Oxalic Acid Pretreatment of Cotton Straw Enhances Its Salt Adsorption and Water Retention Capacity—A Soil-Amending Strategy for Saline Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
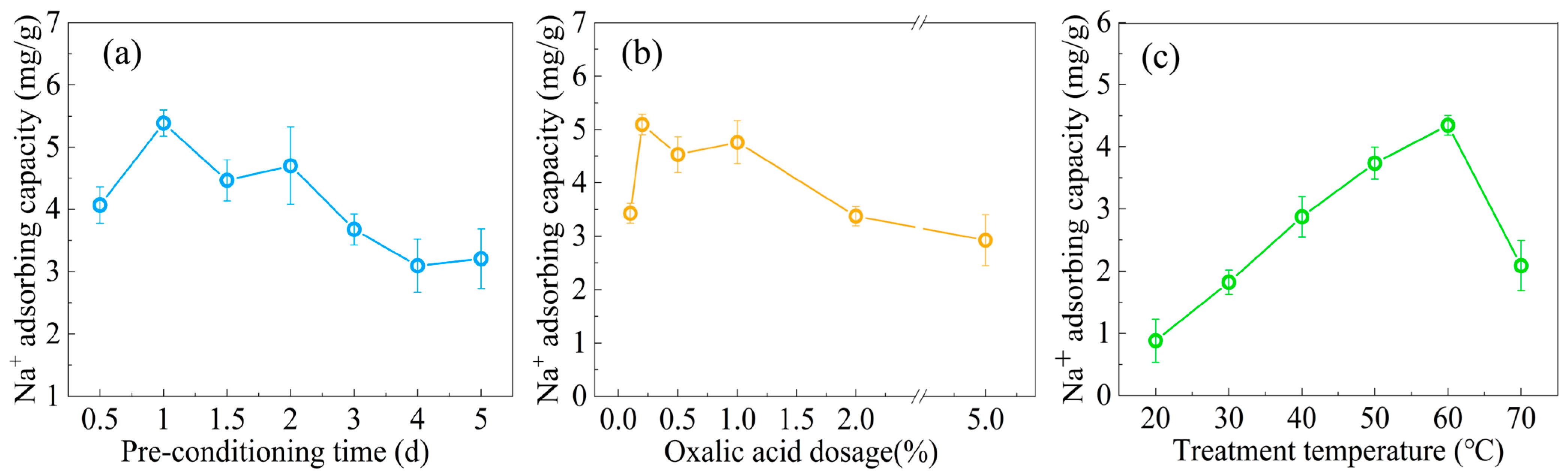
2.2. Optimization of the Preparation Conditions for Oxalic Acid-Modified Cotton Straw Salt-Absorbing Water-Retention Agent (OAC-SR)
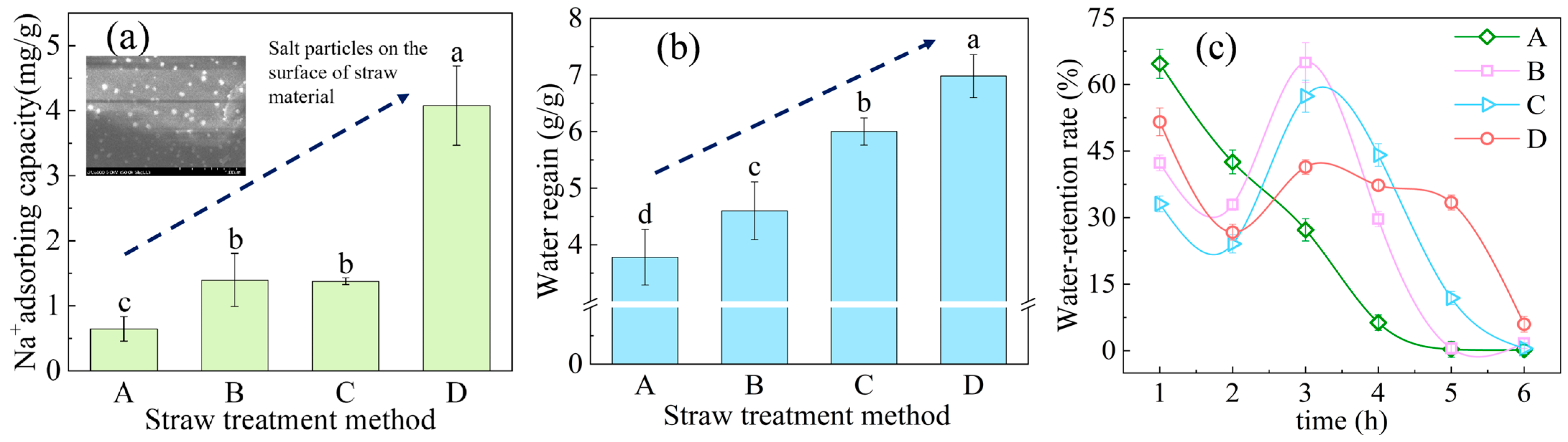
2.3. Verification of the Salt Absorption and Water Retention Capacity of OAC-SR
2.3.1. Na+ Adsorption Capacity of Materials
2.3.2. Water Adsorption Capacity of Materials
2.3.3. Water Retention Comparison Experiment
2.4. Adsorption Experiments
2.4.1. Adsorption Condition Test
2.4.2. Base Cation Adsorption Experiment
2.4.3. Soil Extraction Solution Adsorption Experiment
2.4.4. Soil Phase Adsorption Experiment
2.5. Indoor Potted Plant Experiment
2.6. Field Experiment
2.7. Data Analysis
3. Results
3.1. Optimization of OAC-SR Preparation Conditions
3.2. The Capacity of Salt Absorption and Water Retention of OAC-SR
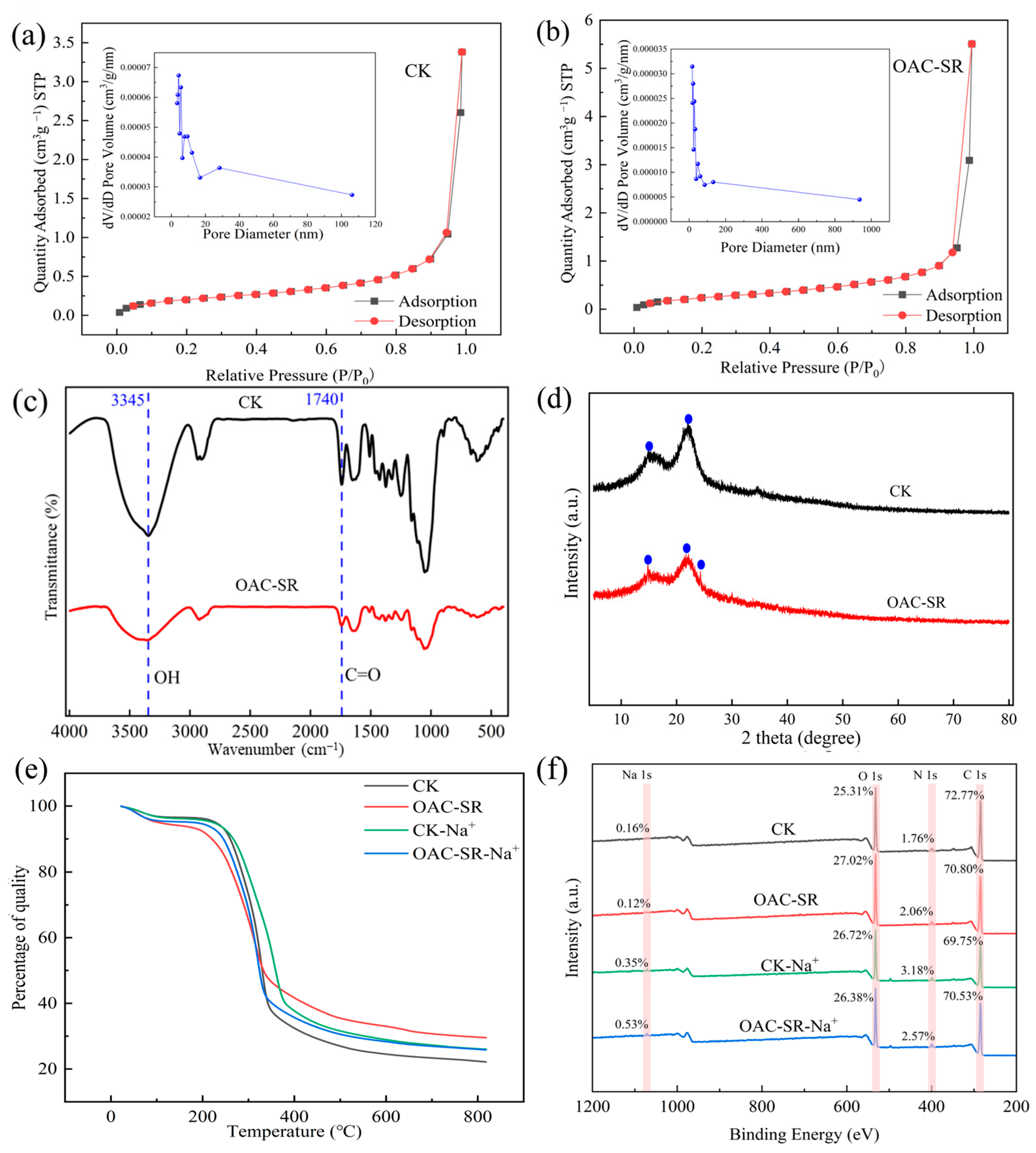
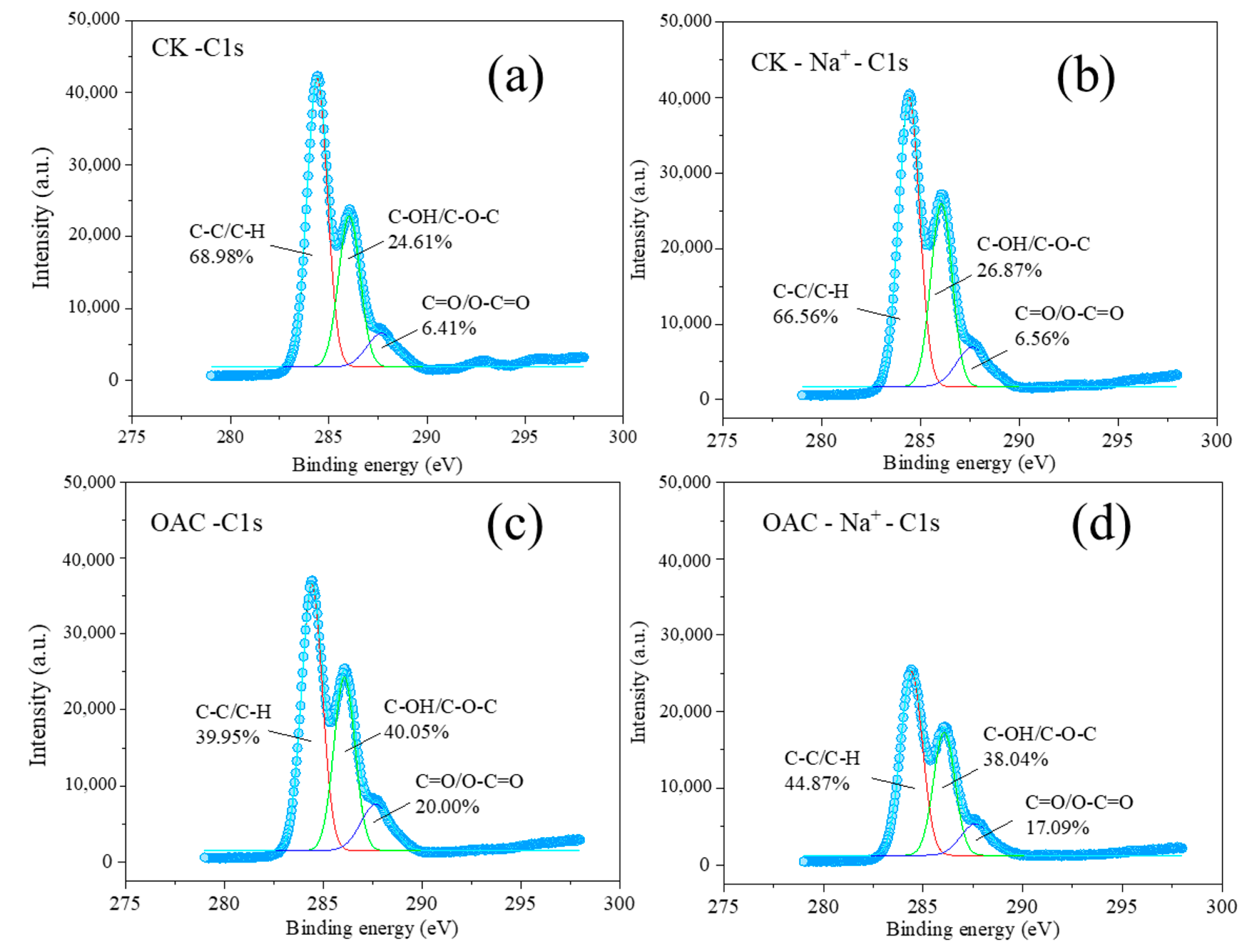
3.3. Effects of Oxalic Acid Treatments on the Structure and Properties of Cotton Straw
3.4. Adsorption Capacities of Materials Studies
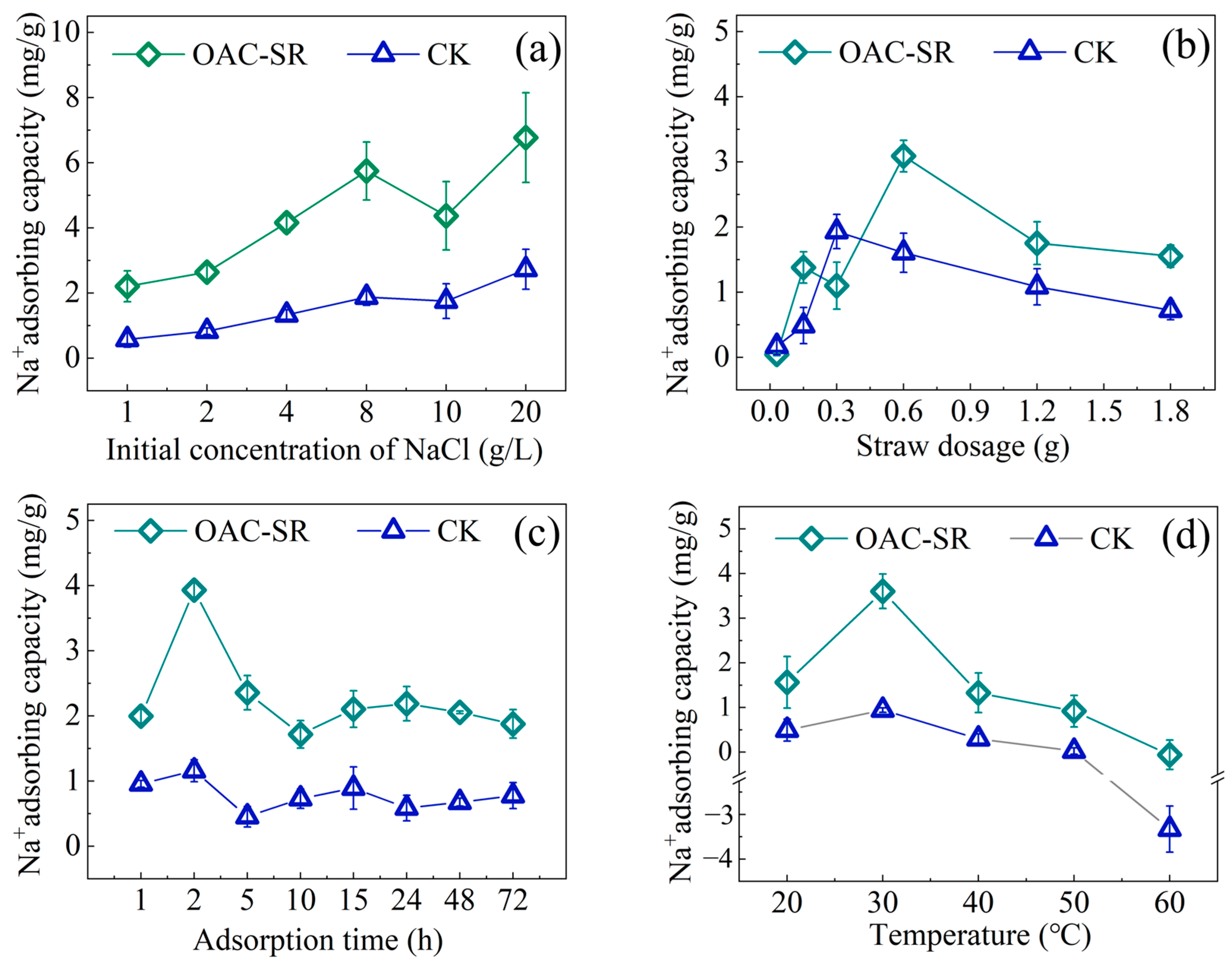
3.4.1. Effect of Adsorption Conditions on Na+ Adsorption of OAC-SR
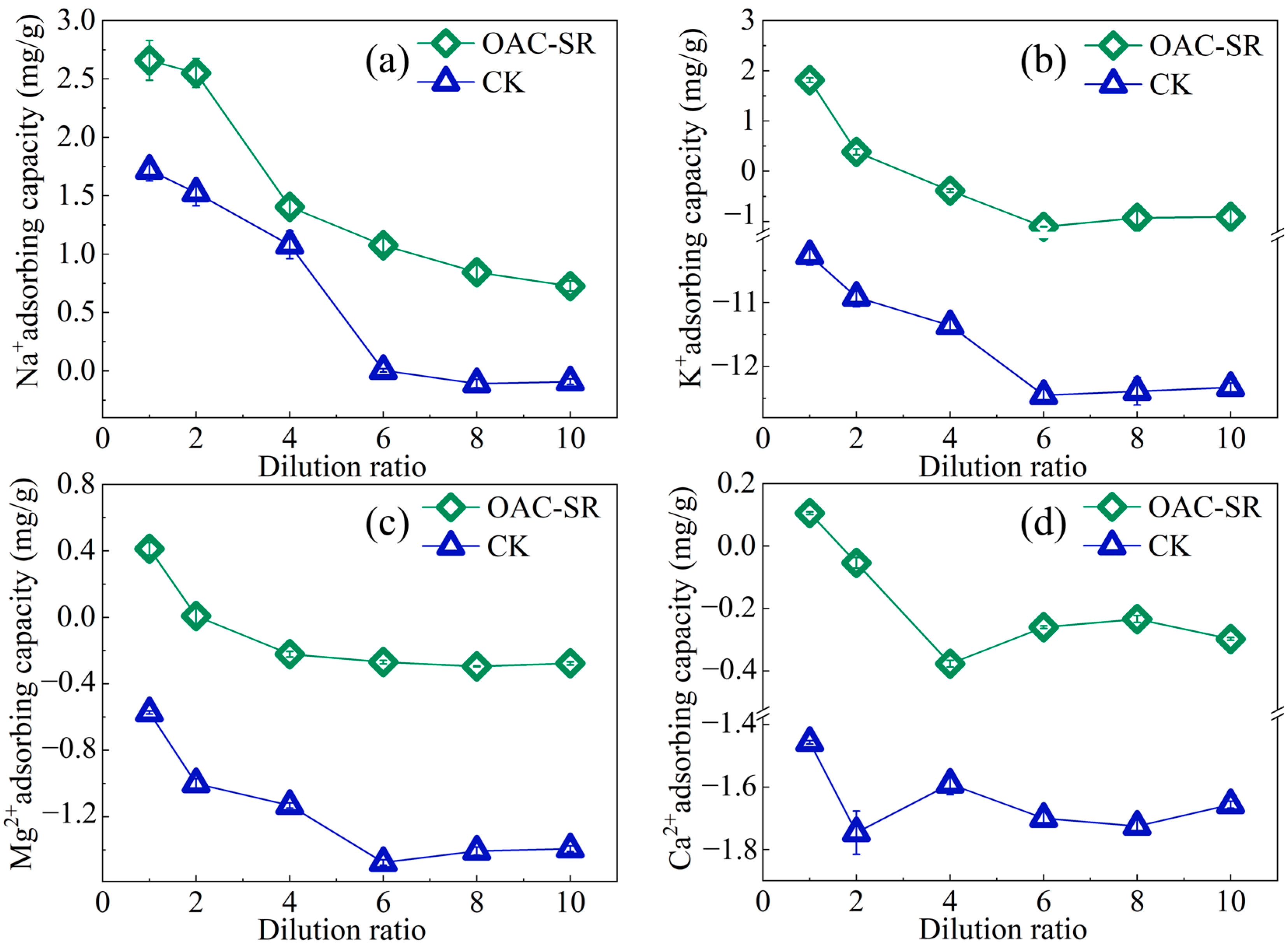
3.4.2. Effects of Cations and Dissolved Organic Matter on Na+ Adsorption of OAC-SR
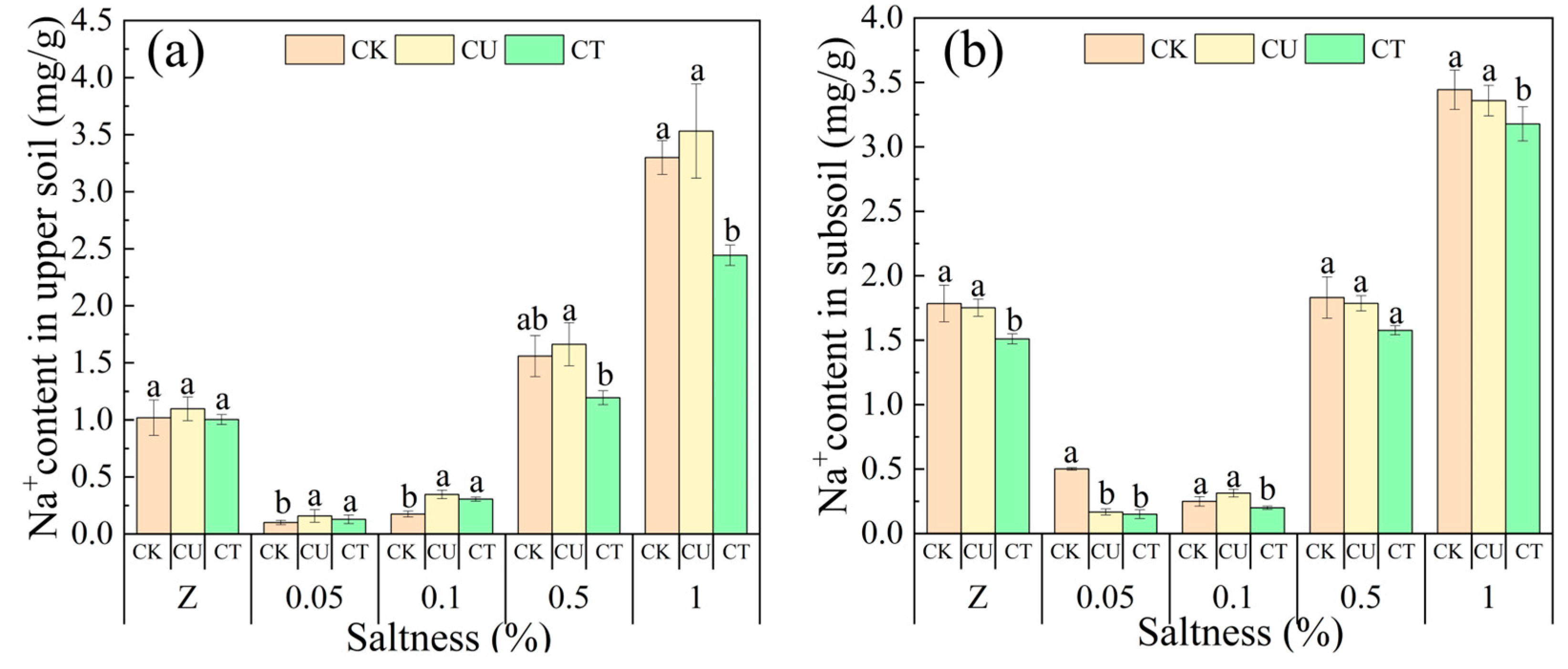
3.5. Salt Barrier Effect of OAC-SR on the Salt Distribution in Soils
3.6. Effects of Indoor Potted Plant Experiments
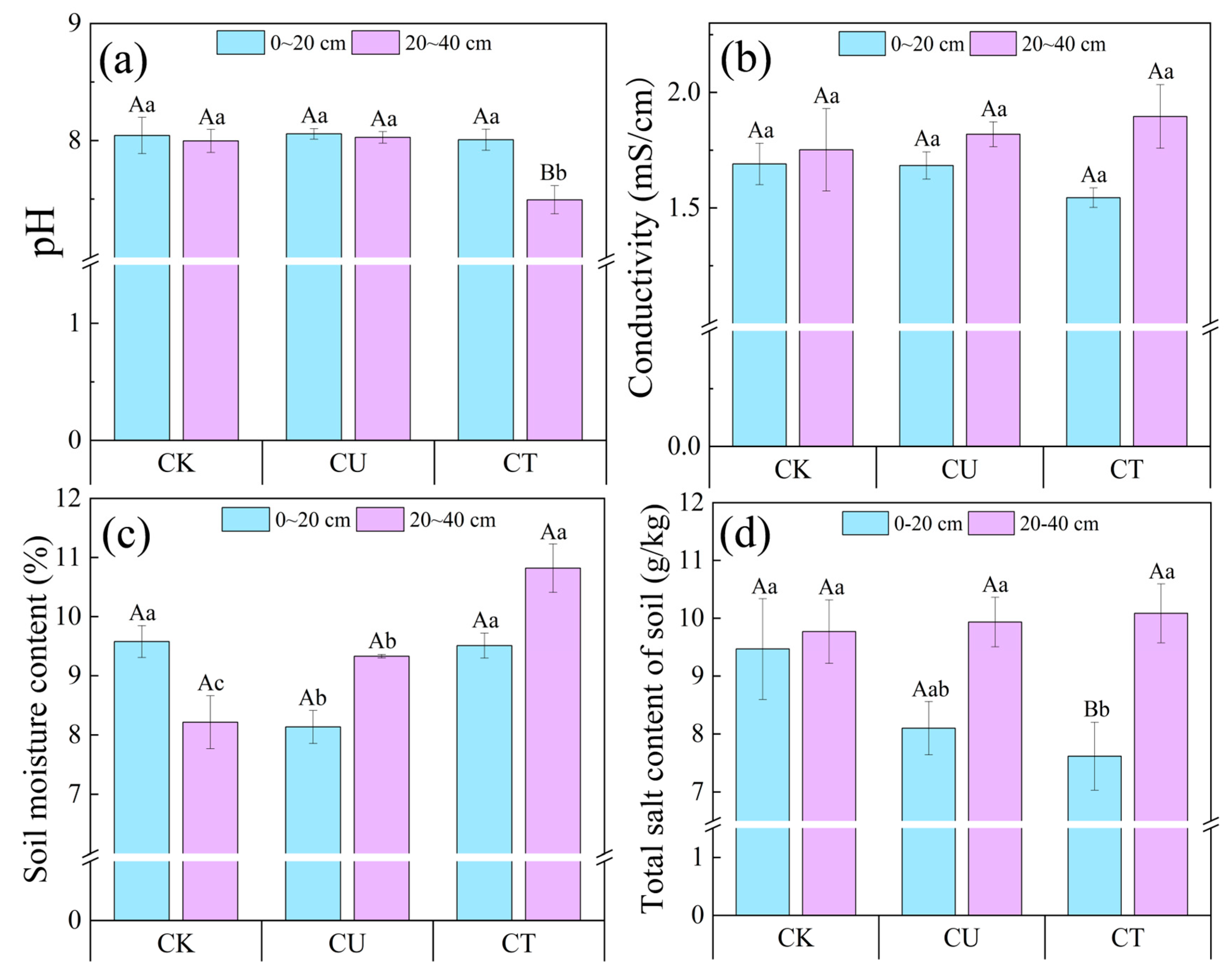
3.6.1. Effect of OAC-SR Addition on Soil Salinity in Potted Plants
3.6.2. Effect of OAC-SR Addition on Soil Moisture in Potted Plants
3.6.3. Effects of OAC-SR Addition on Soil Microorganisms in Potted Plants
3.7. The Field Verification of OAC-SR
4. Discussion
4.1. Analysis of Pore Expansion, Salt Absorption, and Water Retention Properties of OAC-SR Materials
4.2. The Effects of OAC-SR Addition on the Physicochemical Properties of Saline–Alkali Soil
4.3. OAC-SR Addition Alteration of Microbial Ecological Function: From Salt Suppression to Growth Promotion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassani, A.; Azapagic, A.; Shokri, N. Global Predictions of Primary Soil Salinization under Changing Climate in the 21st Century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Lei, S.; Jia, X.; Zhao, C.; Shao, M. A Review of Saline-Alkali Soil Improvements in China: Efforts and Their Impacts on Soil Properties. Agric. Water Manag. 