Study on the Improved Black Soil Structure Under Biological Tillage on Brassica chinensis L. Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Soil Preparation
2.2. Pot Experiment Design and Treatments
2.3. CT Image Acquisition Andprocessing
2.4. Data Analysis
3. Results and Discussion
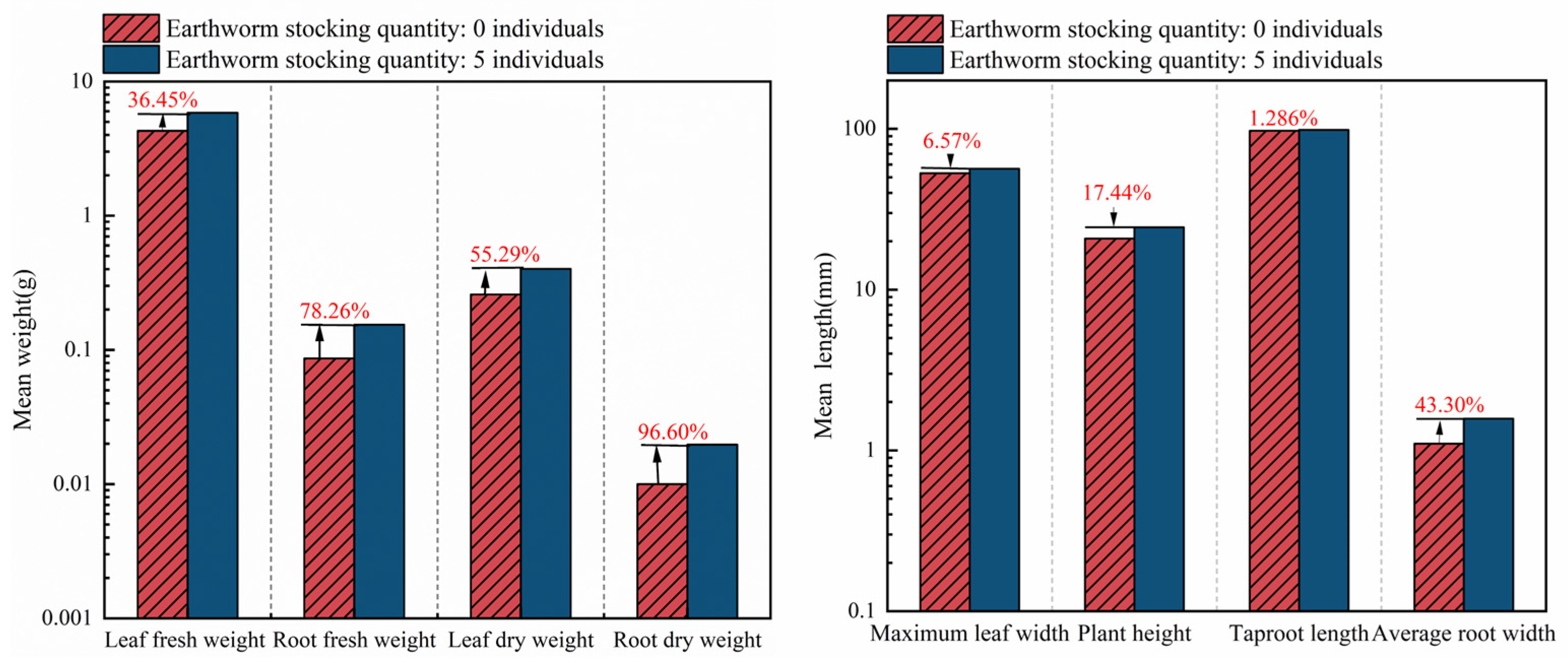
3.1. Effects of Biological Tillage on Brassica Chinensis L. Growth Parameters Under Different Soil Structures
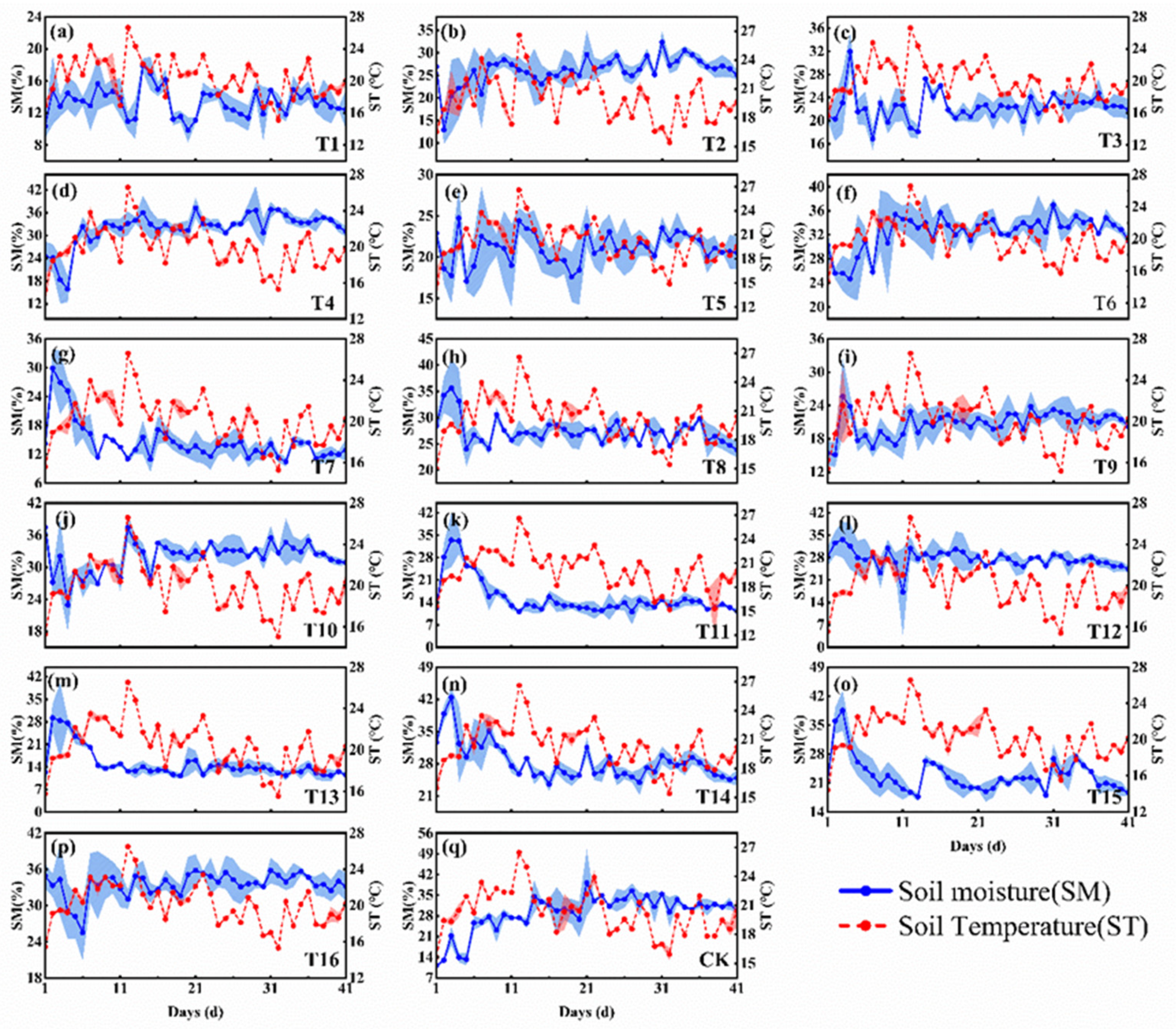
3.2. Effects of Biological Tillage Under Different Food Sources, Soil Moisture, and Soil Compaction Conditions on Soil Water and Temperature
3.3. Effects of Biological Tillage on Biological Characteristics, Root Systems, and Yield of Brassica Chinensis L. Under Different Conditions of Earthworm Food, Soil Moisture, and Soil Compaction
