Early Stress Resilience in Turfgrass: Comparative Germination and Seedling Responses of Lolium perenne L. and Poa pratensis L. Under Osmotic and Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Material
2.2. Germination Tests Under Osmotic and Salinity Stress
2.3. Germination Recovery Assessment
2.4. Statistical Analysis
3. Results
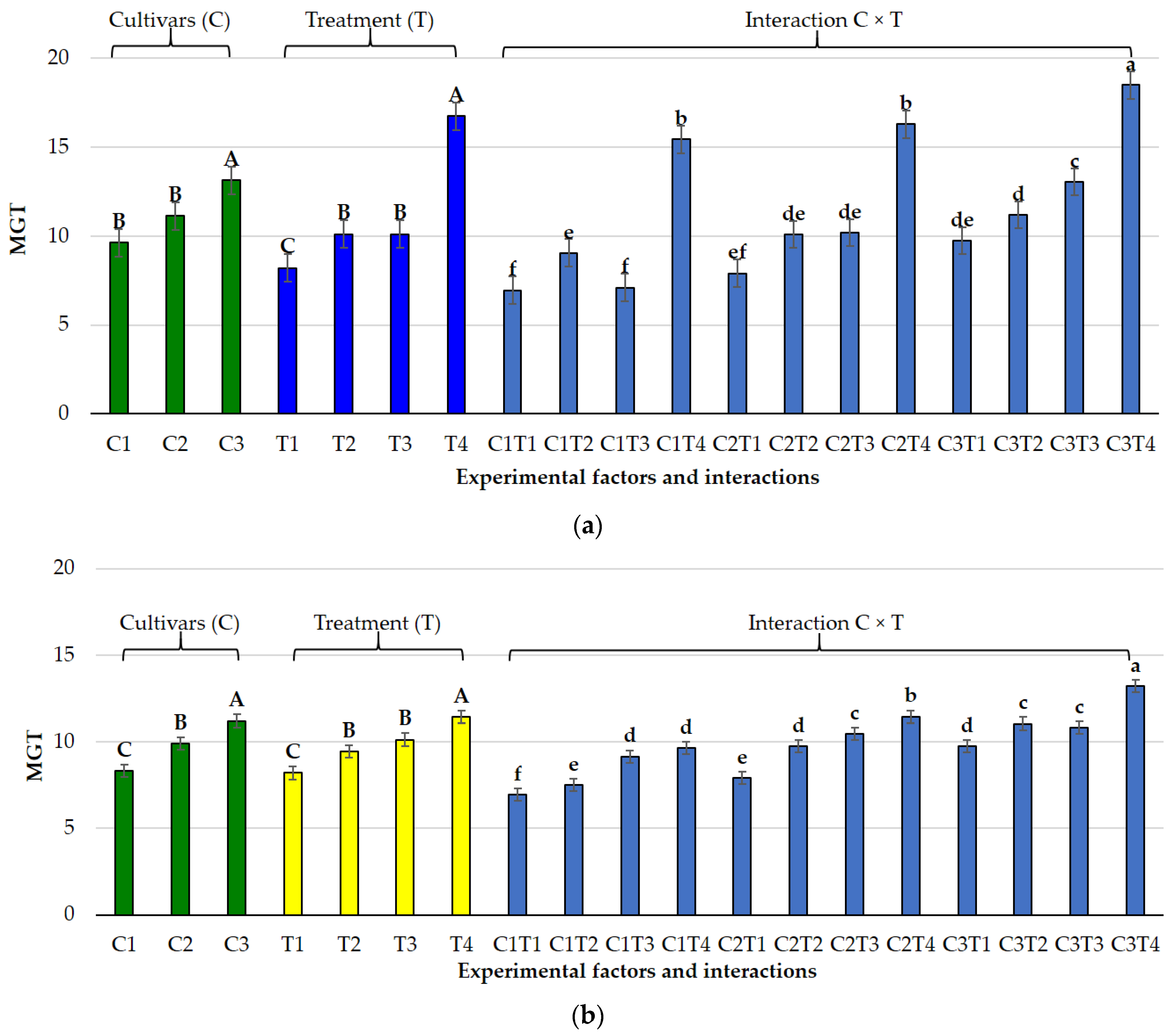
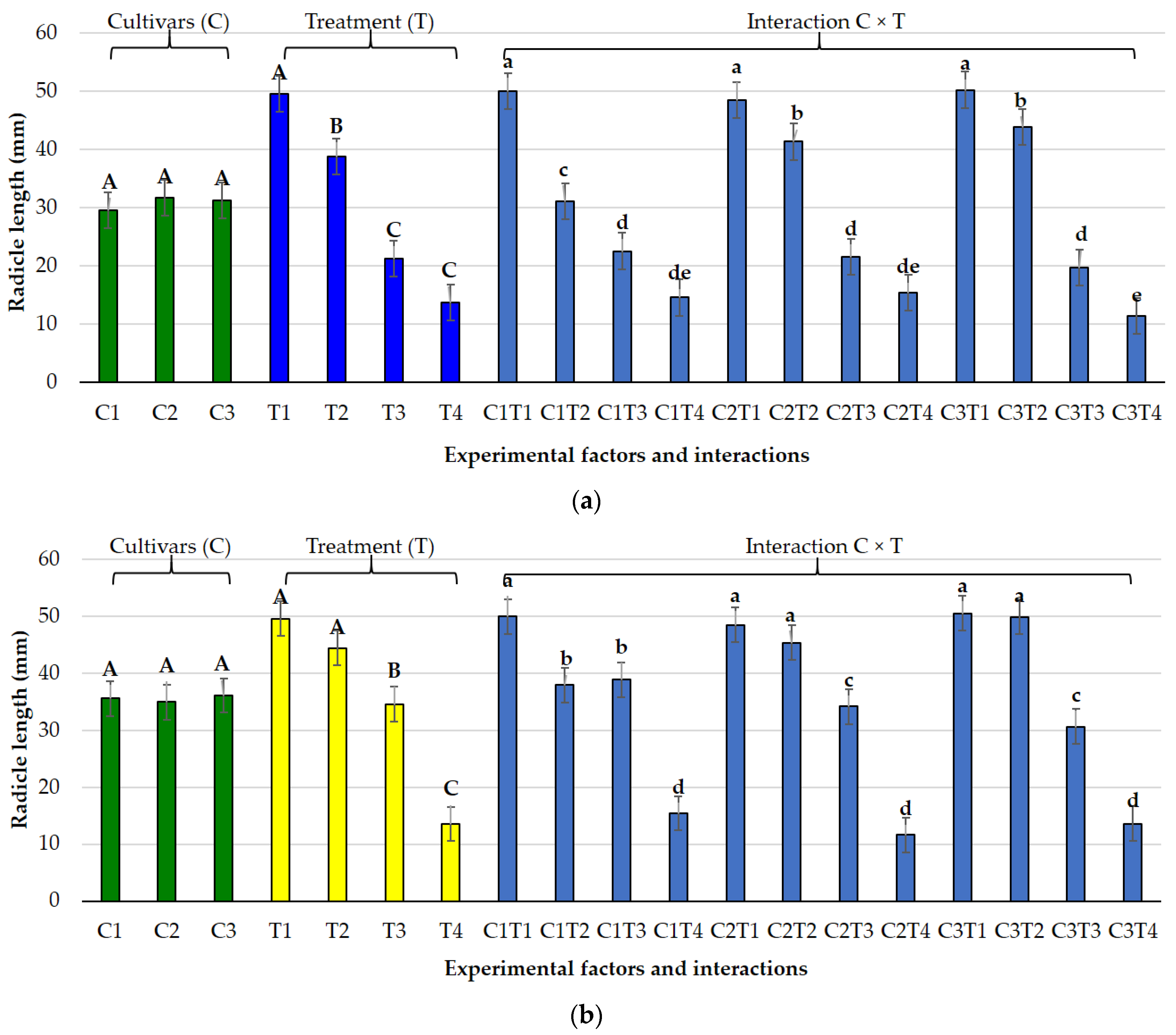
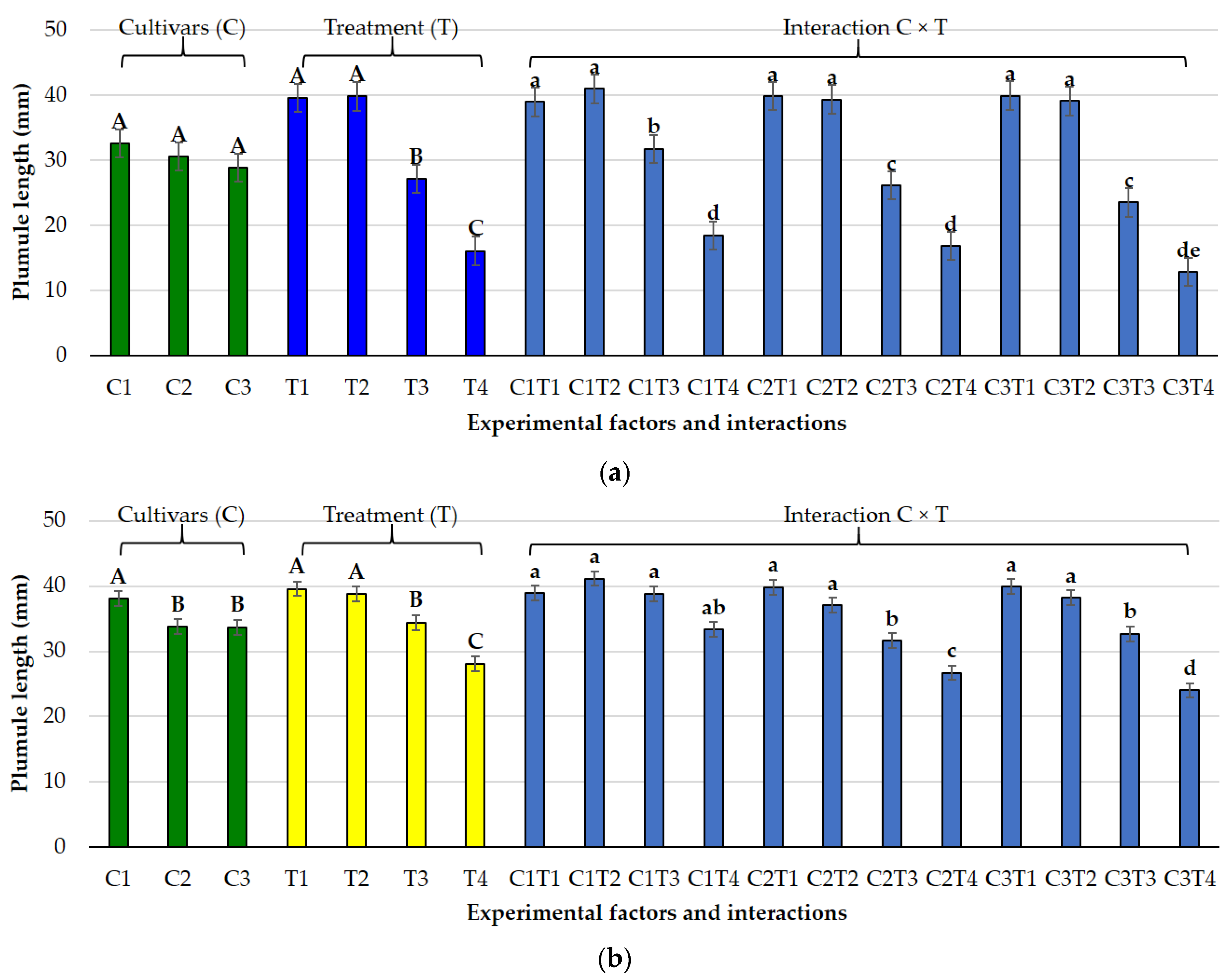
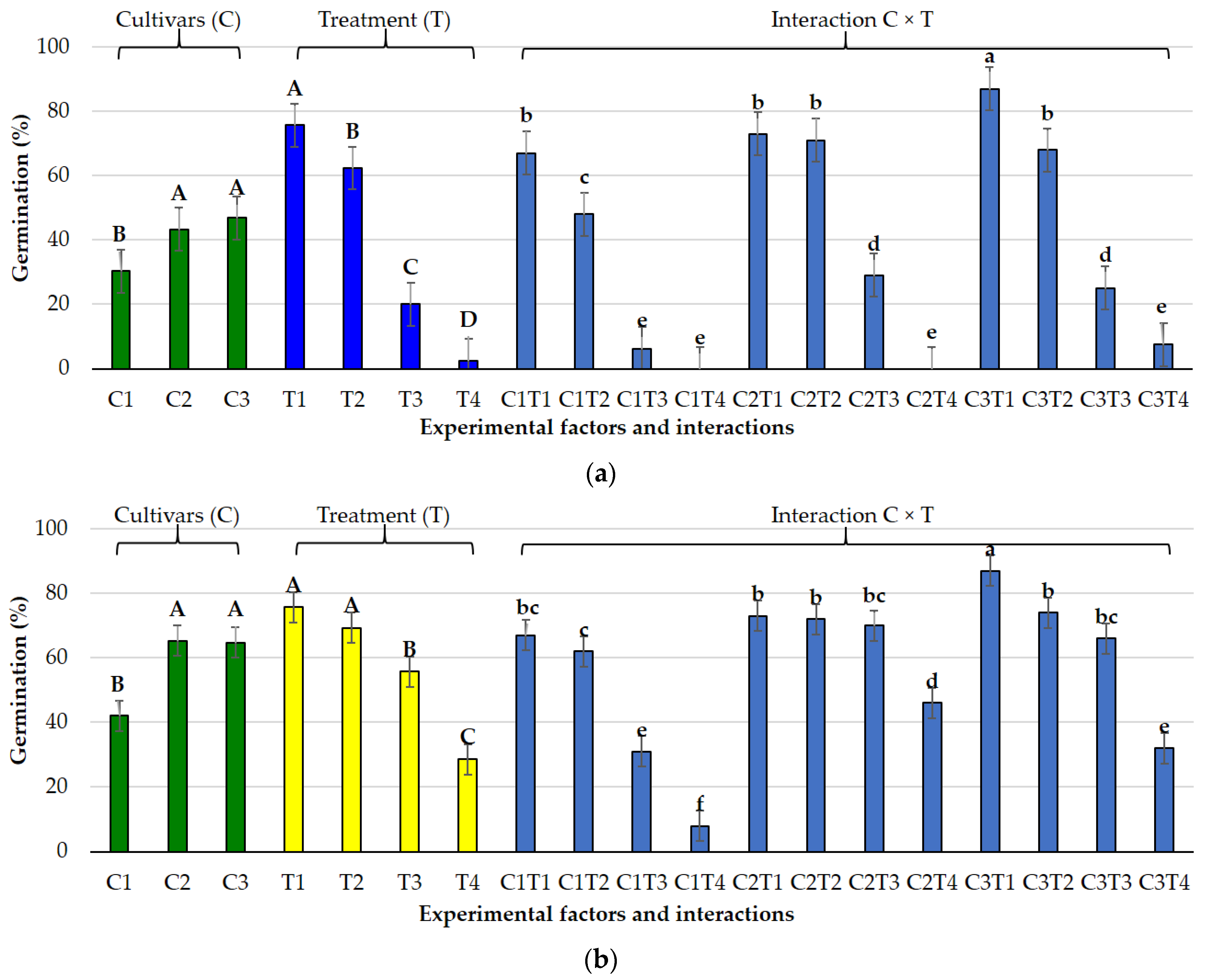
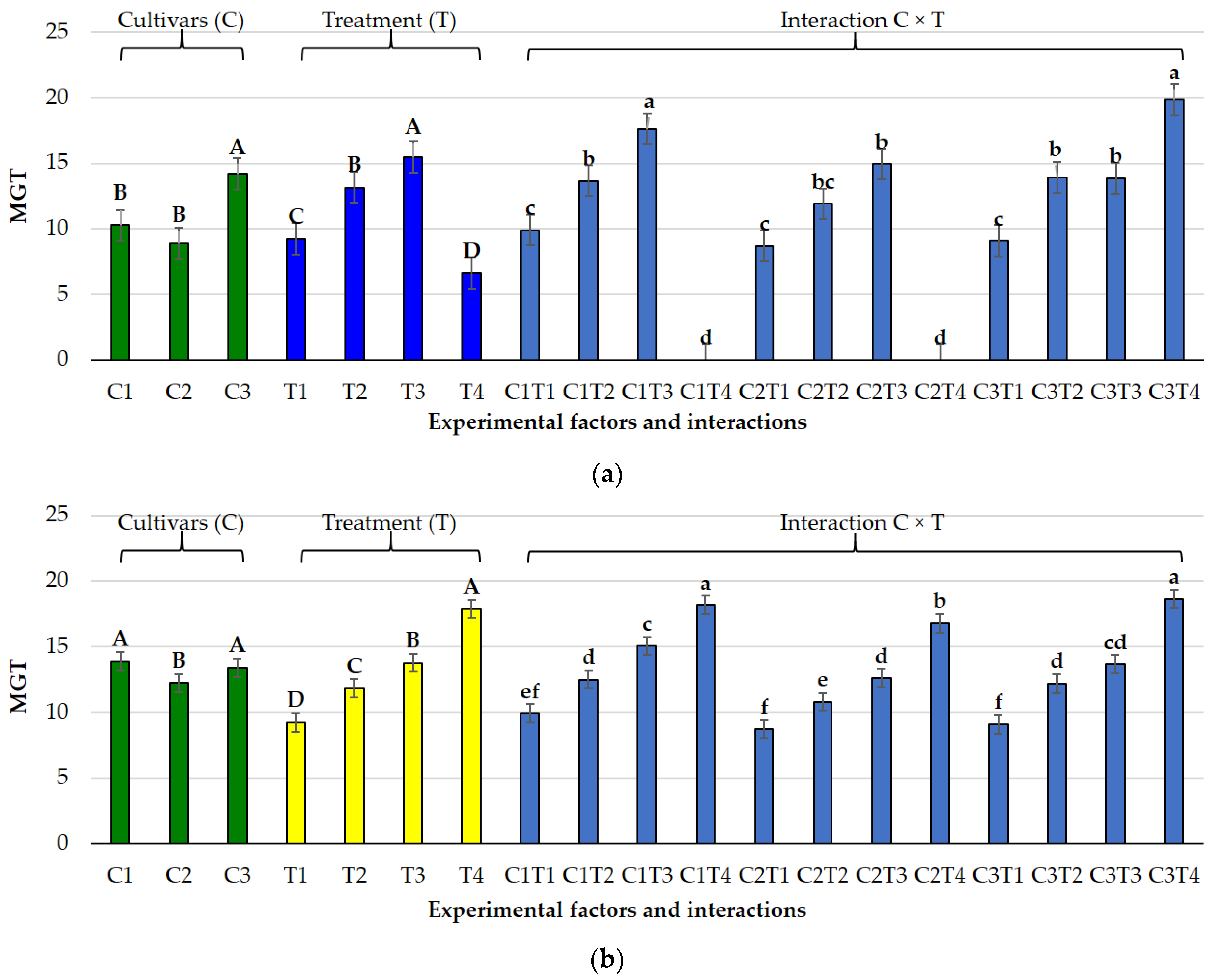
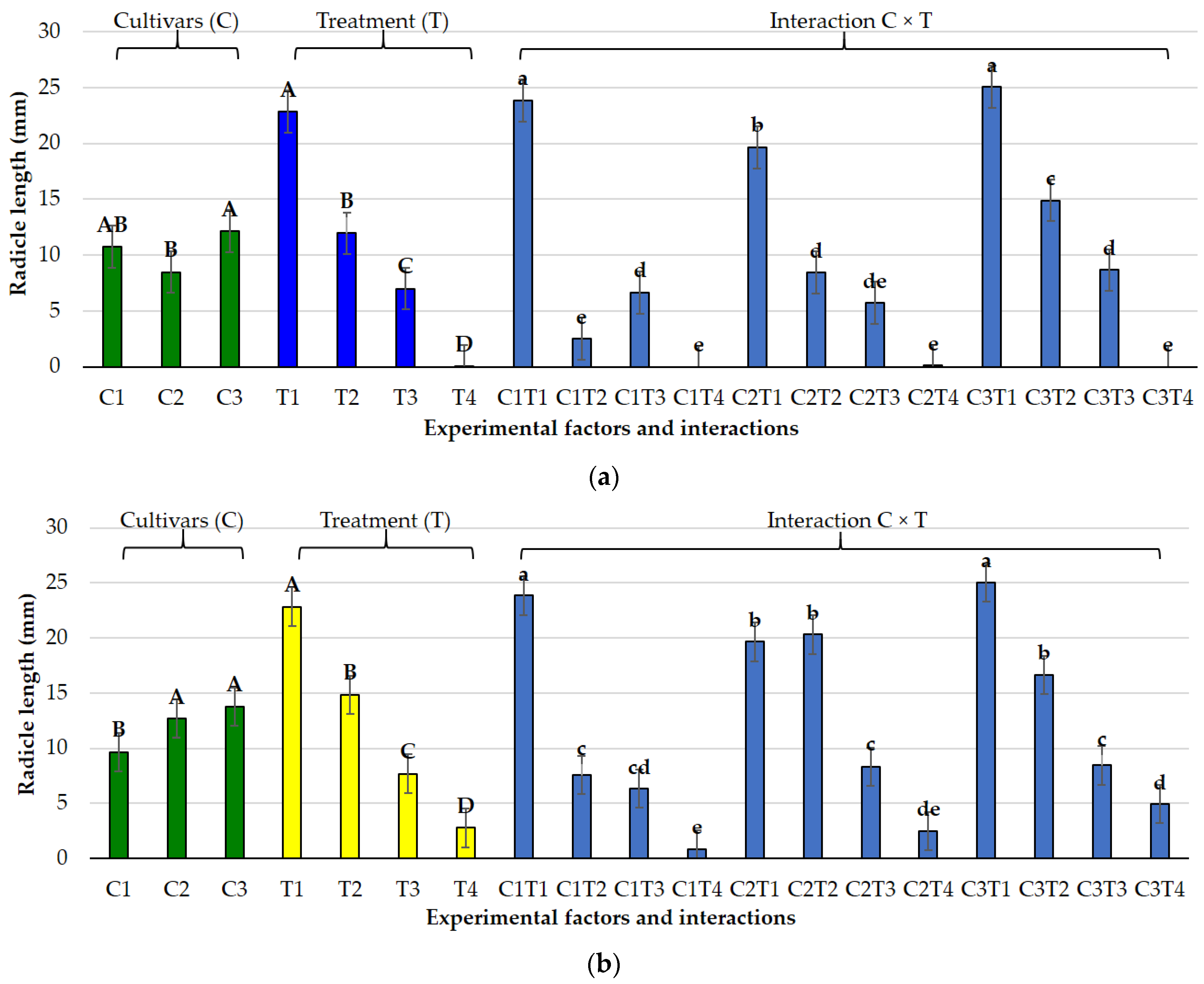
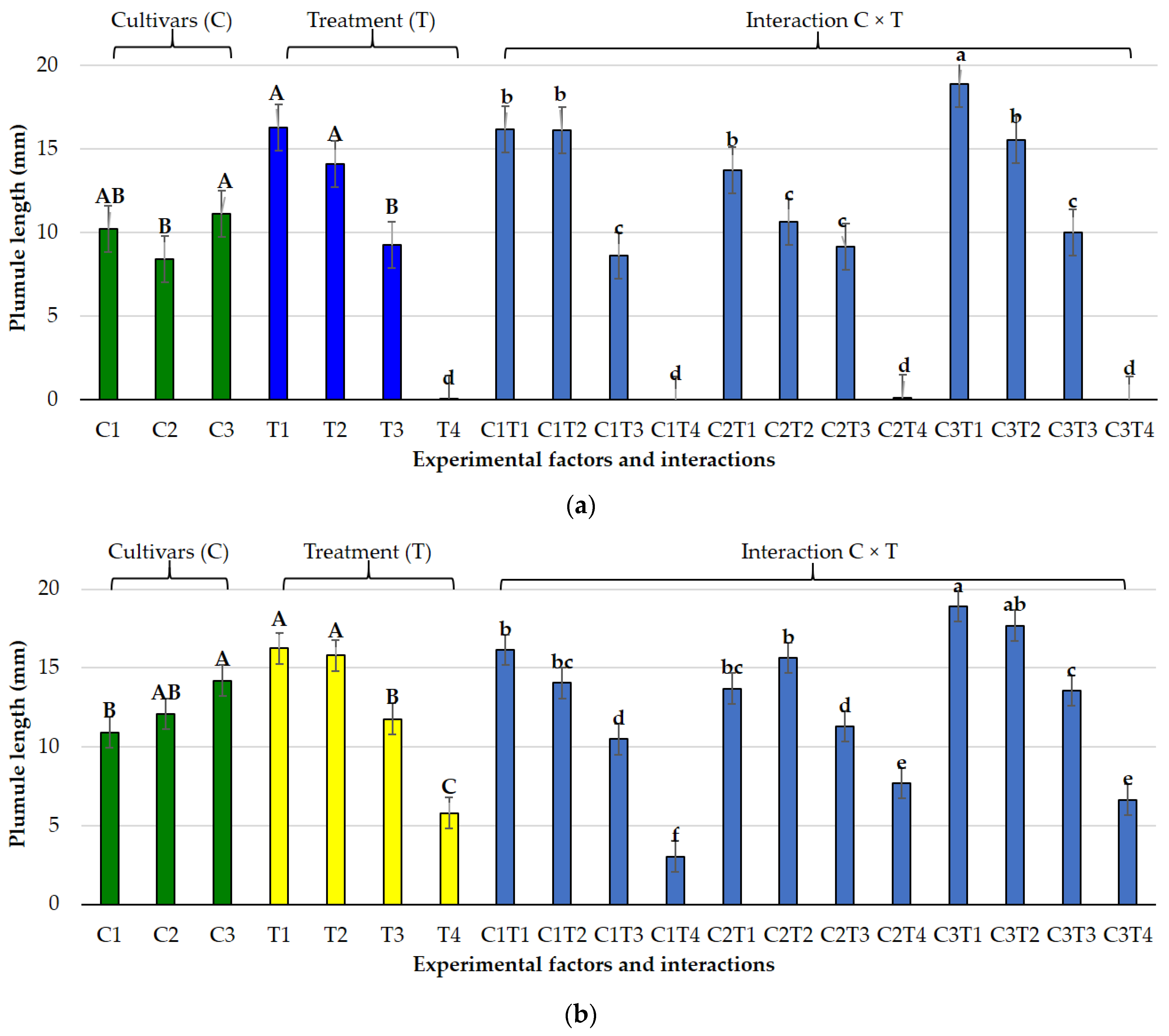
3.1. Effects of Salt and Osmotic Stress on Germination and Seedling Traits
3.2. Species-Specific Responses
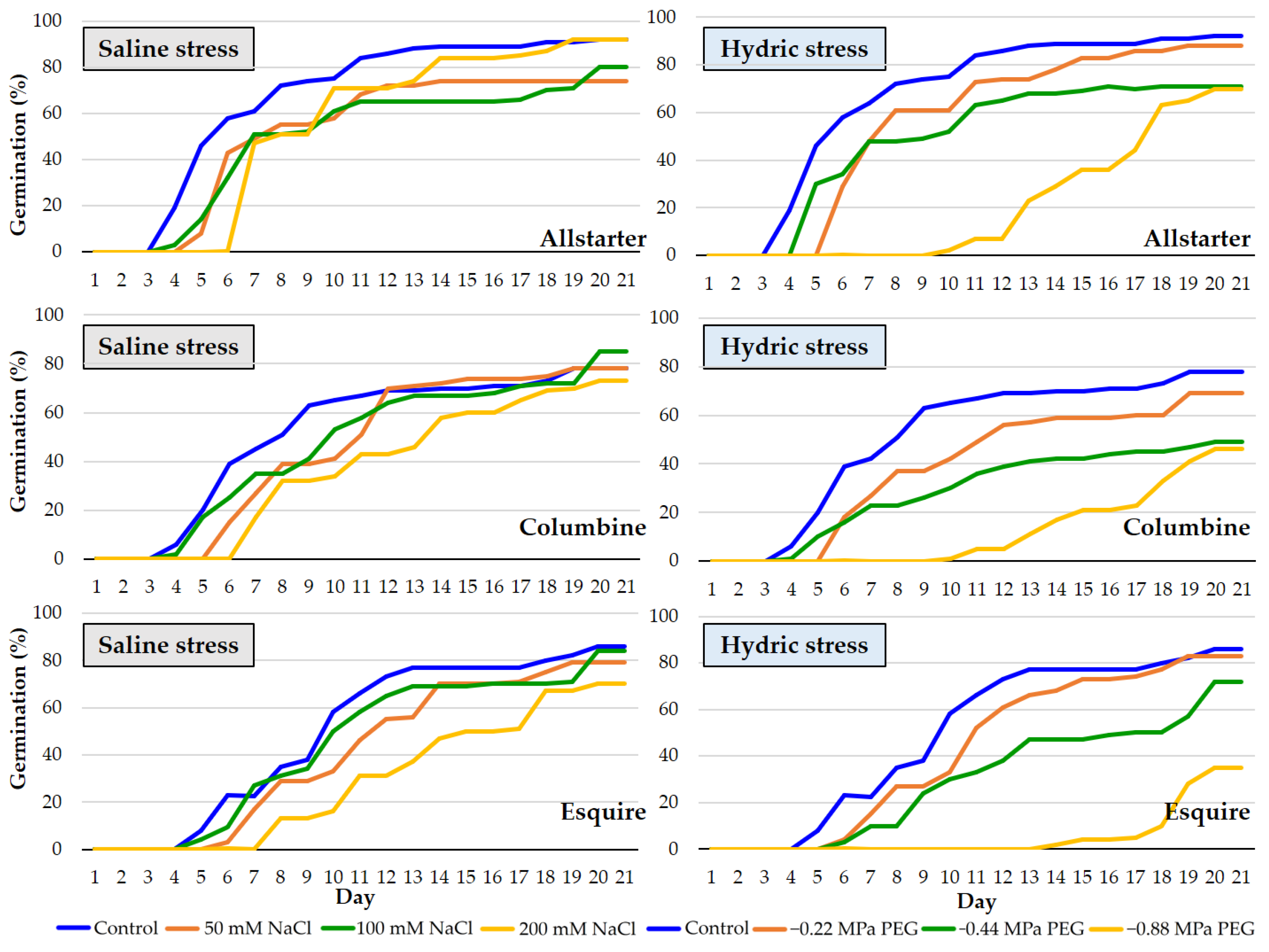
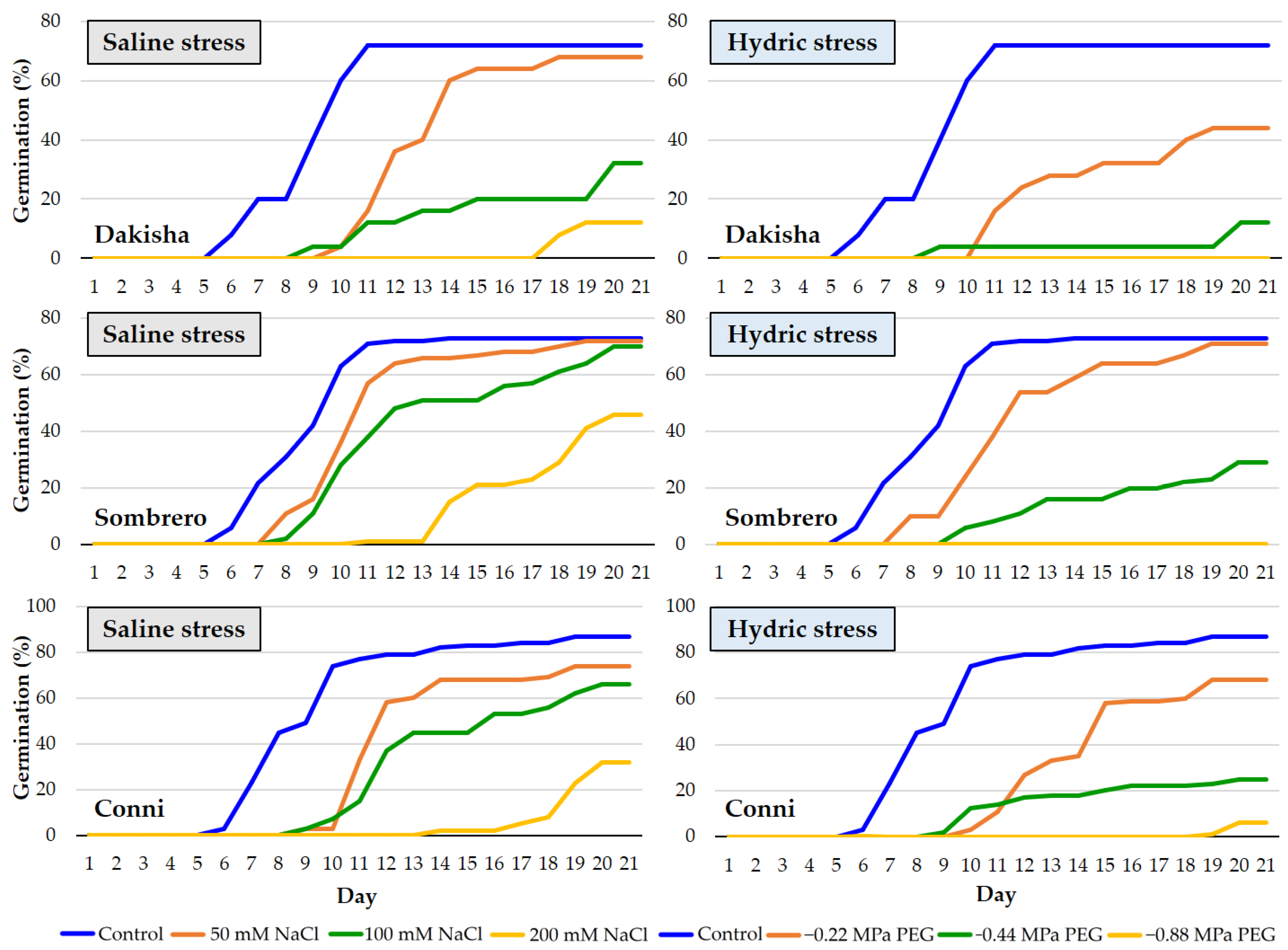
3.2.1. Lolium perenne L.
3.2.2. Poa pratensis L.
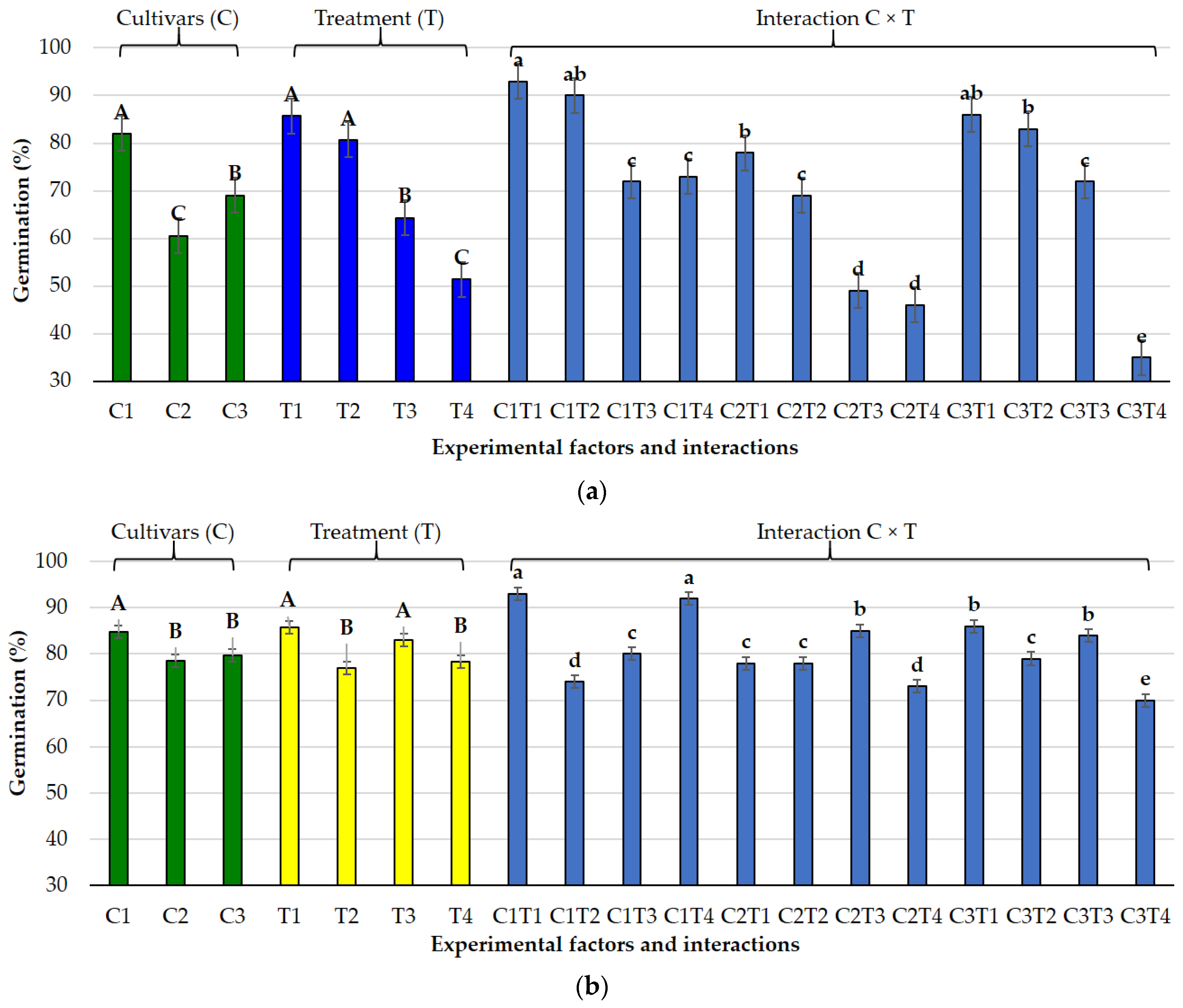
3.3. Germination Indices Under Osmotic and Salt Stress
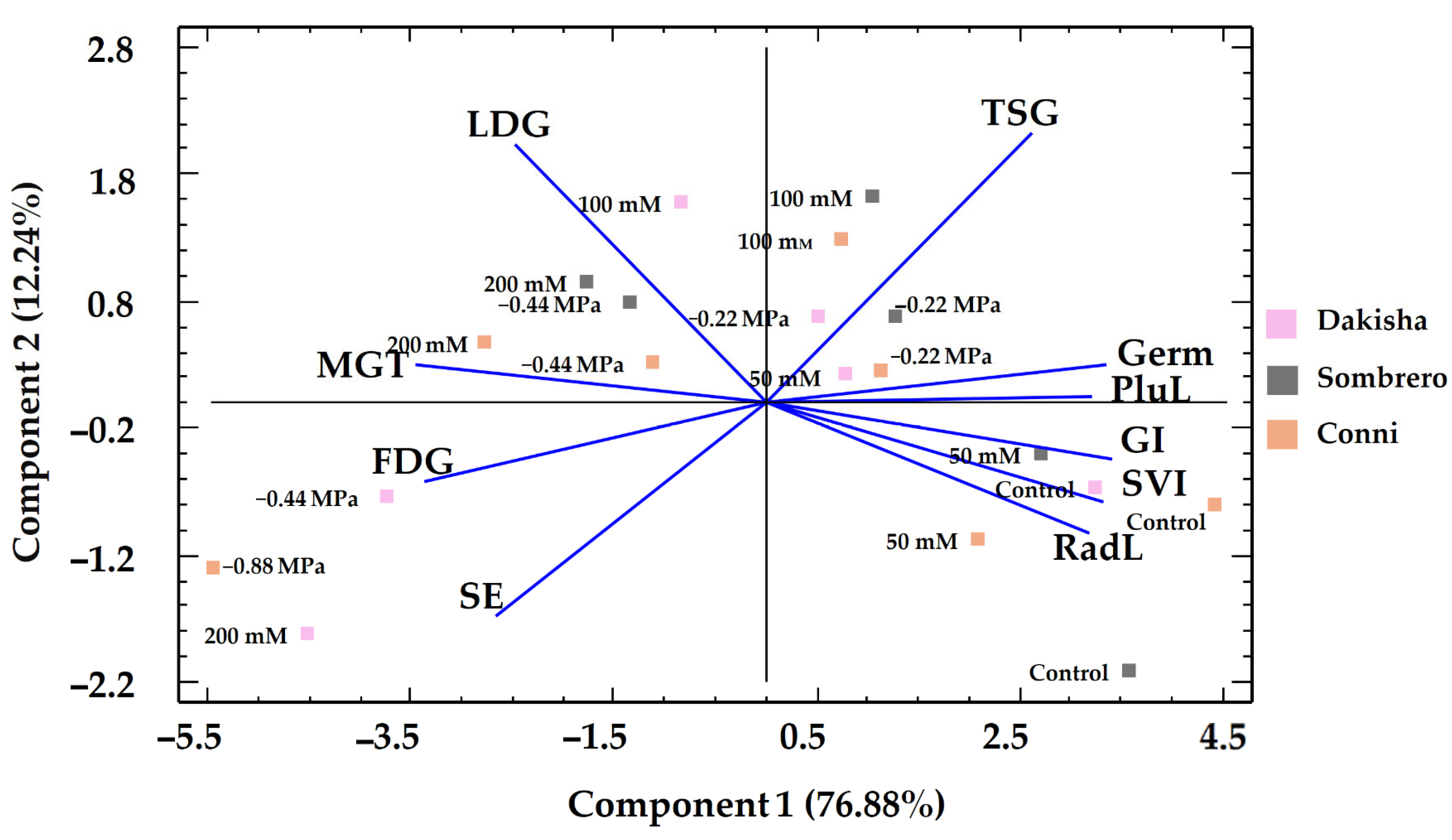
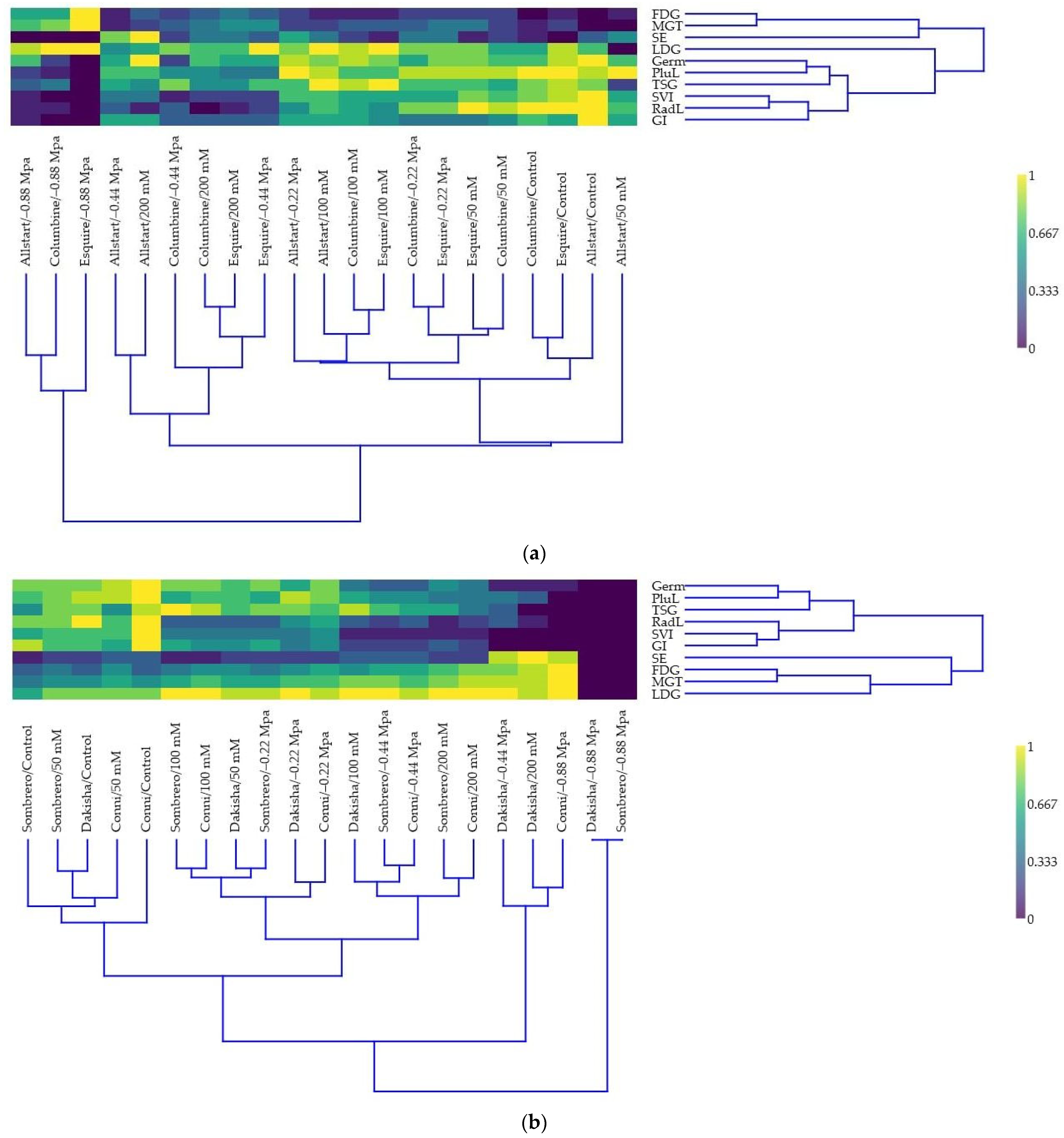
3.4. Multivariate Analysis
3.5. Recovery of Germination
4. Discussion
4.1. Comparative Impact of Osmotic and Salt Stress
4.2. Species-Specific Responses
4.3. Genotypic Variation and Cultivar Performance
4.4. Germination Dynamics and Physiological Interpretation
4.5. Post-Stress Recovery and Adaptive Strategies
4.6. Practical Implications for Turfgrass Selection and Management
4.7. Broader Ecological and Agronomic Context
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No | Cultivar/ Treatment | GI | SE | FDG | LDG | TSG | SVI |
|---|---|---|---|---|---|---|---|
| Effect of the interaction between cultivar and treatment | |||||||
| 1 | C1T1 | 4.0 a | 20.6 c | 4.0 d | 17.3 c | 13.3 a | 74.1 a |
| 2 | C1T2 | 2.8 b | 32.1 b | 6.0 cd | 18.3 b | 12.3 a | 64.9 a |
| 3 | C1T3 | 2.9 b | 54.6 a | 5.0 d | 16.0 d | 11.0 ab | 38.8 c |
| 4 | C1T4 | 1.3 d | 8.4 d | 11.0 b | 19.3 a | 8.3 b | 24.7 d |
| 5 | C2T1 | 2.9 b | 11.1 d | 4.3 d | 17.0 c | 12.8 a | 68.8 a |
| 6 | C2T2 | 2.0 c | 25.2 bc | 6.0 cd | 18.5 b | 12.5 a | 55.6 b |
| 7 | C2T3 | 1.6 d | 17.5 c | 5.8 cd | 18.5 b | 12.8 a | 25.1 d |
| 8 | C2T4 | 0.8 e | 9.4 d | 11.3 b | 19.8 a | 8.5 b | 15.1 e |
| 9 | C3T1 | 2.6 b | 9.4 d | 5.0 d | 19.5 a | 14.5 a | 77.2 a |
| 10 | C3T2 | 2.1 c | 6.1 d | 6.3 cd | 18.8 ab | 12.5 a | 68.9 a |
| 11 | C3T3 | 1.6 d | 20.9 c | 7.5 c | 20.0 a | 12.5 a | 31.3 cd |
| 12 | C3T4 | 0.5 e | 14.5 dc | 15.3 a | 20.0 a | 4.8 c | 8.6 e |
| Effect of cultivar | |||||||
| 1 | Allstart | 2.7 A | 28.9 A | 6.5 B | 17.7 B | 11.2 A | 50.6 A |
| 2 | Columbine | 1.8 AB | 15.8 B | 6.8 B | 18.4 AB | 11.6 A | 41.1 B |
| 3 | Esquire | 1.7 B | 12.7 B | 8.5 A | 19.6 A | 11.1 A | 46.5 AB |
| Effect of treatment | |||||||
| 1 | Control | 3.1 A | 13.7 B | 4.4 C | 17.9 B | 13.5 A | 73.4 A |
| 2 | −0.22 MPa | 2.3 B | 21.1 AB | 6.1 B | 18.2 AB | 12.4 A | 63.1 B |
| 3 | −0.44 MPa | 2.0 C | 31.0 A | 6.1 B | 18.5 AB | 12.1 A | 31.7 C |
| 4 | −0.88 MPa | 0.8 D | 10.7 B | 12.5 A | 19.7 A | 7.2 B | 16.1 D |
| No | Cultivar/ Treatment | GI | SE | FDG | LDG | TSG | SVI |
|---|---|---|---|---|---|---|---|
| Effect of the interaction between cultivar and treatment | |||||||
| 1 | C1T1 | 4.0 a | 20.6 a | 4.0 e | 17.3 a | 13.3 ab | 74.1 a |
