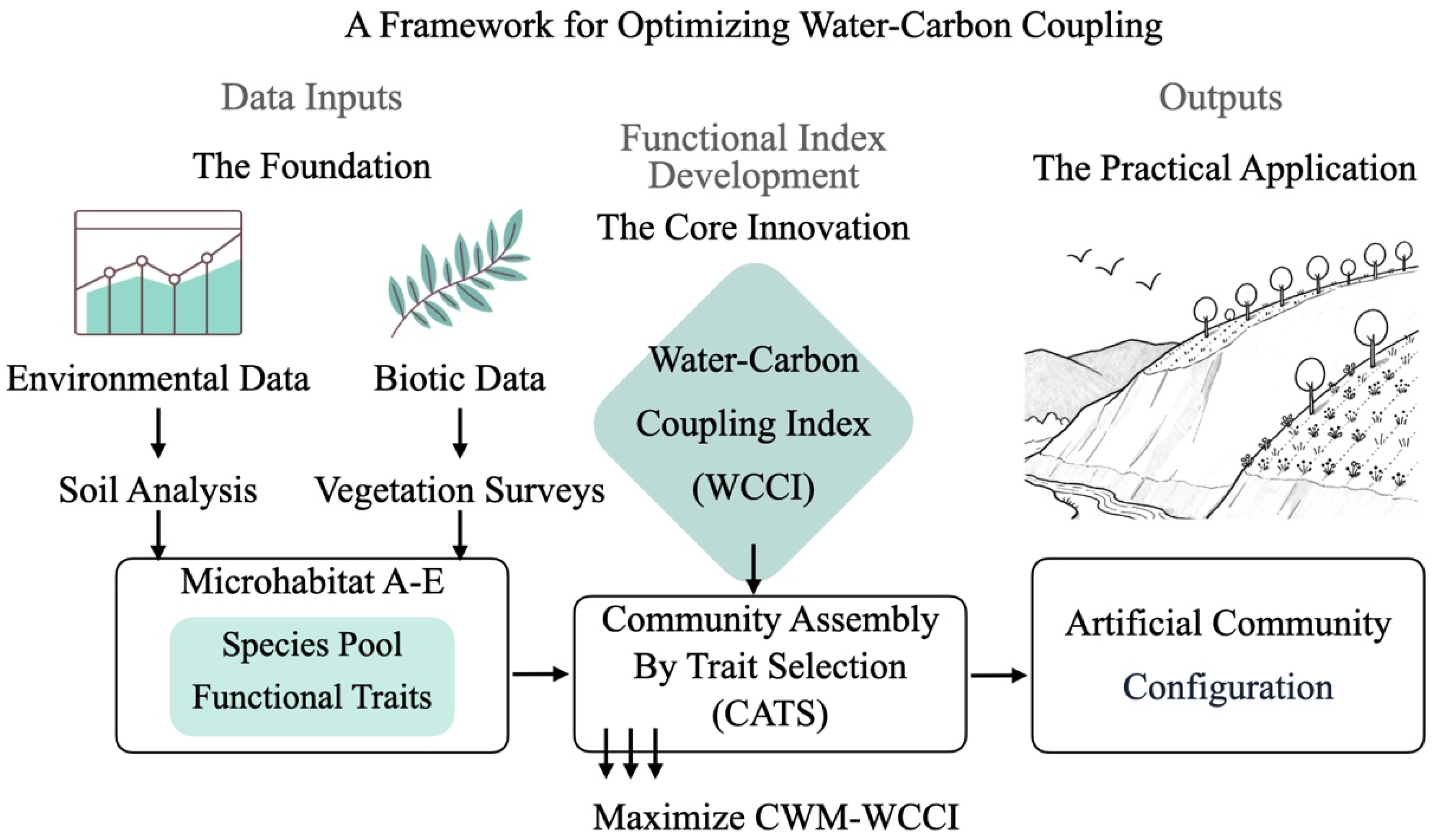
2.4. Objective 3 and 4: The Dual-Filter CATS Optimization Framework
2.4.1. Environmental Filtering and Target Trait Prediction
- (1)
- Filter 1 (Training): We used the randomForest package to train a Random Forest model on the reference ecosystem data (N = 20 plots). This model learned the relationship between environmental predictors (SMC, SOM, TN, TP, E) and the observed CWM traits (CWM-H, CWM-FC, CWM-SLA).
- (2)
- Filter 2 (Prediction): This trained model was then used to predict the “target” CWM trait values for each of the five mining microhabitats (A–E) based on their specific soil conditions.
2.4.2. Optimization: Maximizing WCCI via Linear Programming
- (1)
- Objective:
- (2)
- Subject to Constraints:
- Sum Constraint:
- Abiotic Filter Constraint: The CWM traits of the final community must fall within a strict 10% tolerance window of the “target” values from Filter 2. This ensures microhabitat-specificity.
- Diversity Constraint: The relative abundance of any single species was capped at 30% to prevent mono-dominance.