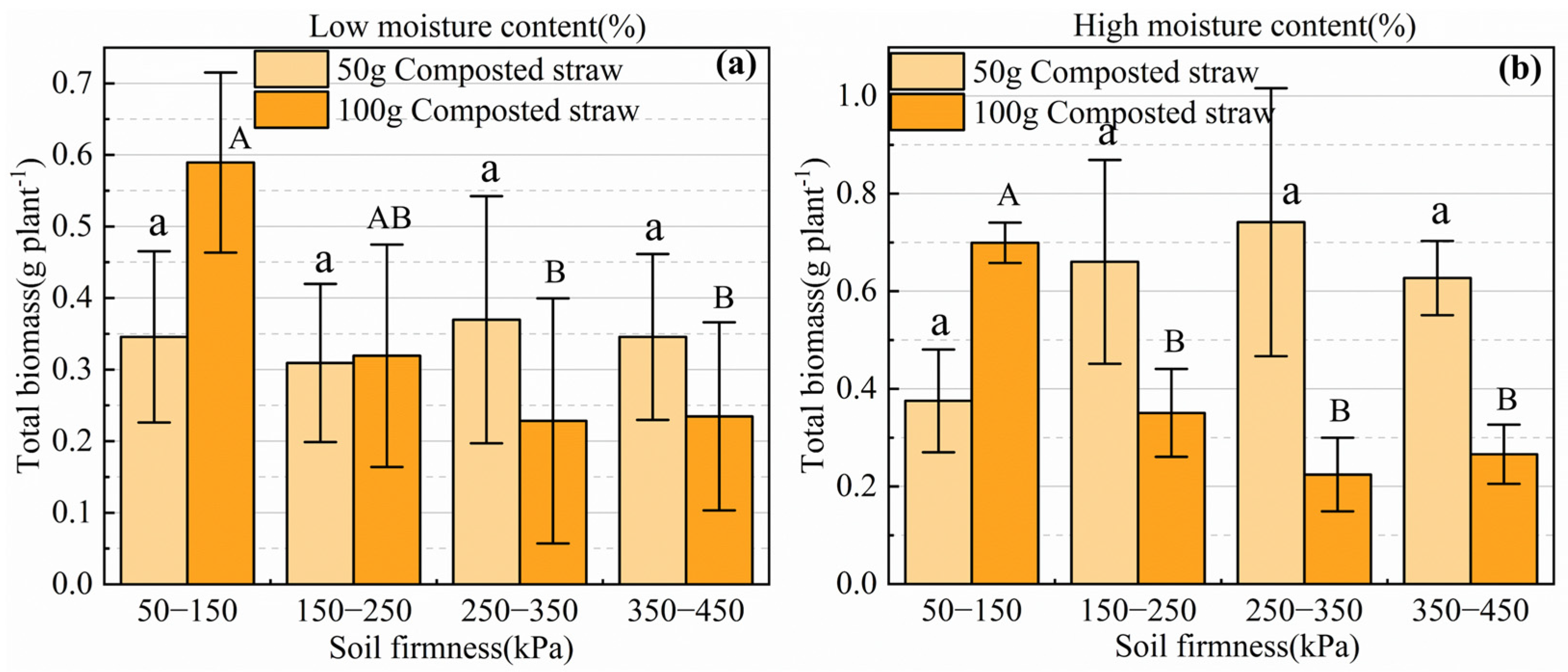
3.3.1. Effects of Biological Tillage on Biomass and Root–Shoot Ratio of Brassica Chinensis L.
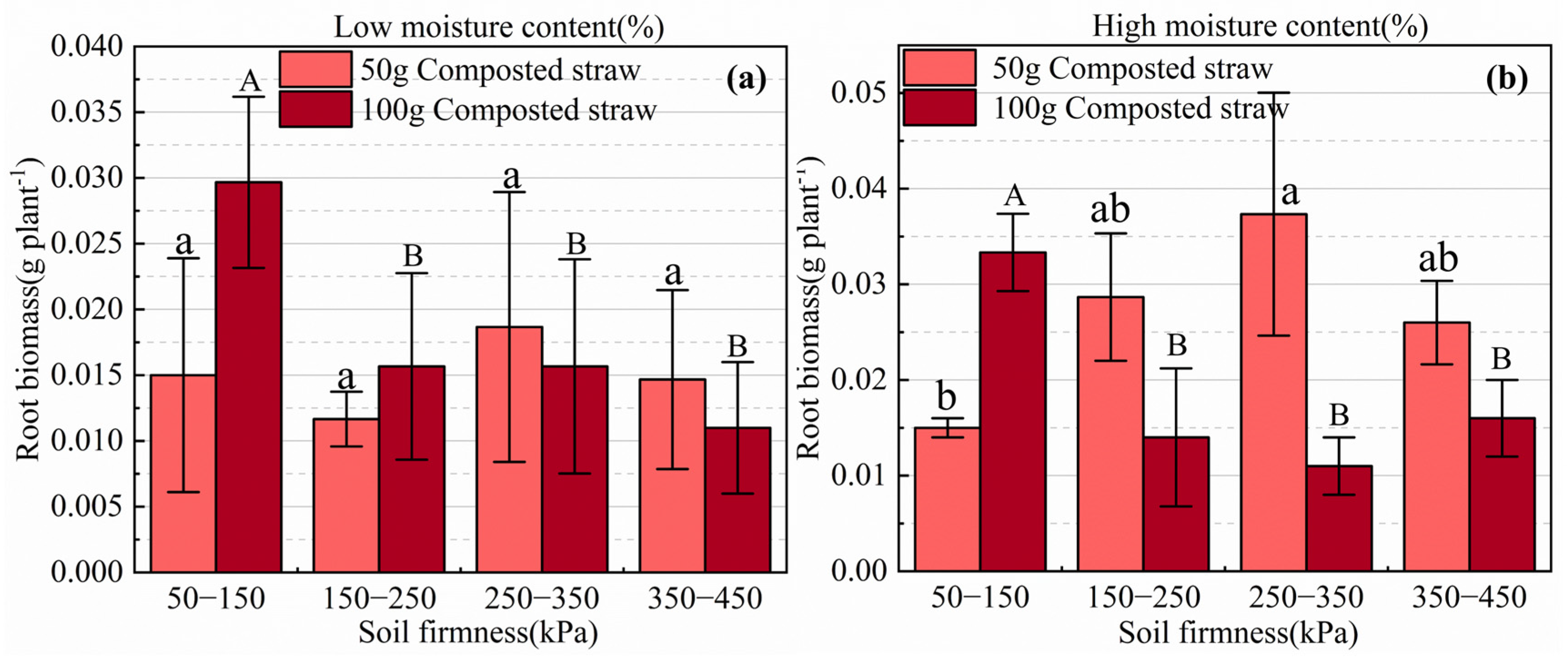
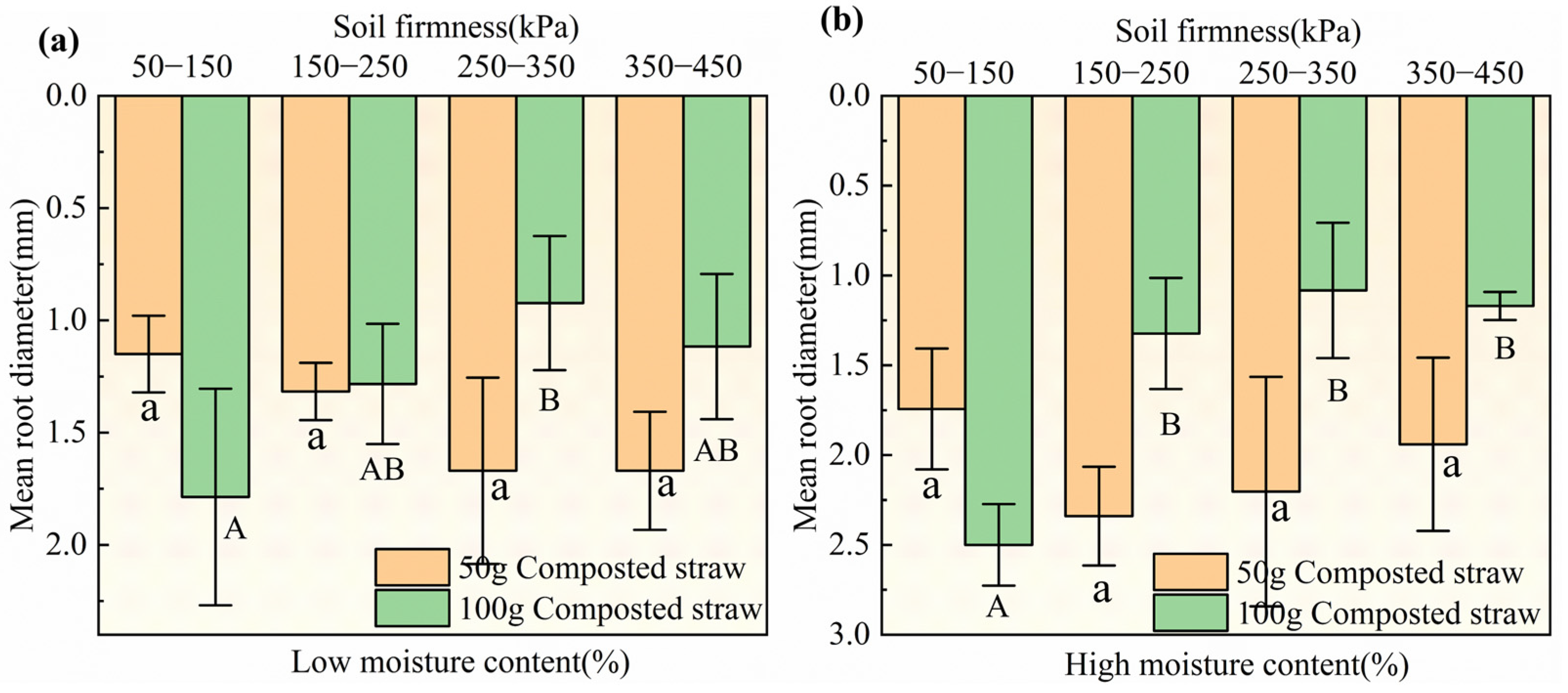
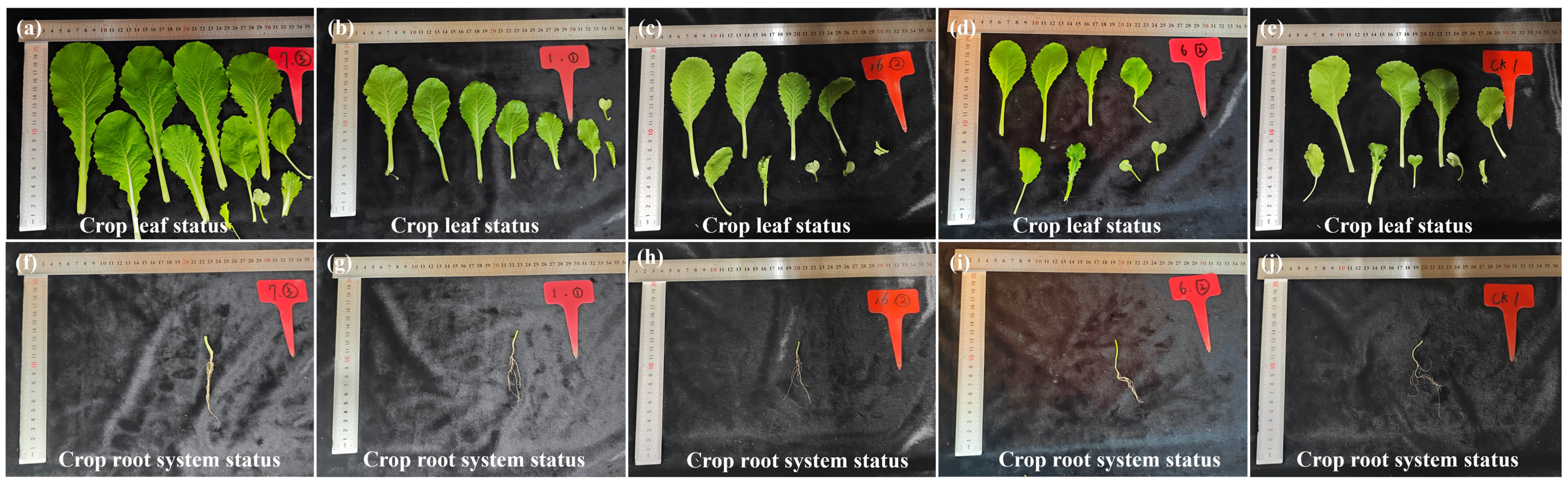
3.3.2. Effects of Biological Tillage on the Root System of Brassica Chinensis L.
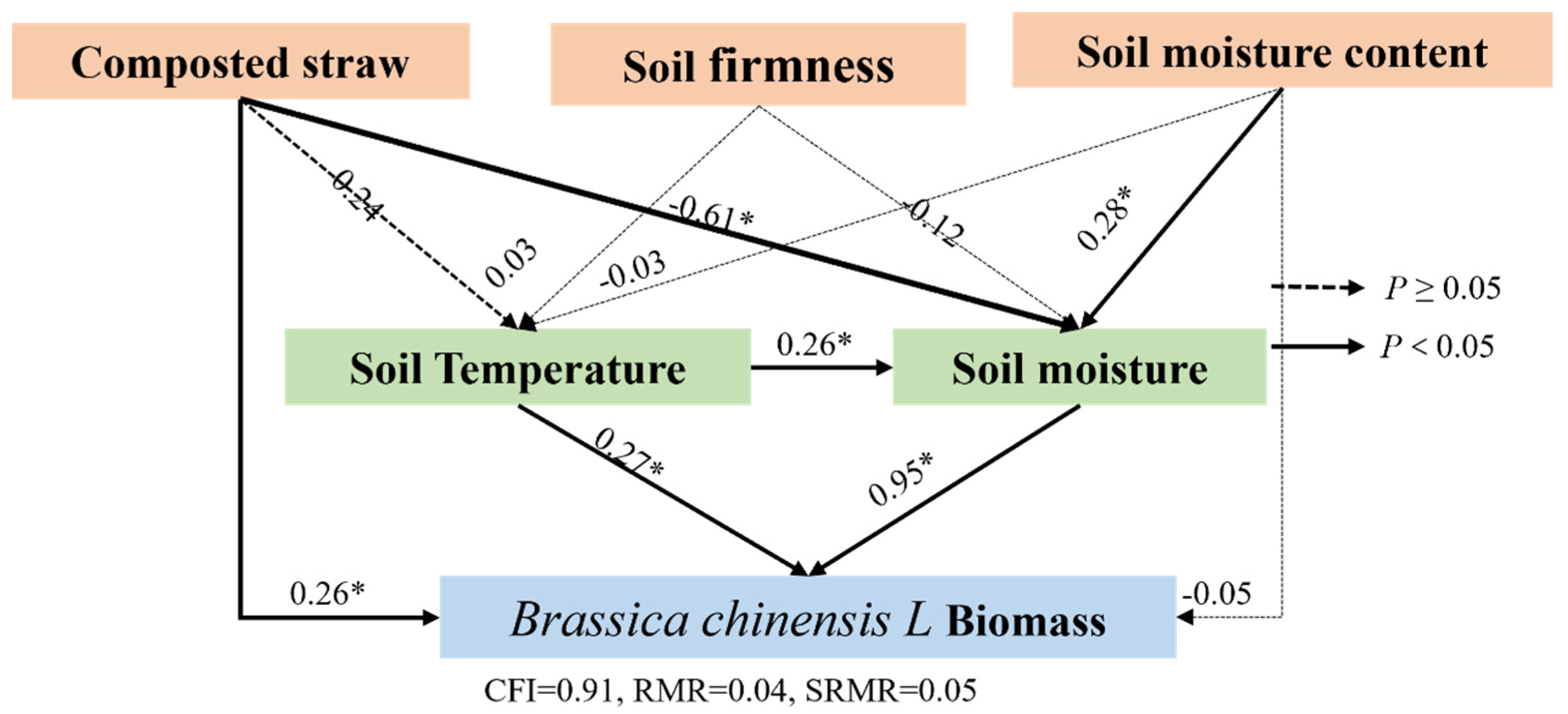
3.3.3. Structural Equation Model (SEM) of Brassica Chinensis L. Biomass Under Composted Straw, Soil Moisture Content, and Soil Compaction
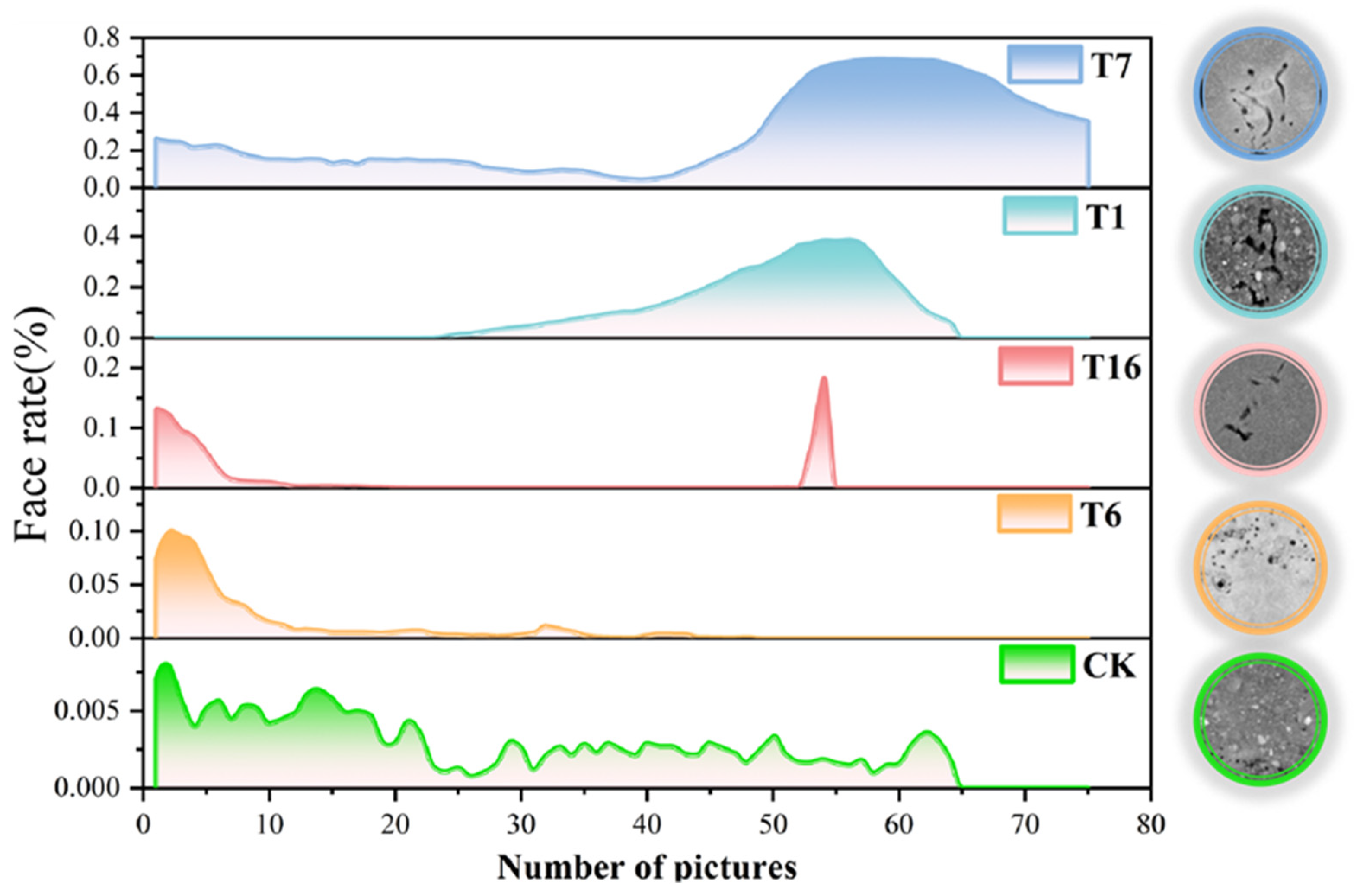
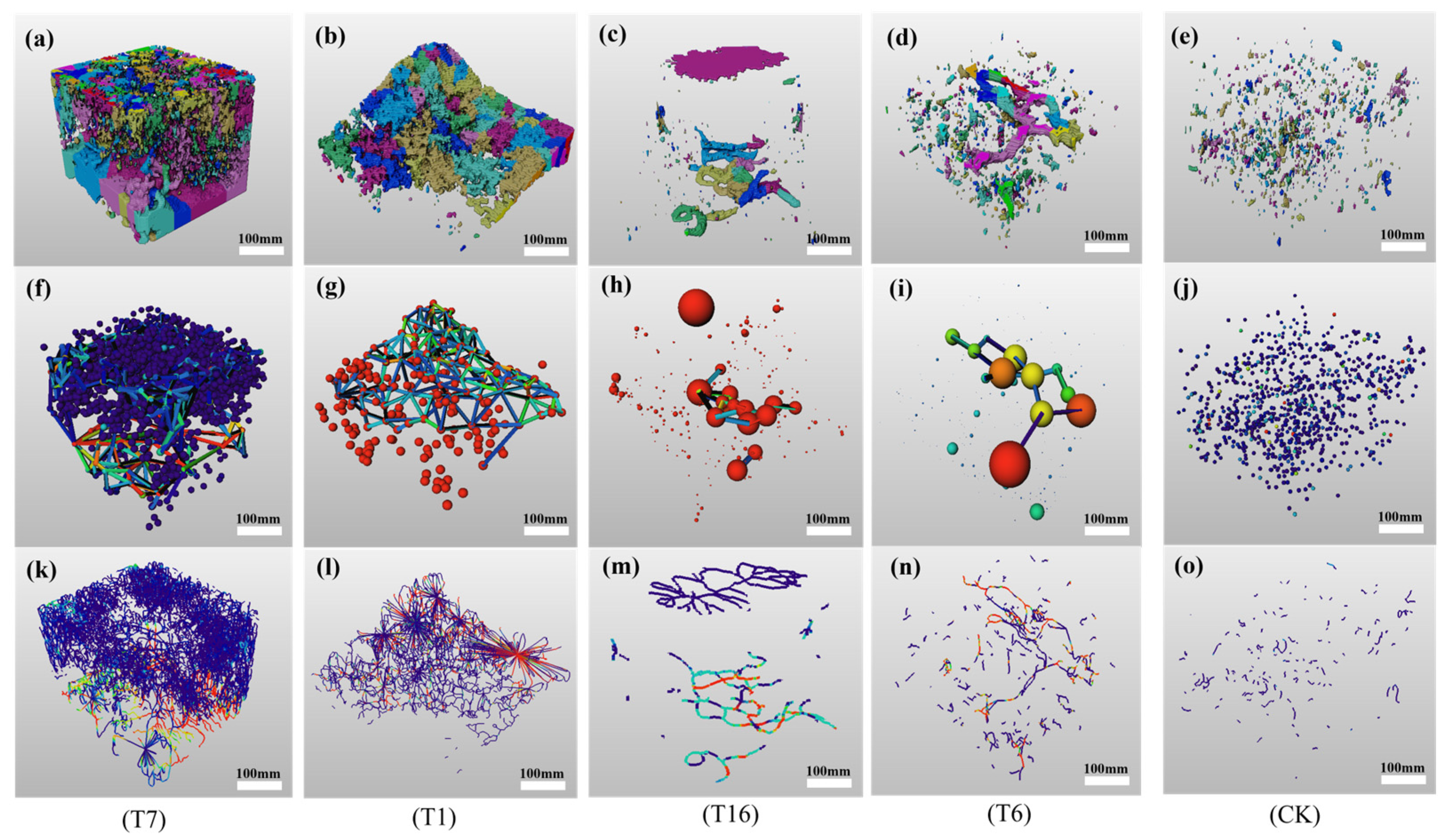
3.4. Effects of Earthworm Biological Tillage on Soil Structure Under Different Conditions of Earthworm Food, Soil Moisture, and Soil Compaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Global Map of Black Soil; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Li, R.; Hu, W.Y.; Jia, Z.J.; Liu, H.Q.; Zhang, C.; Huang, B.; Yang, S.H.; Zhao, Y.G.; Zhao, Y.C.; Shukla, M.K.; et al. Soil degradation: A global threat to sustainable use of black soils. Pedosphere 2025, 35, 264–279. [Google Scholar] [CrossRef]
- Afshar, R.K.; Cabot, P.; Ippolito, J.A.; Dekamin, M.; Reed, B.; Doyle, H.; Fry, J. Corn productivity and soil characteristic alterations following transition from conventional to conservation tillage. Soil Tillage Res. 2022, 220, 105351. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, Y.; Mu, X.; Liu, K.; Li, C. Effects of tillage on soil physical properties and root growth of maize in loam and clay in central China. Plant Soil Environ. 2013, 59, 295–302. [Google Scholar] [CrossRef]
- Yang, J.; He, J.H.; Jia, L.; Gu, H.Y. Integrating metagenomics and metabolomics to study the response of microbiota in black soil degradation. Sci. Total Environ. 2023, 899, 165486. [Google Scholar] [CrossRef]
- Behringer, M.; Koestel, J.; Muys, B.; Wriessnig, K.; Bieringer, M.; Schlögl, M.; Katzensteiner, K. A Long Road to Soil Health Restoration: Earthworms and Soil Structure Show Partial Recovery in 18-Year-Old Forest Skid Trails. Soil Biol. Biochem. 2025, 210, 109953. [Google Scholar] [CrossRef]
- Yakovenko, V.; Gorban, V.; Kotovych, O.; Didur, O.; Poleva, J. Humus Forms, Earthworm Bioturbation and Soil Organic Carbon Storage in Chernozems of the Low-Intensity Land Use of Steppe Zone of Ukraine. Geoderma Reg. 2025, 42, e00988. [Google Scholar] [CrossRef]
