The Influence of Foliar Application of Nod Factors (LCOs) and Microelements on the Growth, Development, and Yield of Peas (Pisum sativum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Experimental Factors
2.3. Preparation of the LCO Extract
2.4. Measurements of Leaf Area and SPAD
2.5. Determination of Gas Exchange Parameters
2.6. Chlorophyll Fluorescence Measurements
2.7. Determination of Nitrogen Content in Parts of Plants
2.8. Determination of the Relative Growth Rate (RGR)
2.9. Determination of Seed Yield and Yield Components
2.10. Statistical Analysis
3. Results
3.1. The Effect of LCOs and/or ME Application on Morphological Traits of Pea
3.2. The Effect of LCOs and/or MEs Application on Physiological Parameters of the Pea
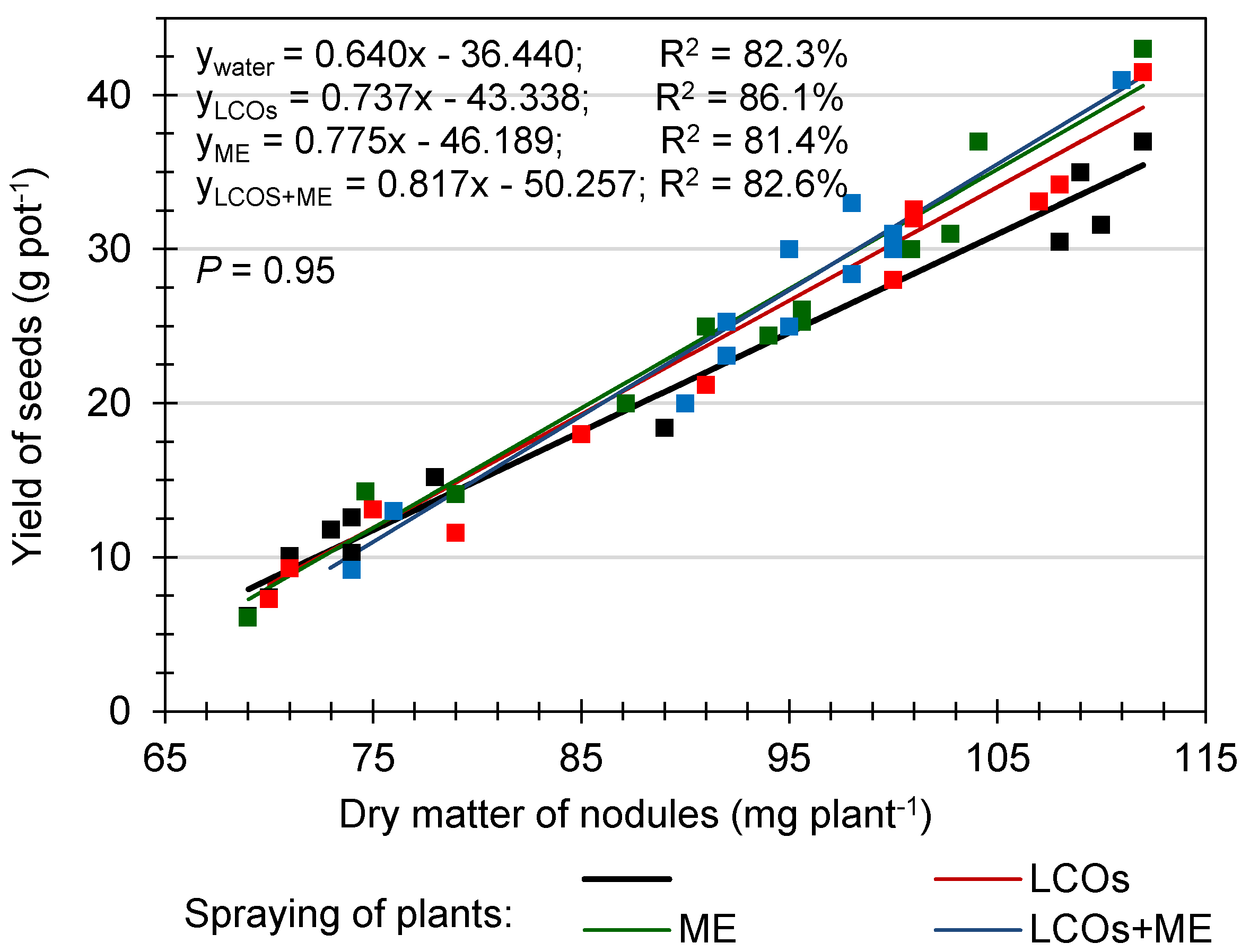
3.3. The Effect of LCOs and/or MEs Application on the Nodulation of the Pea
3.4. The Effect of LCOs and/or MEs Application on Pea Growth and Yield
4. Discussion
5. Conclusions
- The Nod factors and microelements significantly influenced selected physiological parameters of yield formation in pea plants (net photosynthetic rate, RGR, and SPAD).
