Responses of Photosynthetic Activity in Flag Leaves and Spikes as well as Seed Development of Wheat (Triticum aestivum L.) to Artificial Shading
Abstract
1. Introduction
2. Materials and Methods
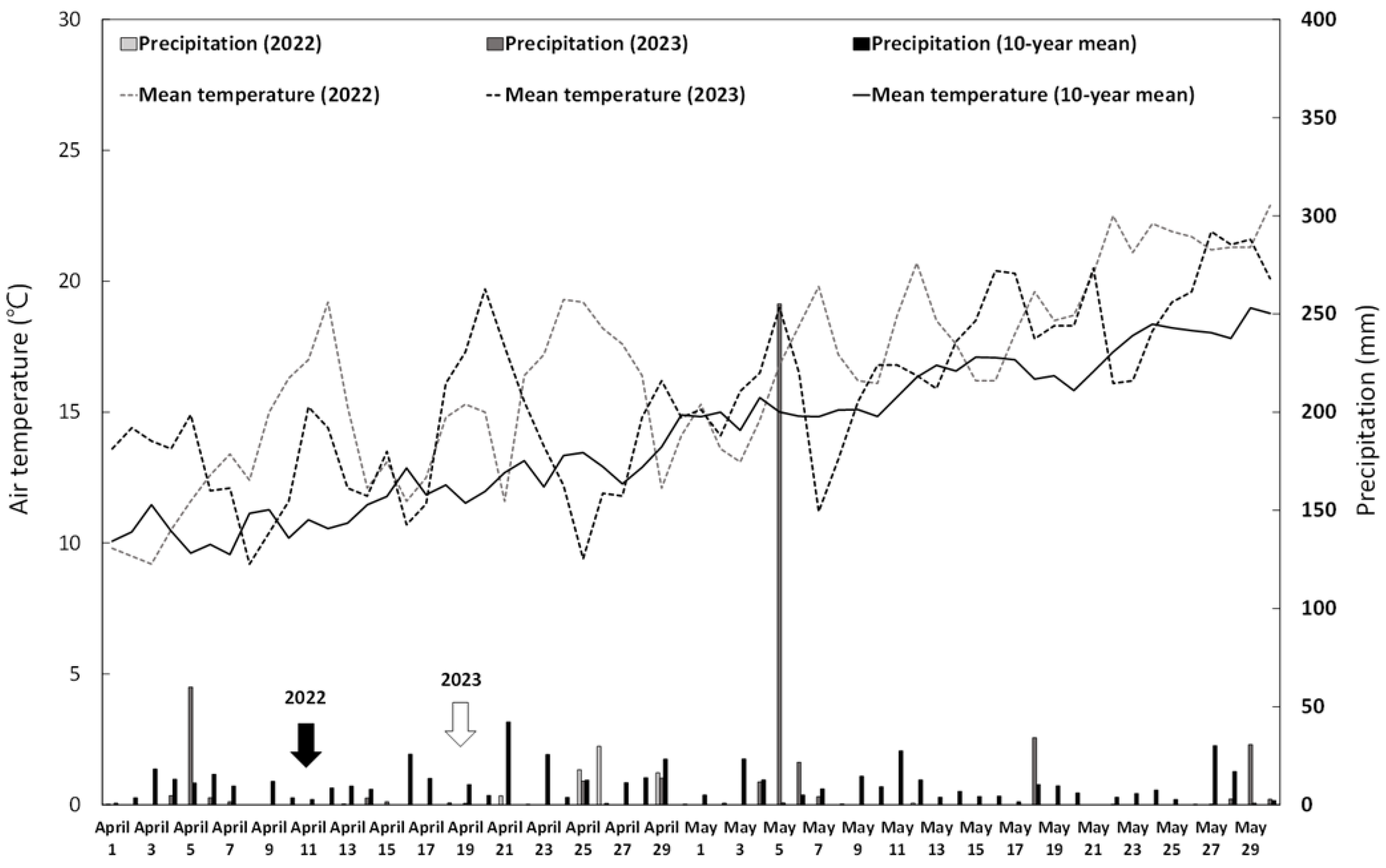
2.1. Experimental Materials and Growth Conditions
2.2. Experimental Design
2.3. Physiological Measurements
2.4. Measurements of Spike and Grain Growth
2.5. Data Analysis
3. Results
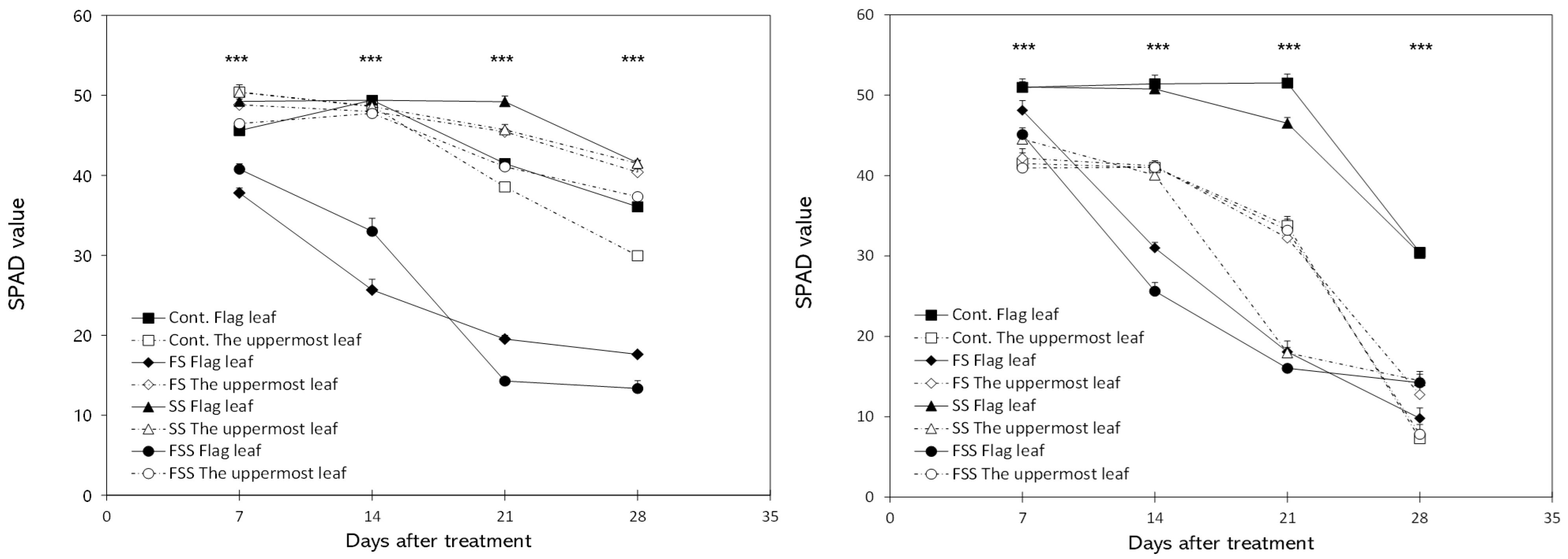
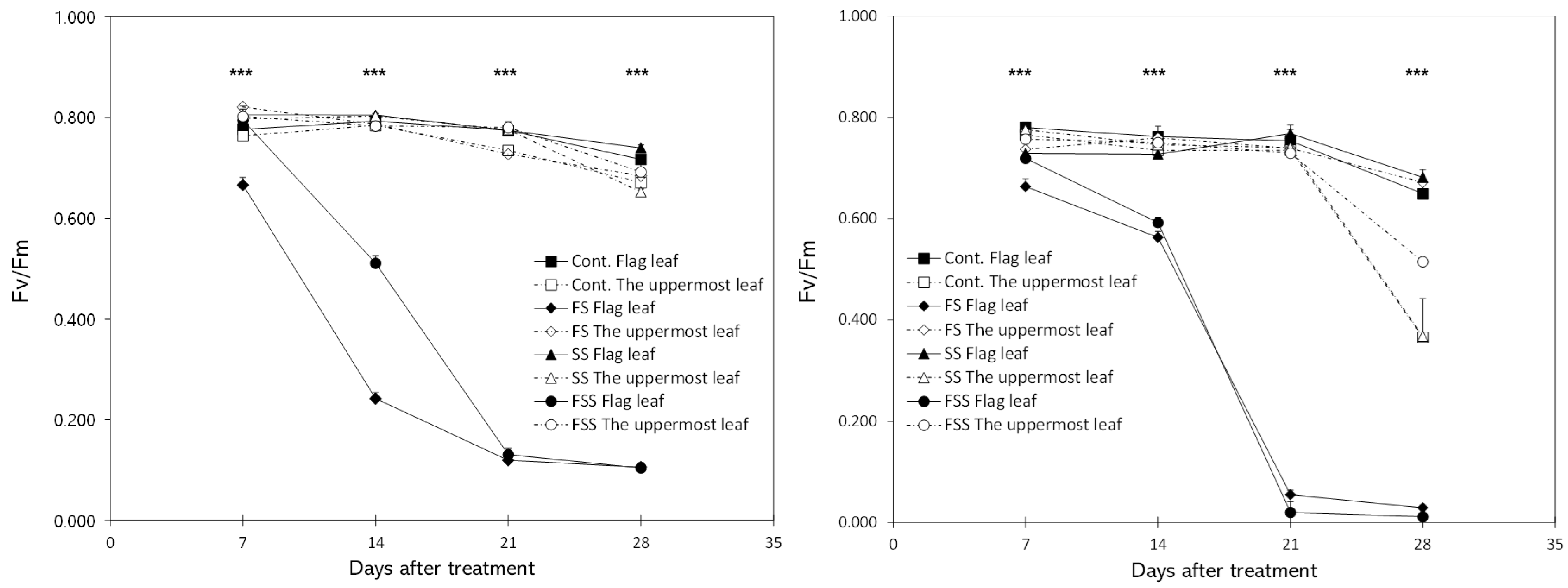
3.1. Leaf Chlorophyll Content and Chlorophyll Fluorescence
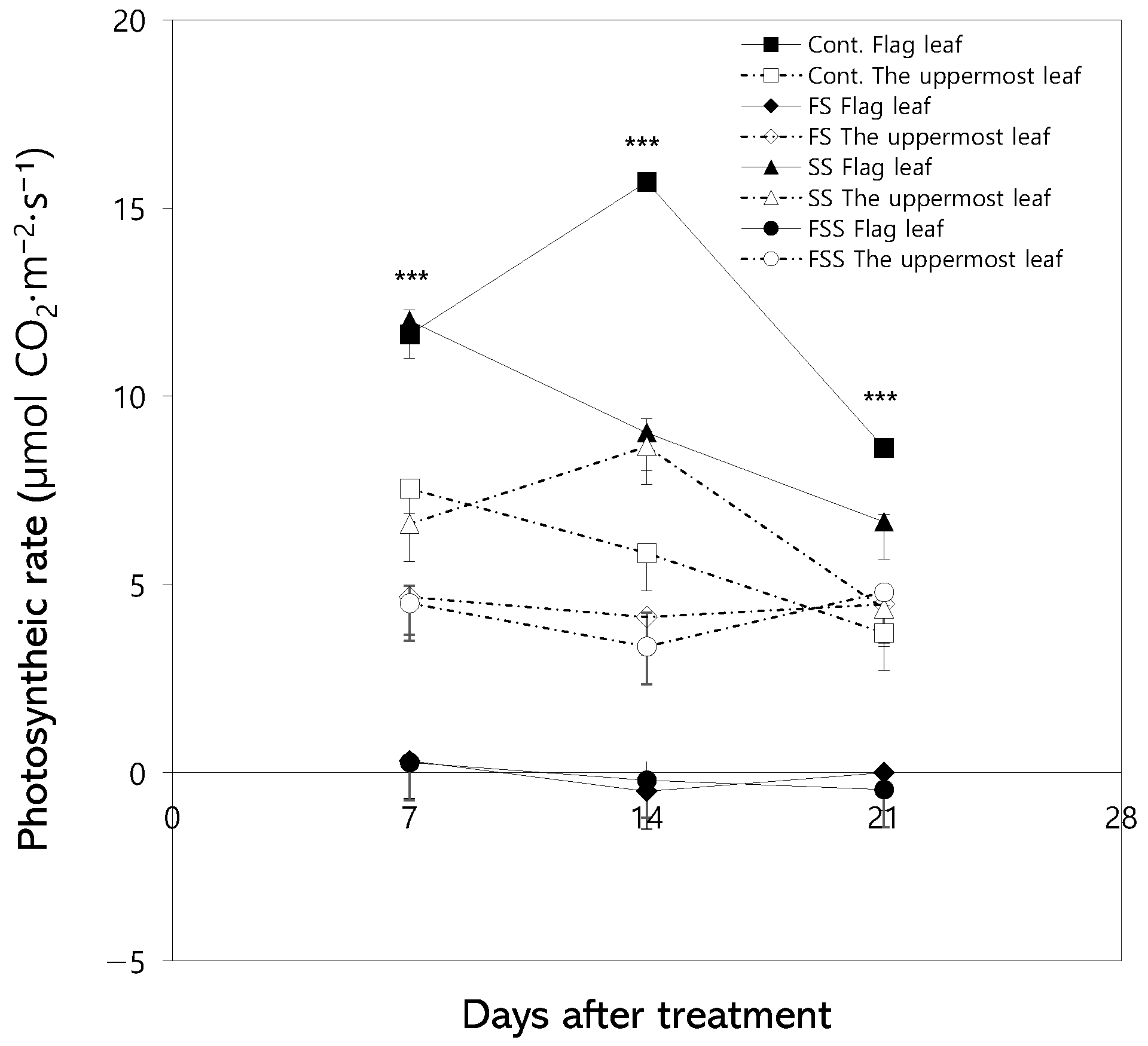
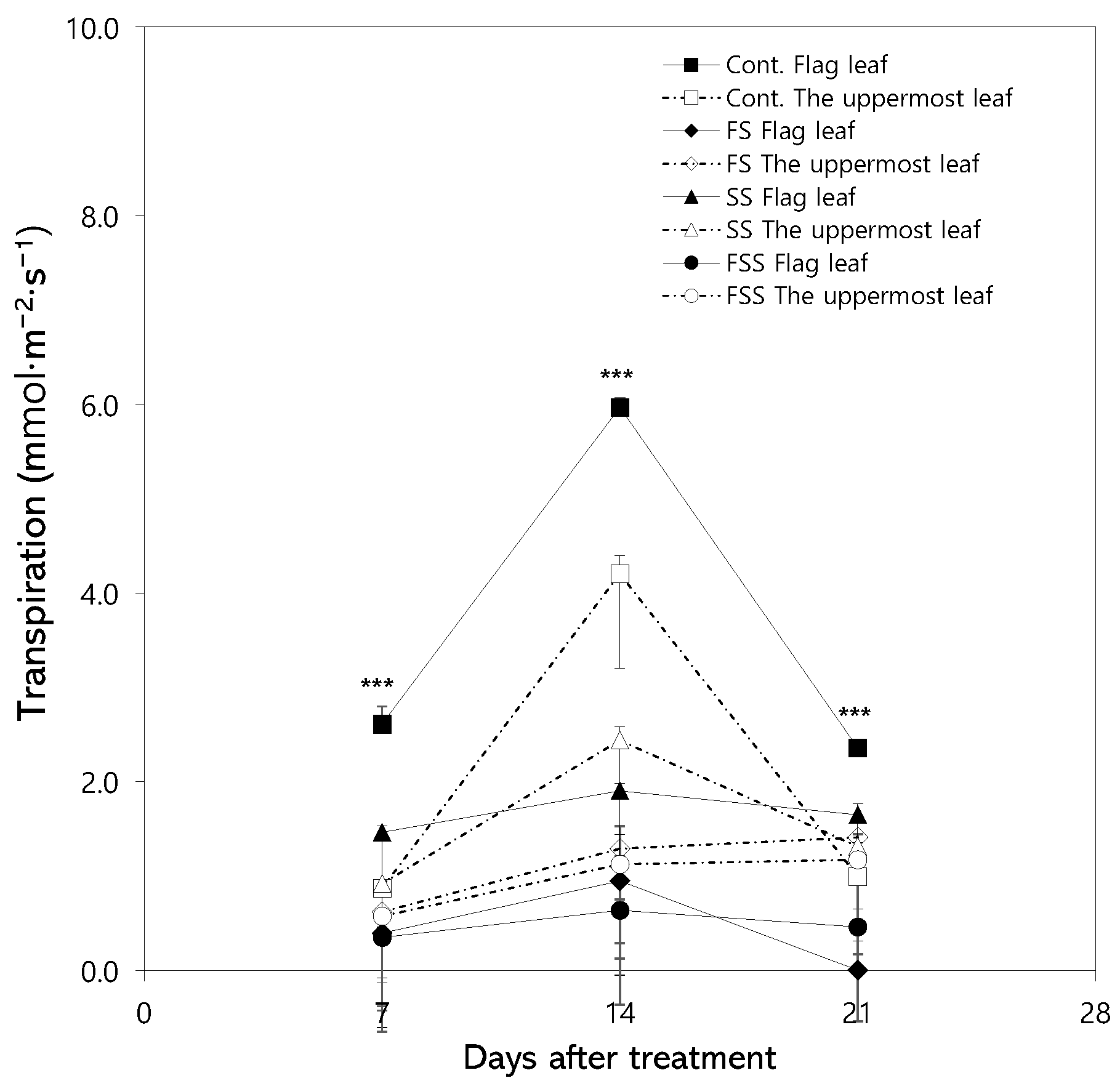
3.2. Changes in Photosynthetic Rate
3.3. Temperature Changes of Spike and Flag Leaf According to Shading Treatments
3.4. Changes in Dry Weight and Relative Growth Rate in Treatments
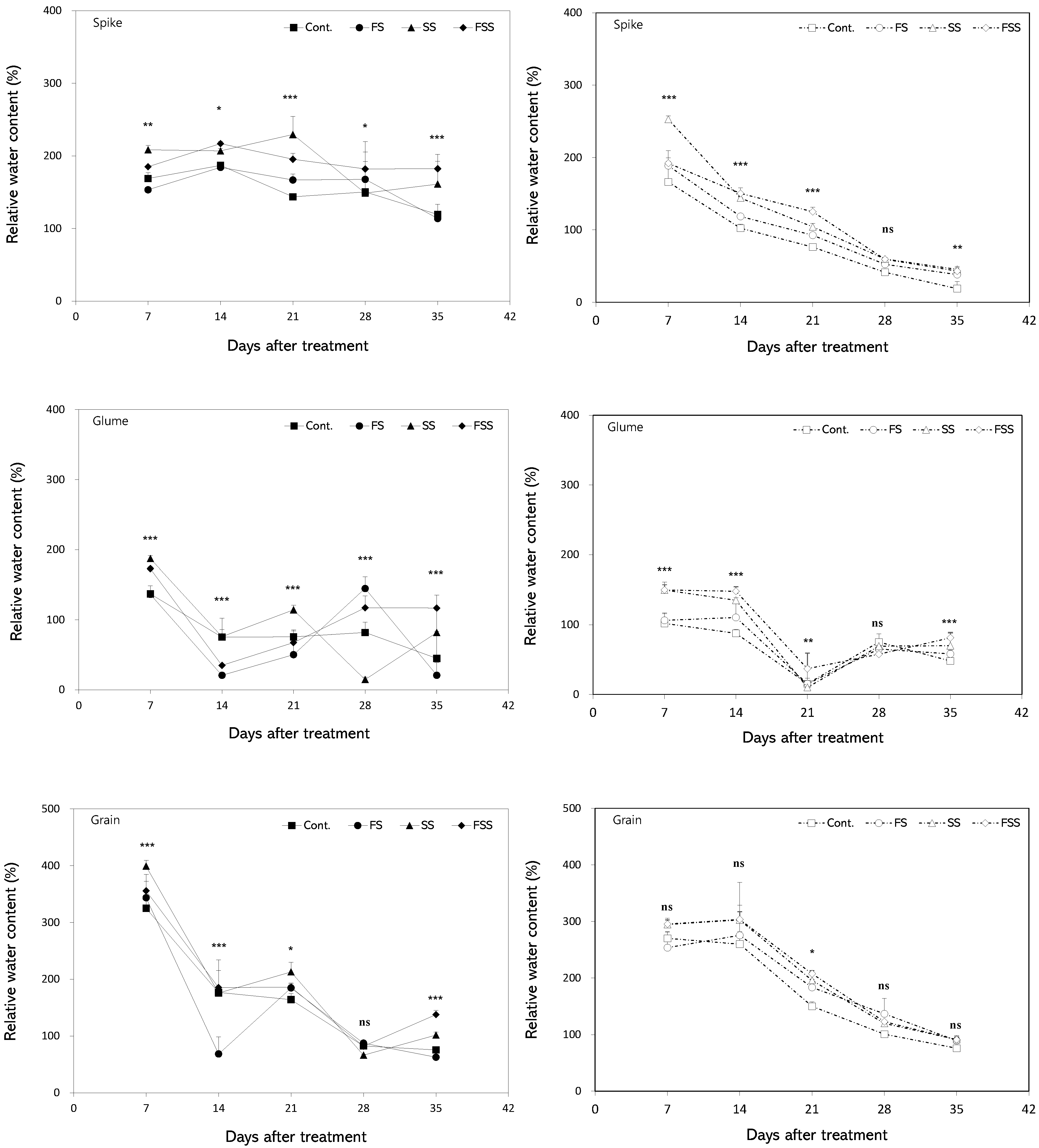
3.5. Relationship Between Relative Water Content and Dry Weight of Yield Components
4. Discussion
4.1. Physiological Responses of Flag Leaves and the Uppermost Leaves to Shading Treatment
4.2. Growth of Spikes and Grains
4.3. Relationship Between Reproductive Organ Growth and the Uppermost Leaves
4.4. Temperature Changes in Spikes, Flag Leaves, and the Uppermost Leaves Due to Shading Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blum, A. Improving wheat grain filling under stress by stem reserve mobilisation. Euphytica 1998, 100, 77–83. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Sylvester-Bradley, R.; Weightman, R.; Snape, J.W. Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Res. 2007, 103, 11–24. [Google Scholar] [CrossRef]
- Molero, G.; Reynolds, M.P. Spike photosynthesis measured at high throughput indicates genetic variation independent of flag leaf photosynthesis. Field Crops Res. 2020, 255, 107866. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Zhang, Y.; Fischer, T.; Zhao, Z.; Zhou, X.; Wang, Z.; Wang, E. The contribution of spike photosynthesis to wheat yield needs to be considered in process-based crop models. Field Crops Res. 2020, 257, 107931. [Google Scholar] [CrossRef]