2.5. Statistical Analysis
3. Results
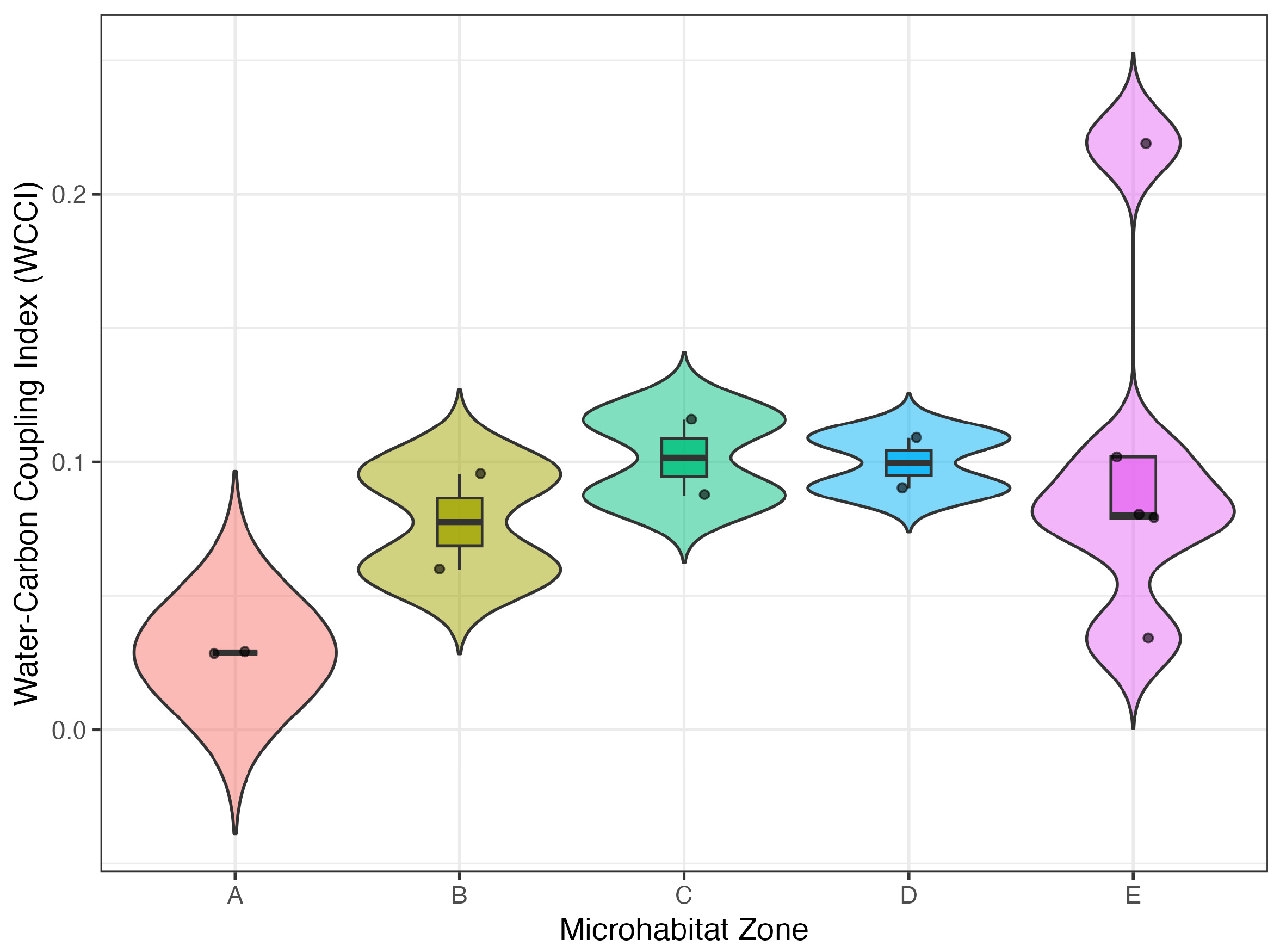
3.1. Objective1: WCCI Is Lowest in Severely Degraded Zones
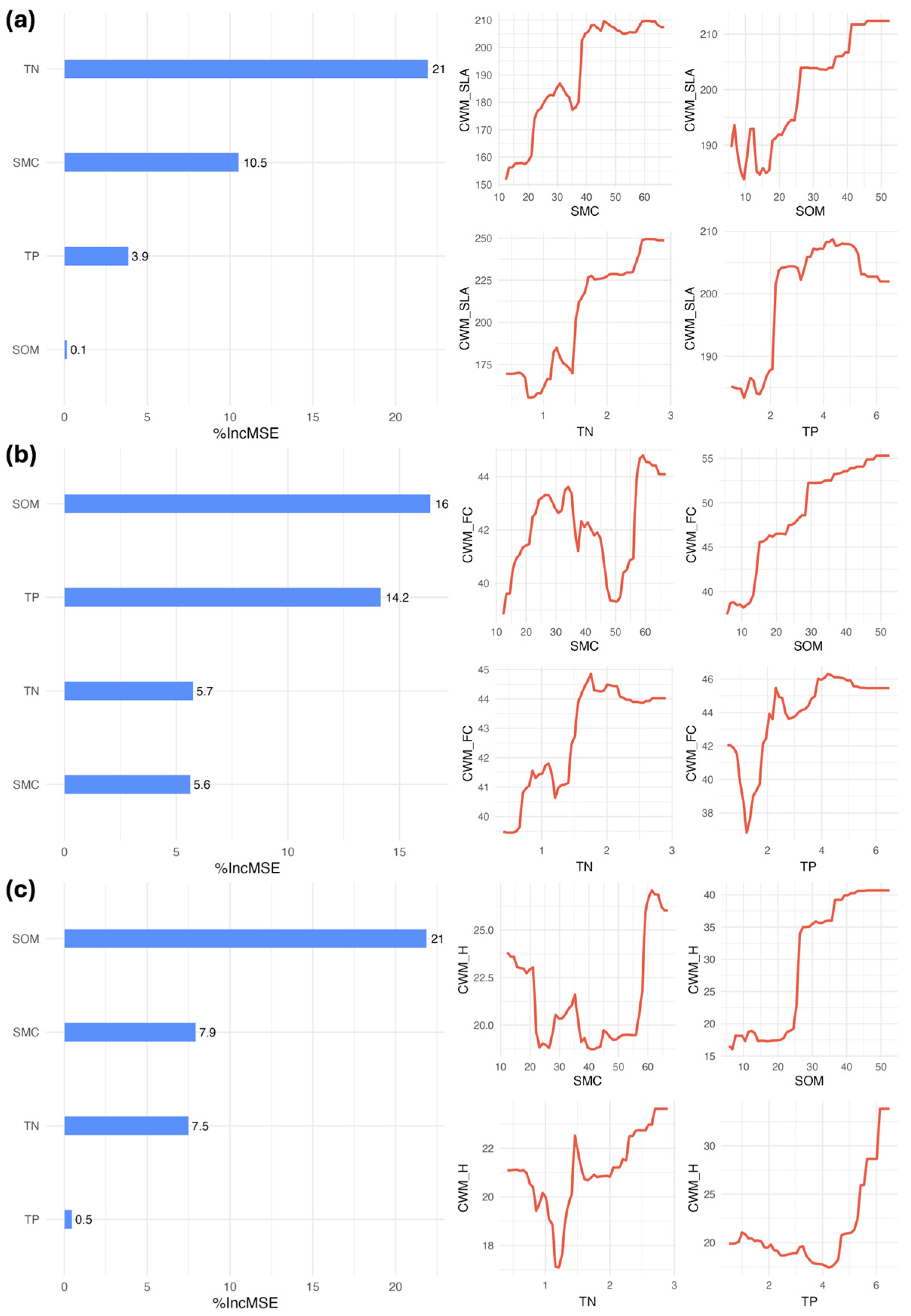
3.2. Objective2: Environmental Filters Drive Trait Distributions
3.3. Objective2: Trait-Environment Filtering in Plant Communities
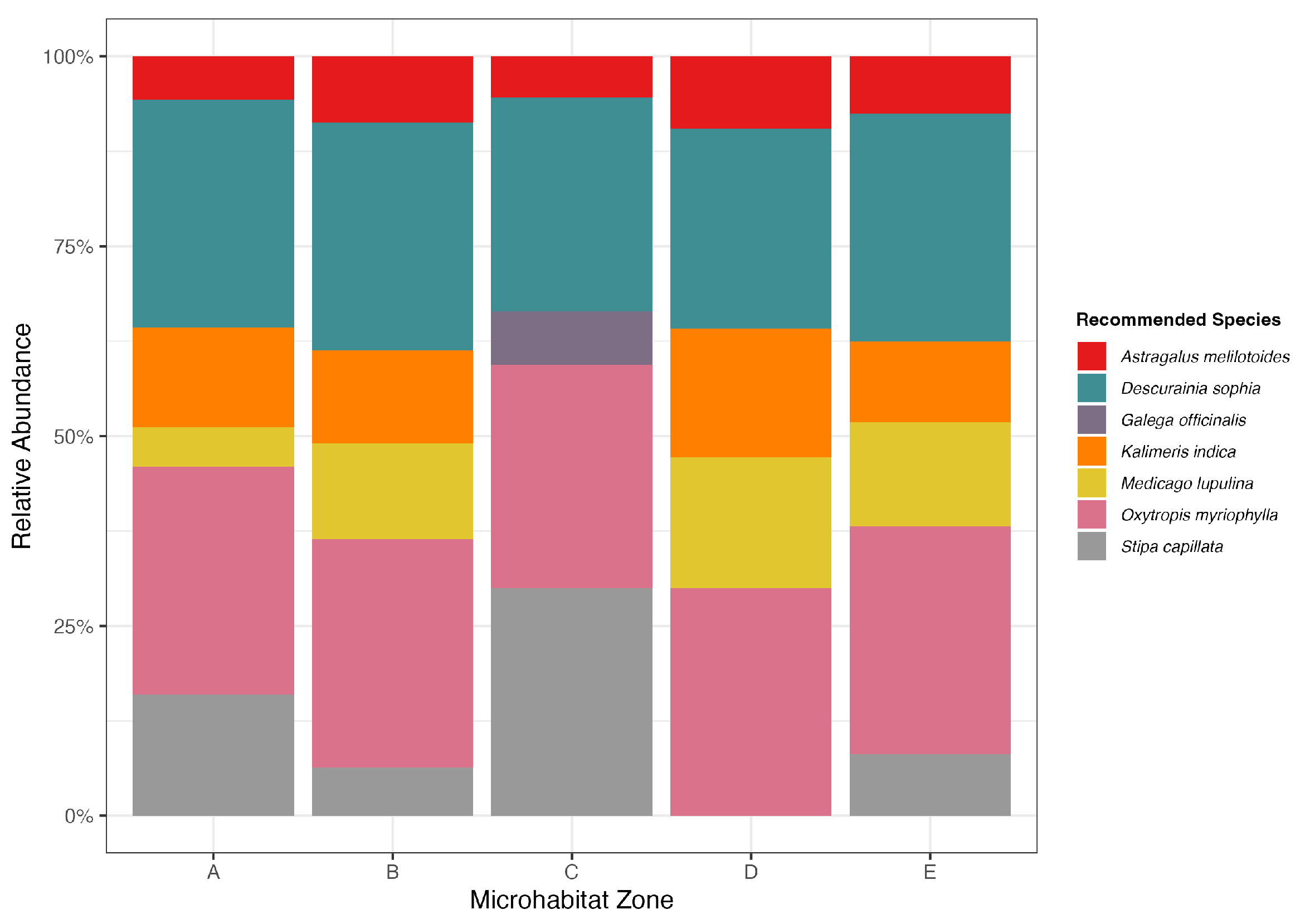
3.4. Objective 3 and 4: Optimized, Zone-Specific Community Assemblages
4. Discussion
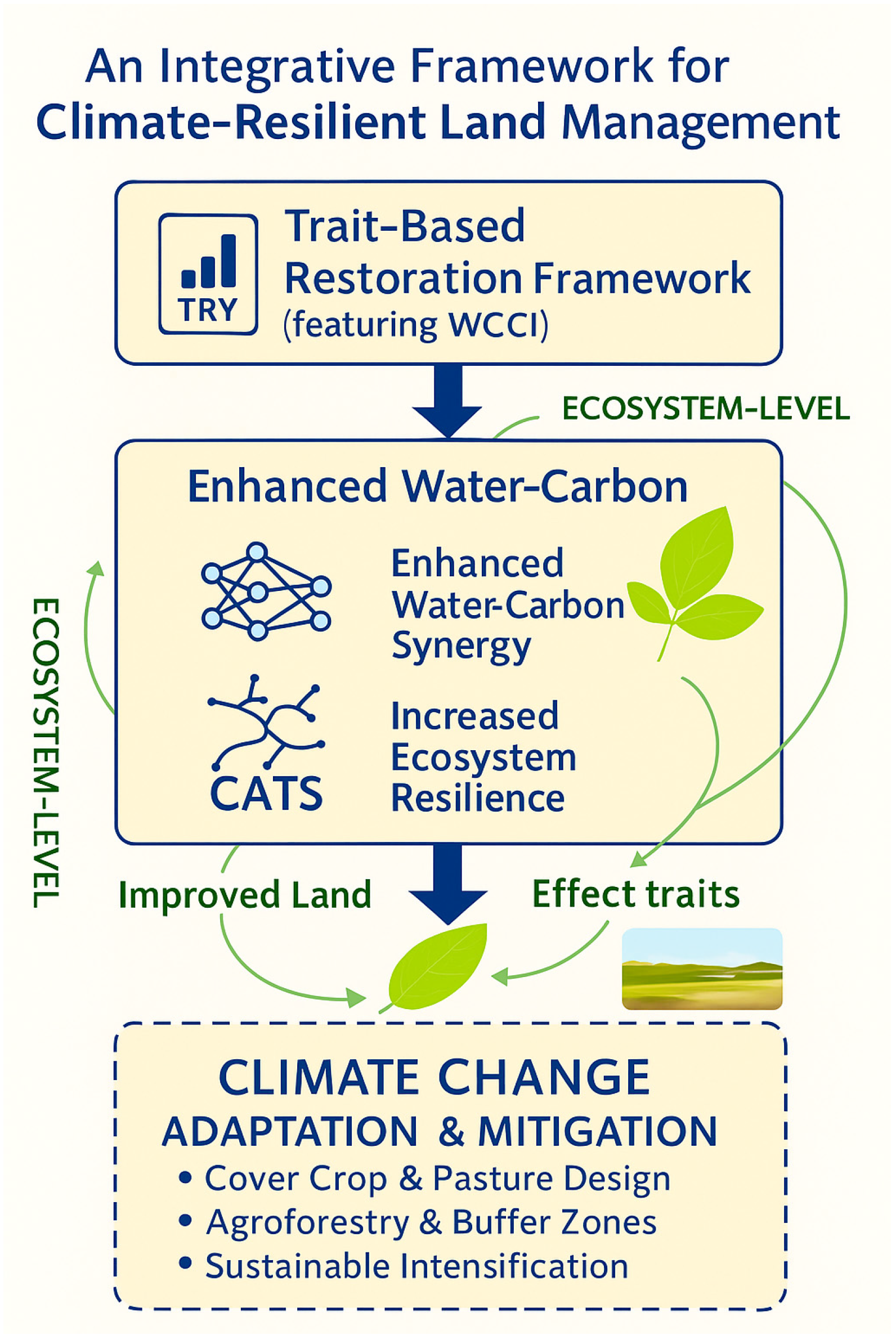
4.1. An Innovative Framework for Optimizing Biogeochemical Function
4.2. The Mechanisms of Functional Optimization: Filters, Keystones, and Soil Feedbacks
4.3. Practical Implications: From Precision Restoration to Sustainable Agronomy
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, G.; Eisenhauer, N.; Terrer, C.; Eldridge, D.J.; Duan, H.; Guirado, E.; Berdugo, M.; Zhou, L.; Liu, S.; Zhou, X.; et al. Resistance of Ecosystem Services to Global Change Weakened by Increasing Number of Environmental Stressors. Nat. Geosci. 2024, 17, 882–888. [Google Scholar] [CrossRef]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and Future Global Transformation of Terrestrial Ecosystems under Climate Change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Khan, U.; Adnan, M.; Younas, W.; Qureshi, N.B.; Yasir, Q.M.; Cai, Q.; Chiu, M.-C. Global Decline of Aquatic and Terrestrial Insects Driven by Climate Change and Anthropogenic Impacts: The Interaction of Multiple Stressors and Disruption of Niche Conservatism. Biol. Conserv. 2025, 308, 111181. [Google Scholar] [CrossRef]
- Scholes, R.; Montanarella, L.; Brainich, A.; Barger, N.; Brink, B.; Cantele, M.; Erasmus, B.; Fisher, J.; Gardner, T.; Holland, T.; et al. Summary for Policymakers of the Assessment Report on Land Degradation and Restoration of the Intergovernmental SciencePolicy Platform on Biodiversity and Ecosystem Services; IPBES: Bonn, Germany, 2018. [Google Scholar]
- Prăvălie, R.; Patriche, C.; Borrelli, P.; Panagos, P.; Roșca, B.; Dumitraşcu, M.; Nita, I.-A.; Săvulescu, I.; Birsan, M.-V.; Bandoc, G. Arable Lands under the Pressure of Multiple Land Degradation Processes. A Global Perspective. Environ. Res. 2021, 194, 110697. [Google Scholar] [CrossRef]
- Hossain, A.; Krupnik, T.J.; Timsina, J.; Mahboob, M.G.; Chaki, A.K.; Farooq, M.; Bhatt, R.; Fahad, S.; Hasanuzzaman, M. Agricultural Land Degradation: Processes and Problems Undermining Future Food Security. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Ali Khan, I., Adnan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–61. ISBN 978-3-030-49731-6. [Google Scholar]