- The foliar application of the Nod factors and the microelements had a positive influence on the number and weight of root nodules, which was correlated with an increase in the weight of vegetative and generative organs of pea plants.
- The foliar application of the Nod factors and the microelements significantly enhanced the growth, development, and yield of pea plants, and the treatments were most effective when the studied preparations were used in combination.
- The increase in the pea yield induced by the LCOs and the microelements can be attributed to the higher number of pods per plant and the higher number of seeds per plant because the number of seeds per pod and the 1000 seed weight did not change significantly.
- It seems that the simultaneous use of a preparation containing LCOs and microelements has a good synergistic effect. Both (a) an increase in the number of nodules and (b) intensification of metabolic processes were observed, which is probably due to (a) the action of the molecular signal and (b) the provision of numerous cofactors for the most important enzymes and proteins involved in or related to the biological nitrogen fixation process. Nitrogenase activity was not determined; however, a significant increase in nitrogen content in plant tissues was observed after LCOs, ME, or LCOs-ME treatment. As a result, an over 30% seed yield increase was obtained, compared to the control group.
- The foliar application of both components was fully successful, which is a promising finding, because this method of application is much more time-flexible and convenient than seed dressing.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schneider, A.V.C. Overview of the Market and Consumption of Puises in Europe. Br. J. Nutr. 2002, 88 (Suppl. S3), 243–250. [Google Scholar] [CrossRef]
- Osiecka, A. Polish National List of Agricultural Plant Varieties; Research Center for Cultivar Testing: Słupia Wielka, Poland, 2014; Part 2. [Google Scholar]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The Contributions of Nitrogen-Fixing Crop Legumes to the Productivity of Agricultural Systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global Inputs of Biological Nitrogen Fixation in Agricultural Systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Baldock, J.; Peoples, M.B. Prospects and Problems of Simple Linear Models for Estimating Symbiotic N2 Fixation by Crop and Pasture Legumes. Plant Soil 2010, 329, 75–89. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- D’Haeze, W.; Holsters, M. Nod Factor Structures, Responses, and Perception during Initiation of Nodule Development. Glycobiology 2002, 12, 79R–105R. [Google Scholar] [CrossRef]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How Rhizobial Symbionts Invade Plants: The Sinorhizobium-Medicago Model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The Molecular Network Governing Nodule Organogenesis and Infection in the Model Legume Lotus japonicus. Nat. Commun. 2010, 1, 10. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume Nodulation: The Host Controls the Party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Siczek, A.; Lipiec, J.; Wielbo, J.; Kidaj, D.; Szarlip, P. Symbiotic Activity of Pea (Pisum sativum) after Application of Nod Factors under Field Conditions. Int. J. Mol. Sci. 2014, 15, 7344–7351. [Google Scholar] [CrossRef] [PubMed]
