Aerial Spray Application of Plant Protection Products for Grapevine Downy Mildew Control: Efficacy and Canopy Deposit Evaluation in Semi-Field Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of PPPs Suitable for Aerial Application
2.2. Experimental Design
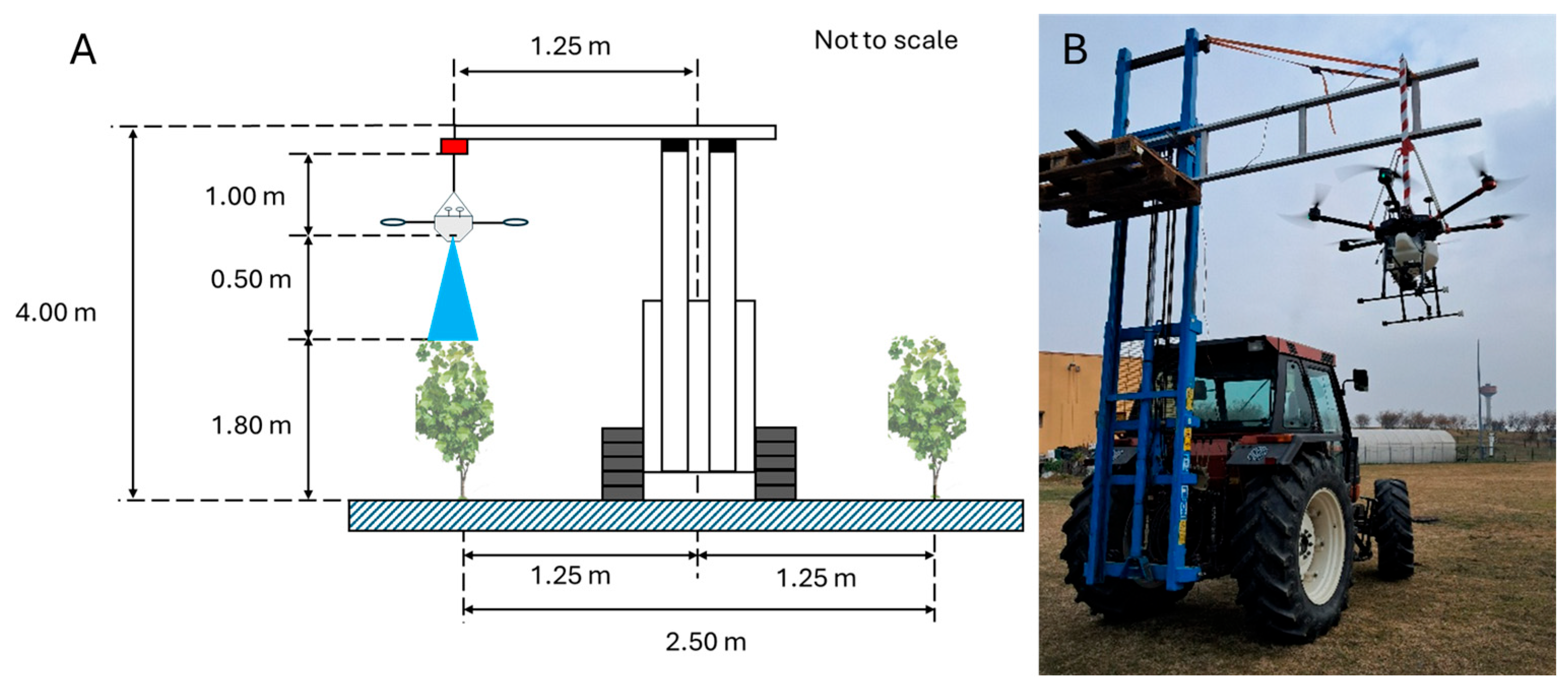
2.2.1. Study Areas Selection
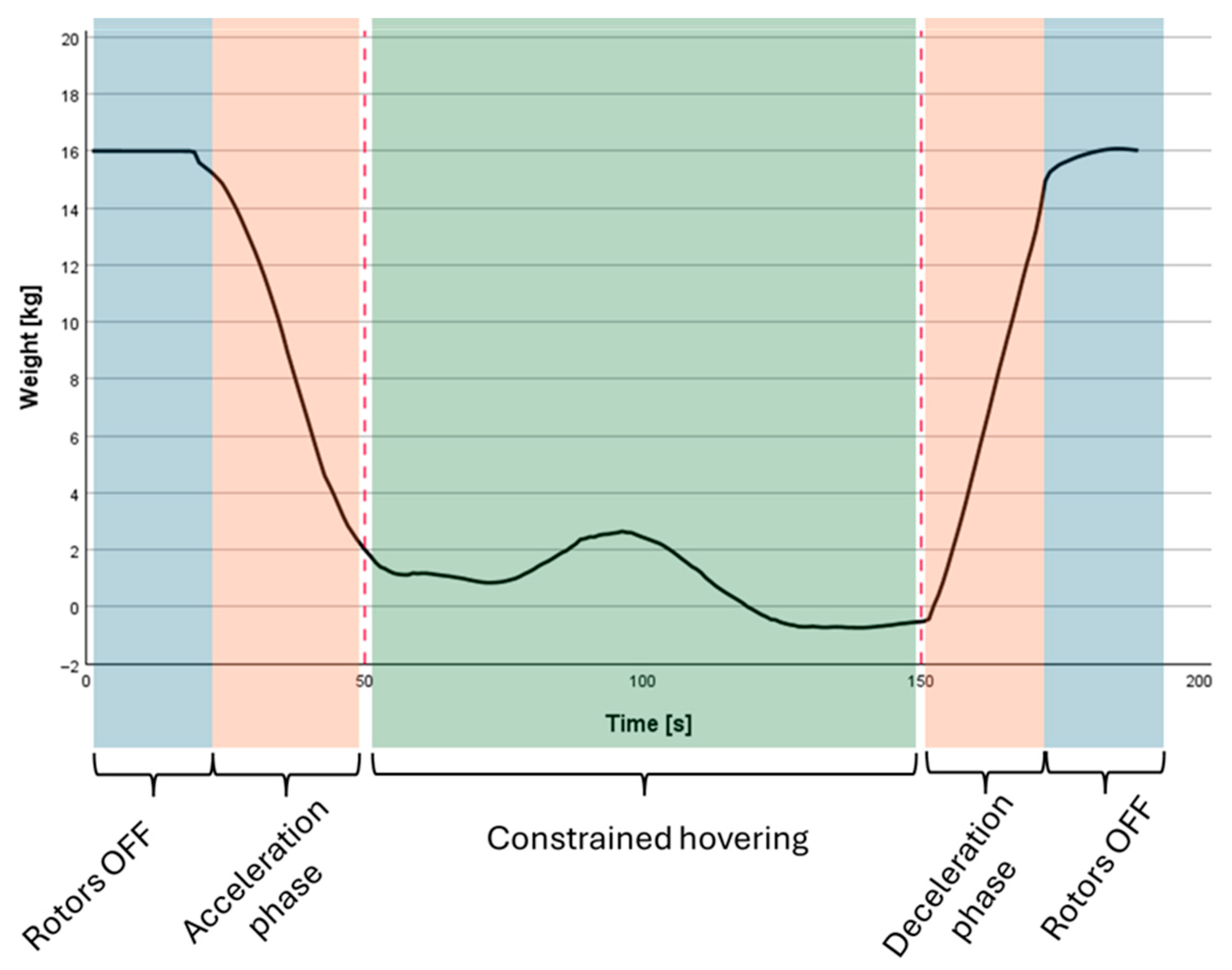
2.2.2. Pesticide Application
2.2.3. Leaf Sample Collection
2.2.4. Evaluation of PPPs Efficacy
2.2.5. Evaluation of PPP Deposits
Validation Method and Analysis of Organic PPPs
Analysis of Inorganic PPPs
2.3. Data and Statistical Analysis
3. Results
3.1. PPP Selection for Aerial Application
Active Ingredients
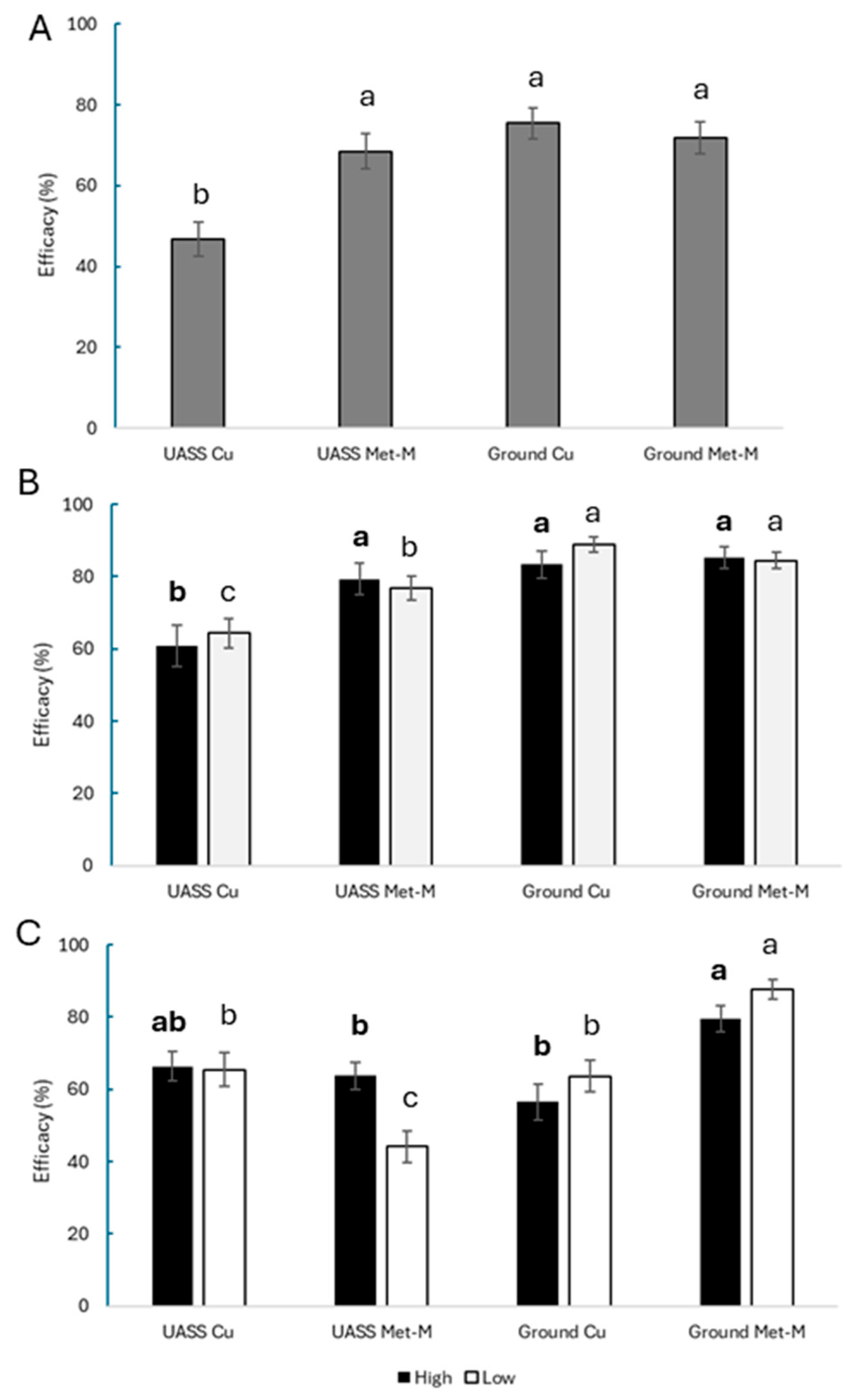
3.2. Treatment Efficacy
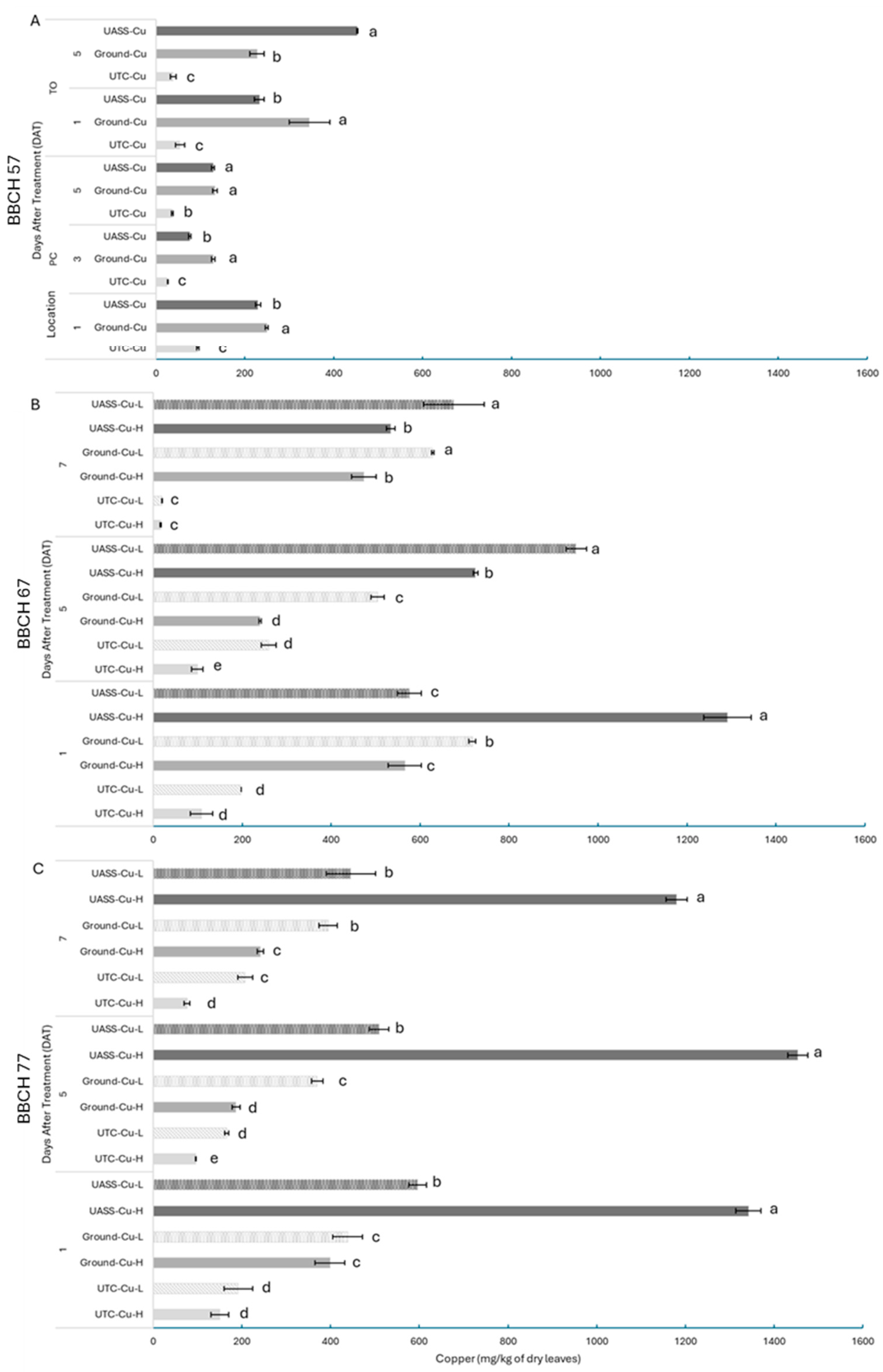
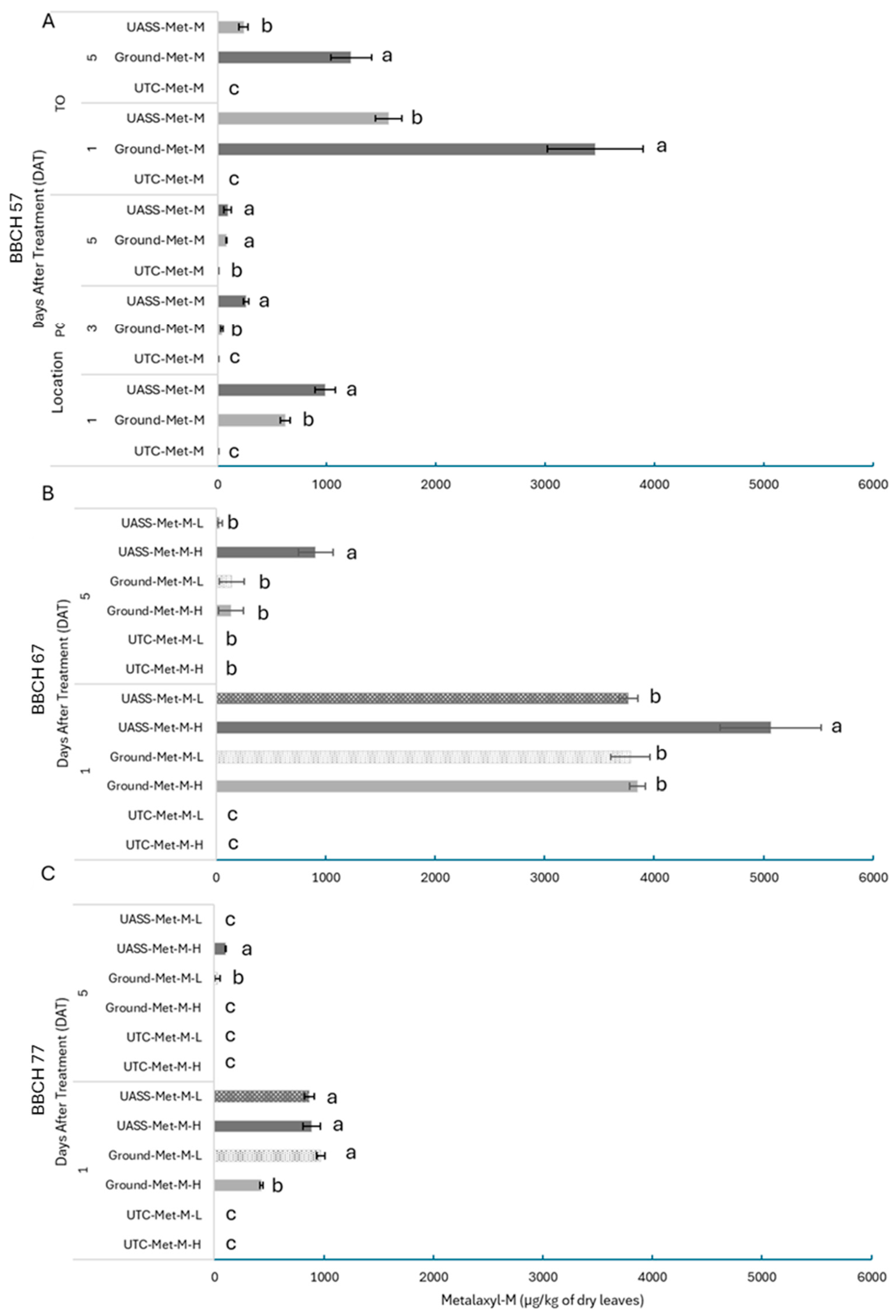
3.3. Fungicide Canopy Deposits
4. Discussion
4.1. State of the Art
4.2. Treatment Efficacy and Deposits
4.3. Limitations and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UAVs | Unmanned Aerial Vehicles |
| UASS | Unmanned Aerial Spray Systems |
| PPPs | Plant Protection Products |
| IPM | Integrated Pest Management |
| UTC | UnTreated Control plot |
| H | High (canopy portion) |
| L | Low (canopy portion) |
| DAT | Days After Treatment |
| QuEChERS | quick, easy, cheap, effective, rugged, and safe |
| HPLC | High-performance liquid chromatography |
| LC-MS/MS | Liquid Chromatography Tandem Mass Spectrometry |
| LOD | Limit of detection |
| Cu | Copper |
| Met-M | Metalaxyl-M |
| UASS-Cu | Cu-based fungicide from simulated aerial spray |
| UASS-Met-M | Met-M-based fungicide from simulated aerial spray |
| Ground-Cu | Cu-based fungicide from ground spray |
| Ground-Met-M | Met-M-based fungicide from ground spray |
| d.w. | Dry weight |
References
- Radoglou-Grammatikis, P.; Sarigiannidis, P.; Lagkas, T.; Moscholios, I. A Compilation of UAV Applications for Precision Agriculture. Comput. Netw. 2020, 172, 107148. [Google Scholar] [CrossRef]