- Racz, I.; Hirişcău, D.; Berindean, I.; Kadar, R.; Muntean, E.; Tritean, N.; Russu, F.; Ona, A.; Muntean, L. The influence of flag leaf removal and its characteristics on main yield components and yield quality indices on wheat. Agronomy 2022, 12, 2545. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Elenein, R.A. The relative contribution of different wheat leaves and awns to the grain yield and its protein content. Z. Acker Pflanzenbau 1977, 144, 1–77. [Google Scholar]
- Evans, L.T.; Wardlaw, I.F. Wheat. In Photoassimilate Distribution in Plants and Crops: Source—Sink Relationships; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 501–518. [Google Scholar]
- Briggs, K.; Aytenfisu, A. Relationships between morphological characters above the flag leaf node and grain yield in spring wheats. Crop Sci. 1980, 20, 350–354. [Google Scholar] [CrossRef]
- Birsin, M.A. Effects of removal of some photosynthetic structures on some yield components in wheat. Tarim. Bilim. Derg. 2005, 11, 364–367. [Google Scholar]
- Kramer, T.; Didden, F.A.M. The influence of awns on grain yield and kernel weight in spring wheat (Triticum aestivum L.). Cereal Res. Commun. 1981, 9, 25–30. [Google Scholar]
- Kichey, T.; Hirel, B.; Heumez, E.; Dubois, F.; Le Gouis, J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crops Res. 2007, 102, 22–32. [Google Scholar] [CrossRef]
- Gaju, O.; Allard, V.; Martre, P.; Snape, J.W.; Heumez, E.; LeGouis, J.; Moreau, D.; Bogard, M.; Griffiths, S.; Orford, S.; et al. Identification of traits to improve the nitrogen-use efficiency of wheat genotypes. Field Crops Res. 2011, 123, 139–152. [Google Scholar] [CrossRef]
