The Effects of Maize–Soybean and Maize–Peanut Intercropping on the Spatiotemporal Distribution of Soil Nutrients and Crop Growth
Abstract
1. Introduction
2. Materials and Methods
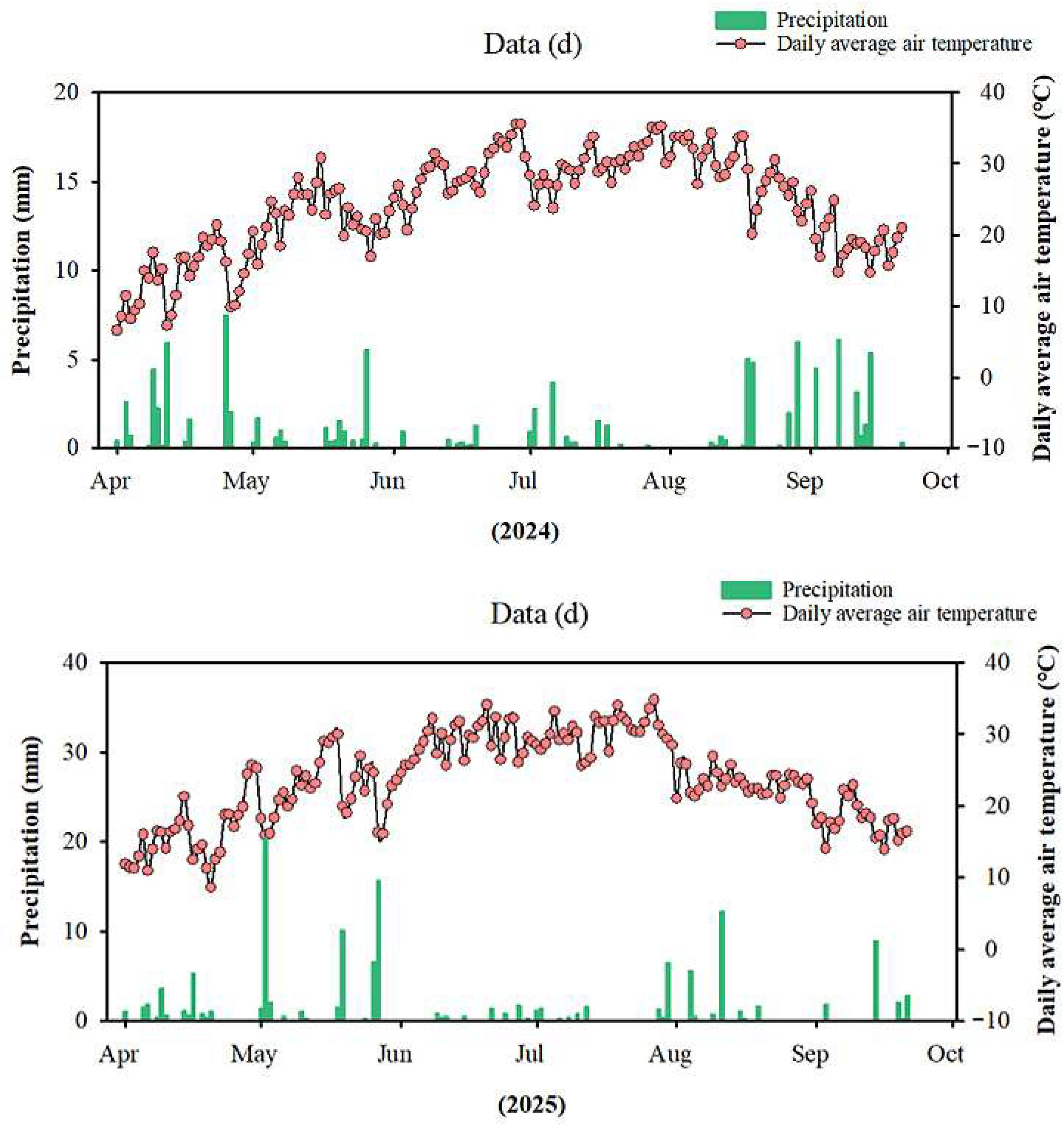
2.1. Experimental Site
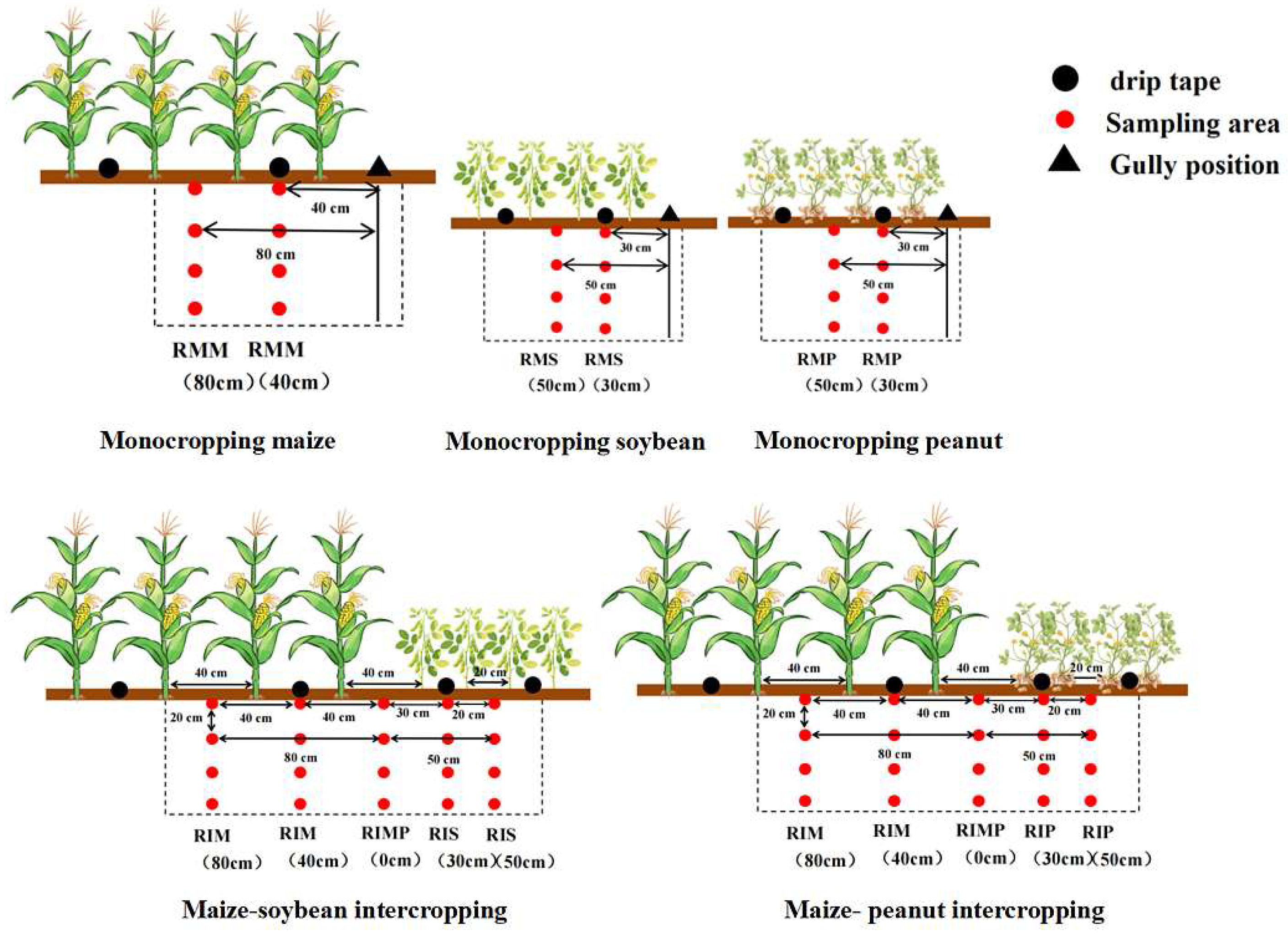
2.2. Experimental Design and Crop Management