| 2 | C1T2 | 2.7 b | 30.6 ab | 5.3 cd | 12.0 a | 6.8 d | 58.4 bc |
| 3 | C1T3 | 2.8 b | 23.7 b | 4.8 d | 20.0 b | 15.3 a | 62.3 b |
| 4 | C1T4 | 2.7 bc | 50.9 a | 7.0 b | 16.5 a | 9.5 c | 44.9 d |
| 5 | C2T1 | 2.9 b | 11.1 b | 4.3 de | 17.0 a | 12.8 ab | 68.8 ab |
| 6 | C2T2 | 2.3 bc | 18.2 b | 6.0 c | 17.0 a | 11.0 bc | 63.6 b |
| 7 | C2T3 | 2.6 b | 16.6 b | 5.0 d | 19.3 a | 14.3 a | 55.8 c |
| 8 | C2T4 | 1.8 d | 23.4 b | 7.0 b | 17.3 a | 10.3 c | 28.2 e |
| 9 | C3T1 | 2.6 b | 9.4 b | 5.0 d | 19.5 a | 14.5 a | 77.2 a |
| 10 | C3T2 | 2.0 b-d | 12.7 b | 6.5 bc | 18.8 a | 12.3 b | 69.2 ab |
| 11 | C3T3 | 2.3 c | 14.2 b | 5.3 d | 20.0 a | 14.8 a | 53.0 c |
| 12 | C3T4 | 1.4 e | 18.7 b | 8.0 a | 19.0 a | 11.0 bc | 26.3 e |
| Effect of cultivar | |||||||
| 1 | Allstart | 3.0 A | 31.3 A | 5.3 B | 16.44 A | 11.2 A | 59.9 A |
| 2 | Columbine | 2.4 AB | 17.3 A | 5.6 B | 17.63 A | 12.1 A | 54.1 A |
| 3 | Esquire | 2.1 AB | 13.5 A | 6.2 A | 19.31 A | 13.1 A | 56.4 A |
| Effect of treatment | |||||||
| 1 | Control | 3.1 A | 13.7 B | 4.4 D | 17.92 AB | 15.5 A | 73.4 A |
| 2 | 50 mM | 2.3 C | 20.5 AB | 5.0 C | 15.92 B | 10.0 B | 63.7 B |
| 3 | 100 mM | 2.5 D | 17.8 B | 5.9 B | 19.75 A | 14.8 A | 57.0 B |
| 4 | 200 mM | 2.0 B | 31.0 A | 7.3 A | 17.58 AB | 10.3 B | 33.1 C |
| No | Cultivar/ Treatment | GI | SE | FDG | LDG | TSG | SVI |
|---|---|---|---|---|---|---|---|
| Effect of the interaction between cultivar and treatment | |||||||
| 1 | C1T1 | 1.9 c | 19.3 c | 6.5 e | 16.0 a | 9.5 a | 26.6 b |
| 2 | C1T2 | 1.0 f | 15.4 d | 10.5 c | 19.0 a | 8.5 a | 13.8 c |
| 3 | C1T3 | 0.1 h | 73.0 a | 16.3 b | 20.0 a | 3.8 c | 0.8 e |
| 4 | C1T4 | 0.0 i | 0.0 g | 0.0 f | 0.0 c | 0.0 d | 0.1 e |
| 5 | C2T1 | 2.2 b | 8.1 f | 6.0 e | 11.8 b | 5.8 c | 24.1 b |
| 6 | C2T2 | 1.6 d | 13.5 e | 8.0 d | 17.5 a | 9.5 a | 13.4 c |
| 7 | C2T3 | 0.5 g | 27.0 b | 11.0 c | 19.0 a | 8.0 b | 4.5 d |
| 8 | C2T4 | 0.0 i | 0.0 g | 0.0 f | 0.0 c | 0.0 d | 0.1 e |
| 9 | C3T1 | 2.6 a | 26.5 b | 6.5 e | 16.8 a | 10.3 a | 38.1 a |
| 10 | C3T2 | 1.3 e | 15.2 d | 11.0 c | 18.8 a | 7.8 b | 20.6 b |
| 11 | C3T3 | 0.6 g | 26.3 b | 11.5 c | 18.5 a | 7.0 b | 5.1 d |
| 12 | C3T4 | 0.1 h | 77.8 a | 19.5 a | 20.0 a | 0.5 d | 0.1 e |
| Effect of cultivar | |||||||
| 1 | Dakisha | 0.7 B | 26.9 B | 8.3 B | 13.8 B | 5.4 B | 10.3 B |
| 2 | Sombrero | 1.1 A | 12.1 C | 6.3 C | 12.1 B | 5.8 B | 10.5 B |
| 3 | Conni | 1.1 A | 36.4 A | 12.1 A | 18.5 A | 6.4 A | 16.0 A |
| Effect of treatment | |||||||
| 1 | Control | 2.2 A | 17.9 C | 6.3 C | 14.8 B | 8.5 A | 29.6 A |
| 2 | −0.22 MPa | 1.3 B | 14.7 D | 9.8 B | 18.4 A | 8.6 A | 15.9 B |
| 3 | −0.44 MPa | 0.4 C | 42.1 A | 12.9 A | 19.2 A | 6.3 B | 3.4 C |
| 4 | −0.88 MPa | 0.0 C | 25.9 B | 6.5 C | 6.7 C | 0.2 C | 0.1 D |
| No | Cultivar/ Treatment | GI | SE | FDG | LDG | TSG | SVI |
|---|---|---|---|---|---|---|---|
| Effect of the interaction between cultivar and treatment | |||||||
| 1 | C1T1 | 1.9 bc | 19.3 b | 6.5 d | 16.0 ab | 9.5 bc | 26.6 b |
| 2 | C1T2 | 1.3 d | 18.2 b | 10.0 c | 17.5 a | 7.5 d | 13.4 c |
| 3 | C1T3 | 0.6 e | 22.9 b | 9.3 c | 19.8 a | 10.5 b | 10.3 c |
| 4 | C1T4 | 0.1 ef | 83.3 a | 18.0 a | 18.5 a | 0.5 h | 0.3 e |
| 5 | C2T1 | 2.2 b | 8.1 b | 6.0 d | 11.8 b | 5.8 f | 24.1 b |
