Simulation of the Fate of Triclosan in a Paddy Soil Co-Contaminated with Graphene Nanomaterials: Enhanced Formation of Bound Residues and Potential Long-Term Risks
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Soil Culture and Sampling
2.3. Determination of Bound Residues
2.4. Fractionation of Bound Residues in Soil Humus
2.5. Distribution of Sequestered and Covalently Bound Residues of Triclosan in Soil
2.6. Distribution of Ether- and Ester-Linked Bound Residues of Triclosan in Soil
2.7. Analysis of Triclosan Metabolites in BR by LC-MS/MS
2.8. Quality Assurance and Quality Control
2.9. Data Analysis
3. Results and Discussion
3.1. Effect of RGO on Total BRs of Triclosan
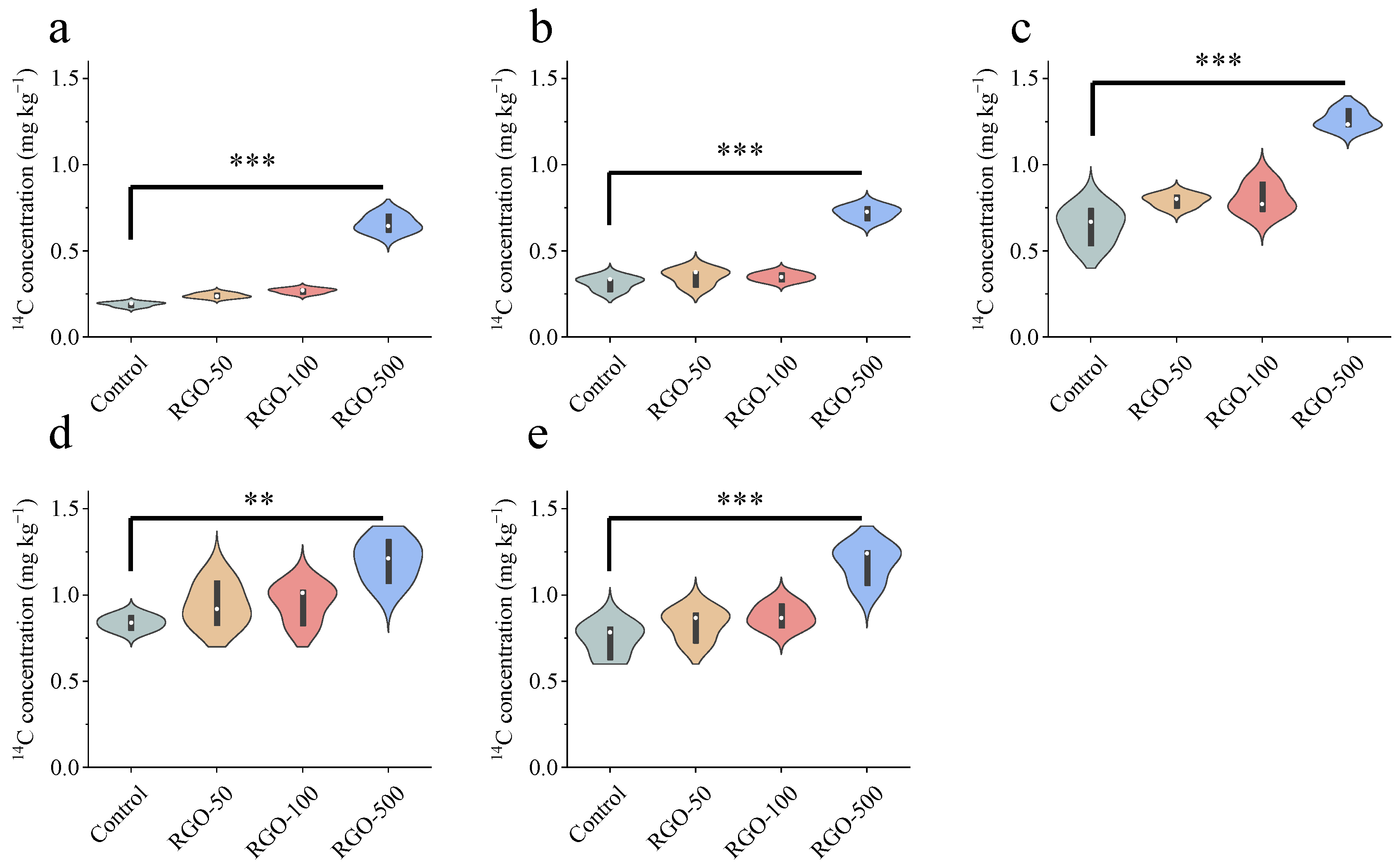
3.2. Effect of RGO on Bound Residues of Triclosan in Humus Fractions
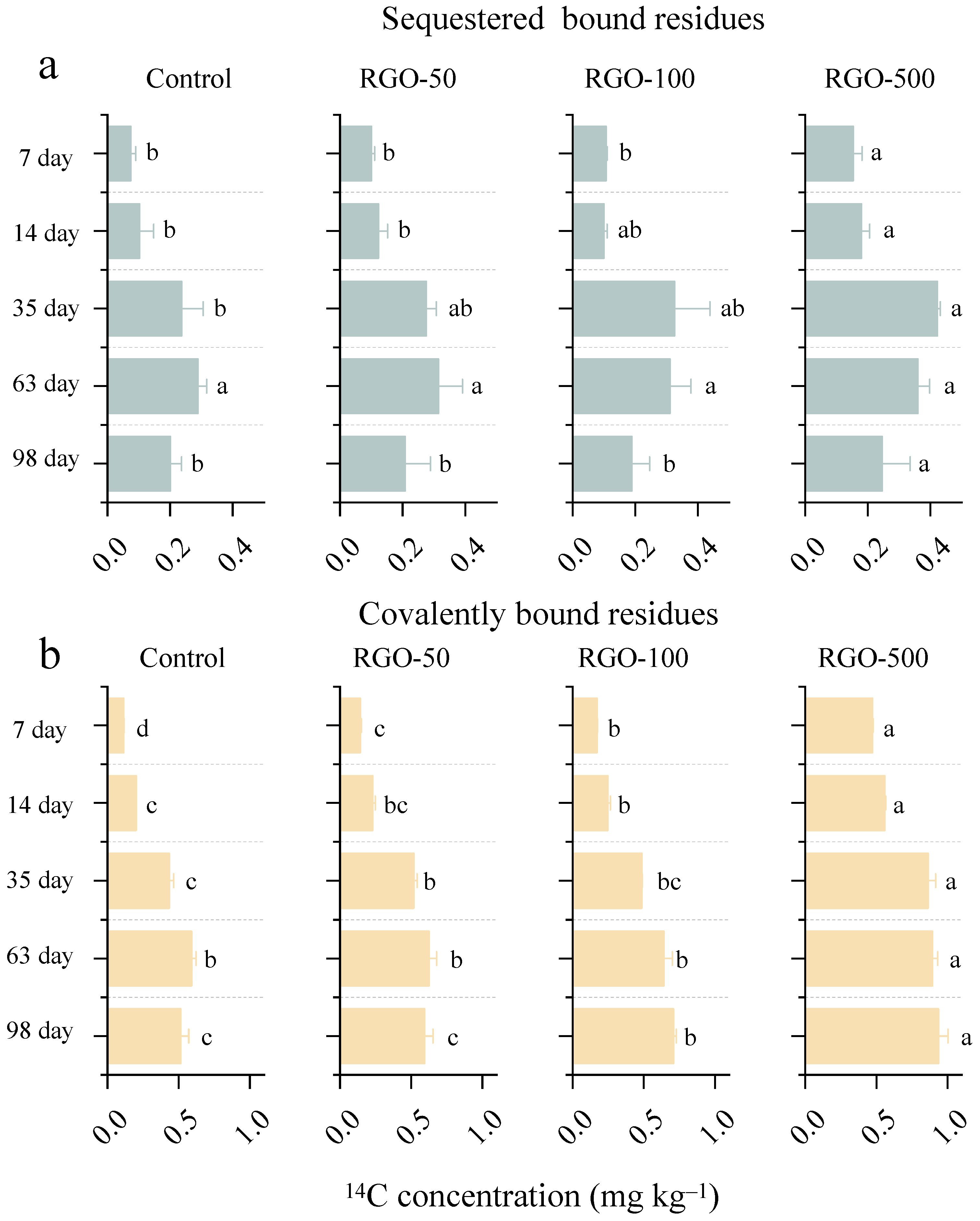
3.3. Effect of RGO on the Distribution of Sequestered and Covalently Bound Residues of Triclosan in Soil
3.4. Effect of RGO on the Distribution of Ether- and Ester-Linked BR of Triclosan in Soil
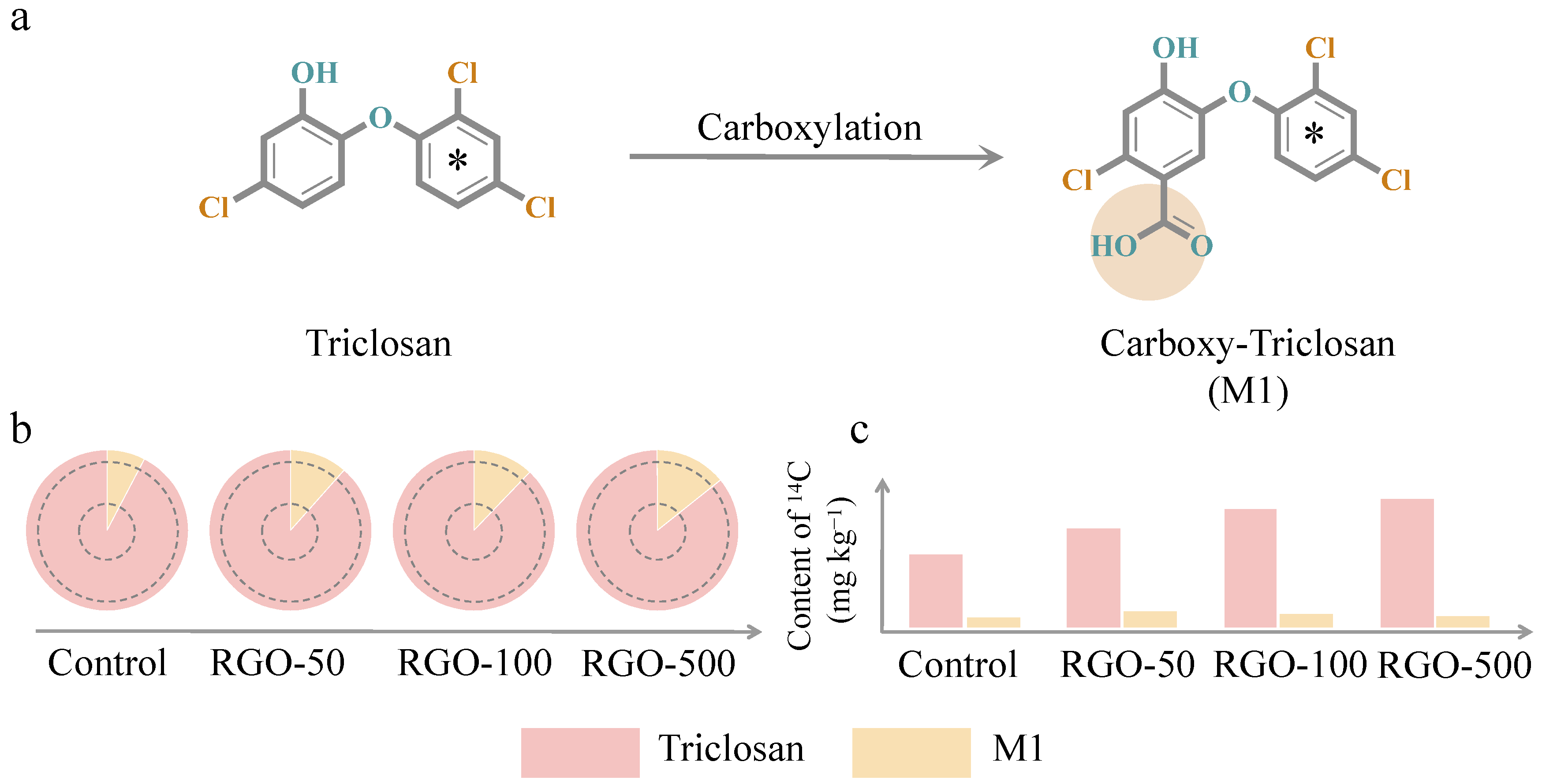
3.5. Effect of RGO on Metabolites of Triclosan in Bound Residues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Feng, H.; Song, B.; Huang, C.; Tang, X. Effect of Exogenous Carbonaceous Materials on the Bioavailability of Organic Pollutants and Their Ecological Risks. Soil Biol. Biochem. 2018, 116, 70–81. [Google Scholar] [CrossRef]
- Thakur, K.; Kandasubramanian, B. Graphene and Graphene Oxide-Based Composites for Removal of Organic Pollutants: A Review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar] [CrossRef]