- Rudd, J.; Roberson, G.; Classen, J. Application of Satellite, Unmanned Aircraft System, and Ground-Based Sensor Data for Precision Agriculture: A Review; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2017. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Lowenberg-Deboer, J. Precision Agriculture and Sustainability. Precis. Agric. 2004, 5, 359–387. [Google Scholar] [CrossRef]
- Tsouros, D.C.; Bibi, S.; Sarigiannidis, P.G. A Review on UAV-Based Applications for Precision Agriculture. Information 2019, 10, 349. [Google Scholar] [CrossRef]
- Olson, D.; Anderson, J. Review on Unmanned Aerial Vehicles, Remote Sensors, Imagery Processing, and Their Applications in Agriculture. Agron. J. 2021, 113, 971–992. [Google Scholar] [CrossRef]
- Grella, M.; Maritano, V.; Barge, P.; Mozzanini, E.; Comba, L.; Lingua, A.; Biglia, A. Development of a New Lab-Methodology to Evaluate Vineyard Spot-Spray UASSs Performance. In Biosystems Engineering Promoting Resilience to Climate Change—AIIA 2024—Mid-Term Conference; Sartori, L., Tarolli, P., Guerrini, L., Zuecco, G., Pezzuolo, A., Eds.; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2025; Volume 586, pp. 723–730. [Google Scholar] [CrossRef]
- EC. Directive (EC) No 128/2009 of the European Parliament and of the Council as regards of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. Off. J. Eur. Union 2009, L309, 71–86. [Google Scholar]
- Imperatore, G.; Ghirardelli, A.; Strinna, L.; Baldoin, C.; Pozzebon, A.; Zanin, G.; Otto, S. Evaluation of a Fixed Spraying System for Phytosanitary Treatments in Heroic Viticulture in North-Eastern Italy. Agriculture 2021, 11, 833. [Google Scholar] [CrossRef]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- EC. Proposal 2022/0196(COD) for a Regulation of the European Parliament and of the Council on the Sustainable Use of Plant Protection Products and Amending Regulation (EU) 2021/2115. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52022PC0305 (accessed on 10 April 2024).
- Pflanzenschutzgesetz vom 6. Februar 2012 (BGBl. I S. 148, 1281), das Zuletzt Durch Artikel 2 Absatz 15 des Gesetzes vom 20. Dezember 2022 (BGBl. I S. 2752) Geändert Worden ist. Available online: https://www.gesetze-im-internet.de/pflschg_2012/BJNR014810012.html (accessed on 10 April 2024).
