Soil Management and Topsoil Quality as Determinants of Residue Decomposition and Nutrient Release in Agroecosystems of the Brazilian Cerrado
Abstract
1. Introduction
2. Materials and Methods
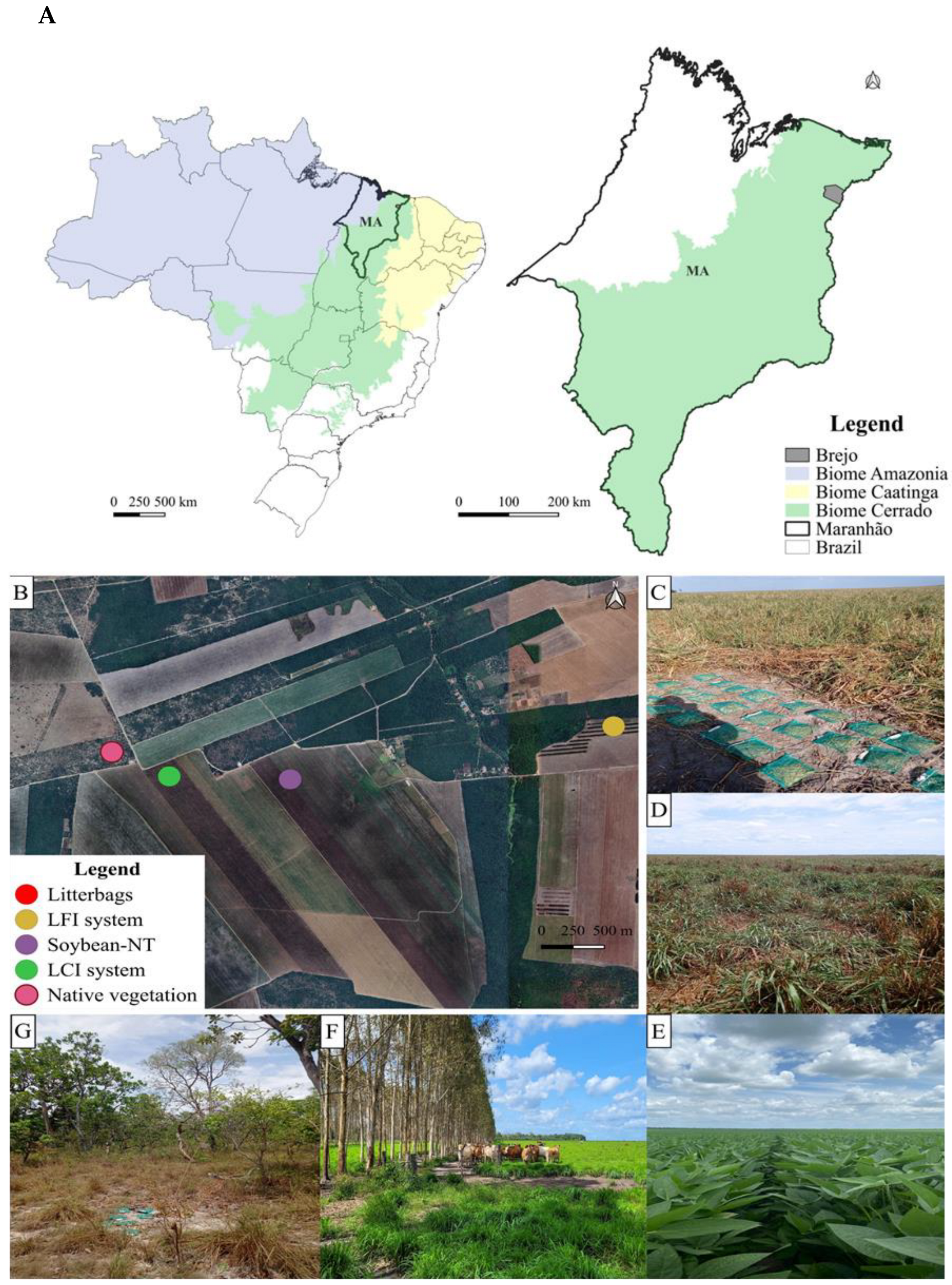
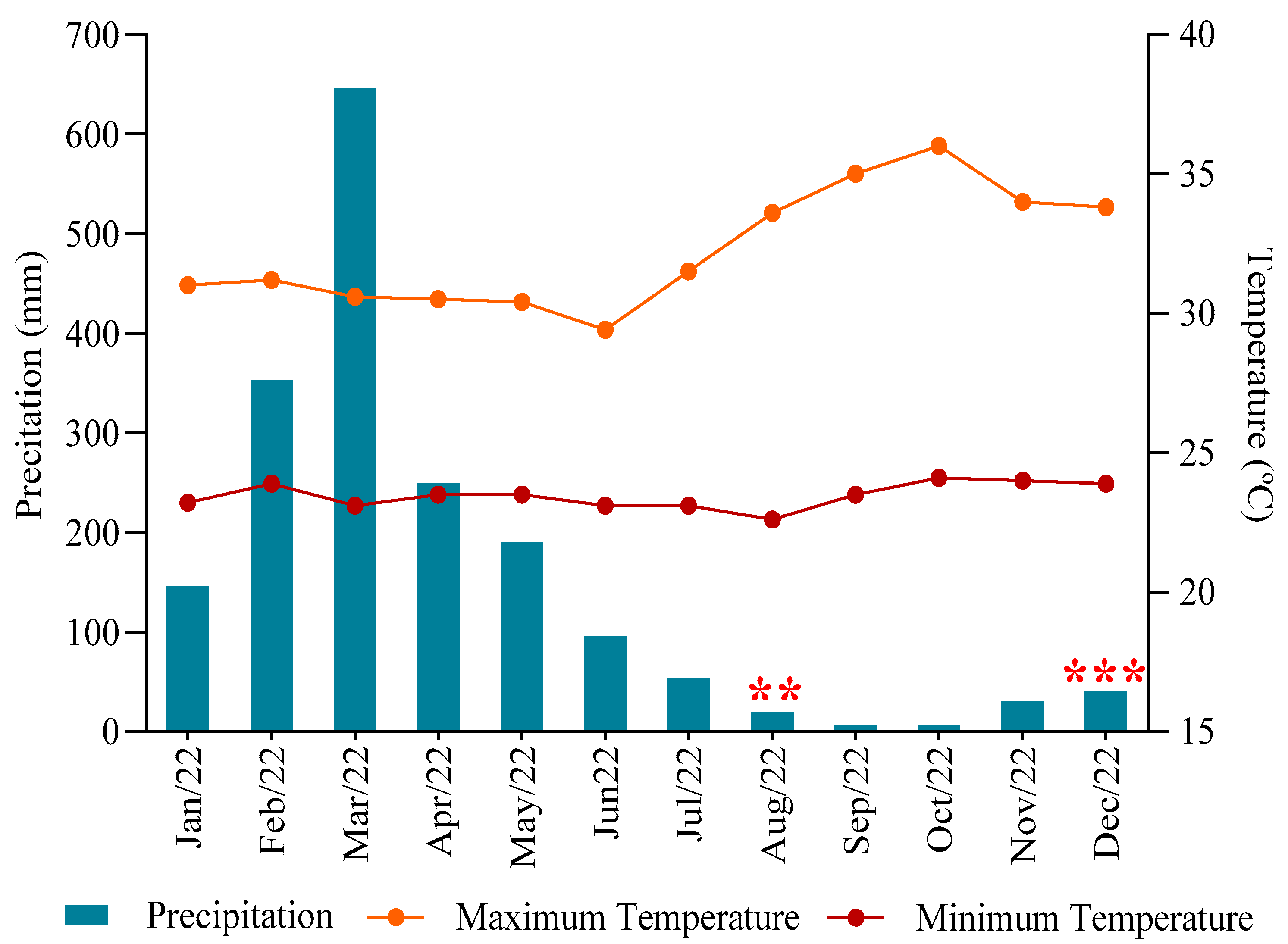
2.1. Characterization and History of Study Sites
2.2. Experimental Design