- Bort, J.; Febrero, A.; Amaro, T.; Araus, J.L. Role of awns in ear water-use efficiency and grain weight in barley. Agronomie 1994, 14, 133–139. [Google Scholar] [CrossRef]
- Gebbing, T.; Schnyder, H. 13C labeling kinetics of sucrose in glumes indicates significant refixation of respiratory CO2 in the wheat ear. Funct. Plant Biol. 2001, 28, 1047–1053. [Google Scholar] [CrossRef]
- Lopes, M.S.; Cortadellas, N.; Kichey, T.; Dubois, F.; Habash, D.Z.; Araus, J.L. Wheat nitrogen metabolism during grain filling: Comparative role of glumes and the flag leaf. Planta 2006, 225, 165–181. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrera-Bosquet, L.; Morcuende, R.; Avice, J.C.; Nogués, S.; Araus, J.L.; Martínez-Carrasco, R.; Pérez, P. Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J. Exp. Bot. 2011, 62, 3957–3969. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Molero, G.; Reynolds, M.P.; Araus, J.L. Relative contribution of shoot and ear photosynthesis to grain filling in wheat under good agronomical conditions assessed by differential organ δ13C. J. Exp. Bot. 2014, 65, 5401–5413. [Google Scholar] [CrossRef]
- Zhu, C.; Zhu, J.; Zeng, Q.; Liu, G.; Xie, Z.; Tang, H.; Cao, J.; Zhao, X. Elevated CO2 accelerates flag leaf senescence in wheat due to ear photosynthesis which causes greater ear nitrogen sink capacity and ear carbon sink limitation. Funct. Plant Biol. 2009, 36, 291–299. [Google Scholar] [CrossRef]
- Kong, L.; Sun, M.; Xie, Y.; Wang, F.; Zhao, Z. Photochemical and antioxidative responses of the glume and flag leaf to seasonal senescence in wheat. Front. Plant Sci. 2015, 6, 358. [Google Scholar] [CrossRef]
- Zhou, B.; Sanz-Sáez, Á.; Elazab, A.; Shen, T.; Sánchez-Bragado, R.; Bort, J.; Serret, M.D.; Araus, J.L. Physiological traits contributed to the recent increase in yield potential of winter wheat from Henan Province, China. J. Integr. Plant Biol. 2014, 56, 492–504. [Google Scholar] [CrossRef]