- Assemblée Nationale. LOI N° 2025-365 Du 23 Avril 2025 Visant à Améliorer Le Traitement Des Maladies Affectant Les Cultures Végétales à L’aide D’aéronefs Télépilotés (1). 2025. Available online: https://www.legifrance.gouv.fr/eli/loi/2025/4/23/AGRX2502891L/jo/texte (accessed on 10 April 2024).
- Decreto Legislativo 14 Agosto 2012, n. 150: (Attuazione Della Direttiva 2009/128/CE Che Istituisce un Quadro per L’azione Comunitaria ai Fini Dell’utilizzo Sostenibile dei Pesticidi), Art. 13: (Irrorazione Aerea). Gazzetta Ufficiale n. 202 del 30 Agosto 2012. Available online: https://www.gazzettaufficiale.it/atto/vediMenuHTML?atto.dataPubblicazioneGazzetta=2012-08-30&atto.codiceRedazionale=012G0171&tipoSerie=serie_generale&tipoVigenza=originario&action=select-all (accessed on 10 April 2024).
- Li, X.; Giles, D.K.; Niederholzer, F.J.; Andaloro, J.T.; Lang, E.B.; Watson, L.J. Evaluation of an Unmanned Aerial Vehicle as a New Method of Pesticide Application for Almond Crop Protection. Pest Manag. Sci. 2021, 77, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Sarri, D.; Martelloni, L.; Rimediotti, M.; Lisci, R.; Lombardo, S.; Vieri, M. Testing a Multi-Rotor Unmanned Aerial Vehicle for Spray Application in High Slope Terraced Vineyard. J. Agric. Eng. 2019, 50, 38–47. [Google Scholar] [CrossRef]
- OECD. Report on the State of the Knowledge—Literature Review on Unmanned Aerial Spray Systems. In Agriculture; OECD Series on Pesticides, No. 105; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Liu, Y.; He, X.; Song, J.; Zeng, A.; Wang, Z. Assessment of Spray Deposition and Losses in an Apple Orchard with an Unmanned Agricultural Aircraft System in China. Trans. ASABE 2020, 63, 619–627. [Google Scholar] [CrossRef]
- Ezin, S.; Sessiz, A. Design, Prototype Manufacturing and Performance of a Drone for Vineyard Spraying. Agric. Eng. Int. CIGR J. 2023, 25, 58–67. [Google Scholar]