2.3. Preparing Litterbags
- Wf = final dry weight (g);
- Wi = initial dry weight (g).
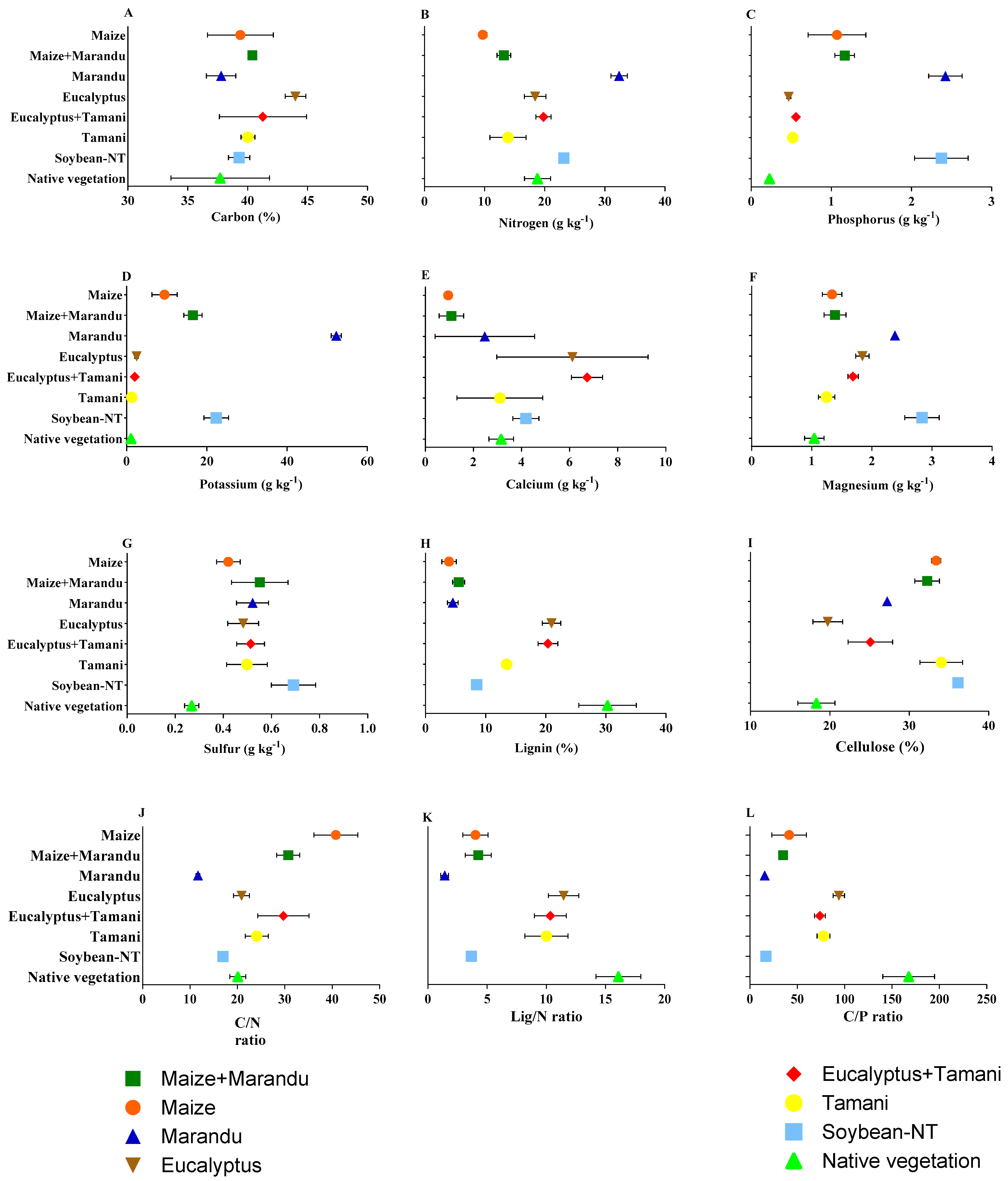
2.4. Chemical Analysis and Chemical Characterization of Residues
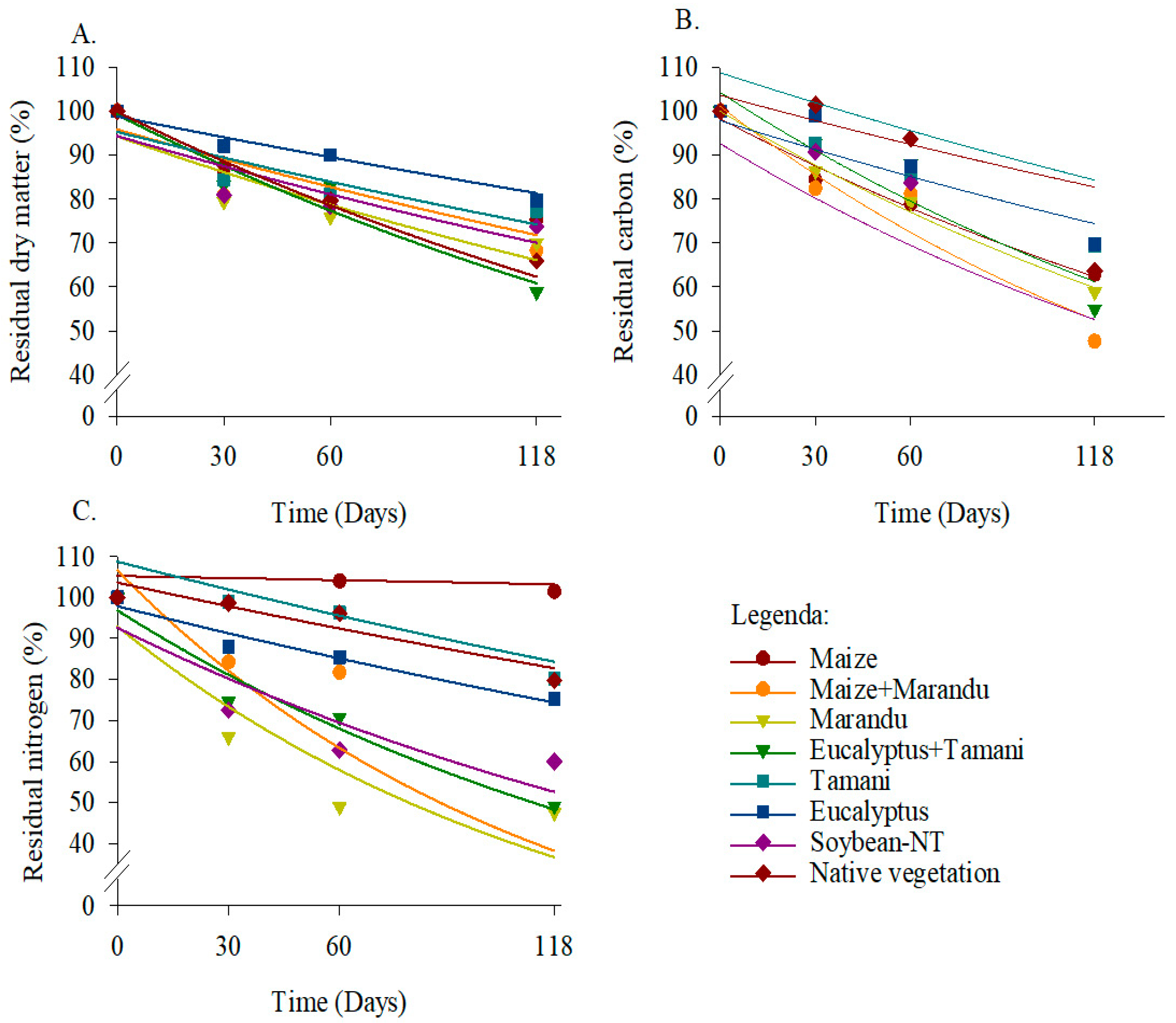
2.5. Decomposition Calculation
- X: is the amount of dry plant matter remaining after time t (days);
- X0: is the initial amount of dry plant matter or a given nutrient;
- k: is the decomposition constant of the residue;
- t: is the elapsed time (days).
- Nreleased = Reased nutrient (kg ha−1);
- Cnutrient = Nutrient content in the straw (g kg−1);
- Mstraw = Straw mass (t ha−1);
- 10 = Coversion factor from t g ha−1 to kg ha−1.