- Serrago, R.A.; Alzueta, I.; Savin, R.; Slafer, G.A. Understanding grain yield responses to source–sink ratios during grain filling in wheat and barley under contrasting environments. Field Crops Res. 2013, 150, 42–51. [Google Scholar] [CrossRef]
- Stamp, P.; Herzog, H. Flag-leaf senescence and grain growth in certain German varieties of spring wheat (Triticum aestivum L.). Z. Pflanzenzuechtung 1976, 77, 330–338. [Google Scholar]
- Gámez, A.L.; Vicente, R.; Sanchez-Bragado, R.; Jauregui, I.; Morcuende, R.; Goicoechea, N.; Aranjuelo, I. Differential flag leaf and ear photosynthetic performance under elevated (CO2) conditions during grain filling period in durum wheat. Front. Plant. Sci. 2020, 11, 587958. [Google Scholar] [CrossRef]
- Barutcular, C.; Yildirim, M.; Koc, M.; Dizlek, H.; Akinci, C.; El Sabagh, A.; Saneoka, H.; Ueda, A.; Islam, M.; Toptas, I.; et al. Quality traits performance of bread wheat genotypes under drought and heat stress conditions. Fresenius Environ. Bull. 2016, 25, 6159–6165. [Google Scholar]
- Araus, J.L.; Alegre, L.; Tapia, L.; Calafell, R. Relationship between leaf structure and gas exchange in wheat leaves at different insertion levels. J. Exp. Bot. 1986, 37, 1323–1333. [Google Scholar] [CrossRef]
- De Simone, V.; Soccio, M.; Borrelli, G.M.; Pastore, D.; Trono, D. Stay-green trait-antioxidant status interrelationship in durum wheat (Triticum durum) flag leaf during post-flowering. J. Plant Res. 2014, 127, 159–171. [Google Scholar] [CrossRef]
- Borrill, P.; Fahy, B.; Smith, A.M.; Uauy, C. Wheat grain filling is limited by grain filling capacity rather than the duration of flag leaf photosynthesis: A case study using NAM RNAi plants. PLoS ONE 2015, 10, e0134947. [Google Scholar] [CrossRef]