- Habibullah, M.S.; Din, B.H.; Tan, S.-H.; Zahid, H. Impact of Climate Change on Biodiversity Loss: Global Evidence. Environ. Sci. Pollut. Res. 2022, 29, 1073–1086. [Google Scholar] [CrossRef]
- Kleespies, M.W.; Hahn-Klimroth, M.; Dierkes, P.W. Perceptions of Biodiversity Loss among Future Decision-Makers in 37 Countries. npj Biodivers. 2024, 3, 21. [Google Scholar] [CrossRef]
- Fu, L.; Huang, J.; Zhang, G.; Han, D.; Ding, L.; Wei, Y.; Liu, X.; Li, C.; Yu, H. Abrupt Loss of Species Richness Caused by Ecosystem Transition. Sci. Bull. 2025, 70, 1523–1532. [Google Scholar] [CrossRef]
- Fu, B.; Liu, Y.; Meadows, M.E. Ecological Restoration for Sustainable Development in China. Natl. Sci. Rev. 2023, 10, nwad033. [Google Scholar] [CrossRef]
- Gann, G. The Language(s) of Ecological Restoration. Ecol. Restor. 2009, 27, 3–4. [Google Scholar] [CrossRef]
- Kremer, K.N.; Bauhus, J. Drivers of Native Species Regeneration in the Process of Restoring Natural Forests from Mono-Specific, Even-Aged Tree Plantations: A Quantitative Review. Restor. Ecol. 2020, 28, 1074–1086. [Google Scholar] [CrossRef]
- Qian, J.; Ji, C.; Yang, J.; Zhao, H.; Wang, Y.; Fu, L.; Liu, Q. The Advantage of Afforestation Using Native Tree Species to Enhance Soil Quality in Degraded Forest Ecosystems. Sci. Rep. 2024, 14, 20022. [Google Scholar] [CrossRef]
- Thomas, E.; Jalonen, R.; Loo, J.; Boshier, D.; Gallo, L.; Cavers, S.; Bordács, S.; Smith, P.; Bozzano, M. Genetic Considerations in Ecosystem Restoration Using Native Tree Species. For. Ecol. Manag. 2014, 333, 66–75. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Alberdi, A.; Morriën, E.; Van Der Putten, W.H.; Rodríguez-Uña, A.; Montoya, D. The Long-Term Restoration of Ecosystem Complexity. Nat. Ecol. Evol. 2020, 4, 676–685. [Google Scholar] [CrossRef]
- Loureiro, N.; Mantuano, D.; Manhães, A.; Sansevero, J. Use of the Trait-Based Approach in Ecological Restoration Studies: A Global Review. Trees 2023, 37, 1287–1297. [Google Scholar] [CrossRef]
- Burylo, M.; Rey, F.; Mathys, N.; Dutoit, T. Plant Root Traits Affecting the Resistance of Soils to Concentrated Flow Erosion. Earth Surf. Process. Landf. 2012, 37, 1463–1470. [Google Scholar] [CrossRef]
- Brudvig, L.A. The Restoration of Biodiversity: Where Has Research Been and Where Does It Need to Go? Am. J. Bot. 2011, 98, 549–558. [Google Scholar] [CrossRef]
- Brancalion, P.H.S.; Niamir, A.; Broadbent, E.; Crouzeilles, R.; Barros, F.S.M.; Almeyda Zambrano, A.M.; Baccini, A.; Aronson, J.; Goetz, S.; Reid, J.L.; et al. Global Restoration Opportunities in Tropical Rainforest Landscapes. Sci. Adv. 2019, 5, eaav3223. [Google Scholar] [CrossRef] [PubMed]