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Bortolli, M.A.; Assmann, T.S.; De Bortolli, B.B.; Maccari, M.; Bernadon, A.; Jamhour, J.; Franzluebbers, A.J.; Soares, A.B.; Severo, I.K. Nutrient Dynamics in Integrated Crop-Livestock Systems: Effects of Stocking Rates and Nitrogen System Fertilization on Litter Decomposition and Release. Agronomy 2024, 14, 2009. [Google Scholar] [CrossRef]
- Carmo, K.B.; Dias, R.; Quadros, P.D.; Berber, G.C.M.; Bourscheidt, M.L.B.; Farias Neto, A.L.; Weber, O.L.S.; Triplett, E.W.; Ferreira, A. Assessment of soil bacterial communities in integrated crop production systems within the Amazon Biome, Brazil: A comparative study. Braz. J. Microbiol. 2024, 55, 2815–2825. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.M.; Santos, R.S.; Chizzotti, F.H.; Bretas, I.L.; Franco, A.L.; Lima, R.P.; Freitas, D.A.F.; Cherubin, M.R.; Cerri, C.E. Crop, livestock, and forestry integration to reconcile soil health, food production, and climate change mitigation in the Brazilian Cerrado: A review. Geoderma Reg. 2024, 37, e00796. [Google Scholar] [CrossRef]
- Funnicelli, M.I.G.; Lima, N.S.M.; Sarini, C.C.F.; Lemos, E.G.M.; Araujo Neto, R.B.; Souza, H.A.; Oliveira Junior, J.O.L.; Sagrilo, E.; Blanco, F.F.; Andrade, H.A.F.; et al. Revealing the Bacteriome in Crop-Livestock-Forest Integration Systems in the Cerrado of MATOPIBA, Brazil. Forests 2025, 16, 626. [Google Scholar] [CrossRef]
- Santos, S.F.C.B.; Souza, H.A.; Nunes, L.A.P.L.; Batista, L.P.; Matos, M.H.M.; Vera, G.S.; Ferreira, A.C.M.; Oliveira Júnior, J.O.L.; Sagrilo, E. Soil fauna diversity in integrated production systems in the Brazilian Cerrado. Rev. Bras. Ciênc. Solo 2024, 48, e0230054. [Google Scholar] [CrossRef]
- Leite, L.F.C.; Freitas, R.C.A.; Sagrilo, E.; Galvão, S.R.S. Decomposition and nutrients release from crop residues placed on a Yellow Latosol in the savanna of the Maranhão State. Rev. Ciênc Agron. 2010, 41, 29–35. [Google Scholar] [CrossRef][Green Version]
- Costa, R.M.; Araujo, E.M.B.; Silva, D.E.O.; Rocha, S.M.B.; Bonifácio, A.; Sousa, R.S.; Pereira, A.P.A.; Medeiros, E.V.; Sagrilo, E.; Oliveira Junior, J.O.L.; et al. Seasonal responses of soil microbial biomass C and enzymatic activity comparing no-tillage and integrated crop-livestock systems. Eur. J. Soil. Biol. 2024, 121, 1–8. [Google Scholar] [CrossRef]
- Silva, A.A.; Lacerda, J.J.J.; Carvalho, S.P.; Ferreira, R.S.; Brito, R.R.; Vogado, R.F.; Araújo Neto, R.B.; Sagrilo, E.; Cavigilli, M.A.; Souza, H.A. Chemical and biological attributes of soil and soybean (Glycine max) yield in integrated systems in the Cerrado of north-east Brazil. Soil. Res. 2024, 62, SR23120. [Google Scholar] [CrossRef]
- Vogado, R.F.; Souza, H.A.; Sagrilo, E.; Brito, L.C.R.; Matias, S.S.R.; Teixeira Neto, M.L.; Oliveira Junior, J.O.; Andrade, H.A.F.; Leite, L.F.C. Soil organic carbon stocjs and fractions under integrated systems and pasture in the Cerrado of Northeast Brazil. Catena 2024, 243, 108–196. [Google Scholar] [CrossRef]
- Reis, J.C.; Rodrigues, G.S.; Barros, I.; Ribeiro, R.R.A.; Garrett, R.D.; Valentim, J.F.; Kamoi, M.Y.T.; Michetti, M.; Wruck, F.J.; Rodrigues-Filho, S.; et al. Integrated crop-livestock systems: A sustainable land-use alternative for food production in the Brazilian Cerrado and Amazon. J. Clean. Prod. 2021, 283, 1–13. [Google Scholar] [CrossRef]
- Brito, L.C.R.; Souza, H.A.; Araújo Neto, R.B.; Azevedo, D.M.P.; Sagrilo, E.; Vogado, R.F.; Carvalho, S.P.; Ferreira, A.C.M.; Cavigelli, M.A. Improved soil fertility, plant nutrition and grain yield of soybean and millet following maize intercropped with forage grasses and crotalaria in the Brazilian savanna. Crop. Pasture Sci. 2023, 74, 438–448. [Google Scholar] [CrossRef]