2.3. Soil Sample Collection
2.4. Soil Sample Analysis
2.5. Measurement of Plant Height and Stem Diameter
2.6. Determination of Dry Matter
2.7. Determination of Yield, Yield Components, and Land Equivalent Ratio (LER)
2.8. Data Analysis
3. Result
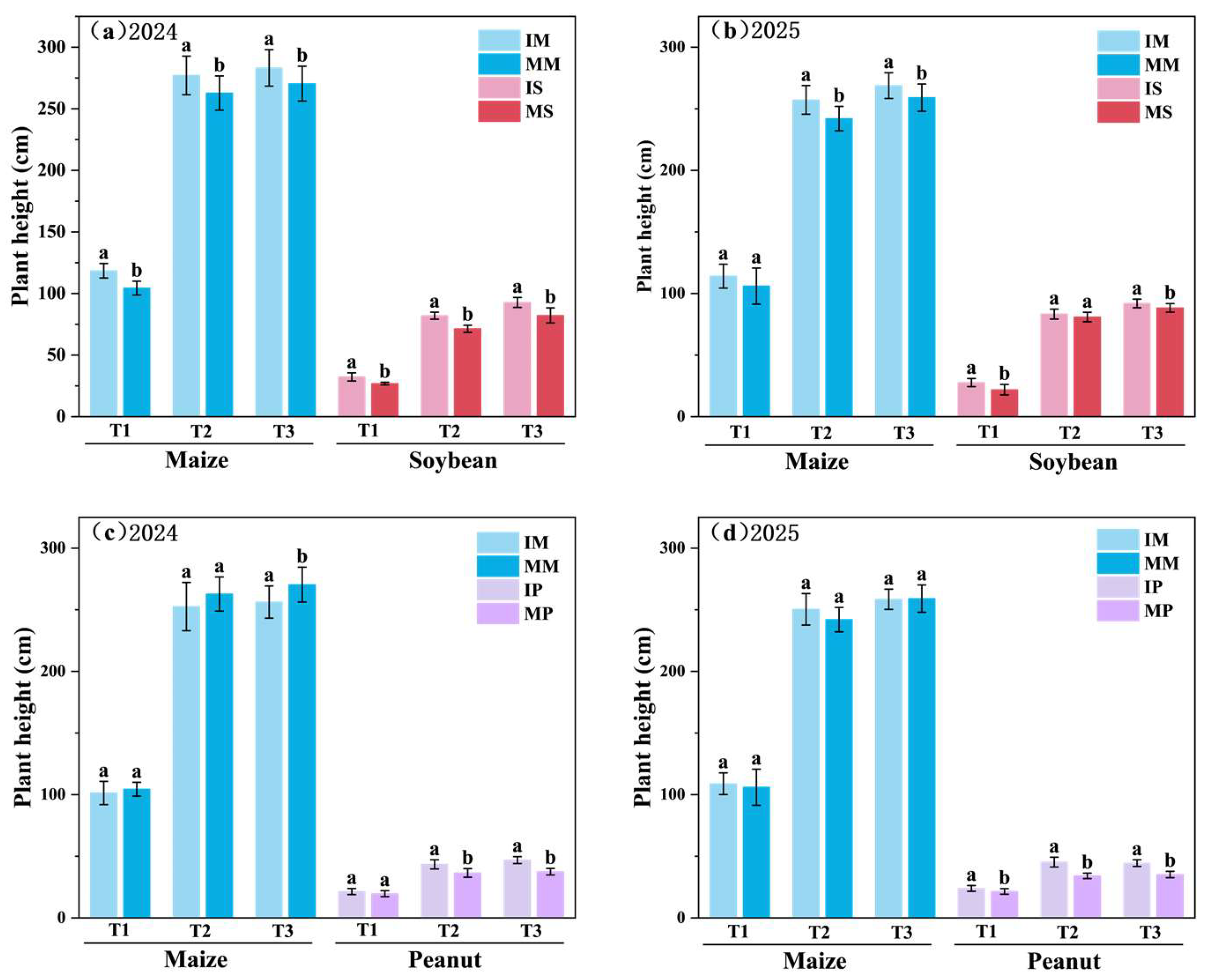
3.1. Crop Plant Height
3.2. Main Stem Thickness