- Ba, Q.; Zhang, L.; Chen, S.; Li, G.; Wang, W. Effects of foliar application of magnesium sulfate on photosynthetic characteristics, dry matter accumulation and its translocation, and carbohydrate metabolism in grain during wheat grain filling. Cereal Res. Commun. 2020, 48, 157–163. [Google Scholar] [CrossRef]
- Du, B.; Wu, J.; Islam, M.S.; Sun, C.; Lu, B.; Wei, P.; Liu, D.; Chen, C. Genome-wide meta-analysis of QTL for morphological related traits of flag leaf in bread wheat. PLoS ONE 2022, 17, e0276602. [Google Scholar] [CrossRef]
- Zhang, C.J.; Chen, G.X.; Gao, X.X.; Chu, C.J. Photosynthetic decline in flag leaves of two field-grown spring wheat cultivars with different senescence properties. S. Afr. J. Bot. 2006, 72, 15–23. [Google Scholar] [CrossRef]
- Rivera-Amado, C.; Molero, G.; Trujillo-Negrellos, E.; Reynolds, M.; Foulkes, J. Estimating organ contribution to grain filling and potential for source upregulation in wheat cultivars with a contrasting source–sink balance. Agronomy 2020, 10, 1527. [Google Scholar] [CrossRef]
- Estrada-Campuzano, G.; Slafer, G.A.; Miralles, D.J. Differences in yield, biomass and their components between triticale and wheat grown under contrasting water and nitrogen environments. Field Crops Res. 2012, 128, 167–179. [Google Scholar] [CrossRef]
- Xue, Q.; Zhu, Z.; Musick, J.T.; Stewart, B.A.; Dusek, D.A. Physiological mechanisms contributing to the increased water-use efficiency in winter wheat under deficit irrigation. J. Plant Physiol. 2006, 163, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, Y.; Chen, J.; Adams, C. Late-season photosynthetic rate and senescence were associated with grain yield in winter wheat of diverse origins. J. Agron. Crop Sci. 2018, 204, 1–12. [Google Scholar] [CrossRef]