| 6 | C2T2 | 1.7 c | 15.9 b | 8.0 dc | 16.8 a | 8.8 c | 25.9 b |
| 7 | C2T3 | 1.5 cd | 12.4 b | 8.5 dc | 19.8 a | 11.3 a | 13.8 c |
| 8 | C2T4 | 0.8 e | 18.5 b | 13.3 b | 19.8 a | 6.5 e | 4.8 d |
| 9 | C3T1 | 2.6 a | 26.5 b | 6.5 d | 16.8 a | 10.3 b | 38.1 a |
| 10 | C3T2 | 1.6 cd | 21.0 b | 10.0 c | 16.0 ab | 6.0 ef | 25.3 b |
| 11 | C3T3 | 1.3 d | 11.6 b | 9.8 c | 19.5 a | 9.8 b | 14.2 c |
| 12 | C3T4 | 0.5 e | 15.8 b | 16.0 a | 20.0 a | 4.0 g | 3.8 d |
| Effect of cultivar | |||||||
| 1 | Dakisha | 1.0 B | 35.9 A | 10.9 A | 17.9 A | 7.0 A | 12.6 B |
| 2 | Sombrero | 1.5 A | 13.7 BC | 8.9 A | 17.0 A | 8.1 A | 17.1 AB |
| 3 | Conni | 1.5 A | 18.7 B | 10.6 A | 18.1 A | 7.5 A | 20.3 A |
| Effect of treatment | |||||||
| 1 | Control | 2.2 A | 17.9 B | 6.3 C | 14.8 B | 8.5 AB | 29.6 A |
| 2 | 50 mM | 1.5 B | 18.4 B | 9.3 B | 16.8 B | 7.4 C | 21.5 B |
| 3 | 100 mM | 1.1 C | 15.6 B | 9.2 B | 19.7 A | 10.5 A | 12.7 C |
| 4 | 200 mM | 0.4 D | 39.2 A | 15.8 A | 19.4 A | 3.7 D | 2.9 D |
References
- Linder, H.P.; Lehmann, C.E.R.; Archibald, S.; Osborne, C.P.; Richardson, D.M. Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biol. Rev. 2018, 93, 1125–1144. [Google Scholar] [CrossRef]
- Gallaher, T.J.; Peterson, P.M.; Soreng, R.J.; Zuloaga, F.O.; Li, D.-Z.; Clark, L.G.; Tyrrell, C.D.; Welker, C.A.D.; Kellogg, E.A.; Teisher, J.K. Grasses through space and time: An overview of the biogeographical and macroevolutionary history of Poaceae. J. Syst. Evol. 2022, 60, 522–569. [Google Scholar] [CrossRef]
- Strömberg, C.A.E. Evolution of Grasses and Grassland Ecosystems. Annu. Rev. Earth Planet. Sci. 2011, 39, 517–544. [Google Scholar] [CrossRef]
- Carbutt, C.; Kirkman, K. Ecological Grassland Restoration—A South African Perspective. Land 2022, 11, 575. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Meng, L.-C.; Huang, Z.; Shi, Z.-H.; Wu, G.-L. Contribution of fine roots mechanical property of Poaceae grasses to soil erosion resistance on the Loess Plateau. Geoderma 2022, 426, 116122. [Google Scholar] [CrossRef]
- Marshall, A.H.; Collins, R.P.; Humphreys, M.W.; Scullion, J. A new emphasis on root traits for perennial grass and legume varieties with environmental and ecological benefits. Food Energy Secur. 2016, 5, 26–39. [Google Scholar] [CrossRef]
- Robbins, P.; Birkenholtz, T. Turfgrass revolution: Measuring the expansion of the American lawn. Land Use Policy 2003, 20, 181–194. [Google Scholar] [CrossRef]
- Beard, J.B.; Green, R.L. The Role of Turfgrasses in Environmental Protection and Their Benefits to Humans. J. Environ. Qual. 1994, 23, 452–460. [Google Scholar] [CrossRef]
- Monteiro, J.A. Ecosystem services from turfgrass landscapes. Urban For. Urban Green. 2017, 26, 151–157. [Google Scholar] [CrossRef]
- Kazemi, F. Effects of drought on ecosystem services of recreational and sport turfgrasses in urban environments. Acta Hortic. 2025, 1429, 265–272. [Google Scholar] [CrossRef]
- Stier, J.C.; Steinke, K.; Ervin, E.H.; Higginson, F.R.; McMaugh, P.E. Turfgrass Benefits and Issues. In Turfgrass: Biology, Use, and Management; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 105–145. [Google Scholar]
- Braun, R.C.; Mandal, P.; Nwachukwu, E.; Stanton, A. The role of turfgrasses in environmental protection and their benefits to humans: Thirty years later. Crop Sci. 2024, 64, 2909–2944. [Google Scholar] [CrossRef]