- Box, E.O. Plant Functional Types and Climate at the Global Scale. J. Veg. Sci. 1996, 7, 309–320. [Google Scholar] [CrossRef]
- Aubin, I.; Munson, A.D.; Cardou, F.; Burton, P.J.; Isabel, N.; Pedlar, J.H.; Paquette, A.; Taylor, A.R.; Delagrange, S.; Kebli, H.; et al. Traits to Stay, Traits to Move: A Review of Functional Traits to Assess Sensitivity and Adaptive Capacity of Temperate and Boreal Trees to Climate Change. Environ. Rev. 2016, 24, 164–186. [Google Scholar] [CrossRef]
- Niklas, K.J.; Shi, P.; Gielis, J.; Schrader, J.; Niinemets, Ü. Editorial: Leaf Functional Traits: Ecological and Evolutionary Implications. Front. Plant Sci. 2023, 14, 1169558. [Google Scholar] [CrossRef]
- Xing, Y.; Deng, S.; Bai, Y.; Wu, Z.; Luo, J. Leaf Functional Traits and Their Influencing Factors in Six Typical Vegetation Communities. Plants 2024, 13, 2423. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Arunachalam, K.; Arunachalam, A.; Alatalo, J.M.; Pandey, R. Assessment of Leaf Morphological, Physiological, Chemical and Stoichiometry Functional Traits for Understanding the Functioning of Himalayan Temperate Forest Ecosystem. Sci. Rep. 2021, 11, 23807. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, D.; He, P.; Cadotte, M.W.; Ye, Q. Linking Plant Functional Traits to Biodiversity under Environmental Change. Biol. Divers. 2024, 1, 22–28. [Google Scholar] [CrossRef]
- Gross, N.; Bagousse-Pinguet, Y.L.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional Trait Diversity Maximizes Ecosystem Multifunctionality. Nat. Ecol. Evol. 2017, 1, 0132. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Strahan, R.T.; Huffman, D.W.; Sánchez Meador, A.J. Using Trait-based Ecology to Restore Resilient Ecosystems: Historical Conditions and the Future of Montane Forests in Western North America. Restor. Ecol. 2017, 25, S135–S146. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Laughlin, D.E. Advances in Modeling Trait-Based Plant Community Assembly. Trends Plant Sci. 2013, 18, 584–593. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Joshi, C.; Van Bodegom, P.M.; Bastow, Z.A.; Fulé, P.Z. A Predictive Model of Community Assembly That Incorporates Intraspecific Trait Variation. Ecol. Lett. 2012, 15, 1291–1299. [Google Scholar] [CrossRef]
- Laughlin, D.C. Applying Trait-Based Models to Achieve Functional Targets for Theory-Driven Ecological Restoration. Ecol. Lett. 2014, 17, 771–784. [Google Scholar] [CrossRef]