- Maydup, M.L.; Antonietta, M.; Guiamet, J.J.; Graciano, C.; López, J.R.; Tambussi, E.A. The contribution of ear photosynthesis to grain filling in bread wheat (Triticum aestivum L.). Field Crops Res. 2010, 119, 48–58. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Effects of shading on morphology, physiology and grain yield of winter wheat. Eur. J. Agron. 2010, 33, 267–275. [Google Scholar] [CrossRef]
- Pires, S.N.; Souza, F.R.d.; Silva, B.E.P.; Fagundes, N.d.S.; Lucho, S.R.; Avila, L.A.d.; Deuner, S. Effects of shading on Metabolism and Grain Yield of Irrigated Rice During Crop Development. Plants 2025, 14, 2491. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Albrizio, R.; Araus, J.L.; Nogués, S. Photosynthetic capacity of field-grown durum wheat under different N availabilities: A comparative study from leaf to canopy. Environ. Exp. Bot. 2009, 67, 145–152. [Google Scholar] [CrossRef]
- Naruoka, Y.; Sherman, J.D.; Lanning, S.P.; Blake, N.K.; Martin, J.M.; Talbert, L.E. Genetic analysis of green leaf duration in spring wheat. Crop Sci. 2012, 52, 99–109. [Google Scholar] [CrossRef]
- Spano, G.; Di Fonzo, N.; Perrotta, C.; Platani, C.; Ronga, G.; Lawlor, D.W.; Napier, J.A.; Shewry, P.R. Physiological characterization of ‘stay green’ mutants in durum wheat. J. Exp. Bot. 2003, 54, 1415–1420. [Google Scholar] [CrossRef]
- Xie, A.; Lv, M.; Zhang, D.; Shi, Y.; Yang, L.; Yang, X.; Du, J.; Sun, L.; Sun, X. Effects of slight shading in summer on the leaf senescence and endogenous hormone and polyamine contents in herbaceous peony. Sci. Rep. 2023, 13, 18714. [Google Scholar] [CrossRef]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Carpel size, grain filling, and morphology determine individual grain weight in wheat. J. Exp. Bot. 2015, 66, 6715–6730. [Google Scholar] [CrossRef]
- Yang, K.; Sun, H.; Liu, M.; Zhu, L.; Zhang, K.; Zhang, Y.; Li, A.; Zhang, H.; Zhu, J.; Liu, X.; et al. Morphological and physiological mechanisms of melatonin on delaying drought-induced leaf senescence in cotton. Int. J. Mol. Sci. 2023, 24, 7269. [Google Scholar] [CrossRef]