- Ignatieva, M. Design for Urban Biodiversity: Applying research on biodiversity of urban lawns into landscape design practice. In Routledge Handbook of Urban Biodiversity; Routledge: London, UK, 2023; pp. 418–440. [Google Scholar]
- Mathew, S. Role of Turfgrass in Urban Landscapes. J. Plant Dev. Sci. 2021, 13, 247–255. [Google Scholar]
- Huang, B.; DaCosta, M.; Jiang, Y. Research Advances in Mechanisms of Turfgrass Tolerance to Abiotic Stresses: From Physiology to Molecular Biology. Crit. Rev. Plant Sci. 2014, 33, 141–189. [Google Scholar] [CrossRef]
- Amombo, E.; Li, H.; Fu, J. Research Advances on Tall Fescue Salt Tolerance: From Root Signaling to Molecular and Metabolic Adjustment. J. Am. Soc. Hortic. Sci. 2017, 142, 337–345. [Google Scholar] [CrossRef]
- Liu, H.; Todd, J.L.; Luo, H. Turfgrass Salinity Stress and Tolerance—A Review. Plants 2023, 12, 925. [Google Scholar] [CrossRef]
- Prijović, M.; Sokolović, D.; Maksimović, J.D.; Maksimović, V.; Milosavljević, D.; Babić, S.; Stepić, M.; Sabovljević, A. Response of Different Perennial Ryegrass Varieties to Water Stress. Agriculture 2025, 15, 917. [Google Scholar] [CrossRef]
- Kazemi, F.; Golzarian, M.R.; Rabbani Kheir Khah, S.M. Quality and Establishment of Some Water-Conserving Turfgrass Species for Sustainable Development and Some Ecosystem Services in Arid Urban Environments. Land 2024, 13, 721. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Y.; Wang, X.; Li, J.; Gao, Y.; Huang, H.; Zhou, Z.; Wang, P.; Zhao, L. Response of seed germination and seedling growth of perennial ryegrass (Lolium perenne L.) to drought, salinity, and pH in Karst regions. Sci. Rep. 2025, 15, 16874. [Google Scholar] [CrossRef]
- Cai, H.; Stewart, A.; Inoue, M.; Yuyama, N.; Hirata, M. Lolium. In Wild Crop Relatives: Genomic and Breeding Resources: Millets and Grasses; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 165–173. [Google Scholar]
- Thorogood, D. Perennial ryegrass (Lolium perenne L.). In Turfgrass Biology, Genetics, Breeding; Casler, M.D., Duncan, R.R., Eds.; Wiley: New York, NY, USA, 2003; pp. 75–105. [Google Scholar]
- Sampoux, J.P.; Baudouin, P.; Bayle, B.; Béguier, V.; Bourdon, P.; Chosson, J.F.; de Bruijn, K.; Deneufbourg, F.; Galbrun, C.; Ghesquière, M.; et al. Breeding perennial ryegrass (Lolium perenne L.) for turf usage: An assessment of genetic improvements in cultivars released in Europe, 1974–2004. Grass Forage Sci. 2013, 68, 33–48. [Google Scholar] [CrossRef]
- Bothe, A.; Westermeier, P.; Wosnitza, A.; Willner, E.; Schum, A.; Dehmer, K.J.; Hartmann, S. Drought tolerance in perennial ryegrass (Lolium perenne L.) as assessed by two contrasting phenotyping systems. J. Agron. Crop Sci. 2018, 204, 375–389. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Chen, J.; Song, H.; Li, S.; Zhao, Y.; Tao, J.; Liu, J. Soil moisture determines horizontal and vertical root extension in the perennial grass Lolium perenne L. growing in Karst soil. Front. Plant Sci. 2019, 10, 629. [Google Scholar] [CrossRef]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Teisher, J.K.; Clark, L.G.; Barberá, P.; Gillespie, L.J.; Zuloaga, F.O. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. J. Syst. Evol. 2017, 55, 259–290. [Google Scholar] [CrossRef]
