LAZY2 and LAZY3 Regulate Rice Root Gravitropism by Affecting Starch Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Analysis of Root Gravitropism
2.3. Starch Granules Staining Assay
2.4. Root System Architecture Analysis
2.5. RNA Extraction and qRT-PCR
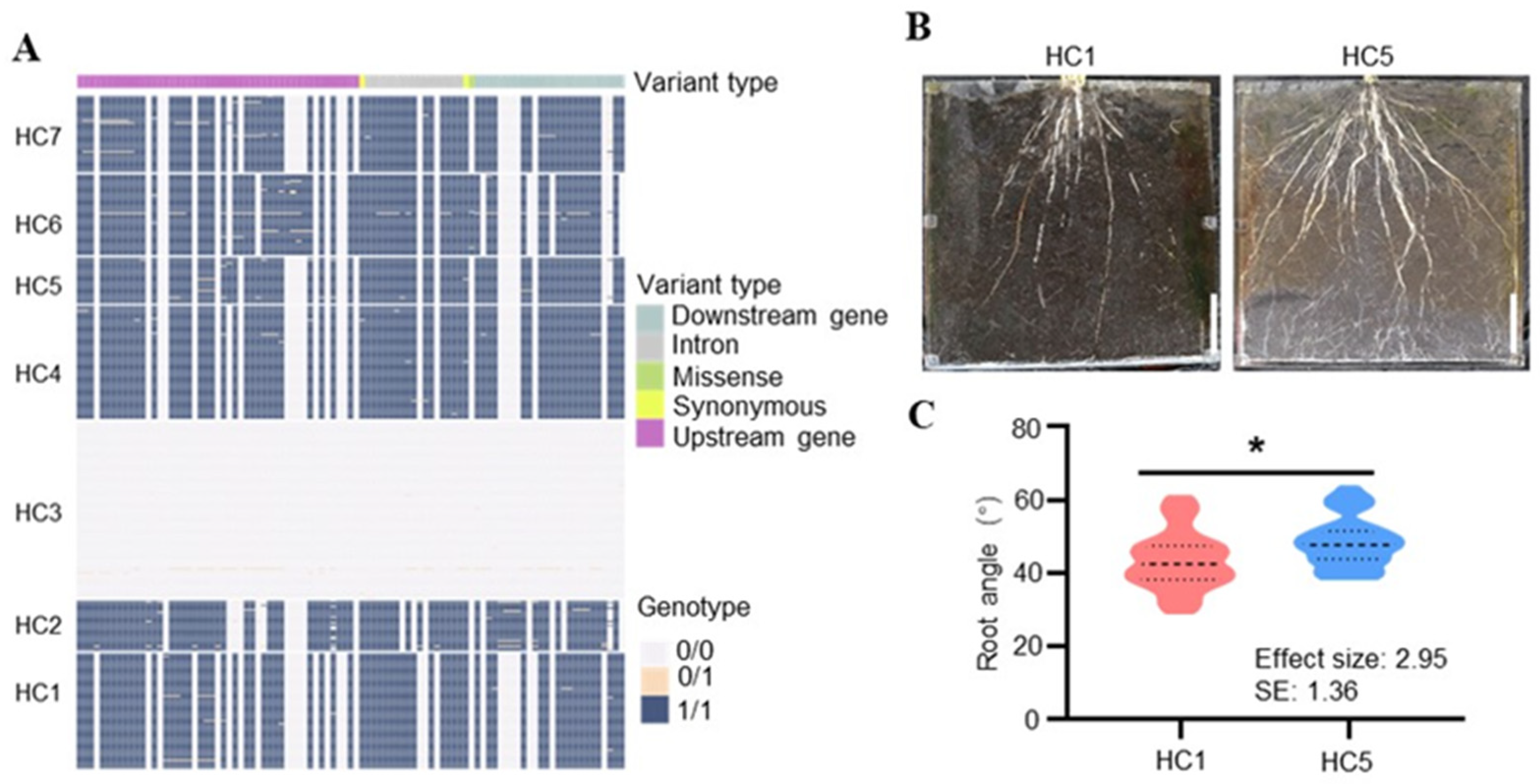
2.6. Haplotype Analysis of LA2 and LA3
2.7. Luciferase Complementation Imaging (LCI) Assay
2.8. Bimolecular Fluorescence Complementation (BiFC) Assay
2.9. Pull-Down Assay
2.10. Co-Immunoprecipitation Assay
3. Results
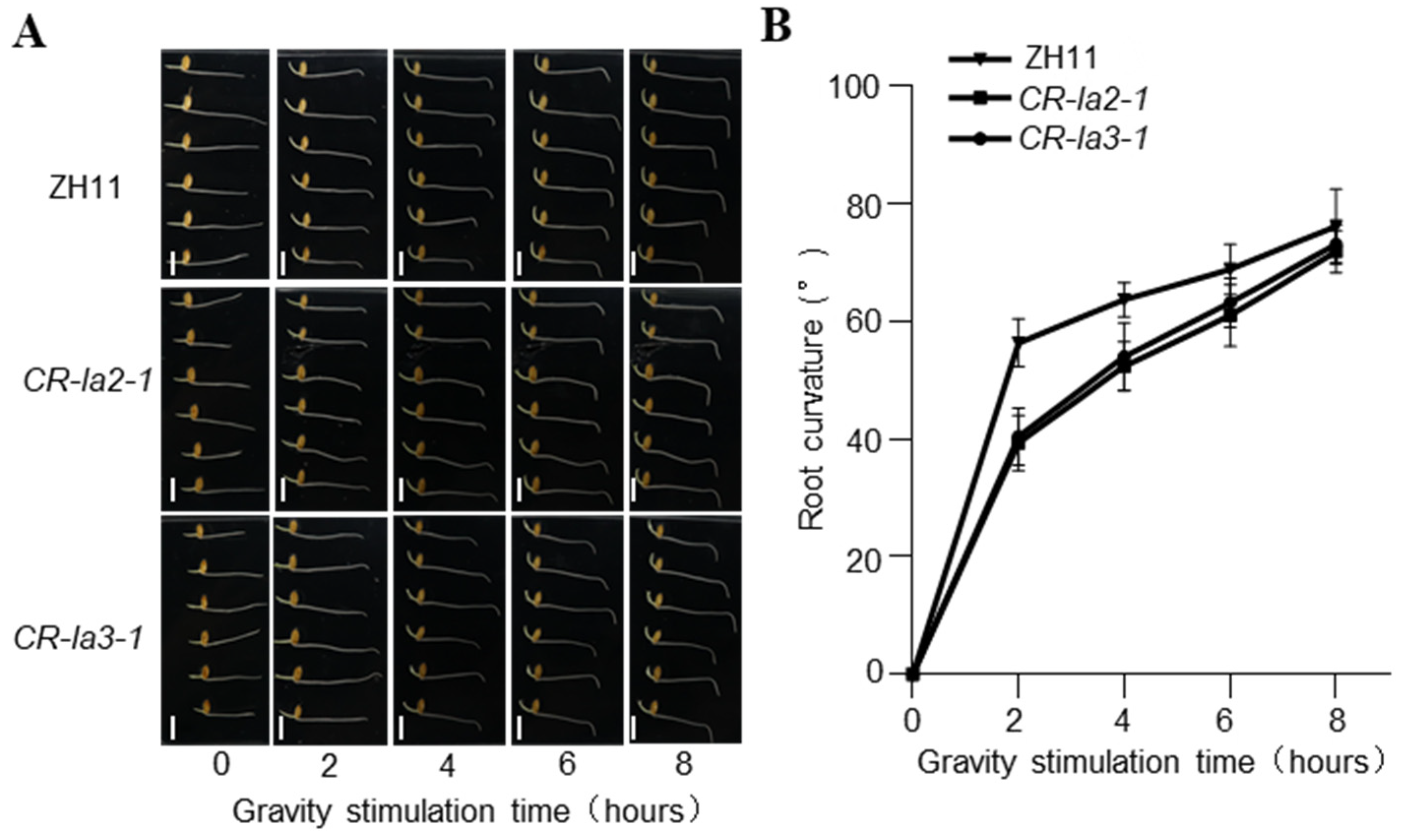
3.1. LA2 and LA3 Regulate Root Gravitropism in Rice
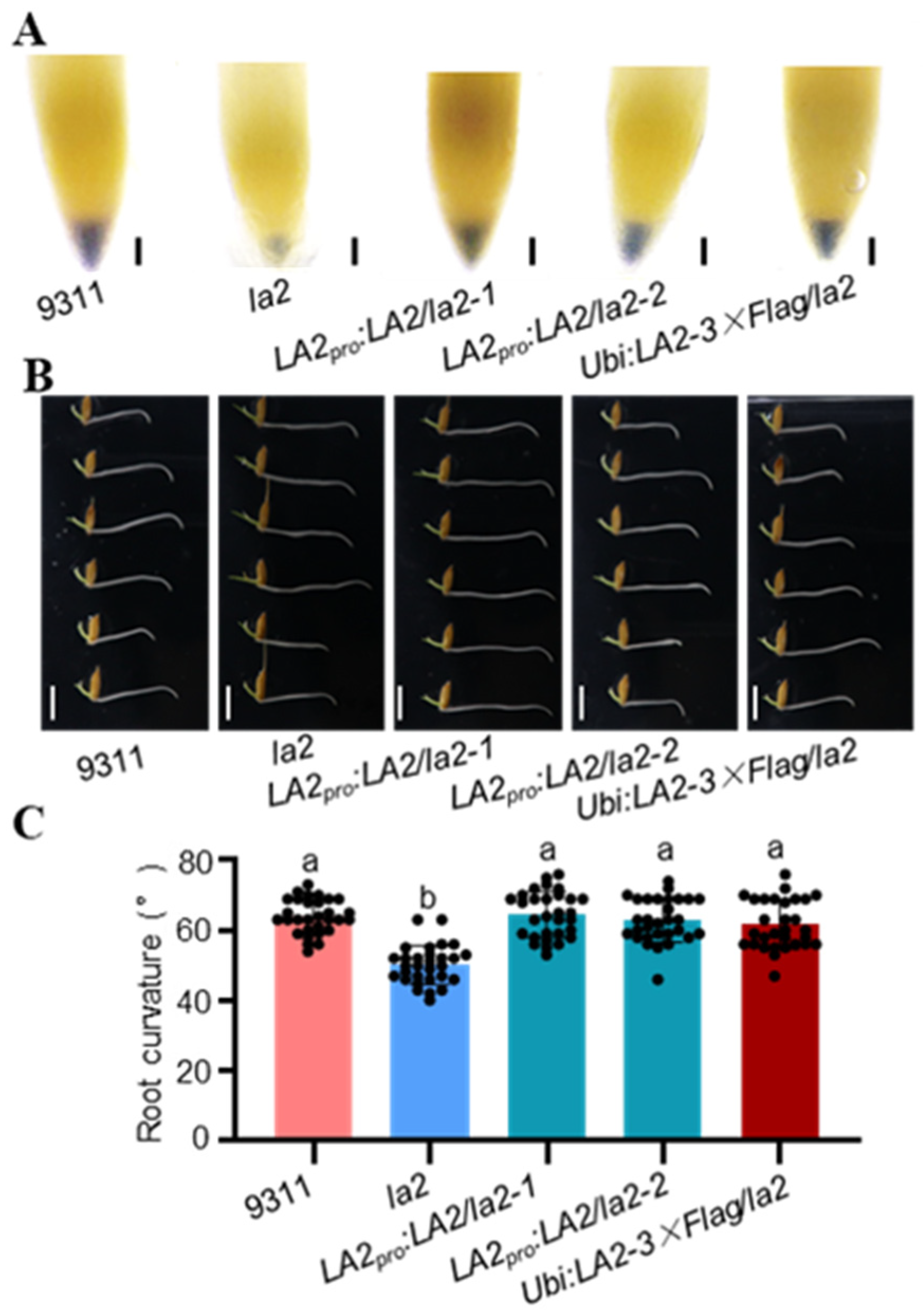
3.2. LA2 and LA3 Regulate Starch Biosynthesis in Rice Root Tips
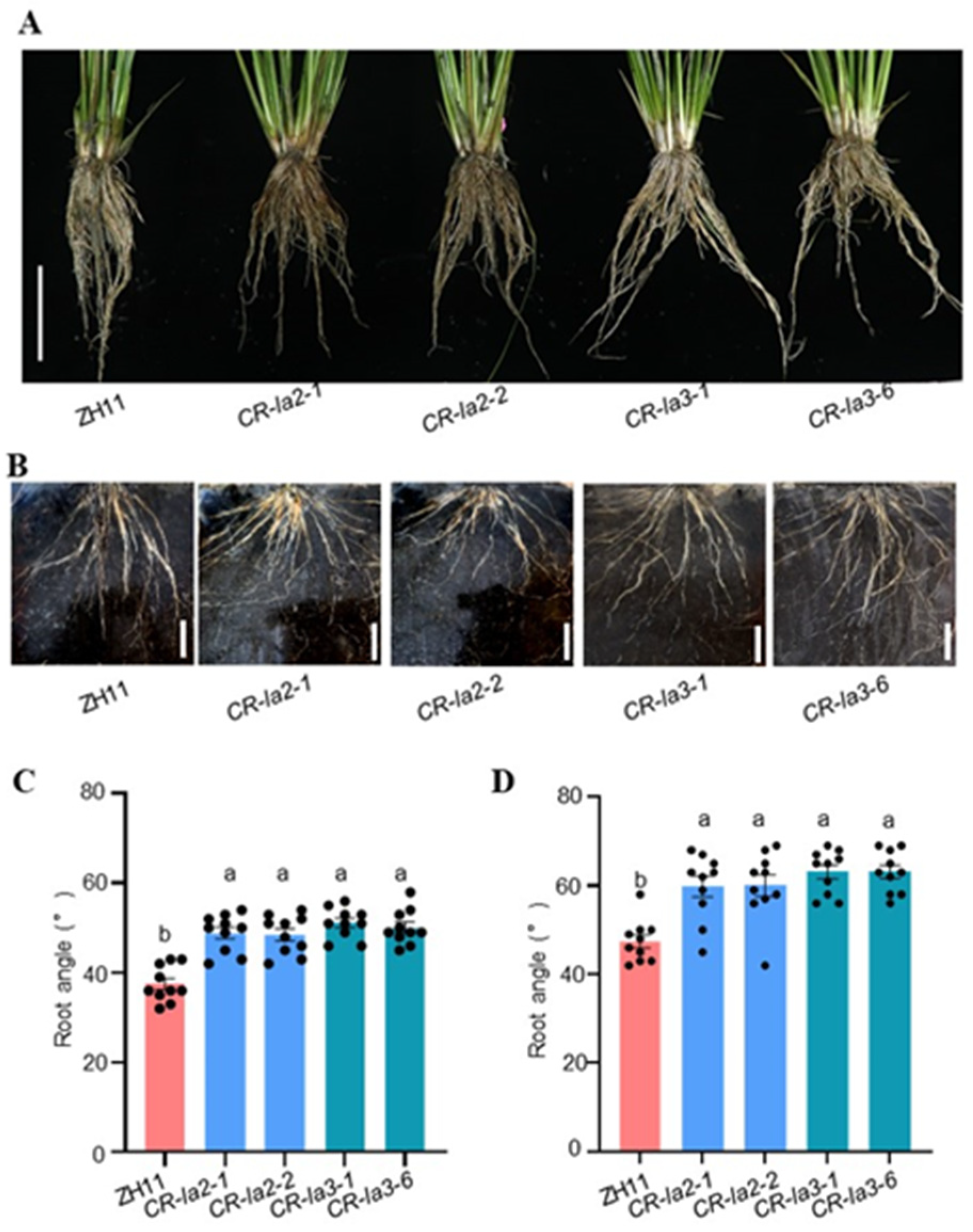
3.3. LA2 and LA3 Regulate Root Architecture in Rice
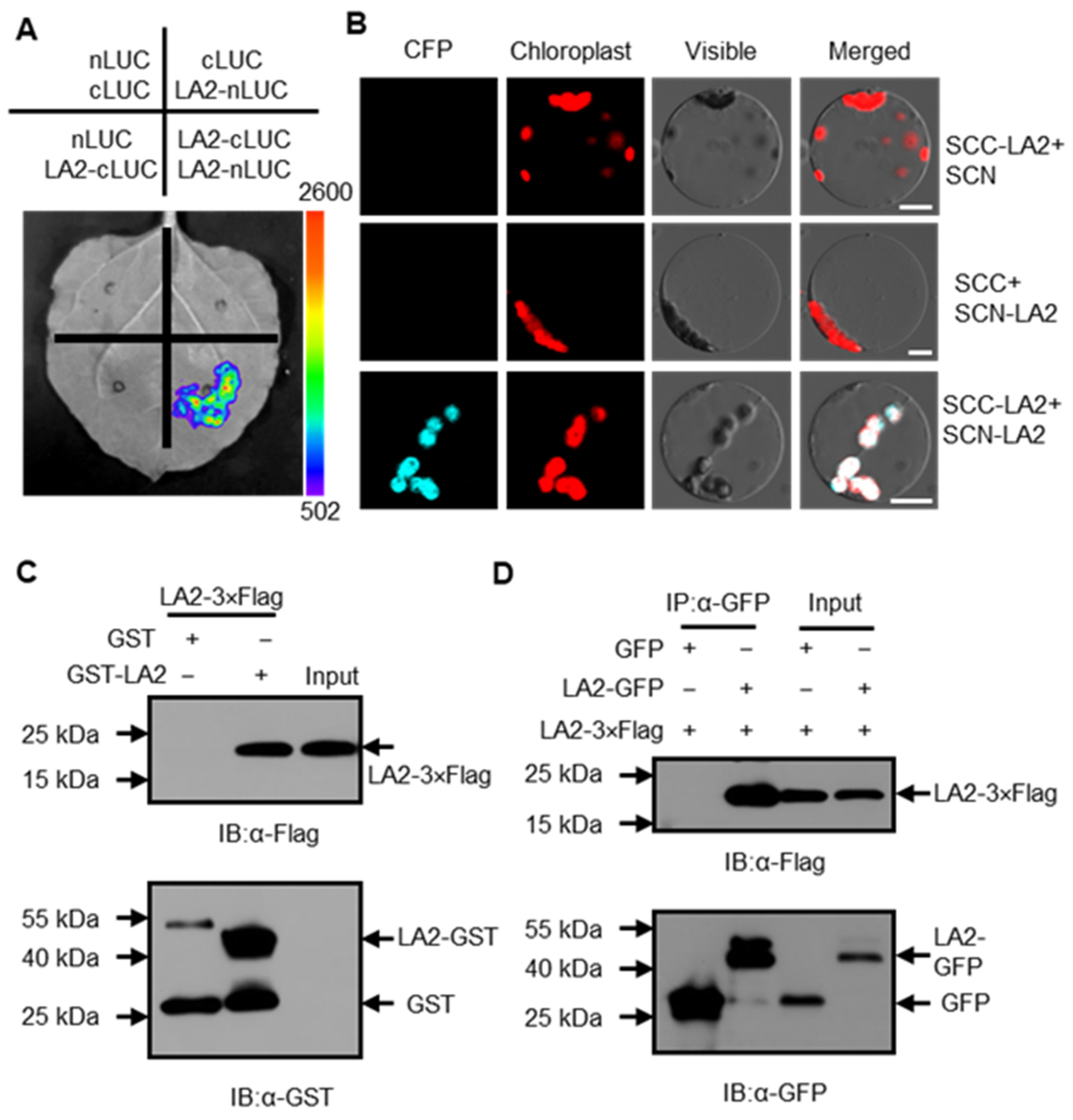
3.4. LA2 Can Form Homodimers In Vivo and In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fusi, R.; Rosignoli, S.; Lou, H.; Sangiorgi, G.; Bovina, R.; Pattem, J.K.; Borkar, A.N.; Lombardi, M.; Forestan, C.; Milner, S.G.; et al. Root angle is controlled by EGT1 in cereal crops employing an antigravitropic mechanism. Proc. Natl. Acad. Sci. USA 2022, 119, e2201350119. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by Deeper Rooting 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Schneider, H.M.; Lor, V.S.N.; Hanlon, M.T.; Perkins, A.; Kaeppler, S.M.; Borkar, A.N.; Bhosale, R.; Zhang, X.; Rodriguez, J.; Bucksch, A.; et al. Root angle in maize influences nitrogen capture and is regulated by calcineurin B-like protein (CBL)-interacting serine/threonine-protein kinase 15 (ZmCIPK15). Plant Cell Environ. 2022, 45, 837–853. [Google Scholar] [CrossRef]
- Huang, G.; Liang, W.; Sturrock, C.J.; Pandey, B.K.; Giri, J.; Mairhofer, S.; Wang, D.; Muller, L.; Tan, H.; York, L.M.; et al. Rice actin binding protein RMD controls crown root angle in response to external phosphate. Nat. Commun. 2018, 9, 2346. [Google Scholar] [CrossRef] [PubMed]
- Kitomi, Y.; Hanzawa, E.; Kuya, N.; Inoue, H.; Hara, N.; Kawai, S.; Kanno, N.; Endo, M.; Sugimoto, K.; Yamazaki, T.; et al. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc. Natl. Acad. Sci. USA 2020, 117, 21242–21250. [Google Scholar] [CrossRef] [PubMed]
- Strohm, A.K.; Baldwin, K.L.; Masson, P.H. Molecular mechanisms of root gravity sensing and signal transduction. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 276–285. [Google Scholar] [CrossRef]
- Jiao, Z.; Du, H.; Chen, S.; Huang, W.; Ge, L. LAZY gene family in plant gravitropism. Front. Plant Sci. 2020, 11, 606241. [Google Scholar] [CrossRef]