2025, 317, 109617. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, G.; Feng, B.; Wang, C.; Luo, Y.; Li, F.; Shen, C.; Ma, D.; Zhang, C.; Zhang, J. Saline-Alkali Land Reclamation Boosts Topsoil Carbon Storage by Preferentially Accumulating Plant-Derived Carbon. Sci. Bull. 2024, 69, 2948–2958. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Tu, W.; Mu, L.; Sun, Z.; Hu, Q.; Yang, Y. Saline Alkali Water Desalination Project in Southern Xinjiang of China: A Review of Desalination Planning, Desalination Schemes and Economic Analysis. Renew. Sustain. Energy Rev. 2019, 113, 109268. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; De Sousa, L. Global Mapping of Soil Salinity Change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Morsy, S.; Elbasyoni, I.S.; Baenziger, S.; Abdallah, A.M. Gypsum Amendment Influences Performance and Mineral Absorption in Wheat Cultivars Grown in Normal and Saline-sodic Soils. J. Agron. Crop Sci. 2022, 208, 675–692. [Google Scholar] [CrossRef]
- Tian, Y.; Xia, R.; Ying, Y.; Lu, S. Desulfurization Steel Slag Improves the Saline-Sodic Soil Quality by Replacing Sodium Ions and Affecting Soil Pore Structure. J. Environ. Manag. 2023, 345, 118874. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Q.; Tian, Z.; Cui, Y.; Liang, Y.; Wang, H. Apply Biochar to Ameliorate Soda Saline-Alkali Land, Improve Soil Function and Increase Corn Nutrient Availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Xing, J.; Li, X.; Li, Z.; Wang, X.; Hou, N.; Li, D. Remediation of Soda-Saline-Alkali Soil through Soil Amendments: Microbially Mediated Carbon and Nitrogen Cycles and Remediation Mechanisms. Sci. Total Environ. 2024, 924, 171641. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.; Pan, Y.; Ma, X.; Ding, F.; Xu, W.; Fu, Y.; Bian, Q.; Kade, M. Effect of Subsurface Drainpipe Parameters on Soil Water and Salt Distribution in a Localized Arid Zone: A Field-Scale Study. Agronomy 2025, 15, 678. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Yuan, J.; Xu, W.; Liu, J. Optimization Study on the Freshwater Production Ratio from the Freezing and Thawing Process of Saline Water with Varied Qualities. Agronomy 2024, 15, 33. [Google Scholar] [CrossRef]
- Feng, L.; Wan, S.; Zhang, Y.; Dong, H. Xinjiang Cotton: Achieving Super-High Yield through Efficient Utilization of Light, Heat, Water, and Fertilizer by Three Generations of Cultivation Technology Systems. Field Crop. Res. 2024, 312, 109401. [Google Scholar] [CrossRef]