- Smytkiewicz, K.; Podleśny, J.; Wielbo, J.; Podleśna, A. The Effect of a Preparation Containing Rhizobial Nod Factors on Pea Morphological Traits and Physiology. Agronomy 2021, 11, 1457. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Nod Factor [Nod Bj V (C18:1, MeFuc)] and Lumichrome Enhance Photosynthesis and Growth of Corn and Soybean. J. Plant Physiol. 2008, 165, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Podleśna, A.; Wielbo, J.; Podleśny, J.; Kidaj, D. Effect of Sulfur and Nod Factors (LCOs) on Some Physiological Features and Yield of Pea (Pisum sativum L.). In Molecular Physiology and Ecophysiology of Sulfur; De Kok, L.J., Hawkesford, M.J., Rennenberg, H., Saito, K., Schnug, E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 221–226. ISBN 978-3-319-20137-5. [Google Scholar]
- Ribbe, M.W.; Hu, Y.; Hodgson, K.O.; Hedman, B. Biosynthesis of Nitrogenase Metalloclusters. Chem. Rev. 2014, 114, 4063–4080. [Google Scholar] [CrossRef]
- Rubio, L.M.; Ludden, P.W. Biosynthesis of the Iron-Molybdenum Cofactor of Nitrogenase. Annu. Rev. Microbiol. 2008, 62, 93–111. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Hoffman, B.M.; Dean, D.R. Mechanism of Mo-Dependent Nitrogenase. Annu. Rev. Biochem. 2009, 78, 701–722. [Google Scholar] [CrossRef]
- Alam, F.; Kim, T.Y.; Kim, S.Y.; Alam, S.S.; Pramanik, P.; Kim, P.J.; Lee, Y.B. Effect of Molybdenum on Nodulation, Plant Yield and Nitrogen Uptake in Hairy Vetch (Vicia villosa Roth). Soil. Sci. Plant Nutr. 2015, 61, 664–675. [Google Scholar] [CrossRef]
- Brear, E.M.; Day, D.A.; Smith, P.M.C. Iron: An Essential Micronutrient for the Legume-Rhizobium Symbiosis. Front. Plant Sci. 2013, 4, 359. [Google Scholar] [CrossRef]
- Rubio, M.C.; James, E.K.; Clemente, M.R.; Bucciarelli, B.; Fedorova, M.; Vance, C.P.; Becana, M. Localization of Superoxide Dismutases and Hydrogen Peroxide in Legume Root Nodules. Mol. Plant Microbe Interact. 2004, 17, 1294–1305. [Google Scholar] [CrossRef]
- Rubio, M.C.; Becana, M.; Sato, S.; James, E.K.; Tabata, S.; Spaink, H.P. Characterization of Genomic Clones and Expression Analysis of the Three Types of Superoxide Dismutases during Nodule Development in Lotus japonicus. Mol. Plant Microbe Interact. 2007, 20, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Kijne, J.W.; Smit, G.; Díaz, C.L.; Lugtenberg, B.J. Lectin-Enhanced Accumulation of Manganese-Limited Rhizobium Leguminosarum Cells on Pea Root Hair Tips. J. Bacteriol. 1988, 170, 2994–3000. [Google Scholar] [CrossRef] [PubMed]
- Preisig, O.; Zufferey, R.; Thöny-Meyer, L.; Appleby, C.A.; Hennecke, H. A High-Affinity Cbb3-Type Cytochrome Oxidase Terminates the Symbiosis-Specific Respiratory Chain of Bradyrhizobium japonicum. J. Bacteriol. 1996, 178, 1532–1538. [Google Scholar] [CrossRef]
- Bolanos, L.; Brewin, N.J.; Bonilla, I. Effects of Boron on Rhizobium-Legume Cell-Surface Interactions and Nodule Development. Plant Physiol. 1996, 110, 1249–1256. [Google Scholar] [CrossRef]