- Caners, R.T.; Lieffers, V.J. Divergent Pathways of Successional Recovery for In Situ Oil Sands Exploration Drilling Pads on Wooded Moderate-Rich Fens in Alberta, Canada. Restor. Ecol. 2014, 22, 657–667. [Google Scholar] [CrossRef]
- Holyoak, M.; Caspi, T.; Redosh, L.W. Integrating Disturbance, Seasonality, Multi-Year Temporal Dynamics, and Dormancy Into the Dynamics and Conservation of Metacommunities. Front. Ecol. Evol. 2020, 8, 571130. [Google Scholar] [CrossRef]
- Bakker, J.D.; Price, J.N.; Henning, J.A.; Batzer, E.E.; Ohlert, T.J.; Wainwright, C.E.; Adler, P.B.; Alberti, J.; Arnillas, C.A.; Biederman, L.A.; et al. Compositional Variation in Grassland Plant Communities. Ecosphere 2023, 14, e4542. [Google Scholar] [CrossRef]
- George, A.B.; Wang, T.; Maslov, S. Functional Convergence in Slow-Growing Microbial Communities Arises from Thermodynamic Constraints. ISME J. 2023, 17, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; He, N.; Yu, K.; Xu, L.; Van Meerbeek, K. Integrating Multiple Plant Functional Traits to Predict Ecosystem Productivity. Commun. Biol. 2023, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.I.; An, R.; Kraft, N.J.B.; Pacala, S.W.; Levine, J.M. Why Ecologists Struggle to Predict Coexistence from Functional Traits. Trends Ecol. Evol. 2025, 40, 147–158. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Brudvig, L.A. Trait–Environment Interactions Affect Plant Establishment Success during Restoration. Ecology 2020, 101, e02971. [Google Scholar] [CrossRef]
- Camps-Valls, G.; Fernández-Torres, M.-Á.; Cohrs, K.-H.; Höhl, A.; Castelletti, A.; Pacal, A.; Robin, C.; Martinuzzi, F.; Papoutsis, I.; Prapas, I.; et al. Artificial Intelligence for Modeling and Understanding Extreme Weather and Climate Events. Nat. Commun. 2025, 16, 1919. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The Global Spectrum of Plant Form and Function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Thakur, M.P.; Van Der Putten, W.H.; Wilschut, R.A.; Veen, G.F.; Kardol, P.; Van Ruijven, J.; Allan, E.; Roscher, C.; Van Kleunen, M.; Bezemer, T.M. Plant–Soil Feedbacks and Temporal Dynamics of Plant Diversity–Productivity Relationships. Trends Ecol. Evol. 2021, 36, 651–661. [Google Scholar] [CrossRef]
- Gornish, E.S.; Campbell, C.; Svejcar, L.; Munson, S.M.; Vaughn, K.; Spaeth, M.K.; Yelenik, S.G.; Wolf, A.; Mitchell, R. Functional Traits Are Used in Restoration Practice: A Response to Merchant et al. (2022). Restor. Ecol. 2023, 31, e13880. [Google Scholar] [CrossRef]
- Merchant, T.K.; Henn, J.J.; De Silva, I.; Van Cleemput, E.; Suding, K.N. Aspirational Goals for the Future of Functional Traits in Restoration: A Response to Gornish et al. (2023). Restor. Ecol. 2025, 33, e14336. [Google Scholar] [CrossRef]