- Ding, H.; Liu, D.; Liu, X.; Li, Y.; Kang, J.; Lv, J.; Wang, G. Photosynthetic and stomatal traits of spike and flag leaf of winter wheat (Triticum aestivum L.) under water deficit. Photosynthetica 2018, 56, 687–697. [Google Scholar] [CrossRef]
- Zeng, H.G.; Yi, K.; Yang, S.F.; Jiang, Y.W.; Mao, P.S.; Yu, Y.; Feng, Y.; Dong, Y.X.; Dou, L.; Li, M.L. Photosynthetic performance of glumes of oat spikelets is more stable for grain-filling stage under drought stress. Plant Physiol. Biochem. 2024, 214, 108890. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, Z.; Huang, G.; Li, Y. Leaf photosynthesis and its temperature response are different between growth stages and N supplies in rice plants. Int. J. Mol. Sci. 2022, 23, 3885. [Google Scholar] [CrossRef]
- Berry, J.; Björkman, O. Photosynthetic response and adaption to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Araus, J.L.; Brown, H.R.; Febrero, A.; Bort, J.; Serret, M.D. Ear photosynthesis, carbon isotope discrimination and the contribution of respiratory CO2 to differences in grain mass in durum wheat. Plant Cell Environ. 1993, 16, 383–392. [Google Scholar] [CrossRef]
- Wardlaw, I.F.; Wrigley, C.W. Heat tolerance in temperate cereals: An overview. Aust. J. Plant Physiol. 1994, 21, 695–703. [Google Scholar] [CrossRef]
- Shrestha, S.; Mahat, J.; Shrestha, J.; Madhav, K.C.; Paudel, K. Influence of high-temperature stress on rice growth and development. A review. Heliyon 2022, 8, e12651. [Google Scholar] [CrossRef]



| Organs | Control | Flag Leaf Shading | Spike Shading | Flag Leaf and Spike Shading | ||||
|---|---|---|---|---|---|---|---|---|
| 14 DAT | 28 DAT | 14 DAT | 28 DAT | 14 DAT | 28 DAT | 14 DAT | 28 DAT | |