- Biglia, A.; Grella, M.; Bloise, N.; Comba, L.; Mozzanini, E.; Sopegno, A.; Pittarello, M.; Dicembrini, E.; Alcatrão, L.E.; Guglieri, G.; et al. UAV-Spray Application in Vineyards: Flight Modes and Spray System Adjustment Effects on Canopy Deposit, Coverage, and off-Target Losses. Sci. Total Environ. 2022, 845, 157292. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Lie, D.; Qiang, L.; Shaolan, H.; Shilai, Y.; Yande, L.; Yongxu, Y.; Haiyang, P. Effects of Citrus Tree-Shape and Spraying Height of Small Unmanned Aerial Vehicle on Droplet Distribution. Int. J. Agric. Biol. Eng. 2016, 9, 45–52. [Google Scholar] [CrossRef]
- Hu, H.; Ren, X.; Ma, X.; Li, H.; Ma, Y.; Wang, D.; Song, X.; Meng, Y.; Ma, Y. Control Effect on Cotton Aphids of Insecticides Sprayed with Unmanned Aerial Vehicles under Different Flight Heights and Spray Volumes. Int. J. Precis. Agric. Aviat. 2021, 4, 44–51. [Google Scholar] [CrossRef]
- Qin, W.-C.; Qiu, B.-J.; Xue, X.-Y.; Chen, C.; Xu, Z.-F.; Zhou, Q.-Q. Droplet Deposition and Control Effect of Insecticides Sprayed with an Unmanned Aerial Vehicle against Plant Hoppers. Crop Prot. 2016, 85, 79–88. [Google Scholar] [CrossRef]
- Poss, B.; Friedel, M.; Bartsch, K.-U.; Stoll, M.; Paraforos, D.S. Investigation of Spraying Applications Using a UAS in Viticulture. In Precision Agriculture’23; Wageningen Academic Publishers: Wageningen, The Netherlands, 2023; pp. 183–188. [Google Scholar] [CrossRef]
- Jaquerod, A.; Dubuis, P.-H. Evaluation of the Performance of UAVs for the Phytosanitary Treatments of Grapevines. Rev. Suisse Vitic. Arboric. Hortic. 2021, 53, 244–250. [Google Scholar]
- Anken, T.; Coupy, G.; Dubuis, P.-H.; Favre, G.; Geiser, H.C.; Gurba, A.; Häni, M.; Hochstrasser, M.; Landis, M.; Maitre, T.; et al. Plant protection treatments in Switzerland using unmanned aerial vehicles: Regulatory framework and lessons learned. Pest Manag. Sci. 2025, 81, 3419–3429. [Google Scholar] [CrossRef]
- Mozzanini, E.; Biglia, A.; Suciu, N.A.; Caffi, T.; Marucco, P.; Gioelli, F.; Gay, P.; Grella, M. Comparative analysis of aerial and ground-based spraying techniques: Coverage efficiency in vineyards. In Proceedings of the 15th European Conference on Precision Agriculture, Barcelona, Spain, 29 June–3 July 2025; pp. 635–641, ISBN 978-90-04-72523-2-5. Available online: https://brill.com/edcollbook-oa/title/71932 (accessed on 1 September 2025).