- Jiang, C.-D.; Gao, H.-Y.; Zou, Q.; Shi, L. Effects of Iron Deficiency on Photosynthesis and Photosystem II Function in Soybean Leaf. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2007, 33, 53–60. [Google Scholar]
- Mukhopadhyay, M.; Ghosh, P.D.; Mondal, T.K. Effect of Boron Deficiency on Photosynthesis and Antioxidant Responses of Young Tea Plantlets. Russ. J. Plant Physiol. 2013, 60, 633–639. [Google Scholar] [CrossRef]
- Imran, M.; Hu, C.; Hussain, S.; Rana, M.S.; Riaz, M.; Afzal, J.; Aziz, O.; Elyamine, A.M.; Farag Ismael, M.A.; Sun, X. Molybdenum-Induced Effects on Photosynthetic Efficacy of Winter Wheat (Triticum aestivum L.) under Different Nitrogen Sources Are Associated with Nitrogen Assimilation. Plant Physiol. Biochem. 2019, 141, 154–163. [Google Scholar] [CrossRef]
- Messant, M.; Hani, U.; Hennebelle, T.; Guérard, F.; Gakière, B.; Gall, A.; Thomine, S.; Krieger-Liszkay, A. Manganese Concentration Affects Chloroplast Structure and the Photosynthetic Apparatus in Marchantia Polymorpha. Plant Physiol. 2023, 192, 356–369. [Google Scholar] [CrossRef]
- Urwat, U.; Ahmad, S.M.; Masi, A.; Ganai, N.A.; Murtaza, I.; Khan, I.; Zargar, S.M. Fe and Zn Stress Induced Gene Expression Analysis Unraveled Mechanisms of Mineral Homeostasis in Common Bean (Phaseolus vulgaris L.). Sci. Rep. 2021, 11, 24026. [Google Scholar] [CrossRef] [PubMed]
- Henriques, F.S. Effects of Copper Deficiency on the Photosynthetic Apparatus of Sugar Beet (Beta vulgaris L.). J. Plant Physiol. 1989, 135, 453–458. [Google Scholar] [CrossRef]
- Fu, C.; Li, M.; Zhang, Y.; Zhang, Y.; Yan, Y.; Wang, Y. Morphology, Photosynthesis, and Internal Structure Alterations in Field Apple Leaves under Hidden and Acute Zinc Deficiency. Sci. Hortic. 2015, 193, 47–54. [Google Scholar] [CrossRef]
- Oliveira, S.L.; Crusciol, C.A.C.; Rodrigues, V.A.; Galeriani, T.M.; Portugal, J.R.; Bossolani, J.W.; Moretti, L.G.; Calonego, J.C.; Cantarella, H. Molybdenum Foliar Fertilization Improves Photosynthetic Metabolism and Grain Yields of Field-Grown Soybean and Maize. Front. Plant Sci. 2022, 13, 887682. [Google Scholar] [CrossRef] [PubMed]
- González-Guerrero, M.; Matthiadis, A.; Sáez, Á.; Long, T.A. Fixating on Metals: New Insights into the Role of Metals in Nodulation and Symbiotic Nitrogen Fixation. Front. Plant Sci. 2014, 5, 45. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Zhang, D.-X.; Zhang, Z.-Y.; Xu, A.; Jiang, Y.-L.; Chen, Z.-C. Metal Nutrition and Transport in the Process of Symbiotic Nitrogen Fixation. Plant Commun. 2024, 5, 100829. [Google Scholar] [CrossRef]
- Ovtsyna, A.O.; Schultze, M.; Tikhonovich, I.A.; Spaink, H.P.; Kondorosi, E.; Kondorosi, A.; Staehelin, C. Nod Factors of Rhizobium Leguminosarum Bv. Viciae and Their Fucosylated Derivatives Stimulate a Nod Factor Cleaving Activity in Pea Roots and Are Hydrolyzed in Vitro by Plant Chitinases at Different Rates. Mol. Plant Microbe Interact. 2000, 13, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants; BBCH Monograf; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001.