- Jing, X.; Li, Q.; Qiao, X.; Chen, J.; Cai, X. Effects of Accumulated Straw Residues on Sorption of Pesticides and Antibiotics in Soils with Maize Straw Return. J. Hazard. Mater. 2021, 418, 126213. [Google Scholar] [CrossRef]
- Maryum, Z.; Luqman, T.; Nadeem, S.; Khan, S.M.U.D.; Wang, B.; Ditta, A.; Khan, M.K.R. An Overview of Salinity Stress, Mechanism of Salinity Tolerance and Strategies for Its Management in Cotton. Front. Plant Sci. 2022, 13, 907937. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Tian, Y.; Wu, Y.; Dong, F.; Xu, J.; Zheng, Y. Isolation and Identification of Potential Allelochemicals from Aerial Parts of Avena fatua L. and Their Allelopathic Effect on Wheat. J. Agric. Food Chem. 2016, 64, 3492–3500. [Google Scholar] [CrossRef]
- Yang, H.; Ma, J.; Rong, Z.; Zeng, D.; Wang, Y.; Hu, S.; Ye, W.; Zheng, X. Wheat Straw Return Influences Nitrogen-Cycling and Pathogen Associated Soil Microbiota in a Wheat–Soybean Rotation System. Front. Microbiol. 2019, 10, 1811. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lei, L.; Gao, Y.; Zhan, E. Effects of Straw Decomposition on Soil Surface Evaporation Resistance and Evaporation Simulation. Plants 2025, 14, 434. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, L. Continuous Straw Returning Enhances the Carbon Sequestration Potential of Soil Aggregates by Altering the Quality and Stability of Organic Carbon. J. Environ. Manag. 2024, 358, 120903. [Google Scholar] [CrossRef]
- Han, W.; He, M. The Application of Exogenous Cellulase to Improve Soil Fertility and Plant Growth Due to Acceleration of Straw Decomposition. Bioresour. Technol. 2010, 101, 3724–3731. [Google Scholar] [CrossRef]
- Zhang, D.; Wan, L.-C.; Wang, M.-X.; Zhou, Z.-F. Bio-Stimulating Effect and Mechanism of Rice Straw Amendment for Enhancing the Elimination of Soil Benzo[a]pyrene. Ecotoxicol. Environ. Saf. 2025, 302, 118570. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.-K.; Wei, L.; Turner, N.C.; Ma, S.-C.; Yang, M.-D.; Wang, T.-C. Improved Straw Management Practices Promote in Situ Straw Decomposition and Nutrient Release, and Increase Crop Production. J. Clean. Prod. 2020, 250, 119514. [Google Scholar] [CrossRef]