| ----------------------------------------------------- °C ---------------------------------------------------------- | ||||||||
| Spike | 24.3 ± 0.4 | 33.1 ± 0.5 | 24.4 ± 0.3 | 32.6 ± 0.3 | 26.5 ± 1.1 | 33.7 ± 0.3 | 24.8 ± 0.3 | 34.8 ± 0.9 |
| Flag leaf | 25.4 ± 0.1 | 38.4 ± 0.9 | 27.8 ± 2.4 | 40.7 ± 1.4 | 25.8 ± 0.4 | 39.4 ± 1.1 | 26.9 ± 1.4 | 40.1 ± 1.8 |
| Uppermost leaf | N.M. † | 39.1 ± 0.6 | N.M. | 37.0 ± 1.2 | N.M. | 36.8 ± 2.3 | N.M. | 39.3 ± 0.5 |
| Treatment | Spike Growth Rate (mg mg−1 d−1) | Glume Growth Rate (mg mg−1 d−1) | Grain Growth Rate (mg mg−1 d−1) |
|---|---|---|---|
| Control | 7.0 ± 0.02 a,† | 2.0 ± 0.04 a | 3.8 ± 0.02 a |
| FS | 6.9 ± 0.03 b | 2.0 ± 0.04 a | 3.8 ± 0.03 a |
| SS | 6.4 ± 0.07 c | 2.1 ± 0.15 a | 3.3 ± 0.05 b |
| FSS | 6.0 ± 0.10 d | 1.6 ± 0.01 b | 2.8 ± 0.03 c |
| p-value | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Hong, S.; Shim, S. Responses of Photosynthetic Activity in Flag Leaves and Spikes as well as Seed Development of Wheat (Triticum aestivum L.) to Artificial Shading. Agronomy 2025, 15, 2577. https://doi.org/10.3390/agronomy15112577
Song K, Hong S, Shim S. Responses of Photosynthetic Activity in Flag Leaves and Spikes as well as Seed Development of Wheat (Triticum aestivum L.) to Artificial Shading. Agronomy. 2025; 15(11):2577. https://doi.org/10.3390/agronomy15112577
Chicago/Turabian StyleSong, Kieun, Sesil Hong, and Sangin Shim. 2025. "Responses of Photosynthetic Activity in Flag Leaves and Spikes as well as Seed Development of Wheat (Triticum aestivum L.) to Artificial Shading" Agronomy 15, no. 11: 2577. https://doi.org/10.3390/agronomy15112577
APA StyleSong, K., Hong, S., & Shim, S. (2025). Responses of Photosynthetic Activity in Flag Leaves and Spikes as well as Seed Development of Wheat (Triticum aestivum L.) to Artificial Shading. Agronomy, 15(11), 2577. https://doi.org/10.3390/agronomy15112577