- Beard, J.B.; Rieke, P.; Turgeon, A.; Vargas, J. Research Report. In Annual Bluegrass (Poa Annua L.): Description, Adaptation, Culture and Control; Michigan State University, Agricultural Experiment Station East Lansing: East Lansing, MI, USA, 1978; pp. 1–32. [Google Scholar]
- Casler, M.D.; Duncan, R.R. Turfgrass Biology, Genetics and Breeding; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Cereti, C.F.; Rossini, F.; Barbetti, B.; Sbrilli, S. Effects of Shading on Lolium perenne and Poa pratensis Turf. Acta Hortic. 2004, 661, 227–231. [Google Scholar] [CrossRef]
- Fustec, J.; Guilleux, J.; Le Corff, J.; Maitre, J.-P. Comparison of Early Development of Three Grasses: Lolium perenne, Agrostis stolonifera and Poa pratensis. Ann. Bot. 2005, 96, 269–278. [Google Scholar] [CrossRef]
- Braun, R.C.; Courtney, L.E.; Patton, A.J. Seed morphology, germination, and seedling vigor characteristics of fine fescue taxa and other cool-season turfgrass species. Crop Sci. 2023, 63, 1613–1627. [Google Scholar] [CrossRef]
- Nwachukwu, E.U.; Fry, J.D.; Domenghini, J.C.; Braun, R.C. Kentucky bluegrass characteristics: Germination and establishment speed, rhizomes, and sod production. Crop Sci. 2025, 65, e70180. [Google Scholar] [CrossRef]
- Folck, A.J.; Bigelow, C.A.; Jiang, Y.; Patton, A. Genotypic variation in germination rate, seedling vigor, and seed phenotype of Kentucky bluegrass cultivars. Crop Sci. 2023, 63, 3065–3078. [Google Scholar] [CrossRef]
- Bushman, B.S.; Robbins, M.D.; Robins, J.G.; Thorsted, K.; Harris, P.; Johnson, P.G. Response to salt stress imposed on cultivars of three turfgrass species: Poa pratensis, Lolium perenne, and Puccinellia distans. Crop Sci. 2020, 60, 1648–1659. [Google Scholar] [CrossRef]
- Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Gozdowski, D.; Małuszyńska, E.; Szydłowska, A. The sensitivity of Lolium perenne and Poa pratensis to salinity and drought during the seed germination and under different photoperiod conditions. Zemdirb. Agric. 2017, 104, 71–78. [Google Scholar] [CrossRef]
- Miao, C.; Zhang, Y.; Bai, X.; Qin, T. Insights into the Response of Perennial Ryegrass to Abiotic Stress: Underlying Survival Strategies and Adaptation Mechanisms. Life 2022, 12, 860. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, W.; Amombo, E.; Hu, L.; Kjorven, J.O.; Chen, L. Mechanisms of Environmental Stress Tolerance in Turfgrass. Agronomy 2020, 10, 522. [Google Scholar] [CrossRef]
- Javaid, M.M.; Mahmood, A.; Alshaya, D.S.; AlKahtani, M.D.F.; Waheed, H.; Wasaya, A.; Khan, S.A.; Naqve, M.; Haider, I.; Shahid, M.A.; et al. Influence of environmental factors on seed germination and seedling characteristics of perennial ryegrass (Lolium perenne L.). Sci. Rep. 2022, 12, 9522. [Google Scholar] [CrossRef]
- Lindsey, A.J.; Schiavon, M.; Unruh, J.B.; Kenworthy, K. Urban Landscapes: Turfgrass Benefits. Grasses 2025, 4, 3. [Google Scholar] [CrossRef]
- Peeters, A. Wild and sown grasses. In Profiles of a Temperate Species Selection: Ecology, Biodiversity and Use; FAO: Rome, Italy; Blackwell Publishing: Hoboken, NJ, USA, 2004. [Google Scholar]