- Butenschoen, O.; Scheu, S.; Eisenhauer, N. Interactive Effects of Warming, Soil Humidity and Plant Diversity on Litter Decomposition and Microbial Activity. Soil Biol. Biochem. 2011, 43, 1902–1907. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Janzen, H.; Ellert, B.H.; Helgason, B.L.; Qian, B.; Zebarth, B.J.; Angers, D.A.; Beyaert, R.P.; Drury, C.F.; Duguid, S.D.; et al. Litter Decay Controlled by Temperature, Not Soil Properties, Affecting Future Soil Carbon. Glob. Change Biol. 2017, 23, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Liu, G.; Zhou, B.; Chen, X.; Wang, Q.; Zhu, H.; Li, Z. Effects of Modified Biochar on Water and Salt Distribution and Water-Stable Macro-Aggregates in Saline-Alkaline Soil. J. Soils Sediments 2021, 21, 2192–2202. [Google Scholar] [CrossRef]
- Du, Z.; Fang, J.; Pang, H.; Cai, Y. Utilising Rice Straw to Prepare a Culture Medium for Synthesizing Lactic Acid Bacteria Biofilms and Regulating Silage Fermentation. Bioresour. Technol. 2025, 438, 133201. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Tiwari, A.; Ghosh, P.; Arora, K.; Sharma, S. Enhanced Lignin Degradation of Paddy Straw and Pine Needle Biomass by Combinatorial Approach of Chemical Treatment and Fungal Enzymes for Pulp Making. Bioresour. Technol. 2023, 368, 128314. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Seo, Y.H.; Terán-Hilares, R.; Rehman, M.S.U.R.; Han, J.-I. Persulfate Based Pretreatment to Enhance the Enzymatic Digestibility of Rice Straw. Bioresour. Technol. 2016, 222, 523–526. [Google Scholar] [CrossRef]
- Shao, Z.; Gnanasekar, P.; Tratnik, N.; Tanguy, N.R.; Guo, X.; Zhu, M.; Qiu, L.; Yan, N.; Chen, H. Low-Temperature Torrefaction Assisted with Solid-State KOH/Urea Pretreatment for Accelerated Methane Production in Wheat Straw Anaerobic Digestion. Bioresour. Technol. 2023, 377, 128940. [Google Scholar] [CrossRef]
- Jing, X.; Chai, X.; Long, S.; Liu, T.; Si, M.; Zheng, X.; Cai, X. Urea/Sodium Hydroxide Pretreatments Enhance Decomposition of Maize Straw in Soils and Sorption of Straw Residues toward Herbicides. J. Hazard. Mater. 2022, 431, 128467. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, E.; Li, T.; Zhang, L.; Wang, Z. High-Value Utilization of Cellulose: Intriguing and Important Effects of Hydrogen Bonding Interactions─A Mini-Review. Biomacromolecules 2024, 25, 6296–6318. [Google Scholar] [CrossRef]
- Kim, I.; Lee, B.; Park, J.-Y.; Choi, S.-A.; Han, J.-I. Effect of Nitric Acid on Pretreatment and Fermentation for Enhancing Ethanol Production of Rice Straw. Carbohydr. Polym. 2014, 99, 563–567. [Google Scholar] [CrossRef]
- Jing, X.; Liu, T.; Chai, X.; Wang, Y.; Zhang, X.; Cai, X. Persulfate Pretreatment Facilitates Decomposition of Maize Straw in Soils and Accumulation of Straw Residues with High Adsorption Capacity. Chem. Eng. J. 2023, 475, 145956. [Google Scholar] [CrossRef]