- EU. Commission Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Union 2018, L150, 1–92. [Google Scholar]
- EC. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, L309, 1–50. [Google Scholar]
- EU. Pesticides Database—European Commission. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 31 March 2025).
- FRAC. FRAC Code List 2024. Fungal Control Agents Sorted by Cross-Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels). Available online: https://www.frac.info/media/kufnaceb/frac-code-list-2024.pdf (accessed on 10 April 2024).
- Muccinelli, M.; Rossi, V. Le Avversità e Il Prontuario Dei Prodotti Fitosanitari. Vite. Il Nuovo Muccinelli; Edagricole: Bologna, Italy, 2021. [Google Scholar]
- PP 1/152 (4) Design and Analysis of Efficacy Evaluation Trials. EPPO Bull. 2012, 42, 367–381. [CrossRef]
- PP 1/181 (5) Conduct and Reporting of Efficacy Evaluation Trials, Including Good Experimental Practice. EPPO Bull. 2022, 52, 4–16. [CrossRef]
- Bove, F.; Savary, S.; Willocquet, L.; Rossi, V. Simulation of Potential Epidemics of Downy Mildew of Grapevine in Different Scenarios of Disease Conduciveness. Eur. J. Plant Pathol. 2020, 158, 599–614. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological Growth Stages of the Grapevine (Vitis vinifera L. ssp. Vinifera)—Codes and Descriptions According to the Extended BBCH Scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Poni, S.; Rebucci, B.; Magnanini, E.; Intrieri, C. Preliminary results on the use of a modified point quadrat method for estimating canopy structure of grapevine training systems. Vitis 1996, 35, 23–28. [Google Scholar]
- Grella, M.; Llop, J.; Marucco, P.; Campos, J.; Balsari, P.; Gioelli, F.; Gil, E. A Decision Support System (DSS) to Reduce the Spray Application Rate in Vineyards: Dosviña®, a Tool for Pesticide Saving. In Advances in Science, Technology & Innovation; Springer: Cham, Switzerland, 2024; pp. 567–570. [Google Scholar] [CrossRef]
- Azimonti, G.; Adell, P.C.; Clementi, E.; Ferentinos, K.P.; Figueroa, C.G.; Grella, M.; Luini, M.; Marucco, P.; Mozzanini, E.; Resecco, M.; et al. PPP Exposure Models for 3-D Orchards Considering Spraying Technologies in Southern Europe. EFSA Support. Publ. 2024, 21, EN-8565. [Google Scholar] [CrossRef]
- ISO 10625; Equipment for Crop Protection—Sprayer Nozzles—Colour Coding for Identification. International Standards Organisation (ISO): Geneva, Switzerland, 2018.
- Biglia, A.; Comba, L.; Alcatrão, L.E.; Sopegno, A.; Messina, C.; Mozzanini, E.; Bloise, N.; Guglieri, G.; Grella, M. Comparison between 60° and 30° Hollow Cone Nozzles for Targeted UAV-Spray Applications in Vineyards. In Precision Agriculture’23; Wageningen Academic Publishers: Wageningen, The Netherlands, 2023; pp. 67–73. [Google Scholar] [CrossRef]
- Biglia, A.; Mozzanini, E.; Gioelli, F.; Sopegno, A.; Alcatrão, L.E.; Suciu, N.A.; Furiosi, M.; Caffi, T.; Gay, P.; Grella, M. Assessing the potential efficacy and efficiency of UASS vine-targeted spray application across growth stages. Smart Agric. Technol. 2025, 12, 101532. [Google Scholar] [CrossRef]
- Bove, F.; Rossi, V. Components of Partial Resistance to Plasmopara viticola Enable Complete Phenotypic Characterization of Grapevine Varieties. Sci. Rep. 2020, 10, 585. [Google Scholar] [CrossRef]
- Taibi, O.; Salotti, I.; Rossi, V. Plant Resistance Inducers Affect Multiple Epidemiological Components of Plasmopara viticola on Grapevine Leaves. Plants 2023, 12, 2938. [Google Scholar] [CrossRef]
- Kennelly, M.M.; Gadoury, D.M.; Wilcox, W.F.; Magarey, P.A.; Seem, R.C. Primary Infection, Lesion Productivity, and Survival of Sporangia in the Grapevine Downy Mildew Pathogen Plasmopara viticola. Phytopathology 2007, 97, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.V.; Hughes, G.; Van Den Bosch, F. The Study of Plant Disease Epidemics; American Phytopathological Society Press: St. Paul, MN, USA, 2007; ISBN 978-0-89054-505-8.