- Crisfield, V.E.; Ficken, C.D.; Allen, B.E.; Jog, S.K.; Bried, J.T. The Potential of Trait Data to Increase the Availability of Bioindicators: A Case Study Using Plant Conservatism Values. Ecol. Appl. 2023, 33, e2866. [Google Scholar] [CrossRef]
- Visscher, A.M.; Vandelook, F.; Fernández-Pascual, E.; Pérez-Martínez, L.V.; Ulian, T.; Diazgranados, M.; Mattana, E. Low Availability of Functional Seed Trait Data from the Tropics Could Negatively Affect Global Macroecological Studies, Predictive Models and Plant Conservation. Ann. Bot. 2022, 130, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, X.; Ran, W.; Zhao, Z.; Su, D.; Song, Y. The Ecological Restoration Strategies in Terrestrial Ecosystems Were Reviewed: A New Trend Based on Soil Microbiomics. Ecol. Evol. 2025, 15, e70994. [Google Scholar] [CrossRef]
- Boretti, A. Evaluating Water Use Efficiency and CO2 Absorption in Plants under Rising Atmospheric Carbon Dioxide Levels. J. Atmos. Sol.-Terr. Phys. 2025, 266, 106409. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Cochard, H.; Delzon, S.; Boivin, T.; Burlett, R.; Cailleret, M.; Corso, D.; Delmas, C.E.L.; De Caceres, M.; Diaz-Espejo, A.; et al. Plant Hydraulics at the Heart of Plant, Crops and Ecosystem Functions in the Face of Climate Change. New Phytol. 2024, 241, 984–999. [Google Scholar] [CrossRef]
- Still, C.J. Trading Water for Carbon. Nat. Geosci. 2018, 11, 702–703. [Google Scholar] [CrossRef]
- Nakad, M.; Sevanto, S.; Domec, J.-C.; Katul, G. Linking the Water and Carbon Economies of Plants in a Drying and Warming Climate. Curr. For. Rep. 2023, 9, 383–400. [Google Scholar] [CrossRef]
- Li, W.; Duveiller, G.; Wieneke, S.; Forkel, M.; Gentine, P.; Reichstein, M.; Niu, S.; Migliavacca, M.; Orth, R. Regulation of the Global Carbon and Water Cycles through Vegetation Structural and Physiological Dynamics. Environ. Res. Lett. 2024, 19, 073008. [Google Scholar] [CrossRef]
- Gentine, P.; Green, J.K.; Guérin, M.; Humphrey, V.; Seneviratne, S.I.; Zhang, Y.; Zhou, S. Coupling between the Terrestrial Carbon and Water Cycles—A Review. Environ. Res. Lett. 2019, 14, 083003. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, W.; Xiao, J.; Zhao, M.; Li, X.; Wu, Y. Recent Weakening of Carbon-Water Coupling in Northern Ecosystems. npj Clim. Atmos. Sci. 2025, 8, 161. [Google Scholar] [CrossRef]
- Sun, P.; Wu, Y.; Xiao, J.; Hui, J.; Hu, J.; Zhao, F.; Qiu, L.; Liu, S. Remote Sensing and Modeling Fusion for Investigating the Ecosystem Water-Carbon Coupling Processes. Sci. Total Environ. 2019, 697, 134064. [Google Scholar] [CrossRef] [PubMed]