- Kumar, N.; Haldar, S.; Saikia, R. Root Exudation as a Strategy for Plants to Deal with Salt Stress: An Updated Review. Environ. Exp. Bot. 2023, 216, 105518. [Google Scholar] [CrossRef]
- Xiang, L.; Chen, X.-T.; Yu, P.-F.; Li, X.-H.; Zhao, H.-M.; Feng, N.-X.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; et al. Oxalic Acid in Root Exudates Enhances Accumulation of Perfluorooctanoic Acid in Lettuce. Environ. Sci. Technol. 2020, 54, 13046–13055. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kapoor, K.K.; Kundu, B.S.; Mehta, R.K. Identification of Organic Acids Produced during Rice Straw Decomposition and Their Role in Rock Phosphate Solubilization. Plant Soil Environ. 2008, 54, 72–77. [Google Scholar] [CrossRef]
- Schneider, L.; Haverinen, J.; Jaakkola, M.; Lassi, U. Solid Acid-Catalyzed Depolymerization of Barley Straw Driven by Ball Milling. Bioresour. Technol. 2016, 206, 204–210. [Google Scholar] [CrossRef]
- Barisik, G.; Isci, A.; Kutlu, N.; Bagder Elmaci, S.; Akay, B. Optimization of Organic Acid Pretreatment of Wheat Straw. Biotechnol. Prog. 2016, 32, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Imman, S.; Arnthong, J.; Burapatana, V.; Champreda, V.; Laosiripojana, N. Effects of Acid and Alkali Promoters on Compressed Liquid Hot Water Pretreatment of Rice Straw. Bioresour. Technol. 2014, 171, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, R.; Luo, Z.; Zhang, C.; Kong, J.; Feng, S. Straw Returning Measures Enhance Soil Moisture and Nutrients and Promote Cotton Growth. Agronomy 2023, 13, 1850. [Google Scholar] [CrossRef]
- Ma, L.; Kong, F.; Wang, Z.; Luo, Y.; Lv, X.; Zhou, Z.; Meng, Y. Growth and Yield of Cotton as Affected by Different Straw Returning Modes with an Equivalent Carbon Input. Field Crop. Res. 2019, 243, 107616. [Google Scholar] [CrossRef]
- Zeng, C.; Ma, Y.; Li, P.; Chen, X.; Liu, H.; Deng, Z.; Mu, R.; Qi, X.; Zhang, Z. Efficient Degradation of Sulfadiazine by UV-Triggered Electron Transfer on Oxalic Acid-Functionalized Corn Straw Biochar for Activating Peroxyacetic Acid: Performance, Mechanism, and Theoretical Calculation. Bioresour. Technol. 2024, 407, 131103. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, Kinetics, and Equilibrium of Methylene Blue Adsorption on Biochar Derived from Eucalyptus Saw Dust Modified with Citric, Tartaric, and Acetic Acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef]