- Adhikari, A.D.; Shrestha, P.; Ghimire, R.; Liu, Z.; Pollock, D.A.; Acharya, P.; Aryal, D.R. Cover crop residue quality regulates litter decomposition dynamics and soil carbon mineralization kinetics in semi-arid cropping systems. Appl. Soil. Ecol. 2024, 193, 105–160. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; An, Y.; Wang, X.; Tong, S.; Wu, H.; Jiang, M.; Guo, Y.; Jiang, L. Factors governing the dynamics of soil organic carbon and nitrogen in wetlands undergoing management changes in a semi-arid region. J. Environ. Manag. 2024, 367, 122005. [Google Scholar] [CrossRef] [PubMed]
- Porre, R.J.; Werf, W.V.D.; Deyn, G.B.; Stomph, T.J.; Hoffland, E. Is litter decomposition enhanced in species mixtures? A meta-analysis. Soil. Biol. Biochem. 2020, 145, 1–16. [Google Scholar] [CrossRef]
- Surigaoge, S.; Yang, H.; Su, Y.; Du, Y.H.; Ren, S.X.; Fornara, D.; Christie, P.; Zhang, W.P.; Li, L. Maize/peanut intercropping has greater synergistic effects and home-field advantages than maize/soybean on straw decomposition. Front. Plant Sci. 2023, 14, 1100842. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Tully, K.L.; Cabrera, M.L.; Dann, C.; Schomberg, H.H.; Timlin, D.; Reberg-Horton, C.; Gaskin, J.; Davis, B.W.; Mirsky, S.B. Effects of moisture and temperature on C and N mineralization from surface-applied cover crop residues. Biol. Fertil. Soils 2021, 57, 485–498. [Google Scholar] [CrossRef]
- Thapa, R.; Tully, K.L.; Reberg-Horton, C.; Cabrera, M.; Davis, B.W.; Fleisher, D.; Gaskin, J.; Hitchcock, R.; Poncet, A.; Schomberg, H.H.; et al. Cover crop residue decomposition in no-till cropping systems: Insights from multi-state on-farm litter bag studies. Agric. Ecosyst. Environ. 2022, 326, 107823. [Google Scholar] [CrossRef]
- Thapa, R.; Cabrera, M.; Schomberg, H.H.; Reberg-Horton, C.; Poffenbarger, H.; Mirsky, S.B. Chemical differences in cover crop residue quality are maintained through litter decay. PLoS ONE 2023, 18, e0289352. [Google Scholar] [CrossRef]
- Hahn, L.; Wamser, A.F.; Wolschick, N.H.; Grando, D.L.; Siqueira, G.N.; Brunetto, G. Garlic yield after decomposition and nutrient release of cover crops under no-tilage and convencional tillage. Res. Bras. Cienc. Solo 2024, 48, e0230134. [Google Scholar] [CrossRef]
- Oelberman, M.; Voroney, R.P. Carbon and nitrogen in a temperate agroforestry system: Using stable isotopes as a tool to understandsoil dynamics. Ecol. Eng. 2007, 29, 342–349. [Google Scholar] [CrossRef]
- Raj, K.K.; Velmurugan, A.; Swarnam, T.P.; Subramani, T.; Adamala, S. Carbon Isotopes in Soil Organic Matter Dynamic Studies. J. Andaman Sci. Assoc. 2020, 25, 1–8. [Google Scholar]
- Raniolo, S.; Ros, L.; Maretto, L.; Gianelle, D.; Camin, F.; Bontempo, L.; Stevanato, P.; Sturaro, E.; Squartini, A.; Rodeghiero, M. Grazing Intensity Accelerates Surface Soil C and N Cycling in Alpine Pastures as Revealed Soil Genes and δ15N Ratio. Sustainability 2025, 7, 2165. [Google Scholar] [CrossRef]
- Furley, P.A. The nature and diversity of neotropical savanna vegetation with particular reference to the Brazilian cerrados. Glob. Ecol. Biogeogr. 1999, 8, 223–241. [Google Scholar] [CrossRef]
- Santos, H.G. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; pp. 1–20. ISBN 978-85-7035-800-4. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Alvares, A.C.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Koppen`s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico de Campinas: Campinas, Brazil, 2001. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual e Métodos de Análise de Solo e Adubação, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Berkeley, CA, USA, 1979; Volume 5. [Google Scholar]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks–a meta-analysis. Glob. Change Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Cottica, R.L.; Lima, E.V.; Andreotti, M.; Moro, E.; Marcon, E. Persistência de palhada e liberação de nutrientes do nabo forrageiro no plantio direto. Pesquisa Agropecuária Bras. 2005, 40, 161–168. [Google Scholar] [CrossRef]