- Yu, M.; He, H.; Cheng, L.; Li, S.; Wan, T.; Qin, J.; Li, J. Bio-Organic Fertilizers Enhance Yield in Continuous Cotton Cropping Systems Through Rhizosphere Microbiota Modulation and Soil Nutrient Improvement. Agronomy 2025, 15, 2238. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Li, Q.; Zhang, L.; Liu, Z.; Zhang, Z.; Chen, Y. The Improved Remediation Effect of the Combined Use of Earthworms with Bacillus subtilis-Loaded Biochar in Ameliorating Soda Saline–Alkali Soil. Microorganisms 2025, 13, 1243. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chen, P.; Liu, Y.; Yin, Z.; Wang, Q.; Xu, S.; Zhang, J.; Bai, B.; Zhou, D.; Liu, Y. Earthworm (Eisenia fetida) Mediated Macropore Network Formation in Black Soil: Decay Straw as a Trigger for Sustainable Tillage. Agriculture 2025, 15, 1397. [Google Scholar] [CrossRef]
- Wen, S.; Shao, M.; Wang, J. Earthworm Burrowing Activity and Its Effects on Soil Hydraulic Properties under Different Soil Moisture Conditions from the Loess Plateau, China. Sustainability 2020, 12, 9303. [Google Scholar] [CrossRef]
- Barron, C.; Clunes, J.; Pinochet, D.; Santelices, M. Does earthworm density change the quality of degraded volcanic soil? Appl. Soil Ecol. 2024, 193, 105154. [Google Scholar] [CrossRef]
- Schon, N.L.; Mackay, A.D.; Gray, R.A.; van Koten, C.; Dodd, M.B. Influence of Earthworm Abundance and Diversity on Soil Structure and the Implications for Soil Services Throughout the Season. Pedobiologia 2017, 62, 41–47. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, N.; Sun, Q.; Horton, R.; Liu, G. The Effect of Macropore Morphology of Actual Anecic Earthworm Burrows on Water Infiltration: A COMSOL Simulation. J. Hydrol. 2023, 618, 129261. [Google Scholar] [CrossRef]
- Hallam, J.; Berdeni, D.; Grayson, R.; Guest, E.J.; Holden, J.; Lappage, M.G.; Prendergast-Miller, M.; Robinson, D.A.; Turner, A.; Leake, J.R.; et al. Effect of earthworms on soil physico-hydraulic and chemical properties, herbage production, and wheat growth on arable land converted to ley. Sci. Total Environ. 2020, 713, 136491. [Google Scholar] [CrossRef]
- Bottinelli, N.; Hallaire, V.; Menasseri-Aubry, S.; Le Guillou, C.; Cluzeau, D. Abundance and stability of belowground earthworm casts influenced by tillage intensity and depth. Soil Tillage Res. 2010, 106, 263–267. [Google Scholar] [CrossRef]
- Hallam, J.; Holden, J.; Robinson, D.A.; Hodson, M.E. Effects of Winter Wheat and Endogeic Earthworms on Soil Physical and Hydraulic Properties. Geoderma 2021, 400, 115126. [Google Scholar] [CrossRef]
- Ma, L.; Shao, M.A.; Fan, J.; Wang, J.; Li, Y. Effects of earthworm (Metaphire guillelmi) density on soil macropore and soil water content in typical Anthrosol soil. Agric. Ecosyst. Environ. 2021, 311, 107338. [Google Scholar] [CrossRef]