- Ji, C.; Xu, M.; Yu, H.; Lv, L.; Zhang, W. Mechanistic Insight into Selective Adsorption and Easy Regeneration of Carboxyl-Functionalized MOFs towards Heavy Metals. J. Hazard. Mater. 2022, 424, 127684. [Google Scholar] [CrossRef]
- Azam, M.A.; Safie, N.E.; Abdul Aziz, M.F. Characterization of Hierarchical Porous Materials. In Advanced Functional Porous Materials; Uthaman, A., Thomas, S., Li, T., Maria, H., Eds.; Engineering Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 407–429. ISBN 978-3-030-85396-9. [Google Scholar]
- Cao, S.; Du, R.; Yan, W.; Zhou, Y. Mitigation of Inhibitory Effect of THP-AD Centrate on Partial Nitritation and Anammox: Insights into Ozone Pretreatment. J. Hazard. Mater. 2022, 431, 128599. [Google Scholar] [CrossRef]
- Zhang, C.; García Meza, J.V.; Zhou, K.; Liu, J.; Song, S.; Zhang, M.; Meng, D.; Chen, J.; Xia, L.; Xiheng, H. Superabsorbent Polymer Used for Saline-Alkali Soil Water Retention. J. Taiwan Inst. Chem. Eng. 2023, 145, 104830. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, Y.; Zhou, F. Tuning Surface Functions by Hydrophilic/Hydrophobic Polymer Brushes. ACS Nano 2025, 19, 11576–11603. [Google Scholar] [CrossRef] [PubMed]
- Rigby, S.P. Do Pores Exist?—Foundational Issues in Pore Structural Characterisation. Foundations 2024, 4, 225–248. [Google Scholar] [CrossRef]
- Lu, H.; Chen, Y.; Dun, C.; Hu, X.; Wang, R.; Cui, P.; Zhang, H.; Zhang, H. Preparation of a Novel Economically Efficient and Environment Friendly Controlled Release Urea from Liquefied Corn Straw and Castor Oil. BMC Chem. 2025, 19, 154. [Google Scholar] [CrossRef]
- Doondani, P.; Gomase, V.; Saravanan, D.; Jugade, R.M. Chitosan Coated Cotton-Straw-Biochar as an Admirable Adsorbent for Reactive Red Dye. Results Eng. 2022, 15, 100515. [Google Scholar] [CrossRef]
- Huang, C.; Mohamed, B.A.; Li, L.Y. Comparative Life-Cycle Assessment of Pyrolysis Processes for Producing Bio-Oil, Biochar, and Activated Carbon from Sewage Sludge. Resour. Conserv. Recycl. 2022, 181, 106273. [Google Scholar] [CrossRef]
- Guan, R.; Li, Y.; Jia, Y.; Jiang, F.; Li, L. Acidified Biochar One-off Application for Saline-Alkali Soil Improvement: A Three-Year Field Trial Evaluating the Persistence of Effects. Ind. Crop. Prod. 2024, 222, 119972. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, H.; Wang, Y.; Biswas, A.; Wai Chau, H.; Liang, J.; Zhang, F.; Bai, Y.; Wu, S.; et al. Targeted Biochar Application Alters Physical, Chemical, Hydrological and Thermal Properties of Salt-Affected Soils under Cotton-Sugarbeet Intercropping. CATENA 2022, 216, 106414. [Google Scholar] [CrossRef]