- Vendramini, J.M.B.; Dubeux, J.C.B.; Silveira, M.L. Nutrient cycling in tropical pasture ecosystems. Rev. Bras. Cienc. Agr. 2014, 9, 308–315. [Google Scholar] [CrossRef]
- Boaretto, E.A.; Raij, V.B.; Silva, C.F.; Chitolina, C.J.; Carmo, S.F.A.C. Amostragem, acondicionamento e preparo de amostras de plantas para análise química. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Embrapa: Brasília, Brazil, 2009. [Google Scholar]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Detmann, E.E.; Silva, L.F.C.; Rocha, G.C.; Palma, M.N.N.; Rodrigues, J.P.P. Métodos para Análise de Alimentos: INCT-Ciência Animal, 2nd ed.; Minas Gerais: Visconde do Rio Branco, Brazil, 2021. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil. Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Wine, R.H. Determination of Lignin and Cellulose in Acid-Detergent Fiber with Permanganate. J. Assoc. Off. Anal. Chem. 1968, 51, 780–785. [Google Scholar] [CrossRef]
- Bataglia, O.C.; Furlani, A.M.C.; Texeira, J.P.F.; Furlani, P.R.; Gallo, J.R. Métodos de Análise Química de Plantas; Instituto Agronômico de Campinas: Campinas, Brazil, 1983; (Boletim Técnico, nº 78). [Google Scholar]
- Thomas, R.J.; Asakawa, N.M. Decomposition of leaf litter from tropical forage grasses and legumes. Soil. Biol. Biochem. 1993, 25, 1351–1361. [Google Scholar] [CrossRef]
- Landsberg, J.J.; Gower, S.T. Applications of Physiological Ecology to Forest Management; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Payton, M.E.; Miller, A.E.; Raun, W.R. Testing statistical hypotheses using standard error bars and confidence intervals. Commun. Soil. Sci. Plant Anal. 2000, 31, 547–551. [Google Scholar] [CrossRef]
- Manly, B.F.J. Multivariate Statistical Methods: An Introduction; Bookman: Porto Alegre, Brazil, 2008. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A.; Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R Package for ANOVA and Experimental Designs. Appl. Math. 2014, 5, 2952–2958. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; Francois, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel and Microsoft Word for Microsoft 365 MSO (Version 2509 Build 16.0.19231.20138) 64-bit; Microsoft Corporation: Redmond, WA, USA, 2023. [Google Scholar]
- Wachendorf, C.; Piepho, H.P.; Beuschel, R. Determination of litter derived C and N in litterbags and soil using stable isotopes prevents overestimation of litter decomposition in alley cropping systems. Pedobiologia 2020, 81–82, 150651. [Google Scholar] [CrossRef]
- Kooch, Y.; Moghimian, N.; Wirth, S.; Noghre, N. Effects of grazing management on leaf litter decomposition and soil microbial activities in northern Iranian rangeland. Geoderma 2020, 361, 114100. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Kleppel, G.S. Microbial community structure in pasture and hayfield soils of the Helderberg region of New York State: A comparison of management strategies. Agroecol. Sustain. Food Syst. 2019, 43, 1031–1053. [Google Scholar] [CrossRef]
- Bachega, L.R.; Bouillet, J.P.; Cássia, P.M.; Saint-André, L.; Bouvet, J.M.; Nouvellon, Y.; Moraes, G.J.L.; Robin, A.; Laclau, J.P. Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the Home Field Advantage hypothesis. For. Ecol. Manag. 2016, 359, 33–43. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Coq, S.; Barantal, S.; Handa, I.T. Leaf traits and decomposition in tropical rainforests: Revisiting some commonly held views and towards a new hypothesis. New Phytol. 2011, 189, 950–965. [Google Scholar] [CrossRef]
- Pitta, C.S.R.; Pelissari, A.; Silveira, A.L.F.; Adami, P.F.; Sartor, L.R.; Assmann, T.S.; Migliorini, F. Decomposition and nitrogen release in areas with and without grazing and its influence on corn. Semin. Agrar. 2013, 34, 905–920. [Google Scholar] [CrossRef]