- Nishimura, T.; Mori, S.; Shikata, H.; Nakamura, M.; Hashiguchi, Y.; Abe, Y.; Hagihara, T.; Yoshikawa, H.Y.; Toyota, M.; Higaki, T.; et al. Cell polarity linked to gravity sensing is generated by LZY translocation from statoliths to the plasma membrane. Science 2023, 381, 1006–1010. [Google Scholar] [CrossRef]
- Chen, J.; Yu, R.; Li, N.; Deng, Z.; Zhang, X.; Zhao, Y.; Qu, C.; Yuan, Y.; Pan, Z.; Zhou, Y.; et al. Amyloplast sedimentation repolarizes LAZYs to achieve gravity sensing in plants. Cell 2023, 186, 4788–4802.e15. [Google Scholar] [CrossRef]
- Taniguchi, M.; Furutani, M.; Nishimura, T.; Nakamura, M.; Fushita, T.; Iijima, K.; Baba, K.; Tanaka, H.; Toyota, M.; Tasaka, M.; et al. The Arabidopsis LAZY1 family plays a key role in gravity signaling within statocytes and in branch angle control of roots and shoots. Plant Cell 2017, 29, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Su, S.H.; Gibbs, N.M.; Jancewicz, A.L.; Masson, P.H. Molecular mechanisms of root gravitropism. Curr. Biol. 2017, 27, 964–972. [Google Scholar] [CrossRef]
- Ottenschlager, I.; Wolff, P.; Wolverton, C.; Bhalerao, R.P.; Sandberg, G.; Ishikawa, H.; Evans, M.; Palme, K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2987–2991. [Google Scholar] [CrossRef]