- Mao, T.; Wang, Y.; Ning, S.; Mao, J.; Sheng, J.; Jiang, P. Assessment of the Effects of Biochar on the Physicochemical Properties of Saline–Alkali Soil Based on Meta-Analysis. Agronomy 2024, 14, 2431. [Google Scholar] [CrossRef]
- Li, S.; Yao, Y.; Yang, M.; Zhang, Y.; Zhang, S.; Shen, T.; Ding, F.; Li, Z.; Liu, W.; Cui, J.; et al. Effects of Different Amendments on Aggregate Stability and Microbial Communities of Coastal Saline–Alkali Soil in the Yellow River Delta. Land Degrad. Dev. 2023, 34, 1694–1707. [Google Scholar] [CrossRef]
- Che, W.; Piao, J.; Gao, Q.; Li, X.; Li, X.; Jin, F. Response of Soil Physicochemical Properties, Soil Nutrients, Enzyme Activity and Rice Yield to Rice Straw Returning in Highly Saline-Alkali Paddy Soils. J. Soil Sci. Plant Nutr. 2023, 23, 4396–4411. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, X.; Gao, J.; Qu, J.; Borjigin, Q.; Meng, T.; Li, D. Improvement of Saline–Alkali Soil and Straw Degradation Efficiency in Cold and Arid Areas Using Klebsiella sp. and Pseudomonas sp. Agronomy 2024, 14, 2499. [Google Scholar] [CrossRef]
- Sekhohola-Dlamini, L.; Tekere, M. Microbiology of Municipal Solid Waste Landfills: A Review of Microbial Dynamics and Ecological Influences in Waste Bioprocessing. Biodegradation 2020, 31, 1–21. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, W.; Yang, D.; Zhang, K.; Zhao, L.; Xie, Z.; Xu, T.; Zhao, Y.; Wang, X.; Pan, X.; et al. Mitigation of Soil Salinization and Alkalization by Bacterium-Induced Inhibition of Evaporation and Salt Crystallization. Sci. Total Environ. 2021, 755, 142511. [Google Scholar] [CrossRef]
- Remmas, N.; Manfe, N.; Zerva, I.; Melidis, P.; Raga, R.; Ntougias, S. A Critical Review on the Microbial Ecology of Landfill Leachate Treatment Systems. Sustainability 2023, 15, 949. [Google Scholar] [CrossRef]
- Meyer-Dombard, D.R.; Bogner, J.E.; Malas, J. A Review of Landfill Microbiology and Ecology: A Call for Modernization With ‘Next Generation’ Technology. Front. Microbiol. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Stamps, B.W.; Lyles, C.N.; Suflita, J.M.; Masoner, J.R.; Cozzarelli, I.M.; Kolpin, D.W.; Stevenson, B.S. Municipal Solid Waste Landfills Harbor Distinct Microbiomes. Front. Microbiol. 2016, 7, 534. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yan, H.; Chen, G.; Yang, S.; Wang, J.; Yang, Y.; Wang, H. Use of Lentinan and Fluopimomide to Control Cotton Seedling Damping-Off Disease Caused by Rhizoctonia Solani. Agriculture 2022, 12, 75. [Google Scholar] [CrossRef]