- Wiewióra, B.; Żurek, G. Amenity Grasses—A Short Insight into Species, Their Applications and Functions. Agronomy 2023, 13, 1164. [Google Scholar] [CrossRef]
- Robins, J.G.; Bushman, B.S. Variation for turfgrass performance in a set of Lolium perenne germplasm evaluated under limited irrigation. Crop Sci. 2023, 63, 705–711. [Google Scholar] [CrossRef]
- Kır, B.; Avcıoglu, R.; Demıroglu, G.; Sımıc, A. Performances of Some Cool Season Turfgrass Species in Mediterranean Environment: I. Lolium perenne L., Festuca arundinacea Schreb., Poa pratensis L., and Agrostis tenuis Sibth. Turk. J. Field Crops 2010, 15, 174–179. [Google Scholar]
- Ben-Gal, A.; Borochov-Neori, H.; Yermiyahu, U.; Shani, U. Is osmotic potential a more appropriate property than electrical conductivity for evaluating whole-plant response to salinity? Environ. Exp. Bot. 2009, 65, 232–237. [Google Scholar] [CrossRef]
- ISTA (International Seed Testing Association). International Rules for Seed Testing; ISTA: Zurich, Switzerland, 2022. [Google Scholar]
- AOSA. Seed Vigor Testing Handbook. In Contribution No. 32 to the Handbook on Seed Testing; Association of Official Seed Analysts (AOSA): Ithaca, NY, USA, 2011. [Google Scholar]
- Georgiu, I.-A.; Ciulca, E.A.; Velicevici, G.; Sestras, R.E.; Boscaiu, M.; Vicente, O.; Sestras, A.F. Eco-Friendly Biotechnological Approaches to Enhance Germination Efficiency in Lavandula angustifolia Mill. Horticulturae 2025, 11, 1339. [Google Scholar] [CrossRef]
- Šerá, B. Methodological contribution on seed germination and seedling initial growth tests in wild plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13164. [Google Scholar] [CrossRef]
- Roman, A.M.; Truta, A.M.; Morar, I.M.; Viman, O.; Dan, C.; Sestras, A.F.; Holonec, L.; Boscaiu, M.; Sestras, R.E. From seed to seedling: Influence of seed geographic provenance and germination treatments on reproductive material represented by seedlings of Robinia pseudoacacia. Sustainability 2022, 14, 5654. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Towards a rational basis for testing seed quality. In Seed Production; Hebblethwaite, P.D., Ed.; Butterworths: London, UK, 1980; pp. 605–635. [Google Scholar]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of Germination—Aid in Selection and Evaluation for Seedling Emergence and Vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Pujol, J.A.; Calvo, J.F.; Ramírez-Díaz, L. Recovery of Germination from Different Osmotic Conditions by Four Halophytes from Southeastern Spain. Ann. Bot. 2000, 85, 279–286. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4–9. [Google Scholar]
- Mircea, D.M.; Calone, R.; Shakya, R.; Saavedra, M.F.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Use of Multivariate Analysis in Screening for Drought Tolerance in Ornamental Asteraceae Species. Agronomy 2023, 13, 687. [Google Scholar] [CrossRef]
- Rao, N.K.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Nowell, D.; Larinde, M. Manual of Seed Handling in Genebanks; Bioversity International: Rome, Italy, 2006; p. 148. [Google Scholar]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; Cabi Wallingford UK: Wallingford, UK, 2017; pp. 24–63. [Google Scholar]
- Cox, K.D.; Kenworthy, K.E.; Rios, E.F.; Unruh, J.B.; Chandra, A.; Erickson, J. The suitability of polyethylene glycol for inducing drought symptoms in zoysiagrass. Int. Turfgrass Soc. Res. J. 2025, 1–8. [Google Scholar] [CrossRef]