- Band, L.R.; Wells, D.M.; Larrieu, A.; Sun, J.; Middleton, A.M.; French, A.P.; Brunoud, G.; Sato, E.M.; Wilson, M.H.; Peret, B.; et al. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc. Natl. Acad. Sci. USA 2012, 109, 4668–4673. [Google Scholar] [CrossRef]
- Ge, L.; Chen, R. Negative gravitropism in plant roots. Nat. Plants 2016, 2, 16155. [Google Scholar] [CrossRef]
- Yoshihara, T.; Spalding, E.P. LAZY genes mediate the effects of gravity on auxin gradients and plant architecture. Plant Physiol. 2017, 175, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Sack, F.D. Plant gravity sensing. Int. Rev. Cytol. 1991, 127, 193–252. [Google Scholar] [PubMed]
- Kiss, J.Z.; Guisinger, M.M.; Miller, A.J.; Stackhouse, K.S. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 1997, 38, 518–525. [Google Scholar] [CrossRef]
- Kiss, J.; Wright, J.; Caspar, T. Gravitropismin roots of intermediate-starch mutants of Arabidopsis. Physiol. Plant 1996, 97, 237–244. [Google Scholar] [CrossRef] [PubMed]
- MacCleery, S.A.; Kiss, J.Z. Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol. 1999, 120, 183–192. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Hertel, R.; Sack, F.D. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 1989, 177, 198–206. [Google Scholar] [CrossRef]