- Peng, S.; Huang, Y.; Ouyang, S.; Huang, J.; Shi, Y.; Tong, Y.J.; Zhao, X.; Li, N.; Zheng, J.; Zheng, J.; et al. Efficient Solid Phase Microextraction of Organic Pollutants Based on Graphene Oxide/Chitosan Aerogel. Anal. Chim. Acta 2022, 1195, 339462. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhou, Q.; Xu, J.; Zeng, H.; Hu, X.; Ouyang, S. The Joint Environmental Fates and Ecotoxicity of Graphene-Family Nanomaterials and Co-Existing Contaminants in the Aquatic Environment: A Review. Crit. Rev. Environ. Sci. Technol. 2025, 55, 215–240. [Google Scholar] [CrossRef]
- Lu, L.; Chen, B. Biochar-Amendment-Reduced Cotransport of Graphene Oxide Nanoparticles and Dimethyl Phthalate in Saturated Porous Media. Sci. Total Environ. 2020, 705, 135094. [Google Scholar] [CrossRef]
- Sun, Y.; Teng, Y.; Li, R.; Wang, X.; Zhao, L. Microbiome Resistance Mediates Stimulation of Reduced Graphene Oxide to Simultaneous Abatement of 2,2′,4,4′,5-Pentabromodiphenyl Ether and 3,4-Dichloroaniline in Paddy Soils. J. Hazard. Mater. 2024, 465, 133121. [Google Scholar] [CrossRef]
- Weber, A.A.; Yang, X.; Mennillo, E.; Ding, J.; Watrous, J.D.; Jain, M.; Chen, S.; Karin, M.; Tukey, R.H. Lactational Delivery of Triclosan Promotes Non-Alcoholic Fatty Liver Disease in Newborn Mice. Nat. Commun. 2022, 13, 4313–4346. [Google Scholar] [CrossRef]
- Bradley, P.M.; Journey, C.A.; Romanok, K.M.; Barber, L.B.; Buxton, H.T.; Foreman, W.T.; Furlong, E.T.; Glassmeyer, S.T.; Hladik, M.L.; Iwanowicz, L.R. Expanded Target-Chemical Analysis Reveals Extensive Mixed-Organic-Contaminant Exposure in US Streams. Environ. Sci. Technol. 2017, 51, 4792–4802. [Google Scholar] [CrossRef]
- Gupta, N.; Kaur, A.; Talwar, A.; Sud, D. A Review on Triclosan: Persistance, Detection, and Remediation in Waste Streams. Water Air Soil Pollut. 2024, 235, 641. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; An, C.; Huang, C.; Weger, H.; Zhao, S.; Zhou, Y.; Rosendahl, S. Insights into the Toxicity of Triclosan to Green Microalga Chlorococcum sp. Using Synchrotron-Based Fourier Transform Infrared Spectromicroscopy: Biophysiological Analyses and Roles of Environmental Factors. Environ. Sci. Technol. 