- Pornaro, C.; Serena, M.; Macolino, S.; Leinauer, B. Drought Stress Response of Turf-Type Perennial Ryegrass Genotypes in a Mediterranean Environment. Agronomy 2020, 10, 1810. [Google Scholar] [CrossRef]
- Thompson, G.L.; Kao-Kniffin, J. Applying Biodiversity and Ecosystem Function Theory to Turfgrass Management. Crop Sci. 2017, 57, S-238–S-248. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Rue, K.; Zhang, Q. Impact of seed priming on early growth of Kentucky bluegrass (Poa pratensis L.) under saline, waterlogging, and combined saline-waterlogging stresses. Grass Res. 2025, 5, e018. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of Seed Dormancy and Germination Mechanisms in a Changing Environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Adetunji, T.L.; Varghese, B.; Sershen; Pammenter, N.W. Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives. Agronomy 2021, 11, 2369. [Google Scholar] [CrossRef]
- Charif, K.; Mzabri, I.; Chetouani, M.; Khamou, L.; Boukroute, A.; Kouddane, N.; Berrichi, A. Germination of some turfgrass species used in the green spaces in eastern Morocco. Mater. Today Proc. 2019, 13, 713–719. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Rue, K. Glycinebetaine Seed Priming Improved Osmotic and Salinity Tolerance in Turfgrasses. HortScience 2012, 47, 1171–1174. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-Sowing Seed Treatment—A Shotgun Approach to Improve Germination, Plant Growth, and Crop Yield Under Saline and Non-Saline Conditions. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2005; Volume 88, pp. 223–271. [Google Scholar]
- Ben Youssef, R.; Jelali, N.; Boukari, N.; Albacete, A.; Martinez, C.; Alfocea, F.P.; Abdelly, C. The Efficiency of Different Priming Agents for Improving Germination and Early Seedling Growth of Local Tunisian Barley under Salinity Stress. Plants 2021, 10, 2264. [Google Scholar] [CrossRef]
- Briske, D.D.; Fuhlendorf, S.D.; Smeins, F.E. Vegetation dynamics on rangelands: A critique of the current paradigms. J. Appl. Ecol. 2003, 40, 601–614. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.D.; Egea-Gilabert, C.; Conesa, E.; Ochoa, J.; Vicente, M.J.; Franco, J.A.; Bañon, S.; Martínez, J.J.; Fernández, J.A. The Importance of Ion Homeostasis and Nutrient Status in Seed Development and Germination. Agronomy 2020, 10, 504. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.; Masud, A.A.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Aamlid, T.S.; Nesheim, L.; Pettersen, T.; Enger, F.; Vesterbukt, P. Poa pratensis or Lolium perenne for establishment and overseeding of Scandinavian football (soccer) pitches. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 32–43. [Google Scholar] [CrossRef]
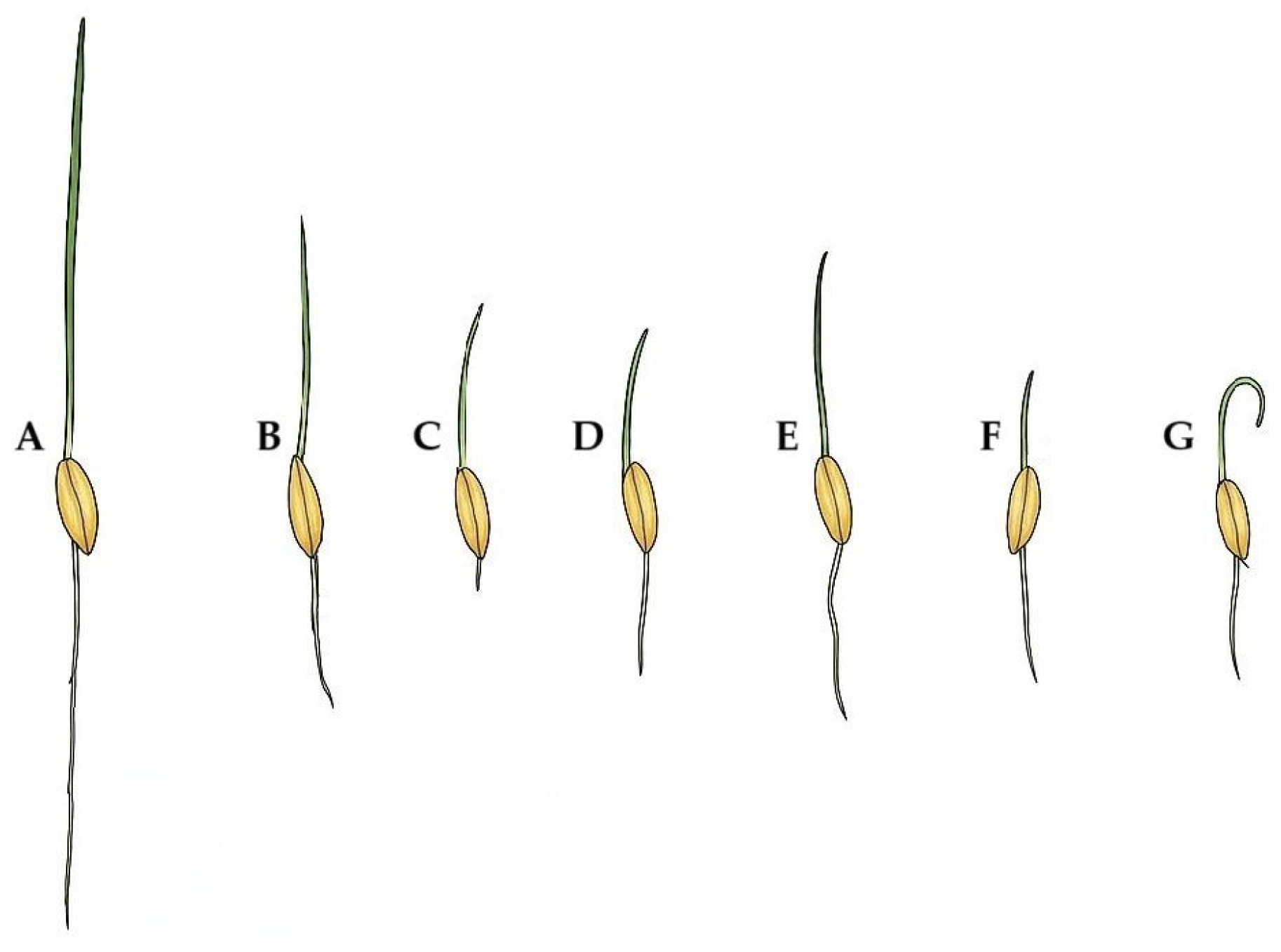

| Species/ Cultivar | Treatment | Normal and Abnormal Seedlings Category (%) 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| A 2 | B | C | D | E | F | G | ||
| L. perenne | ||||||||
| Allstarter | Control | 96.5 ± 2.8 a | 0.0 | 0.5 | 2.2 | 0.0 | 0.0 | 0.8 |
| Osmotic stress | 88.3 ± 3.1 b | 0.0 | 1.2 | 9.7 | 0.0 | 0.0 | 0.8 | |