- Novick, K.A.; Ficklin, D.L.; Stoy, P.C.; Williams, C.A.; Bohrer, G.; Oishi, A.C.; Papuga, S.A.; Blanken, P.D.; Noormets, A.; Sulman, B.N.; et al. The Increasing Importance of Atmospheric Demand for Ecosystem Water and Carbon Fluxes. Nat. Clim. Change 2016, 6, 1023–1027. [Google Scholar] [CrossRef]
- El Masri, B.; Schwalm, C.; Huntzinger, D.N.; Mao, J.; Shi, X.; Peng, C.; Fisher, J.B.; Jain, A.K.; Tian, H.; Poulter, B.; et al. Carbon and Water Use Efficiencies: A Comparative Analysis of Ten Terrestrial Ecosystem Models under Changing Climate. Sci. Rep. 2019, 9, 14680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Zhang, L.; Liang, X.; Yuan, W. Coupled Models of Water and Carbon Cycles from Leaf to Global: A Retrospective and a Prospective. Agric. For. Meteorol. 2024, 358, 110229. [Google Scholar] [CrossRef]
- Peters, W.; Van Der Velde, I.R.; Van Schaik, E.; Miller, J.B.; Ciais, P.; Duarte, H.F.; Van Der Laan-Luijkx, I.T.; Van Der Molen, M.K.; Scholze, M.; Schaefer, K.; et al. Increased Water-Use Efficiency and Reduced CO2 Uptake by Plants during Droughts at a Continental Scale. Nat. Geosci. 2018, 11, 744–748. [Google Scholar] [CrossRef]
- Bacelar, E.L.V.A.; Moutinho-Pereira, J.M.; Gonçalves, B.M.C.; Brito, C.V.Q.; Gomes-Laranjo, J.; Ferreira, H.M.F.; Correia, C.M. Water Use Strategies of Plants under Drought Conditions. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–170. ISBN 978-3-642-32652-3. [Google Scholar]
- McDowell, N.G.; Sapes, G.; Pivovaroff, A.; Adams, H.D.; Allen, C.D.; Anderegg, W.R.L.; Arend, M.; Breshears, D.D.; Brodribb, T.; Choat, B.; et al. Mechanisms of Woody-Plant Mortality under Rising Drought, CO2 and Vapour Pressure Deficit. Nat. Rev. Earth Environ. 2022, 3, 294–308. [Google Scholar] [CrossRef]
- Beugnon, R.; Albert, G.; Hähn, G.; Yu, W.; Haider, S.; Hättenschwiler, S.; Davrinche, A.; Rosenbaum, B.; Gauzens, B.; Eisenhauer, N. Improving Forest Ecosystem Functions by Optimizing Tree Species Spatial Arrangement. Nat. Commun. 2025, 16, 6286. [Google Scholar] [CrossRef]
- Wikum, D.A.; Shanholtzer, G.F. Application of the Braun-Blanquet Cover-Abundance Scale for Vegetation Analysis in Land Development Studies. Environ. Manag. 1978, 2, 323–329. [Google Scholar] [CrossRef]
- ISO 11272:2017; Soil Quality—Determination of Dry Bulk Density. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 17892-1:2014; Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 1: Determination of Water Content. International Organization for Standardization: Geneva, Switzerland, 2014.