2018, 52, 2295–2306. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Wang, C.; Ying, Z.; Huo, M.; Yang, W. Effective Column Adsorption of Triclosan from Pure Water and Wastewater Treatment Plant Effluent by Using Magnetic Porous Reduced Graphene Oxide. J. Hazard. Mater. 2020, 386, 121942. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fang, L.; Cai, P.; Huang, Q.; Chen, H.; Liang, W.; Rong, X. Influence of Extracellular Polymeric Substances (EPS) on Cd Adsorption by Bacteria. Environ. Pollut. 2011, 159, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Kang, J.; Moon, J.; Um, B.; Lee, C.; Jeong, S.; Park, S. Removal of Triclosan from Aqueous Solution via Adsorption by Kenaf-Derived Biochar: Its Adsorption Mechanism Study via Spectroscopic and Experimental Approaches. J. Environ. Chem. Eng. 2021, 9, 106343. [Google Scholar] [CrossRef]
- Quan, B.; Li, X.; Zhang, H.; Zhang, C.; Ming, Y.; Huang, Y.; Xi, Y.; Weihua, X.; Yunguo, L.; Tang, Y. Technology and Principle of Removing Triclosan from Aqueous Media: A Review. Chem. Eng. J. 2019, 378, 122185. [Google Scholar] [CrossRef]
- Nie, E.; Xu, L.; Chen, Y.; Chen, Y.; Lu, Y.; Zhang, S.; Yu, Z.; Li, Q.X.; Ye, Q.; Wang, H. Effects of Reduced Graphene Oxide Nanomaterials on Transformation of 14C-Triclosan in Soils. Sci. Total Environ. 2024, 946, 173858. [Google Scholar] [CrossRef]
- Luks, A.; Zegarski, T.; Nowak, K.M.; Miltner, A.; Kästner, M.; Matthies, M.; Schmidt, B.; Schäffer, A. Fate of Pendimethalin in Soil and Characterization of Non-Extractable Residues (NER). Sci. Total Environ. 2021, 753, 141870. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.; Dai, Y.; Zhu, Q.; Wu, Y.; Lin, X. Soil Drying Legacy Does Not Affect Phenanthrene Fate in Soil but Modifies Bacterial Community Response. Environ. Pollut. 2023, 331, 121909. [Google Scholar] [CrossRef]