- Wang, L.; Guo, M.; Li, Y.; Ruan, W.; Mo, X.; Wu, Z.; Sturrock, C.J.; Yu, H.; Lu, C.; Peng, J.; et al. LARGE ROOT ANGLE1, encoding OsPIN2, is involved in root system architecture in rice. J. Exp. Bot. 2018, 69, 385–397. [Google Scholar] [CrossRef]
- Xu, K.; Lou, Q.; Wang, D.; Li, T.; Chen, S.; Li, T.; Luo, L.; Chen, L. Overexpression of a novel small auxin-up RNA gene, OsSAUR11, enhances rice deep rootedness. BMC Plant Biol. 2023, 23, 319. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Wu, L.; Shao, Y.; Wu, Y.; Mao, C. Functional divergence of PIN1 paralogous genes in rice. Plant Cell Physiol. 2019, 60, 2720–2732. [Google Scholar] [CrossRef]
- Giri, J.; Bhosale, R.; Huang, G.; Pandey, B.K.; Parker, H.; Zappala, S.; Yang, J.; Dievart, A.; Bureau, C.; Ljung, K.; et al. Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate. Nat. Commun. 2018, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, B.; Zhou, Y.; He, S.; Tang, S.; Lu, X.; Xie, Q.; Chen, S.; Zhang, J. E3 ubiquitin ligase SOR1 regulates ethylene response in rice root by modulating stability of AUX/IAA protein. Proc. Natl. Acad. Sci. USA 2018, 115, 4513–4518. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, G.K.; Rosignoli, S.; Guo, L.; Vardanega, I.; Imani, J.; Altmuller, J.; Milner, S.G.; Balzano, R.; Nagel, K.A.; Pflugfelder, D.; et al. Enhanced gravitropism 2 encodes a sterile alpha motif-containing protein that controls root growth angle in barley and wheat. Proc. Natl. Acad. Sci. USA 2021, 118, e2101526118. [Google Scholar] [CrossRef]
- Huang, L.; Wang, W.; Zhang, N.; Cai, Y.; Liang, Y.; Meng, X.; Yuan, Y.; Li, J.; Wu, D.; Wang, Y. LAZY2 controls rice tiller angle through regulating starch biosynthesis in gravity-sensing cells. New Phytol. 2021, 231, 1073–1087. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, L.; Song, Y.; Yuan, Y.; Xu, S.; Wang, X.; Liang, Y.; Zhou, J.; Liu, G.; Li, J.; et al. LAZY3 interacts with LAZY2 to regulate tiller angle by modulating shoot gravity perception in rice. Plant Biotechnol. J. 2023, 21, 1217–1228. [Google Scholar] [CrossRef]
- Pal, P.; Modi, M.; Ravichandran, S.; Yennamalli, R.M.; Priyadarshini, R. DNA-binding properties of YBAB, a putative nucleoid-associated protein from Caulobacter crescentus. Front. Microbiol. 2021, 12, 733344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, G.; Wang, X.; Zhang, X.; Friml, J. Evolution of fast root gravitropism in seed plants. Nat. Commun. 2019, 10, 3480. [Google Scholar] [CrossRef]