- PP 1/031(3) Efficacy Evaluation of Fungicide Plasmopara Viticola. EPPO Bull. 2008, 31, 313–317. [CrossRef]
- Lehotay, S.; Mastovska, K.; Lightfield, A. Use of Buffering Other Means to Improve Results of Problematic Pesticides in a Fast Easy Method for Residue Analysis of Fruits Vegetables. J. AOAC Int. 2005, 88, 615–629. [Google Scholar] [CrossRef]
- Balkan, T.; Kara, K. Dissipation Kinetics of Some Pesticides Applied Singly or in Mixtures in/on Grape Leaf. Pest Manag. Sci. 2023, 79, 1234–1242. [Google Scholar] [CrossRef]
- Marsala, R.Z.; Capri, E.; Russo, E.; Bisagni, M.; Colla, R.; Lucini, L.; Gallo, A.; Suciu, N.A. First Evaluation of Pesticides Occurrence in Groundwater of Tidone Valley, an Area with Intensive Viticulture. Sci. Total Environ. 2020, 736, 139730. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in Plant Protection: Current Situation and Prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Giles, D.; Billing, R. Deployment and Performance of a UAV for Crop Spraying. Chem. Eng. Trans. 2015, 44, 307–312. [Google Scholar] [CrossRef]
- Sánchez-Fernández, L.; Barrera-Báez, M.; Martínez-Guanter, J.; Pérez-Ruiz, M. Reducing Environmental Exposure to PPPs in Super-High Density Olive Orchards Using UAV Sprayers. Front. Plant Sci. 2024, 14, 1272372. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Tian, Y.; Yang, Z.; Li, Z.; Lyu, S.; Song, S.; Sun, D. Research on a UAV Spray System Combined with Grid Atomized Droplets. Front. Plant Sci. 2024, 14, 1286332. [Google Scholar] [CrossRef]
- Meng, Y.; Su, J.; Song, J.; Chen, W.-H.; Lan, Y. Experimental Evaluation of UAV Spraying for Peach Trees of Different Shapes: Effects of Operational Parameters on Droplet Distribution. Comput. Electron. Agric. 2020, 170, 105282. [Google Scholar] [CrossRef]
- Sassu, A.; Psiroukis, V.; Bettucci, F.; Ghiani, L.; Fountas, S.; Gambella, F. Unmanned Aerial System Plant Protection Products Spraying Performance Evaluation on a Vineyard. Precis. Agric. 2024, 25, 2082–2112. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Mo, J. The Application of Unmanned Aircraft Systems to Plant Protection in China. Precis. Agric. 2018, 19, 278–292. [Google Scholar] [CrossRef]
- Xiongkui, H.; Bonds, J.; Herbst, A.; Langenakens, J. Recent Development of Unmanned Aerial Vehicle for Plant Protection in East Asia. Int. J. Agric. Biol. Eng. 2017, 10, 18–30. [Google Scholar]
- Wang, G.; Lan, Y.; Yuan, H.; Qi, H.; Chen, P.; Ouyang, F.; Han, Y. Comparison of Spray Deposition, Control Efficacy on Wheat Aphids and Working Efficiency in the Wheat Field of the Unmanned Aerial Vehicle with Boom Sprayer and Two Conventional Knapsack Sprayers. Appl. Sci. 2019, 9, 218. [Google Scholar] [CrossRef]
- Gibbs, J.; Peters, T.M.; Heck, L.P. Comparison of Droplet Size, Coverage, and Drift Potential from UAV Application Methods and Ground Application Methods on Row Crops. Trans. ASABE 2021, 64, 819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Sun, Z.; Bird, N.; Gu, Y.; Xu, Y.; Zhang, Z.; Wu, X. Effects of Tank-Mix Adjuvants on Physicochemical Properties and Dosage Delivery at Low Dilution Ratios for Unmanned Aerial Vehicle Application in Paddy Fields. Pest Manag. Sci. 2022, 78, 1582–1593. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, M.; Sun, Z.; Li, L.; Shang, H.-Y.; Xi, W.; Li, B.; Li, Y.; Xu, Y.; Wu, X.-M. Using Tank-Mix Adjuvant Improves the Physicochemical Properties and Dosage Delivery to Reduce the Use of Pesticides in Unmanned Aerial Vehicles for Plant Protection in Wheat. Pest Manag. Sci. 2022, 78, 2512–2522. [Google Scholar] [CrossRef]
- Menechini, W.; Maggi, M.F.; Jadoski, S.O.; Leite, C.D.; Camicia, R.d.M. Aerial and Ground Application of Fungicide in Corn Second Crop on Disease Control. Eng. Agríc. 2017, 37, 116–127. [Google Scholar] [CrossRef]
- Wongsuk, S.; Qi, P.; Wang, C.; Zeng, A.; Sun, F.; Yu, F.; Zhao, X.; Xiongkui, H. Spray Performance and Control Efficacy against Pests in Paddy Rice by UAV-Based Pesticide Application: Effects of Atomization, UAV Configuration and Flight Velocity. Pest Manag. Sci. 2024, 80, 2072–2084. [Google Scholar] [CrossRef]
- Mogili, U.R.; Deepak, B.B.V.L.; Syam Sundar, P.; Esram, R. Effects of UAV Flight Parameters Over Droplet Distribution in Pesticide Spraying. In Applications of Computational Methods in Manufacturing and Product Design; Deepak, B.B.V.L., Parhi, D.R.K., Biswal, B.B., Jena, P.C., Eds.; Springer Nature: Singapore, 2022; pp. 633–642. [Google Scholar] [CrossRef]
- Hellman, E.W. Grapevine structure and function. In Oregon Viticulture; Oregon State University Press: Corvallis, OR, USA, 2003; pp. 5–19. [Google Scholar]
- Viret, O.; Siegfried, W.; Holliger, E.; Raisigl, U. Comparison of Spray Deposits and Efficacy against Powdery Mildew of Aerial and Ground-Based Spraying Equipment in Viticulture. Crop Prot. 2003, 22, 1023–1032. [Google Scholar] [CrossRef]
- Wong, F.P.; Wilcox, W.F. Comparative Physical Modes of Action of Azoxystrobin, Mancozeb, and Metalaxyl Against Plasmopara Viticola (Grapevine Downy Mildew). Plant Dis. 2001, 85, 649–656. [Google Scholar] [CrossRef]
- Klittich, C.J.R. Fungicide Mobility and the Influence of Physical Properties. In Retention, Uptake, and Translocation of Agrochemicals in Plants; ACS Symposium Series; American Chemical Societ: Washington, DC, USA, 2014; Volume 1171, pp. 95–109. ISBN 978-0-8412-2972-3. [Google Scholar] [CrossRef]
- Rippa, M.; Battaglia, V.; Cermola, M.; Sicignano, M.; Lahoz, E.; Mormile, P. Monitoring of the Copper Persistence on Plant Leaves Using Pulsed Thermography. Environ. Monit. Assess. 2022, 194, 160. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, P.; Soto-Gómez, D.; De La Calle, I.; López-Periago, J.E.; Paradelo, M. Rainfall-Induced Removal of Copper-Based Spray Residues from Vines. Ecotoxicol. Environ. Saf. 2016, 132, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Grella, M.; Marucco, P.; Zwertvaegher, I.; Gioelli, F.; Bozzer, C.; Biglia, A.; Manzone, M.; Caffini, A.; Fountas, S.; Nuyttens, D.; et al. The Effect of Fan Setting, Air-Conveyor Orientation and Nozzle Configuration on Airblast Sprayer Efficiency: Insights Relevant to Trellised Vineyards. Crop Prot. 2022, 155, 105921. [Google Scholar] [CrossRef]
- Schmidt, K. Application of Plant Protection Products by Helicopter in Germany (Legislation, Requirements, Guidelines, Use in Vineyards and Forests, Drift). EPPO Bull. 1996, 26, 117–122. [Google Scholar] [CrossRef]
- Caffi, T.; Legler, S.E.; González-domínguez, E.; Rossi, V. Effect of Temperature and Wetness Duration on Infection by Plasmopara Viticola and on Post-Inoculation Efficacy of Copper. Eur. J. Plant Pathol. 2016, 144, 737–750. [Google Scholar] [CrossRef]
- Caffi, T. La difesa fitosanitaria in viticoltura: Attualità e prospettive. In Proceedings of the Bilancio Fitosanitario Viticolo 2024, Susegana, Italy, 29 November 2024; Available online: https://www.crea.gov.it/web/viticoltura-e-enologia/-/disponibili-gli-atti-del-bilancio-fitosanitario-viticolo-2024 (accessed on 1 September 2025).