| Saline stress | 95.3 ± 2.7 a | 0.0 | 0.9 | 3.1 | 0.0 | 0.0 | 0.8 | |
| Columbine | Control | 92.9 ± 3.4 a | 0.0 | 0.4 | 6.8 | 0.0 | 0.0 | 0.0 |
| Osmotic stress | 89.3 ± 3.9 b | 0.0 | 0.4 | 8.6 | 0.0 | 0.0 | 1.7 | |
| Saline stress | 92.0 ± 3.0 a | 0.0 | 2.2 | 5.5 | 0.0 | 0.0 | 0.4 | |
| Esquire | Control | 95.4 ± 2.8 a | 0.0 | 0.0 | 4.3 | 0.0 | 0.0 | 0.3 |
| Osmotic stress | 85.9 ± 4.1 b | 0.0 | 0.1 | 10.1 | 0.0 | 0.0 | 3.9 | |
| Saline stress | 91.3 ± 2.7 ab | 0.0 | 0.8 | 2.4 | 0.0 | 0.0 | 5.4 | |
| P. pratensis | ||||||||
| Dakisha | Control | 84.0 ± 4.1 a | 0.0 | 3.7 | 12.3 | 0.0 | 0.0 | 0.0 |
| Osmotic stress | 79.6 ± 4.4 b | 0.0 | 0.3 | 20.1 | 0.0 | 0.0 | 0.0 | |
| Saline stress | 80.7 ± 3.7 b | 0.0 | 5.6 | 13.7 | 0.0 | 0.0 | 0.0 | |
| Sombrero | Control | 80.4 ± 4.3 a | 0.0 | 1.4 | 18.2 | 0.0 | 0.0 | 0.0 |
| Osmotic stress | 80.2 ± 4.5 a | 0.0 | 2.8 | 17.0 | 0.0 | 0.0 | 0.0 | |
| Saline stress | 79.7 ± 4.7 a | 0.0 | 2.1 | 18.2 | 0.0 | 0.0 | 0.0 | |
| Conni | Control | 84.1 ± 3.6 a | 0.0 | 2.0 | 13.9 | 0.0 | 0.0 | 0.0 |
| Osmotic stress | 77.5 ± 4.8 c | 0.0 | 4.2 | 13.3 | 0.0 | 0.0 | 5.0 | |
| Saline stress | 80.7 ± 3.3 b | 0.0 | 1.8 | 17.5 | 0.0 | 0.0 | 0.1 | |
| Treatment | L. perenne | P. pratensis | ||||
|---|---|---|---|---|---|---|
| Allstarter | Columbine | Esquire | Dakisha | Sombrero | Conni | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 mM | 0 | 0 | 0 | 21.21 a | 5.56 b | 0 |
| −0.22 MPa | 16.67 a | 0 | 11.11 b | 41.25 ab | 14.29 b | 13.33 bc |
| 100 mM | 0 | 4.17 a | 0 | 30.30 ab | 9.52 b | 4.17 c |
| −0.44 MPa | 8.33 a | 7.88 a | 11.11 b | 60.51 a | 47.55 ab | 28.03 bc |
| 200 mM | 0 | 0 | 20.83 b | 52.95 ab | 38.97 ab | 23.81 bc |
| −0.88 MPa | 31.67 a | 32.74 a | 60.19 a | 56.67 ab | 77.78 a | 70.77 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craciun, L.; Bacharach Sánchez, R.J.; Mircea, D.M.; Sapiña-Solano, A.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Early Stress Resilience in Turfgrass: Comparative Germination and Seedling Responses of Lolium perenne L. and Poa pratensis L. Under Osmotic and Salt Stress. Agronomy 2025, 15, 2719. https://doi.org/10.3390/agronomy15122719
Craciun L, Bacharach Sánchez RJ, Mircea DM, Sapiña-Solano A, Sestras RE, Boscaiu M, Sestras AF, Vicente O. Early Stress Resilience in Turfgrass: Comparative Germination and Seedling Responses of Lolium perenne L. and Poa pratensis L. Under Osmotic and Salt Stress. Agronomy. 2025; 15(12):2719. https://doi.org/10.3390/agronomy15122719
Chicago/Turabian StyleCraciun, Ligia, Rodolfo J. Bacharach Sánchez, Diana M. Mircea, Adrián Sapiña-Solano, Radu E. Sestras, Monica Boscaiu, Adriana F. Sestras, and Oscar Vicente. 2025. "Early Stress Resilience in Turfgrass: Comparative Germination and Seedling Responses of Lolium perenne L. and Poa pratensis L. Under Osmotic and Salt Stress" Agronomy 15, no. 12: 2719. https://doi.org/10.3390/agronomy15122719
APA StyleCraciun, L., Bacharach Sánchez, R. J., Mircea, D. M., Sapiña-Solano, A., Sestras, R. E., Boscaiu, M., Sestras, A. F., & Vicente, O. (2025). Early Stress Resilience in Turfgrass: Comparative Germination and Seedling Responses of Lolium perenne L. and Poa pratensis L. Under Osmotic and Salt Stress. Agronomy, 15(12), 2719. https://doi.org/10.3390/agronomy15122719