- Favarato, L.F.; Souza, J.L.; Guarçoni, R.C.; Fornazier, M.J.; Martins, A.G. Persistência e liberação de nutrientes de diferentes palhadas no sistema de plantio direto orgânico de milho verde. In Impacto, Excelência e Produtividade das Ciências Agrárias no Brasil, 4th ed.; Atena: Ponta Grossa, Brazil, 2020; pp. 26–41. [Google Scholar] [CrossRef]
- Pereira, F.C.B.L.; Mello, L.M.M.; Pariz, C.M.; Mendonça, V.Z.; Yano, É.H.; Miranda, E.E.V.; Crusciol, C.A.C. Autumn maize intercropped with tropical forages: Crop residues, nutrient cycling, subsequent soybean and soil quality. Rev. Bras. Cienc. Solo 2016, 40, e0150003. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; Korthals, G.W.; Brussaard, L.; Mainardi, G.; Deyn, G.B. Litter quality drives nitrogen release, and agricultural management (organic vs. conventional) drives carbon loss during litter decomposition in agro-ecosystems. Soil. Biol. Biochem. 2021, 153, 1–9. [Google Scholar] [CrossRef]
- Constantinides, M.; Fownes, J.H. Nitrogen mineralization patterns of leaf-twig mixtures from tropical leguminous trees. Agrofor. Syst. 1993, 24, 223–231. [Google Scholar] [CrossRef]
- Seneviratne, G. Litter quality and nitrogen release in tropical agriculture: A synthesis. Biol. Fertil. Soils 2000, 31, 60–64. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H. Lixiviação, acumulação e liberação de nitrogênio em serapilheira em decomposição. Ecol. Bull. 1981, 33, 163–178. [Google Scholar]
- Ferreira, P.A.A.; Girotto, E.; Trentin, G.; Miotto, A.; Melo, G.W.; Ceretta, C.A.; Kaminski, J.; Del, F.B.K.; Marchezan, C.; Silva, L.O.S.; et al. Biomass decomposition and nutrientrelease from black oat and hairy vetch residues deposited in a vineyard. Rev. Bras. Cienc. Solo 2014, 38, 1621–1632. [Google Scholar] [CrossRef]
- Pereira, D.G.C.; Portugal, A.F.; Giustolin, T.A.; Maia, V.M.; Megda, M.X.V.; Kondo, M.K. Litter decomposition and nutrient release in diferent land use systems in the Brazilian semi-arid region. Catena 2023, 231, 107345. [Google Scholar] [CrossRef]
- Jiang, Y.; Fuzhong, W.U.; Qiuxia, W.U.; Siqi, W.U.; Jingjing, Z.H.U.; Xiangyin, N.I. Drought effects on nitrogen and phosphorus releases from litter vary between arid and humid areas: A meta-analysis. Pedosphere 2025, 35, 182–192. [Google Scholar] [CrossRef]
- Yan, G.; Han, S.; Liu, G.; Xing, Y.; Wang, Q. Litter quality mediated the effect of nitrogen addition and precipitation reduction on the release and immobilization of plant litter nitrogen and phosphorus. Can. J. Soil. Sci. 2022, 102, 263–275. [Google Scholar] [CrossRef]
- Maia, R.S.; Vasconcelos, S.S.; Carvalho, C.J.R. Frações de fósforo e simbiose micorriza em floresta secundária em resposta a disponibilidade de água e nutrientes na Amazônia oriental. Acta Amaz. 2015, 45, 255–264. [Google Scholar] [CrossRef]
- Pereira, N.D.; Martins, W.B.R.; Andrade, V.M.S.; Oliveira, F.A. Influência da remoção de serrapilheira no teor de fósforo e potássio na Amazônia Oriental. Rev. Bras. Cienc. Agr. 2017, 12, 3380–3385. [Google Scholar] [CrossRef]
- Sousa, I.R.L.; Pauletto, D.; Lopes, L.S.S.; Rode, R.; Peleja, V.L.; Freitas, B.B. Taxa de decomposição foliar de espécies utilizadas em sistemas agroflorestais. Rev. Verde De Agroecol. Desenvolv. Sustentável 2020, 2, 118–126. [Google Scholar] [CrossRef]
- Rebêlo, A.G.M.; Capucho, H.L.V.; Pauletto, D.; Dantas, E.F. Nutrient stock and rate decomposition in agro-forestry systems in the municipality of Belterra–Pará. Ciênc. Florest. 2022, 32, 1876–1893. [Google Scholar] [CrossRef]