- Assoenologi; Unione Italiana Vini; Istituto di Servizi per il Mercato Agricolo Alimentare. Relazione Vendemmiale. 2024. Available online: https://www.ismeamercati.it/flex/cm/pages/ServeAttachment.php/L/IT/D/1%252F4%252F1%252FD.2efb8ff2687c6c6e0442/P/BLOB%3AID%3D13248/E/pdf?mode=download (accessed on 1 September 2025).
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara Viticola: A Review of Knowledge on Downy Mildew of Grapevine and Effective Disease Management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- Scholthof, K.B. The disease triangle: Pathogens, the environment and society. Nat. Rev. Microbiol. 2007, 5, 152–156. [Google Scholar] [CrossRef]
- Ivezić, A.; Trudić, B.; Stamenković, Z.; Kuzmanović, B.; Perić, S.; Ivošević, B.; Buđen, M.; Petrović, K. Drone-Related Agrotechnologies for Precise Plant Protection in Western Balkans: Applications, Possibilities, and Legal Framework Limitations. Agronomy 2023, 13, 2615. [Google Scholar] [CrossRef]
- Resecco, M.; Prieto, S.; Mozzanini, E.; Bucci, L.; Biglia, A.; Romagnolo, S.; Grella, M.; Gonella, E. Viability and Efficacy Evaluation of Entomopathogenic Nematodes Applied with Two Spray Application Techniques under Controlled Lab-Conditions. Acta Hortic. 2025, 1433, 57–64. [Google Scholar] [CrossRef]
- Faers, M.A. Future Suspension Concentrate Formulation Design for Emerging Very-Low Volume and Precision Application Technologies. In Proceedings of the 15th European Conference of Precision Agriculture, Barcelona, Spain, 29 June–3 July 2025; pp. 232–238, ISBN 978-90-04-72522-5. [Google Scholar]
- Li, L.; Hu, Z.; Liu, Q.; Yi, T.; Han, P.; Zhang, R.; Pan, L. Effect of Flight Velocity on Droplet Deposition and Drift of Combined Pesticides Sprayed Using an Unmanned Aerial Vehicle Sprayer in a Peach Orchard. Front. Plant Sci. 2022, 13, 981494. [Google Scholar] [CrossRef]
- Qi, P.; Zhang, L.; Wang, Z.; Han, H.; Müller, J.; Li, T.; Wang, C.; Huang, Z.; He, M.; Liu, Y.; et al. Effect of Operational Parameters of Unmanned Aerial Vehicle (UAV) on Droplet Deposition in Trellised Pear Orchard. Drones 2023, 7, 57. [Google Scholar] [CrossRef]
- Yan, X.; Wang, M.; Zhu, Y.; Shi, X.; Liu, X.; Chen, Y.; Xu, J.; Yang, D.; Yuan, H. Effect of Aviation Spray Adjuvant on Improving Control of Fusarium Head Blight and Reducing Mycotoxin Contamination in Wheat. Agriculture 2021, 11, 1284. [Google Scholar] [CrossRef]
- Chen, P.; Lan, Y.; Huang, X.; Qi, H.; Wang, G.; Wang, J.; Wang, L.; Xiao, H. Droplet Deposition and Control of Planthoppers of Different Nozzles in Two-Stage Rice with a Quadrotor Unmanned Aerial Vehicle. Agronomy 2020, 10, 303. [Google Scholar] [CrossRef]
- Aru, F.; Gertsis, A.; Vellidis, G.; Morari, F. Investigation of Spraying Efficiency of an Aerial Spraying System in a Super-High Density Olive Grove in Greece. In Precision Agriculture’23; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 357–363. [Google Scholar] [CrossRef]
- Lopes, L.d.L.; Cunha, J.P.A.R.d.; Nomelini, Q.S.S. Use of Unmanned Aerial Vehicle for Pesticide Application in Soybean Crop. AgriEngineering 2023, 5, 2049–2063. [Google Scholar] [CrossRef]
- Richardson, B.; Rolando, C.A.; Kimberley, M.O.; Strand, T.M. Spray Application Efficiency from a Multi-Rotor Unmanned Aerial Vehicle Configured for Aerial Pesticide Application. Trans. ASABE 2019, 62, 1447–1453. [Google Scholar] [CrossRef]
- Li, X.; Giles, D.K.; Andaloro, J.T.; Long, R.; Lang, E.B.; Watson, L.J.; Qandah, I. Comparison of UAV and Fixed-Wing Aerial Application for Alfalfa Insect Pest Control: Evaluating Efficacy, Residues, and Spray Quality. Pest Manag. Sci. 2021, 77, 4980–4992. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, R.; Yu, M.; Liu, Y.; Ma, Y.; Guo, X.; Gu, Y.; Formstone, C.; Xu, Y.; Wu, X. Enhanced Dosage Delivery of Pesticide under Unmanned Aerial Vehicle Condition for Peanut Plant Protection: Tank-Mix Adjuvants and Formulation Improvement. Pest Manag. Sci. 2024, 80, 1632–1644. [Google Scholar] [CrossRef]
- Grimalt, S.; Dehouck, P. Review of Analytical Methods for the Determination of Pesticide Residues in Grapes. J. Chromatogr. A 2016, 1433, 1–23. [Google Scholar] [CrossRef]
- Turkoz-Bakirci, G. Evaluation of Pesticide Residues in Brined Vine Leaves by Liquid Chromatography Coupled to Tandem Mass Spectrometry. Fresenius Environ. Bull. 2018, 27, 4543–4558. [Google Scholar]
- Wang, W.; Teng, P.; Liu, F.; Fan, T.; Peng, Q.; Wang, Z.; Hou, T. Residue Analysis and Risk Assessment of Oxathiapiprolin and Its Metabolites in Cucumbers under Field Conditions. J. Agric. Food Chem. 2019, 67, 12904–12910. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.; Pandey, D.; Shukla, S. Multiresidue Analysis and Probabilistic Dietary Risk Assessment of 241 Pesticides in Wheatgrass (Triticum sp.) Using LC–MS/MS in Combination with QuEChERS Extraction. Biomed. Chromatogr. 2022, 36, e5411. [Google Scholar] [CrossRef] [PubMed]
- Balkan, T.; Kara, K.; Yılmaz, Ö.; Kızılarslan, M.; Özbek, Ö.F. Determination of Pesticide Residues in Almonds by LC-MS/MS and GC-MS: A Study of Method Validation and Matrix Effects. Food Chem. Adv. 2023, 3, 100442. [Google Scholar] [CrossRef]
- Su, Y.; Lu, J.; Liu, J.; Li, F.; Wang, N.; Lei, H.; Shen, X. Optimization of a QuEChERS–LC–MS/MS Method for 51 Pesticide Residues Followed by Determination of the Residue Levels and Dietary Intake Risk Assessment in Foodstuffs. Food Chem. 2024, 434, 137467. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed; SANTE/12682/2019 Rev. 0; European Commission: Brussels, Belgium, 2019; Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 15 April 2024).