- Wang, S.; Su, Y.; Cheng, M.; Wang, Q.; Wu, X.; Wang, Y.; Sun, F.; Wang, R.; Ji, R. Fate of Bisphenol A (BPA) in a Flooded Soil-Rice System. J. Hazard. Mater. 2023, 459, 132177. [Google Scholar] [CrossRef]
- Schaffer, A.; Kastner, M.; Trapp, S. A Unified Approach for Including Non-Extractable Residues (NER) of Chemicals and Pesticides in the Assessment of Persistence. Environ. Sci. Eur. 2018, 30, 51. [Google Scholar] [CrossRef]
- Nie, E.; Lin, Z.; Chen, Y.; Chen, Y.; Zhang, S.; Yu, Z.; Ye, Q.; Wang, H.; Yang, Z. Reduced Graphene Oxide Modulates Bioaccumulation, Persistence, and Metabolic Transformation of 14C-Triclosan in a Soil-Radish System. J. Hazard. Mater. 2025, 139278. [Google Scholar] [CrossRef]
- Claßen, D.; Siedt, M.; Nguyen, K.T.; Ackermann, J.; Schaeffer, A. Formation, Classification and Identification of Non-Extractable Residues of 14C-Labelled Ionic Compounds in Soil. Chemosphere 2019, 232, 164–170. [Google Scholar] [CrossRef]
- Cao, S.; Wang, S.; Zhao, Y.; Wang, L.; Ma, Y.; Schaffer, A.; Ji, R. Fate of Bisphenol S (BPS) and Characterization of Non-Extractable Residues in Soil: Insights into Persistence of BPS. Environ. Int. 2020, 143, 105908. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Liang, A.; Xu, X.; Hao, Y.; Shang, H.; Li, C.; Cai, Z.; Han, L.; Zhao, J.; et al. Commonly Used Engineered Nanomaterials Improve Soil Health via Suppressing Soil-Borne Fusarium and Positively Altering Soil Microbiome. ACS ES&T Eng. 2024, 4, 915–927. [Google Scholar] [CrossRef]
- Das, P.; Davis, K.; Penton, C.R.; Westerhoff, P.; Bi, Y. Impacts of Graphitic Nanofertilizers on Nitrogen Cycling in a Sandy, Agricultural Soil. J. Nanopart. Res. 2022, 24, 120. [Google Scholar] [CrossRef]
- Nie, E.; Chen, Y.; Gao, X.; Chen, Y.; Ye, Q.; Wang, H. Uptake, Translocation and Accumulation of 14C-Triclosan in Soil-Peanut Plant System. Sci. Total Environ. 2020, 724, 138165. [Google Scholar] [CrossRef] [PubMed]