- Merchant, M.; Kahali, T.; Kumawat, D.K.; Mande, S.S.; Sar, P. Understanding the Structure and Function of Landfill Microbiome Through Genomics. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2024; pp. 669–695. ISBN 978-0-443-13320-6. [Google Scholar]
- Li, S.; Li, L.; Wang, Z.; Sun, J.; Zhang, H. Impacts of Corn Straw Compost on Rice Growth and Soil Microflora under Saline–Alkali Stress. Agronomy 2023, 13, 1525. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, J.; Yu, X.; Ma, D.; Hu, S.; Shen, T. Effect of Deep Straw Return under Saline Conditions on Soil Nutrient and Maize Growth in Saline–Alkali Land. Agronomy 2023, 13, 707. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Liu, Y.; Niu, Z.; Li, Y. Identifying Change in Spatial Accumulation of Soil Salinity in an Inland River Watershed, China. Sci. Total Environ. 2018, 621, 177–185. [Google Scholar] [CrossRef]


| Name | pH | Conductivity (mS/cm) | Na+ Content (mg/g) | |||
|---|---|---|---|---|---|---|
| Upper | Lower | Upper | Lower | Upper | Lower | |
| CK | 7.70 ± 0.09 a | 7.69 ± 0.07 a | 2.37 ± 0.03 a | 2.76 ± 0.06 b | 0.14 ± 0.01 a | 0.44 ± 0.01 a |
| CU | 7.69 ± 0.15 a | 7.67 ± 0.09 a | 2.37 ± 0.03 a | 2.88 ± 0.02 a | 0.13 ± 0.02 a | 0.48 ± 0.03 a |
| CT | 7.68 ± 0.09 a | 7.72 ± 0.04 a | 2.31 ± 0.04 a | 2.59 ± 0.03 c | 0.14 ± 0.01 a | 0.38 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Sun, M.; Zhao, Z.; Wen, L.; Du, Y.; Sun, X.; Jing, X.; Zhang, F. Oxalic Acid Pretreatment of Cotton Straw Enhances Its Salt Adsorption and Water Retention Capacity—A Soil-Amending Strategy for Saline Soil. Agronomy 2025, 15, 2657. https://doi.org/10.3390/agronomy15112657
Guo C, Sun M, Zhao Z, Wen L, Du Y, Sun X, Jing X, Zhang F. Oxalic Acid Pretreatment of Cotton Straw Enhances Its Salt Adsorption and Water Retention Capacity—A Soil-Amending Strategy for Saline Soil. Agronomy. 2025; 15(11):2657. https://doi.org/10.3390/agronomy15112657
Chicago/Turabian StyleGuo, Changshuai, Mengyao Sun, Zhihui Zhao, Le Wen, Yingzi Du, Xianxian Sun, Xudong Jing, and Fenghua Zhang. 2025. "Oxalic Acid Pretreatment of Cotton Straw Enhances Its Salt Adsorption and Water Retention Capacity—A Soil-Amending Strategy for Saline Soil" Agronomy 15, no. 11: 2657. https://doi.org/10.3390/agronomy15112657
APA StyleGuo, C., Sun, M., Zhao, Z., Wen, L., Du, Y., Sun, X., Jing, X., & Zhang, F. (2025). Oxalic Acid Pretreatment of Cotton Straw Enhances Its Salt Adsorption and Water Retention Capacity—A Soil-Amending Strategy for Saline Soil. Agronomy, 15(11), 2657. https://doi.org/10.3390/agronomy15112657