- Capowiez, Y.; Bottinelli, N.; Jouquet, P. Quantitative Estimates of Burrow Construction and Destruction, by Anecic and Endogeic Earthworms in Repacked Soil Cores. Appl. Soil Ecol. 2014, 74, 46–50. [Google Scholar] [CrossRef]
- Arrázola-Vásquez, E.; Larsbo, M.; Capowiez, Y.; Taylor, A.; Sandin, M.; Iseskog, D.; Keller, T. Earthworm Burrowing Modes and Rates Depend on Earthworm Species and Soil Mechanical Resistance. Appl. Soil Ecol. 2022, 178, 104568. [Google Scholar] [CrossRef]
- Seibutis, V.; Tamošiūnas, K.; Deveikytė, I.; Kadžienė, G.; Semaškienė, R. Earthworm Population Response to Simplified Tillage and Shortened Crop Rotations in a Central Lithuanian Cambisol: A Five-Year Study. Agriculture 2025, 15, 366. [Google Scholar] [CrossRef]
- Hossen, M.; Khan, M.; Azad, M.; Hashem, M.; Bhuiyan, M.; Rahman, M. Effects of Moisture Content on the Quality of Vermicompost Produced from Cattle Manure. Bangladesh J. Anim. Sci. 2022, 51, 40–46. [Google Scholar] [CrossRef]
- Hu, Y.; Kou, T.; Cong, M.; Jia, Y.; Yan, H.; Huang, X.; Yang, Z.; An, S.; Jia, H. Grassland Degradation-Induced Soil Organic Carbon Loss Associated with Micro-Food Web Simplification. Soil Biol. Biochem. 2025, 201, 109659. [Google Scholar] [CrossRef]
- Budhathoki, S.; Lamba, J.; Srivastava, P.; Williams, C.; Arriaga, F.; Karthikeyan, K.G. Impact of land use and tillage practice on soil macropore characteristics inferred from X-ray computed tomography. Catena 2022, 210, 105886. [Google Scholar] [CrossRef]
- Turunen, M.; Hyvaluoma, J.; Keskin, R.; Kaseva, J.; Nikama, J.; Reunamo, A.; Rasa, K. Pore structure of wastewater sludge chars and their water retention impacts in different soils. Biosyst. Eng. 2021, 206, 6–18. [Google Scholar] [CrossRef]
- Niu, X.L.; Cheng, Y.F.; Feng, X.P.; Zhao, W.; Zhang, X.; Du, M.J.; Gu, Y.F. The effects of Eisenia fetida and metaphire guillelmi on the soil micro-food web in a microcosm experiment. PLoS ONE 2023, 18, e0290282. [Google Scholar] [CrossRef]
- Chaoui, H.; Keener, H.M. Separating earthworms from organic media using an electric field. Biosyst. Eng. 2008, 100, 409–421. [Google Scholar] [CrossRef][Green Version]
- Elliott, E.T.; Cambardella, C.A. Physical Separation of Soil Organic Matter. Agric. Ecosyst. Environ. 1991, 34, 407–419. [Google Scholar] [CrossRef]
- Halko, S.; Vershkov, O.; Horák, J.; Lezhenkin, O.; Boltianska, L.; Kucher, A.; Suprun, O.; Miroshnyk, O.; Nitsenko, V. Efficiency of Combed Straw Harvesting Technology Involving Straw Decomposition in the Soil. Agriculture 2023, 13, 655. [Google Scholar] [CrossRef]