- Campolino, M.L.; dos Santos, T.T.; Lana, U.G.D.; Gomes, E.A.; Guilhen, J.H.S.; Pastina, M.M.; Coelho, A.M.; de Sousa, S.M. Crop type determines the relation between root system architecture and microbial diversity indices in different phosphate fertilization conditions. Field Crops Res. 2023, 295, 108893. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, D. The plasticity of root systems in response to external phosphate. Int. J. Mol. Sci. 2020, 21, 5955. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Huang, L.; Chen, J.; Wang, W. LAZY2 and LAZY3 Regulate Rice Root Gravitropism by Affecting Starch Accumulation. Agronomy 2025, 15, 2639. https://doi.org/10.3390/agronomy15112639
Song H, Huang L, Chen J, Wang W. LAZY2 and LAZY3 Regulate Rice Root Gravitropism by Affecting Starch Accumulation. Agronomy. 2025; 15(11):2639. https://doi.org/10.3390/agronomy15112639
Chicago/Turabian StyleSong, Haoran, Linzhou Huang, Jiaze Chen, and Wenguang Wang. 2025. "LAZY2 and LAZY3 Regulate Rice Root Gravitropism by Affecting Starch Accumulation" Agronomy 15, no. 11: 2639. https://doi.org/10.3390/agronomy15112639
APA StyleSong, H., Huang, L., Chen, J., & Wang, W. (2025). LAZY2 and LAZY3 Regulate Rice Root Gravitropism by Affecting Starch Accumulation. Agronomy, 15(11), 2639. https://doi.org/10.3390/agronomy15112639