- Podleśny, J.; Wielbo, J.; Podleśna, A.; Kidaj, D. Usefulness of Nod Preparation (LCOs) Use to Presowable Dressing of Pea Seeds. J. Res. Appl. Agric. Eng. 2013, 58, 124–129. [Google Scholar]
- Evans, G. The Quantative Analysis of Plant Growth; University of California Press: Oakland, CA, USA, 1972. [Google Scholar]
- Maj, D.; Wielbo, J.; Marek-Kozaczuk, M.; Martyniuk, S.; Skorupska, A. Pretreatment of Clover Seeds with Nod Factors Improves Growth and Nodulation of Trifolium Pratense. J. Chem. Ecol. 2009, 35, 479–487. [Google Scholar] [CrossRef]
- Souleimanov, A.; Prithiviraj, B.; Smith, D.L. The Major Nod Factor of Bradyrhizobium Japonicum Promotes Early Growth of Soybean and Corn. J. Exp. Bot. 2002, 53, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Almaraz, J.; Zhou, X.; Souleimanov, A.; Smith, D. Gas Exchange Characteristics and Dry Matter Accumulation of Soybean Treated with Nod Factors. J. Plant Physiol. 2007, 164, 1391–1393. [Google Scholar] [CrossRef]
- Nandhini, D.U.; Somasundaram, E.; Amanullah, M.M. Effect of Rhizobial Nod Factors (Lipochitooligosaccharide) on Seedling Growth of Blackgram under Salt Stress. Legume Res. 2018, 41, 159–162. [Google Scholar]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of Nutrient Deficiency in Maize and Tomato Plants by in Vivo Chlorophyll a Fluorescence Measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Schwinghamer, T.; Souleimanov, A.; Dutilleul, P.; Smith, D. Supplementation with Solutions of Lipo-Chitooligosacharide Nod Bj V (C18:1, MeFuc) and Thuricin 17 Regulates Leaf Arrangement, Biomass, and Root Development of Canola (Brassica napus L.). Plant Growth Regul. 2016, 78, 31–41. [Google Scholar] [CrossRef]
- Almaraz, J.J.; Mabood, F.; Zhou, X.; Souleimanov, A.; Smith, D.L. Effect of Nod Factor Sprays on Soybean Growth and Productivity under Field Conditions. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011, 61, 228–234. [Google Scholar] [CrossRef]
- Podleśny, J.; Wielbo, J.; Podleśna, A.; Kidaj, D. The Pleiotropic Effects of Extract Containing Rhizobial Nod Factors on Pea Growth and Yield. Open Life Sci. 2014, 9, 396–409. [Google Scholar] [CrossRef]
- Gao, J.-P.; Chiu, C.H. Micronutrients: Minor yet Crucial for Symbiotic Nitrogen Fixation. Plant Commun. 2025, 6, 101345. [Google Scholar] [CrossRef] [PubMed]
- Yeremko, L.; Czopek, K.; Staniak, M.; Marenych, M.; Hanhur, V. Role of Environmental Factors in Legume-Rhizobium Symbiosis: A Review. Biomolecules 2025, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Karn, N.; Sharma, A.K. A Comprehensive Review on Role Of Micronutrients In Legumes. J. Surv. Fish. Sci. 2022, 8, 381–387. [Google Scholar] [CrossRef]
- Subasinghe, S.; Dayatilake, G.A.; Senaratne, R. Effect of B, CO and MO on Nodulation, Growth and Yield of Cowpea (Vigna unguiculata). Trop. Agric. Res. Ext. 2003, 6, 108. [Google Scholar] [CrossRef]