- Li, M.; Zhang, K.R.; Yan, Z.Q.; Liu, L.; Kang, E.Z.; Kang, X.M. Soil Water Content Shapes Microbial Community Along Gradients of Wetland Degradation on the Tibetan Plateau. Front. Microbiol. 2022, 13, 824267. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Zhou, S.; Long, K.; Xie, B.; Ai, C.; Yan, C. Investigation of key morphological parameters of pores in different grades of asphalt mixture based on CT scanning technology. Constr. Build. Mater. 2024, 434, 136770. [Google Scholar] [CrossRef]
- Zi, J.J.; Liu, T.; Zhang, W.; Pan, X.H.; Ji, H.; Zhu, H.H. Quantitatively Characterizing Sandy Soil Structure Altered by MICP Using Multi-Level Thresholding Segmentation Algorithm. J. Rock. Mech. Geotech. 2024, 16, 4285–4299. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, J.M.; Shan, L.Y.; Li, Z.W. Characterization and Design of Three-Dimensional Skeleton Structure of Asphalt Mixture Based on Network Science. Constr. Build. Mater. 2024, 419, 135497. [Google Scholar] [CrossRef]
- Li, S.X.; Zheng, X.Q.; Yuan, D.W.; Zhang, J.Q.; He, Q.Y.; Lü, W.G.; Tao, X.B. Effects of Biological Tillage on Physicochemical Properties, Soil Enzyme Activity, and Growth and Quality of Brassica oleracea var. italica. Chin. J. Eco-Agric. 2012, 20, 1018–1023. [Google Scholar] [CrossRef]
- Levasseur, A.; Chauvat, M.; Bohm, V.; Cardinael, P.; Le Mer, G.; Peulon-Agasse, V.; Forey, E. Experimental Evidence That Soil Fauna Drives Plant Root Exudation Patterns. Soil Biol. Biochem. 2025, 205, 109771. [Google Scholar] [CrossRef]
- Sutri, M.; Kuu, A.; Escuer-Gatius, J.; Konsap, K.; Shanskiy, M.; Reintam, E.; Ivask, M. Earthworm Community Structure Under Different Land-Use Systems Across Various Soil Conditions. Appl. Soil Ecol. 2025, 211, 106151. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Y.; Tian, H.; Jia, H.; Guo, M. Effects of Straw Returning and Residue Cleaner on the Soil Moisture Content, Soil Temperature, and Maize Emergence Rate in China’s Three Major Maize Producing Areas. Sustainability 2019, 11, 5796. [Google Scholar] [CrossRef]
- Hafez, E.M.; Omara, A.E.D.; Alhumaydhi, F.A.; El-Esawi, M.A. Minimizing Hazard Impacts of Soil Salinity and Water Stress on Wheat Plants by Soil Application of Vermicompost and Biochar. Physiol. Plant 2021, 172, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, B.; Li, J.; Lian, S.; Zhang, J.; Shi, S. Effects of Deficit-Regulated Irrigation on Root-Growth Dynamics and Water-Use Efficiency of Winter Wheat in a Semi-Arid Area. Water 2024, 16, 2678. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Hong, Y.; Li, C.H.; Lu, C.Y.; He, Y.H.; Shao, J.J.; Sun, X.Y.; Wang, C.Y.; Liu, R.Q.; Liu, H.Y.; et al. Responses of Biomass Allocation to Multi-Factor Global Change: A Global Synthesis. Agric. Ecosyst. Environ. 2020, 304, 107115. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.Q.; Ma, J.; Li, X.H.; Cao, J.F.; Zhou, J.; Wu, L.M.; Zhao, P.Y.; Cao, W.D. Co-Incorporating Green Manure and Crop Straw Increases Crop Productivity and Improves Soil Quality with Low Greenhouse-Gas Emissions in a Crop Rotation. Crop J. 2024, 12, 1233–1241. [Google Scholar] [CrossRef]
- Shohag, M.J.I.; Tian, S.F.; Sriti, N.; Liu, G.D. Enhancing Bok Choy Growth Through Synergistic Effects of Hydrogel and Different Nitrogen Fertilizer Forms. Sci. Hortic.-Amst. 2024, 336, 113400. [Google Scholar] [CrossRef]
- Ni, H.; Hu, H.; Zohner, C.M.; Huang, W.; Chen, J.; Sun, Y.; Ding, J.; Zhou, J.; Yan, X.; Zhang, J.; et al. Effects of Winter Soil Warming on Crop Biomass Carbon Loss from Organic Matter Degradation. Nat. Commun. 2024, 15, 8847. [Google Scholar] [CrossRef]
- Liu, C.; Tong, F.; Yan, L.; Zhou, H.; Hao, S. Effect of Porosity on Soil-Water Retention Curves: Theoretical and Experimental Aspects. Geofluids 2020, 2020, 6671479. [Google Scholar] [CrossRef]
- Frazão, J.; de Goedé, R.G.M.; Capowiez, Y.; Pulleman, M.M. Soil structure formation and organic matter distribution as affected by earthworm species interactions and crop residue placement. Geoderma 2019, 338, 453–463. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Al-Taey, D.K.A.; Al-Mammori, H.Z. Earthworms and Eco-Consequences: Considerations to Soil Biological Indicators and Plant Function: A Review. Acta Ecol. Sin. 2021, 41, 512–523. [Google Scholar] [CrossRef]
- Jarvis, N.J. A review of non-equilibrium water flow and solute transport in soil macropores: Principles, controlling factors and consequences for water quality. Eur. J. Soil Sci. 2020, 71, 279–302. [Google Scholar] [CrossRef]
- Capowiez, Y.; Cadoux, S.; Bouchand, P.; Roger-Estrade, J.; Richard, G.; Boizard, H. Experimental evidence for the role of earthworms in compacted soil regeneration based on field observations and results from a semi-field experiment. Soil Biol. Biochem. 2009, 41, 711–717. [Google Scholar] [CrossRef]
- Pierret, A.; Capowiez, Y.; Belzunces, L.; Moran, C.J. 3D reconstruction and quantification of macropores using X-ray computed tomography and image analysis. Geoderma 2002, 106, 247–271. [Google Scholar] [CrossRef]