- Nie, E.; Chen, Y.; Lu, Y.; Xu, L.; Zhang, S.; Yu, Z.; Ye, Q.; Wang, H. Reduced Graphene Oxide Accelerates the Dissipation of 14C-Triclosan in Paddy Soil via Adsorption Interactions. Chemosphere 2022, 307, 136125. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Wang, W.; Zhou, D.; Wang, L.; Corvini, P.F.X.; Ji, R. Fate of Lower-Brominated Diphenyl Ethers (LBDEs) in a Red Soil–Application of 14C-Labelling. Sci. Total Environ. 2020, 721, 137735. [Google Scholar] [CrossRef]
- Junge, T.; Meyer, K.C.; Ciecielski, K.; Adams, A.; Schaffer, A.; Schmidt, B. Characterization of Non-Extractable 14C- and 13C-Sulfadiazine Residues in Soil Including Simultaneous Amendment of Pig Manure. J. Environ. Sci. Health B 2011, 46, 137–149. [Google Scholar] [CrossRef]
- Chen, K.; Yin, C.; Li, J. Influence of Activated Carbon on Fate of 14C-Sulfamethoxazole and 14C-Acetaminophen in Soil. J. Labelled Compd. Radiopharm. 2025, 68, e4152. [Google Scholar] [CrossRef]
- Zhou, W.; Shan, J.; Jiang, B.; Wang, L.; Feng, J.; Guo, H.; Ji, R. Inhibitory Effects of Carbon Nanotubes on the Degradation of 14C-2,4-Dichlorophenol in Soil. Chemosphere 2013, 90, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Kästner, M.; Nowak, K.M.; Miltner, A.; Trapp, S.; Schäffer, A. Classification and Modelling of Nonextractable Residue (NER) Formation of Xenobiotics in Soil-A Synthesis. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2107–2171. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; Zhang, Z.; Song, Y.; Cao, W.; Guo, J.; Zhou, G.; Rui, Y.; Liu, L.; Xing, B. Carbon Nanomaterials Alter Plant Physiology and Soil Bacterial Community Composition in a Rice-Soil-Bacterial Ecosystem. Environ. Pollut. 2018, 232, 123–136. [Google Scholar] [CrossRef]