- Khan, N.; Tariq, M.; Ullah, K.; Muhammad, D.; Khan, I.; Rahatullah, K.; Ahmed, N.; Ahmed, S. The Effect of Molybdenum and Iron on Nodulation, Nitrogen Fixation and Yield of Chickpea Genotypes (Cicer arietinum L). IOSR J. Agric. Vet. Sci. 2014, 7, 63–79. [Google Scholar] [CrossRef]
- Parry, F.A.; Chattoo, M.A.; Magray, M.; Ganie, S.A.; Dar, Z.M.; Masood, A. Effect of Different Levels of Sulphur and Boron on Growth and Nodulationof Garden Pea (Pisum sativum L.). Legume Res. 2016, 39, 466–469. [Google Scholar]
- Qasim, M.; Khan, Z.U.D.; Syed, H.R.; Mehmood, F. Effect of Exogenous Supply of Boron on Nodule Development in Pea (Pisum sativum L.). Pak. J. Bot. 2011, 43, 2115–2118. [Google Scholar]
- Singh Grewal, H. Zinc Influences Nodulation, Disease Severity, Leaf Drop and Herbage Yield of Alfalfa Cultivars. Plant Soil 2001, 234, 47–59. [Google Scholar] [CrossRef]
- Wahab, A.M.A.; Abd-Alla, M.H.; El-Enany, A.E. Stimulation of Nodulation, Nitrogen Fixation and Plant Growth of Faba Bean by Cobalt and Copper Additions. In Fertilizers and Environment, Proceedings of the International Symposium “Fertilizers and Environment”, Salamanca, Spain, 26–29 September 1994; Rodriguez-Barrueco, C., Ed.; Springer: Dordrecht, The Netherlands, 1996; pp. 127–130. ISBN 978-94-009-1586-2. [Google Scholar]
- Vieira, R.F.; Cardoso, E.J.B.N.; Vieira, C.; Cassini, S.T.A. Foliar Application of Molybdenum in Common Bean. III. Effect on Nodulation. J. Plant Nutr. 1998, 21, 2153–2161. [Google Scholar] [CrossRef]
- Vieira, R.F.; Cardoso, E.J.B.N.; Vieira, C.; Cassini, S.T.A. Foliar Application of Molybdenum in Common Beans. I. Nitrogenase and Reductase Activities in a Soil of High Fertility. J. Plant Nutr. 1998, 21, 169–180. [Google Scholar] [CrossRef]
- Tang, C.; Robson, A.D.; Dilworth, M.J. The Role of Iron in Nodulation and Nitrogen Fixation in Lupinus angustifolius L. New Phytol. 1990, 114, 173–182. [Google Scholar] [CrossRef]
- Kamran, A.; Naveed, I.; Jahan, S.; Komal, L.; Siddiqui, M.H.; Alamri, S.; Khalil, A. Boron Bioavailability Enhanced by Foliar Applied Fulvic Acid to Improve Grain Yield and Quality of Fine Basmati Rice. Sci. Rep. 2025, 15, 30862. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Hayat, S.; Alam, P. Foliar Application of Copper Oxide Nanoparticles Increases the Photosynthetic Efficiency and Antioxidant Activity in Brassica Juncea. J. Food Qual. 2022, 2022, 5535100. [Google Scholar] [CrossRef]
- Wang, X.; Deng, S.; Zhou, Y.; Long, J.; Ding, D.; Du, H.; Lei, M.; Chen, C.; Tie, B.Q. Application of Different Foliar Iron Fertilizers for Enhancing the Growth and Antioxidant Capacity of Rice and Minimizing Cadmium Accumulation. Environ. Sci. Pollut. Res. Int. 2021, 28, 7828–7839. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Baninasab, B.; Ghobadi, C.; Khoshgoftarmanesh, A.H. Zinc Soil Application Enhances Photosynthetic Capacity and Antioxidant Enzyme Activities in Almond Seedlings Affected by Salinity Stress. Photosynthetica 2016, 54, 267–274. [Google Scholar] [CrossRef]