| Soil Moisture Content (%) | Temperature (°C) | PH | Soil Bulk Density (g·cm3) | Clay (g·kg−1) | Sand (g·kg−1) | Silt (g·kg−1) |
|---|---|---|---|---|---|---|
| 19.6–21.4 | 23.2–24.7 | 6.8–7.9 | 1.6–2.1 | 107.1 3 | 823 7 | 69.9 6 |
| Soil aggregate fractionation | >2 mm | 1–2 mm | 0.5–1 mm | 0.25–0.5 mm | <0.25 mm | |
| 1 | 4.33 | 10.69 | 9.91 | 7.06 | 68.02 | |
| 2 | 65.58 | 2.47 | 3.09 | 0.30 | 28.56 | |
| 3 | 12.26 | 6.77 | 6.78 | 7.05 | 67.15 | |
| Total Nitrogen (TN) g/kg | Total Phosphorus (TP) g/kg | Total Potassium (TK) g/kg | Alkaline Hydrolyzable Nitrogen (AHN) mg/kg | Available Phosphorus (AP) mg/kg | Available Potassium (AK) mg/kg | Total Organic Carbon (TOC)% |
| 9.456 | 2.711 | 21.018 | 638.37 | 152.60 | 1426.95 | 8.55 |
| 9.736 | 2.565 | 21.297 | 658.43 | 145.25 | 1414.20 | 9.53 |
| 10.016 | 2.744 | 19.563 | 650.75 | 151.15 | 1462.50 | 8.11 |
| Level | Factor A Composted Straw | Factor B Soil Moisture Content | Factor C Soil Firmness |
|---|---|---|---|
| 1 | 50 g | 9–16% | 50–150 kPa |
| 2 | 100 g | 16–23% | 150–250 kPa |
| 3 | 23–30% | 250–350 kPa | |
| 4 | 30–37% | 350–450 kPa |
| Case | Factor A | Factor B | Factor C | Experimental Parameter Composted Straw (g) | Soil Moisture Content (%) | Soil Firmness (kPa) |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 50 g | 9–16% | 50–150 kPa |
| 2 | 1 | 1 | 3 | 50 g | 9–16% | 250–350 kPa |
| 3 | 1 | 2 | 2 | 50 g | 16–23% | 150–250 kPa |
| 4 | 1 | 2 | 4 | 50 g | 16–23% | 350–450 kPa |
| 5 | 1 | 3 | 2 | 50 g | 23–30% | 150–250 kPa |
| 6 | 1 | 3 | 4 | 50 g | 23–30% | 350–450 kPa |
| 7 | 1 | 4 | 1 | 50 g | 30–37% | 50–150 kPa |
| 8 | 1 | 4 | 3 | 50 g | 30–37% | 250–350 kPa |
| 9 | 2 | 1 | 2 | 100 g | 9–16% | 150–250 kPa |
| 10 | 2 | 1 | 4 | 100 g | 9–16% | 350–450 kPa |
| 11 | 2 | 2 | 1 | 100 g | 16–23% | 50–150 kPa |
| 12 | 2 | 2 | 3 | 100 g | 16–23% | 250–350 kPa |
| 13 | 2 | 3 | 1 | 100 g | 23–30% | 50–150 kPa |
| 14 | 2 | 3 | 3 | 100 g | 23–30% | 250–350 kPa |
| 15 | 2 | 4 | 2 | 100 g | 30–37% | 150–250 kPa |
| 16 | 2 | 4 | 4 | 100 g | 30–37% | 350–450 kPa |
| A | B | C | A | B | C | A | B | C | |
|---|---|---|---|---|---|---|---|---|---|
| Aboveground Biomass | Root biomass | Total Biomass | |||||||
| k1 | 102.7416667 | 97.72333333 | 111.9233333 | 0.020875 | 0.01525 | 0.02325 | 0.471833333 | 0.30975 | 0.502416667 |
| k2 | 94.46291667 | 97.14916667 | 98.88666667 | 0.018291667 | 0.01775 | 0.0175 | 0.364 | 0.37575 | 0.409916667 |
| k3 | 86.8075 | 94.42083333 | 0.0165 | 0.020666667 | 0.394333333 | 0.391 | |||
| k4 | 112.7291667 | 89.17833333 | 0.028833333 | 0.016916667 | 0.591833333 | 0.368333333 | |||