- Kebede, E. Contribution, utilization, and improvement of legumes-driven biological nitrogen fixation in agricultural systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Islam, M.A.; Sarkar, D.; Alam, M.R.; Jahangir, M.M.R.; Ali, M.O.; Sarker, D.; Hossain, H.; Sarker, A.; Gaber, A.; Maitra, S.; et al. Legumes in conservation agriculture: A sustainable approach in rice-based ecology of the Eastern Indo-Gangetic Plain of South Asia− an overview. Technol. Agron. 2023, 3, 1–17. [Google Scholar] [CrossRef]
- Godinho, T.O.; Caldeira, M.V.W.; Rocha, J.H.T.; Caliman, J.P.; Trazzi, P.A. Quantificação de biomassa e nutrientes na serapilheira acumulada em trecho de floresta estacional semidecidual submontana, ES. Cerne 2014, 20, 11–20. [Google Scholar] [CrossRef]
- Sousa, D.M.G.D.; Lobato, E. Cerrado: Correção do Solo E Adubação, 2nd ed.; Embrapa: Brasília, Brazil, 2004. [Google Scholar]
- Dias-Filho, M.B. Degradação de Pastagens: Processos, Causas e Estratégias de Recuperação; Embrapa Amazônia Oriental: Belém, Brazil, 2014. [Google Scholar]

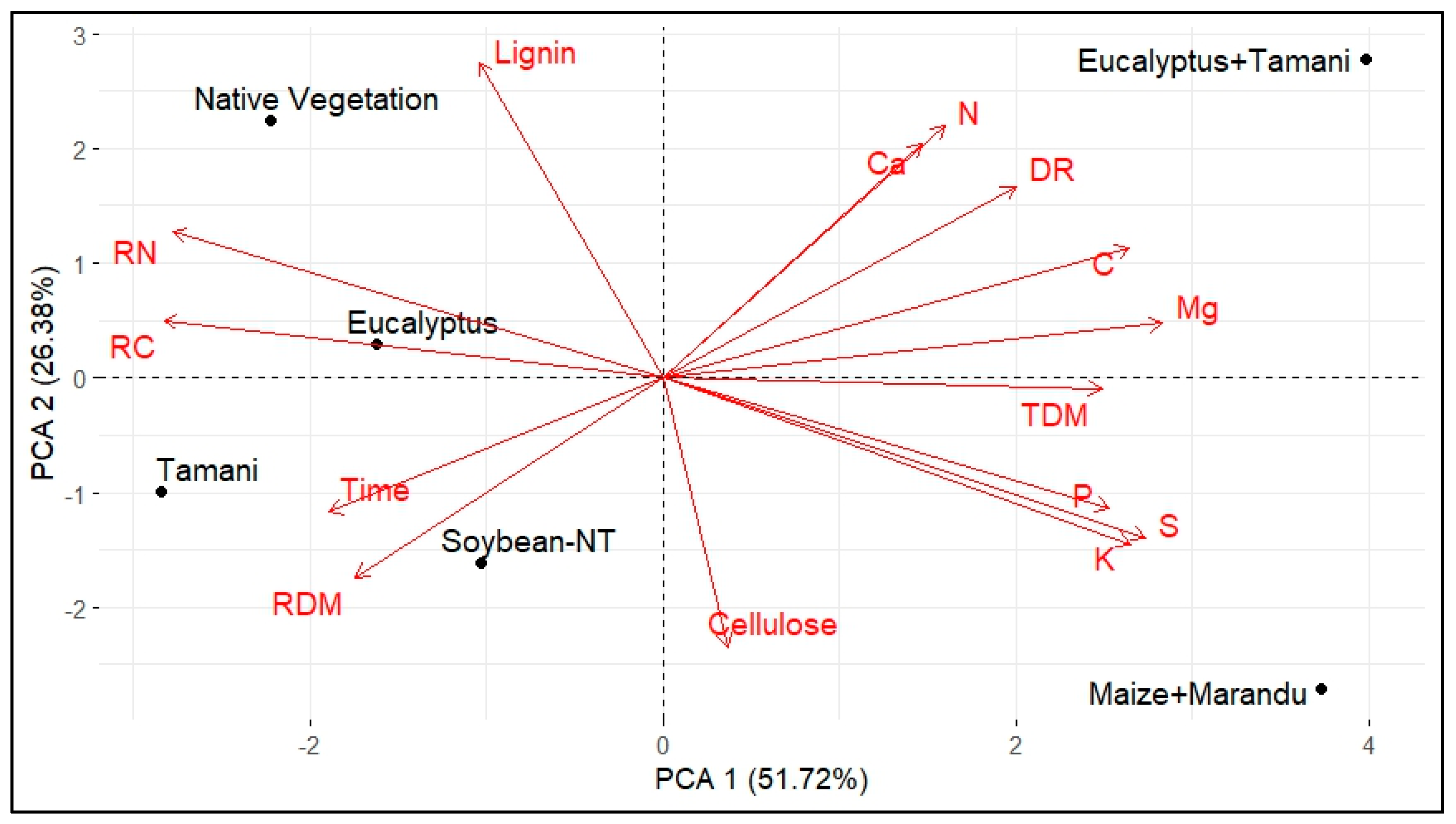
| Soil Attributes | CLI | LFI | Soybean-NT | Native Vegetation |
|---|---|---|---|---|
| pH (CaCl2) | 4.67 | 5.07 | 5.10 | 4.23 |
| TN (g kg−1) | 1.56 | 3.10 | 2.06 | 1.31 |
| S (mg dm−3) | 2.99 | 5.68 | 3.42 | 5.20 |
| P (mg dm−3) | 36.78 | 6.87 | 26.34 | 3.10 |
| K+ (cmolc dm−3) | 0.17 | 0.19 | 0.06 | 0.02 |
| Ca (cmolc dm−3) | 0.85 | 2.24 | 1.75 | 0.28 |
| Mg (cmolc dm−3) | 0.77 | 1.81 | 1.12 | 0.37 |
| Al+3 (cmolc dm−3) | 0.02 | 0.03 | 0.07 | 0.53 |
| H+Al (cmolc dm−3) | 2.57 | 4.90 | 3.95 | 4.67 |
| SB (cmolc dm−3) | 1.79 | 4.25 | 2.93 | 0.67 |
| CEC (cmolc dm−3) | 4.36 | 9.15 | 6.88 | 5.33 |
| SB (%) | 40.97 | 46.64 | 42.62 | 12.14 |
| m (%) | 0.85 | 0.70 | 2.35 | 45.65 |
| OXC (mg g−3) | 0.74 | 1.84 | 1.45 | 0.64 |
| TOC (g kg−1) | 7.95 | 18.60 | 6.56 | 15.36 |
| Litters | C | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|---|
| kg ha−1 | |||||||
| Maize + Marandu | 150.13 | 22.12 | 3.61 | 32.67 | 2.07 | 3.66 | 3.47 |
| Eucalyptus + Tamani | 256.96 | 121.08 | 2.41 | 16.01 | 17.94 | 4.93 | 2.37 |
| Tamani | 67.71 | 32.63 | 0.79 | 4.11 | 2.12 | 1.12 | 1.16 |
| Eucalyptus | 115.71 | 46.98 | 2.31 | 6.63 | 10.49 | 2.81 | 0.98 |
| Soybean-NT | 51.92 | 30.16 | 1.58 | 11.22 | 2.69 | 2.22 | 1.89 |
| Native vegetation | 54.26 | 33.87 | 0.51 | 3.59 | 2.76 | 1.14 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, D.C.d.; Medeiros, J.C.; Dalla Rosa, J.; Sagrilo, E.; Jesús Lacerda, J.J.d.; Oliveira Júnior, J.O.L.d.; Tavares, R.d.K.O.; Andrade, H.A.F.d.; Souza, H.A.d. Soil Management and Topsoil Quality as Determinants of Residue Decomposition and Nutrient Release in Agroecosystems of the Brazilian Cerrado. Agronomy 2025, 15, 2687. https://doi.org/10.3390/agronomy15122687
Sousa DCd, Medeiros JC, Dalla Rosa J, Sagrilo E, Jesús Lacerda JJd, Oliveira Júnior JOLd, Tavares RdKO, Andrade HAFd, Souza HAd. Soil Management and Topsoil Quality as Determinants of Residue Decomposition and Nutrient Release in Agroecosystems of the Brazilian Cerrado. Agronomy. 2025; 15(12):2687. https://doi.org/10.3390/agronomy15122687
Chicago/Turabian StyleSousa, Daiane Conceição de, João Carlos Medeiros, Jaqueline Dalla Rosa, Edvaldo Sagrilo, Julian Junio de Jesús Lacerda, José Oscar Lustosa de Oliveira Júnior, Rita de Kássia Oliveira Tavares, Hosana Aguiar Freitas de Andrade, and Henrique Antunes de Souza. 2025. "Soil Management and Topsoil Quality as Determinants of Residue Decomposition and Nutrient Release in Agroecosystems of the Brazilian Cerrado" Agronomy 15, no. 12: 2687. https://doi.org/10.3390/agronomy15122687
APA StyleSousa, D. C. d., Medeiros, J. C., Dalla Rosa, J., Sagrilo, E., Jesús Lacerda, J. J. d., Oliveira Júnior, J. O. L. d., Tavares, R. d. K. O., Andrade, H. A. F. d., & Souza, H. A. d. (2025). Soil Management and Topsoil Quality as Determinants of Residue Decomposition and Nutrient Release in Agroecosystems of the Brazilian Cerrado. Agronomy, 15(12), 2687. https://doi.org/10.3390/agronomy15122687