- Renaud-Gentié, C.; Dieu, V.; Thiollet-Scholtus, M.; Mérot, A. Addressing Organic Viticulture Environmental Burdens by Better Understanding Interannual Impact Variations. Int. J. Life Cycle Assess. 2020, 25, 1307–1322. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, J.; Shu, X.; Kong, W.; Yang, M. Multi-Elements Determination in Medical and Edible Alpinia Oxyphylla and Morinda Officinalis and Their Decoctions by ICP-MS. Chemosphere 2016, 164, 430–435. [Google Scholar] [CrossRef]
- Vezzulli, F.; Fontanella, M.C.; Lambri, M.; Beone, G.M. Specialty and High-Quality Coffee: Discrimination through Elemental Characterization via ICP-OES, ICP-MS, and ICP-MS/MS of Origin, Species, and Variety. J. Sci. Food Agric. 2023, 103, 4303–4316. [Google Scholar] [CrossRef]
- Santos, A.P.M.; Segura-Muñoz, S.I.; Nadal, M.; Schuhmacher, M.; Domingo, J.L.; Martinez, C.A.; Magosso Takayanagui, A.M. Traffic-Related Air Pollution Biomonitoring with Tradescantia Pallida (Rose) Hunt. Cv. Purpurea Boom in Brazil. Environ. Monit. Assess. 2015, 187, 39. [Google Scholar] [CrossRef]
- Gava, A.; Emer, C.D.; Ficagna, E.; Fernandes de Andrade, S.; Fuentefria, A.M. Occurrence and Impact of Fungicides Residues on Fermentation during Wine Production—A Review. Food Addit. Contam. Part A 2021, 38, 943–961. [Google Scholar] [CrossRef]
- Hummes, A.P.; Bortoluzzi, E.C.; Tonini, V.; da Silva, L.P.; Petry, C. Transfer of Copper and Zinc from Soil to Grapevine-Derived Products in Young and Centenarian Vineyards. Water Air Soil Pollut. 2019, 230, 150. [Google Scholar] [CrossRef]
- Giner Martínez-Sierra, J.; Galilea San Blas, O.; Marchante Gayón, J.M.; García Alonso, J.I. Sulfur Analysis by Inductively Coupled Plasma-Mass Spectrometry: A Review. Spectrochim. Acta Part B At. Spectrosc. 2015, 108, 35–52. [Google Scholar] [CrossRef]

| Vineyard Location | Phenological Stage | PPP Application Date | Cumulative Precipitation (mm) over 7 DAT | Average Daily Temperature (°C) over 7 DAT |
|---|---|---|---|---|
| Piacenza | BBCH 57 | 22 May | 101.2 | 18.8 |
| Grugliasco | BBCH 57 | 21 May | 45.6 | 17.1 |
| Grugliasco | BBCH 67 | 13 June | 11.0 | 21.4 |
| Grugliasco | BBCH 77 | 16 July | 1.2 | 27.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furiosi, M.; Triachini, S.; Beone, G.M.; Fontanella, M.C.; Gaaied, S.; Arbi, G.; Lomadze, A.; Grella, M.; Mozzanini, E.; Dicembrini, E.; et al. Aerial Spray Application of Plant Protection Products for Grapevine Downy Mildew Control: Efficacy and Canopy Deposit Evaluation in Semi-Field Trials. Agronomy 2025, 15, 2703. https://doi.org/10.3390/agronomy15122703
Furiosi M, Triachini S, Beone GM, Fontanella MC, Gaaied S, Arbi G, Lomadze A, Grella M, Mozzanini E, Dicembrini E, et al. Aerial Spray Application of Plant Protection Products for Grapevine Downy Mildew Control: Efficacy and Canopy Deposit Evaluation in Semi-Field Trials. Agronomy. 2025; 15(12):2703. https://doi.org/10.3390/agronomy15122703
Chicago/Turabian StyleFuriosi, Margherita, Sara Triachini, Gian Maria Beone, Maria Chiara Fontanella, Sonia Gaaied, Ghada Arbi, Anastasia Lomadze, Marco Grella, Eric Mozzanini, Emilio Dicembrini, and et al. 2025. "Aerial Spray Application of Plant Protection Products for Grapevine Downy Mildew Control: Efficacy and Canopy Deposit Evaluation in Semi-Field Trials" Agronomy 15, no. 12: 2703. https://doi.org/10.3390/agronomy15122703
APA StyleFuriosi, M., Triachini, S., Beone, G. M., Fontanella, M. C., Gaaied, S., Arbi, G., Lomadze, A., Grella, M., Mozzanini, E., Dicembrini, E., Languasco, L., Fittipaldi Broussard, M., Nassi, L., Caffi, T., & Suciu, N. A. (2025). Aerial Spray Application of Plant Protection Products for Grapevine Downy Mildew Control: Efficacy and Canopy Deposit Evaluation in Semi-Field Trials. Agronomy, 15(12), 2703. https://doi.org/10.3390/agronomy15122703