| R | 8.27875 | 25.92166667 | 22.745 | 0.002583333 | 0.013583333 | 0.006333333 | 0.107833333 | 0.282083333 | 0.134083333 |
| Influence Factors (from Large to Small) | B > C > A | B > C > A | B > C > A | ||||||
| Optimal Combination | A1B4C1 | A1B4C1 | A1B4C1 | ||||||
| Root shoot ratio | Mean root diameter | Taproot length | |||||||
| k1 | 0.045960974 | 0.05532323 | 0.047462601 | 1.754166667 | 1.256666667 | 1.795 | 102.7416667 | 97.72333333 | 111.9233333 |
| k2 | 0.05640854 | 0.050656619 | 0.045596167 | 1.398333333 | 1.4725 | 1.565833333 | 94.46291667 | 97.14916667 | 98.88666667 |
| k3 | 0.045216981 | 0.060541836 | 1.5225 | 1.47 | 86.8075 | 94.42083333 | |||
| k4 | 0.053541105 | 0.051138425 | 2.053333333 | 1.474166667 | 112.7291667 | 89.17833333 | |||
| R | 0.010447567 | 0.010107343 | 0.014945669 | 0.355833333 | 0.796666667 | 0.325 | 8.27875 | 25.92166667 | 22.745 |
| Influence Factors (from Large to Small) | C > A > B | B > A > C | B > C > A | ||||||
| Optimal Combination | A2B1C3 | A1B4C1 | A1B4C1 | ||||||
| Dispose | Volume Fraction | Label Volume (µm3) | Mask Volume (µm3) | Label Voxel Count | Mask Voxel Count |
|---|---|---|---|---|---|
| T7 | 0.2884249 | 2.7 × 1023 | 9.4 × 1023 | 3,714,177 | 12,877,500 |
| T1 | 0.123758 | 1.3 × 1023 | 10.8 × 1023 | 1,679,644 | 13,572,000 |
| T16 | 0.009291 | 9.7 × 1021 | 10.4 × 1023 | 77,880 | 8,382,010 |
| T6 | 0.004650 | 7.5 × 1021 | 16.0 × 1023 | 84,511 | 18,173,750 |
| CK | 0.00194 | 2.4 × 1021 | 12.2 × 1023 | 22,653 | 11,660,836 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Chen, P.; Yin, Z.; Xu, S.; Liu, Y.; Wang, Q.; Wang, Z.; Ye, J. Study on the Improved Black Soil Structure Under Biological Tillage on Brassica chinensis L. Yield. Agronomy 2025, 15, 2532. https://doi.org/10.3390/agronomy15112532
Wu B, Chen P, Yin Z, Xu S, Liu Y, Wang Q, Wang Z, Ye J. Study on the Improved Black Soil Structure Under Biological Tillage on Brassica chinensis L. Yield. Agronomy. 2025; 15(11):2532. https://doi.org/10.3390/agronomy15112532
Chicago/Turabian StyleWu, Baoguang, Pu Chen, Zhipeng Yin, Shun Xu, Yuping Liu, Qiuju Wang, Zhenyu Wang, and Junting Ye. 2025. "Study on the Improved Black Soil Structure Under Biological Tillage on Brassica chinensis L. Yield" Agronomy 15, no. 11: 2532. https://doi.org/10.3390/agronomy15112532
APA StyleWu, B., Chen, P., Yin, Z., Xu, S., Liu, Y., Wang, Q., Wang, Z., & Ye, J. (2025). Study on the Improved Black Soil Structure Under Biological Tillage on Brassica chinensis L. Yield. Agronomy, 15(11), 2532. https://doi.org/10.3390/agronomy15112532