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 25–114. [Google Scholar]
- Wen, A.; Feng, M. Progress in the study of 137Cs method for soil erosion in China. J. Soil Water Conserv. 1997, 13, 61–64. [Google Scholar]
- Hou, J.; Wu, M.; Feng, H. Applying Trait-Based Modeling to Achieve Functional Targets during the Ecological Restoration of an Arid Mine Area. Agronomy 2022, 12, 2833. [Google Scholar] [CrossRef]
- Li, J. Global Patterns of Plant Functional Traits and Their Relationships to Climate. Commun. Biol. 2024, 7, 1136. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Pearson, K. Note on Regression and Inheritance in the Case of Two Parents. Proc. R. Soc. Lond. 1895, 58, 240–242. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Metropolis, N.; Ulam, S. The Monte Carlo Method. J. Am. Stat. Assoc. 1949, 44, 335–341. [Google Scholar] [CrossRef]
- Asefa, M.; Cao, M.; Zhang, G.; Ci, X.; Li, J.; Yang, J. Environmental Filtering Structures Tree Functional Traits Combination and Lineages across Space in Tropical Tree Assemblages. Sci. Rep. 2017, 7, 132. [Google Scholar] [CrossRef]
- Li, Y.; Shipley, B.; Price, J.N.; Dantas, V.D.L.; Tamme, R.; Westoby, M.; Siefert, A.; Schamp, B.S.; Spasojevic, M.J.; Jung, V.; et al. Habitat Filtering Determines the Functional Niche Occupancy of Plant Communities Worldwide. J. Ecol. 2018, 106, 1001–1009. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Yu, Y.; Huang, J.; Zhou, Z.; Zeng, J.; Chen, P.; Xiao, F.; He, Z.; Yan, Q. Ecological Stability of Microbial Communities in Lake Donghu Regulated by Keystone Taxa. Ecol. Indic. 2022, 136, 108695. [Google Scholar] [CrossRef]
- Yan, P.; He, N.; Fernández-Martínez, M.; Yang, X.; Zuo, Y.; Zhang, H.; Wang, J.; Chen, S.; Song, J.; Li, G.; et al. Plant Acquisitive Strategies Promote Resistance and Temporal Stability of Semiarid Grasslands. Ecol. Lett. 2025, 28, e70110. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Xi, N.; Adler, P.B.; Chen, D.; Wu, H.; Catford, J.A.; Van Bodegom, P.M.; Bahn, M.; Crawford, K.M.; Chu, C. Relationships between Plant–Soil Feedbacks and Functional Traits. J. Ecol. 2021, 109, 3411–3423. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going Underground: Root Traits as Drivers of Ecosystem Processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, D.C.; Chalmandrier, L.; Joshi, C.; Renton, M.; Dwyer, J.M.; Funk, J.L. Generating Species Assemblages for Restoration and Experimentation: A New Method That Can Simultaneously Converge on Average Trait Values and Maximize Functional Diversity. Methods Ecol. Evol. 2018, 9, 1764–1771. [Google Scholar] [CrossRef]
- McCormack, M.L.; Guo, D.; Iversen, C.M.; Chen, W.; Eissenstat, D.M.; Fernandez, C.W.; Li, L.; Ma, C.; Ma, Z.; Poorter, H.; et al. Building a Better Foundation: Improving Root-Trait Measurements to Understand and Model Plant and Ecosystem Processes. New Phytol. 2017, 215, 27–37. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, L.; Cong, C.; Deal, B.; Wang, Y. A Dynamic and Spatially Explicit Modeling Approach to Identify the Ecosystem Service Implications of Complex Urban Systems Interactions. Ecol. Indic. 2019, 102, 426–436. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhang, Y.; Hou, J.; Tai, Y.; Huang, X.; Guo, X.; Wu, H.; Xing, C. Optimizing Water–Carbon Coupling Through a Trait-Based Framework Integrating WCCI and Dual-Filter CATS Model. Agronomy 2025, 15, 2733. https://doi.org/10.3390/agronomy15122733
Wu S, Zhang Y, Hou J, Tai Y, Huang X, Guo X, Wu H, Xing C. Optimizing Water–Carbon Coupling Through a Trait-Based Framework Integrating WCCI and Dual-Filter CATS Model. Agronomy. 2025; 15(12):2733. https://doi.org/10.3390/agronomy15122733
Chicago/Turabian StyleWu, Shaoyang, Yan Zhang, Jian Hou, Yang Tai, Xiaohui Huang, Xiaochen Guo, Hailong Wu, and Chen Xing. 2025. "Optimizing Water–Carbon Coupling Through a Trait-Based Framework Integrating WCCI and Dual-Filter CATS Model" Agronomy 15, no. 12: 2733. https://doi.org/10.3390/agronomy15122733
APA StyleWu, S., Zhang, Y., Hou, J., Tai, Y., Huang, X., Guo, X., Wu, H., & Xing, C. (2025). Optimizing Water–Carbon Coupling Through a Trait-Based Framework Integrating WCCI and Dual-Filter CATS Model. Agronomy, 15(12), 2733. https://doi.org/10.3390/agronomy15122733