- Braun, K.E.; Luks, A.; Schmidt, B. Fate of the 14C-Labeled Herbicide Prosulfocarb in a Soil and in a Sediment-Water System. J. Environ. Sci. Health B 2017, 52, 122–130. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Y.; Wang, Z.; Ye, Q.; Wang, H. Fate Characterization of Bound Residues of 14C-Pyraoxystrobin in Soils. Chemosphere 2021, 263, 128023. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Liu, X.; Zhang, X.; Liu, X.; Dong, L.; Li, P.; Xue, M.; Li, B.; Xia, G. Critical Impact of Biochar on Hydroxyl Radical Generation During Humin Oxidation. Chem. Eng. J. 2024, 500, 157479. [Google Scholar] [CrossRef]
- Jespersen, C.; Trapp, S.; Kästner, M. Non-Extractable Residues (NER) in Persistence Assessment: Effect on the Degradation Half-Life of Chemicals. Environ. Sci. Eur. 2024, 36, 206. [Google Scholar] [CrossRef]
- Mulder, I.; Siemens, J.; Sentek, V.; Amelung, W.; Smalla, K.; Jechalke, S. Quaternary Ammonium Compounds in Soil: Implications for Antibiotic Resistance Development. Rev. Environ. Sci. Bio/Technol. 2018, 17, 159–185. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Jiang, B.; Yang, X.; Nastold, P.; Kolvenbach, B.; Wang, L.; Ma, Y.; Corvini, P.F.; Ji, R. Fate of Tetrabromobisphenol A (TBBPA) and Formation of Ester- and Ether-Linked Bound Residues in an Oxic Sandy Soil. Environ. Sci. Technol. 2015, 49, 12758–12765. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Hou, S.; Lin, H.; Chen, S.; Wang, Q.; Wei, H.; Zhou, J.; Zhuo, S. Thiophene-Diketopyrrolopyrrole-Based Polymer Derivatives/Reduced Graphene Oxide Composite Materials as Organic Anode Materials for Lithium-Ion Batteries. Chem. Eng. J. 2022, 438, 135540. [Google Scholar] [CrossRef]
- O'Neill, J.S.; Hoyle, N.P.; Robertson, J.B.; Edgar, R.S.; Beale, A.D.; Peak-Chew, S.Y.; Day, J.; Costa, A.; Frezza, C.; Causton, H.C. Eukaryotic Cell Biology is Temporally Coordinated to Support the Energetic Demands of Protein Homeostasis. Nat. Commun. 2020, 11, 4706. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Dong, B.; Yan, H.; Tang, F.; Yu, Y. Characterization of a Bacterial Strain Capable of Degrading DDT Congeners and Its Use in Bioremediation of Contaminated Soil. J. Hazard. Mater. 2010, 184, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kalathoor, R.; Zeiner, M.; Schmidt, B.; Schäffer, A.; Schwarzbauer, J. First Evidence for Covalent Linkage of Acidic Metabolites of Metalaxyl and DDT as Non-Extractable Pesticide Residues in Soil and Sediment. Environ. Chem. Lett. 2015, 13, 431–437. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Pan, X.; Yang, M.; Wang, Z.; Yu, J.; Wang, H.; Yang, Z.; Xiao, H.; Nie, E. Simulation of the Fate of Triclosan in a Paddy Soil Co-Contaminated with Graphene Nanomaterials: Enhanced Formation of Bound Residues and Potential Long-Term Risks. Agronomy 2025, 15, 2658. https://doi.org/10.3390/agronomy15112658
Hu Y, Pan X, Yang M, Wang Z, Yu J, Wang H, Yang Z, Xiao H, Nie E. Simulation of the Fate of Triclosan in a Paddy Soil Co-Contaminated with Graphene Nanomaterials: Enhanced Formation of Bound Residues and Potential Long-Term Risks. Agronomy. 2025; 15(11):2658. https://doi.org/10.3390/agronomy15112658
Chicago/Turabian StyleHu, Yishun, Xuanyun Pan, Mengdie Yang, Zegang Wang, Jiageng Yu, Haiyan Wang, Zhen Yang, Huan Xiao, and Enguang Nie. 2025. "Simulation of the Fate of Triclosan in a Paddy Soil Co-Contaminated with Graphene Nanomaterials: Enhanced Formation of Bound Residues and Potential Long-Term Risks" Agronomy 15, no. 11: 2658. https://doi.org/10.3390/agronomy15112658
APA StyleHu, Y., Pan, X., Yang, M., Wang, Z., Yu, J., Wang, H., Yang, Z., Xiao, H., & Nie, E. (2025). Simulation of the Fate of Triclosan in a Paddy Soil Co-Contaminated with Graphene Nanomaterials: Enhanced Formation of Bound Residues and Potential Long-Term Risks. Agronomy, 15(11), 2658. https://doi.org/10.3390/agronomy15112658