- Gao, D.; Ran, C.; Zhang, Y.; Wang, X.; Lu, S.; Geng, Y.; Guo, L.; Shao, X. Effect of Different Concentrations of Foliar Iron Fertilizer on Chlorophyll Fluorescence Characteristics of Iron-Deficient Rice Seedlings under Saline Sodic Conditions. Plant Physiol. Biochem. 2022, 185, 112–122. [Google Scholar] [CrossRef]
- Netto, A.T.; Campostrini, E.; Oliveira, J.G.D.; Bressan-Smith, R.E. Photosynthetic Pigments, Nitrogen, Chlorophyll a Fluorescence and SPAD-502 Readings in Coffee Leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance Index as a Sensitive Indicator of Water Stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Tunc, C.E.; von Wirén, N. Hidden Aging: The Secret Role of Root Senescence. Trends Plant Sci. 2025, 30, 553–564. [Google Scholar] [CrossRef]
- Sun, J.; Ye, M.; Peng, S.; Li, Y. Nitrogen Can Improve the Rapid Response of Photosynthesis to Changing Irradiance in Rice (Oryza sativa L.) Plants. Sci. Rep. 2016, 6, 31305. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The Nitrogen Cost of Photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S. The Amount of Nitrogen Used for Photosynthesis Modulates Molecular Evolution in Plants. Mol. Biol. Evol. 2018, 35, 1616–1625. [Google Scholar] [CrossRef] [PubMed]


| Spraying Variant | Height of Plants (cm) | Leaf Area (cm2 Plant−1) | Number of Leaves Per Plant |
|---|---|---|---|
| H2O | 49.2 a * | 379 a | 15.4 a |
| LCOs | 54.0 b | 414 b | 15.7 a |
| MEs | 54.7 b | 394 b | 14.9 a |
| LCOs + MEs | 56.5 b | 426 b | 17.6 b |
| Mean | 53.6 | 393.2 | 15.9 |
| F | 38.4 | 45.1 | 13.9 |
| df | 3 | 3 | 3 |
| Gas Exchange Parameters and SPAD | Spraying of Plants | Mean | F | df | |||
|---|---|---|---|---|---|---|---|
| H2O | LCOs | MEs | LCOs + MEs | ||||
| Pn (µmol CO2 m−2 s−1) | 11.5 a * | 13.1 c | 12.8 b | 13.6 c | 12.7 | 8.38 | 3 |
| E (mmol H2O m−2 s−1) | 5.42 a | 7.23 c | 6.37 b | 7.14 c | 6.54 | 10.1 | 3 |
| Gs (mmol H2O m−2 s−1) | 742 a | 864 b | 856 b | 775 a | 809 | 537.2 | 3 |
| WUE (µmol CO2 mmol−1 H2O) | 2.12 a | 1.81 a | 2.01 a | 1.90 a | 1.96 | 7.45 | 3 |
| SPAD | 438 a | 492 b | 475 b | 489 b | 473 | 42.41 | 3 |
| Chlorophyll Fluorescence Parameters | Spraying of Plants | Mean | F | df | |||
|---|---|---|---|---|---|---|---|
| H2O | LCOs | MEs | LCOs + MEs | ||||
| Fo | 576 b * | 389 a | 411 a | 391 a | 442 | 9.89 | 3 |
| Fm | 2143 a | 2214 a | 2246 a | 2235 a | 2209 | 10.15 | 3 |
| Fv/Fm | 0.732 a | 0.825 b | 0.817 b | 0.825 b | 0.799 | 2.39 | 3 |
| Pindex (PI) | 4.24 a | 5.12 b | 5.23 b | 5.26 b | 4.96 | 6.61 | 3 |
| Tfm | 580 b | 530 a | 540 a | 530 a | 545 | 5.67 | 3 |
| Area | 46,100 a | 52,300 b | 50,400 b | 52,600 b | 50,350 | 81.46 | 3 |
| Description | Spraying of Plants | Mean | F | df | |||
|---|---|---|---|---|---|---|---|
| H2O | LCOs | MEs | LCOs + MEs | ||||
| BBCH 60 | |||||||
| Number of root nodules per plant | 88.4 a * | 96.2 b | 96.5 b | 105.6 c | 96.7 | 173.28 | 3 |
| Dry matter of root nodules (mg per plant) | 81.1 a | 90.1 b | 87.8 b | 94.4 c | 88.5 | 145.12 | 3 |
| Dry matter of one nodule (mg) | 0.92 a | 0.94 a | 0.91 a | 0.89 a | 0.91 | 0.16 | 3 |
| N concentration in above-ground part (%) | 2.31 a | 2.72 b | 2.54 c | 2.71 b | 2.57 | 17,7 | 3 |
| BBCH 75 | F | df | |||||
| Number of root nodules per plant | 74.6 a | 78.5 ab | 80.1 ab | 86.4 b | 79.9 | 85.61 | 3 |
| Dry matter of root nodules (mg per plant) | 80.4 a | 84.4 a | 84.0 a | 85.6 a | 83,6 | 18.36 | 3 |
| Dry matter of one nodule (mg) | 1.08 a | 1.08 a | 1.05 a | 0.99 a | 1.05 | 1.37 | 3 |
| BBCH 90 | F | df | |||||
| N concentration in seeds (%) | 3.42 a | 3.84 b | 3.68 c | 3.84 b | 3.69 | 24.4 | 3 |
| BBCH | Spraying of Plants | Mean | F | df | |||
|---|---|---|---|---|---|---|---|
| H2O | LCOs | MEs | LCOS + MEs | ||||
| Above-ground part | |||||||
| 00–60 | 0.448 a * | 0.515 b | 0.516 b | 0.558 b | 0.510 | 19.24 | 3 |
| 60–75 | 1.579 a | 1.749 b | 1.612 a | 1.742 b | 1.671 | 54.28 | 3 |
| 75–89 | 1.162 a | 1.148 a | 1.124 a | 1.394 b | 1.157 | 47.34 | 3 |
| Roots | F | df | |||||
| 00–60 | 0.224 a | 0.264 b | 0.265 b | 0.305 c | 0.265 | 16.1 | |
| 60–75 | 0.682 a | 0.746 b | 0.668 a | 0.741 b | 0.710 | 18.2 | 3 |
| 75–89 | −4.619 b | −3.213 a | −3.140 a | −3.026 a | −3.500 | 5.9 | 3 |
| Description | Spraying of Plants | Mean | F | df | |||
|---|---|---|---|---|---|---|---|
| H2O | LCOs | ME | LCOs + ME | ||||
| Number of pods per plant | 5.08 a * | 6.14 b | 5.52 b | 5.94 b | 5.67 | 19.11 | 3 |
| Number of seeds per pod | 4.07 a | 3.70 a | 3.96 a | 4.05 a | 3.94 | 0.94 | 3 |
| Number of seeds per plant | 20.7 a | 22.7 b | 21.9 b | 24.1 c | 22.3 | 24.58 | 3 |
| Weight of 1000 seeds (g) | 216 a | 212 a | 216 a | 218 a | 215 | 2.29 | 3 |
| Yield of seeds (g per pot) | 19.2 a | 23.5 b | 21.9 b | 25.4 c | 22.5 | 65.59 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podleśny, J.; Wielbo, J.; Podleśna, A.; Klikocka, H.; Kidaj, D. The Influence of Foliar Application of Nod Factors (LCOs) and Microelements on the Growth, Development, and Yield of Peas (Pisum sativum L.). Agronomy 2025, 15, 2536. https://doi.org/10.3390/agronomy15112536
Podleśny J, Wielbo J, Podleśna A, Klikocka H, Kidaj D. The Influence of Foliar Application of Nod Factors (LCOs) and Microelements on the Growth, Development, and Yield of Peas (Pisum sativum L.). Agronomy. 2025; 15(11):2536. https://doi.org/10.3390/agronomy15112536
Chicago/Turabian StylePodleśny, Janusz, Jerzy Wielbo, Anna Podleśna, Hanna Klikocka, and Dominika Kidaj. 2025. "The Influence of Foliar Application of Nod Factors (LCOs) and Microelements on the Growth, Development, and Yield of Peas (Pisum sativum L.)" Agronomy 15, no. 11: 2536. https://doi.org/10.3390/agronomy15112536
APA StylePodleśny, J., Wielbo, J., Podleśna, A., Klikocka, H., & Kidaj, D. (2025). The Influence of Foliar Application of Nod Factors (LCOs) and Microelements on the Growth, Development, and Yield of Peas (Pisum sativum L.). Agronomy, 15(11), 2536. https://doi.org/10.3390/agronomy15112536