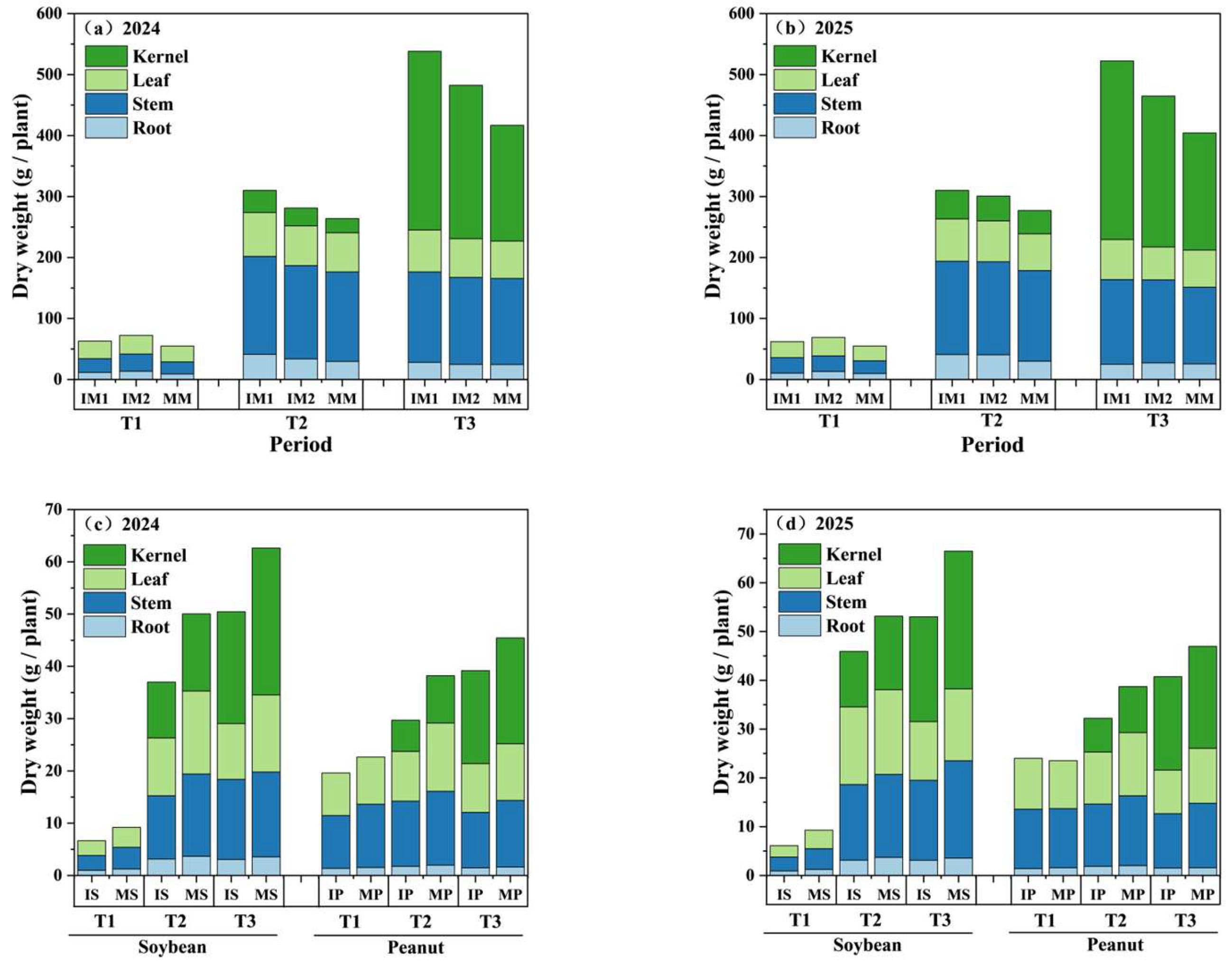
3.3. Dry Matter Accumulation (DMA)
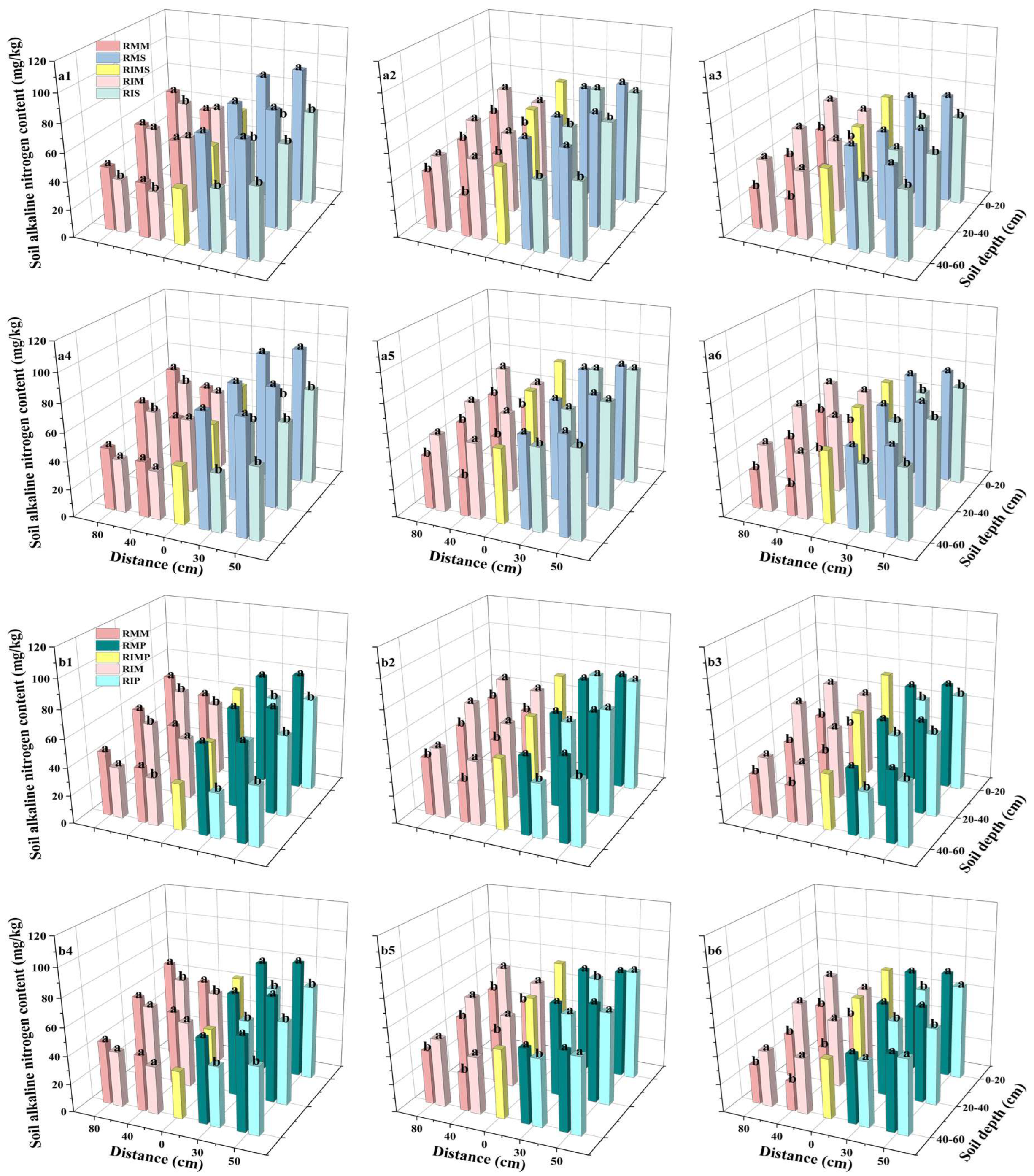
3.4. Soil Alkaline-Hydrolyzable Nitrogen (AN) Content
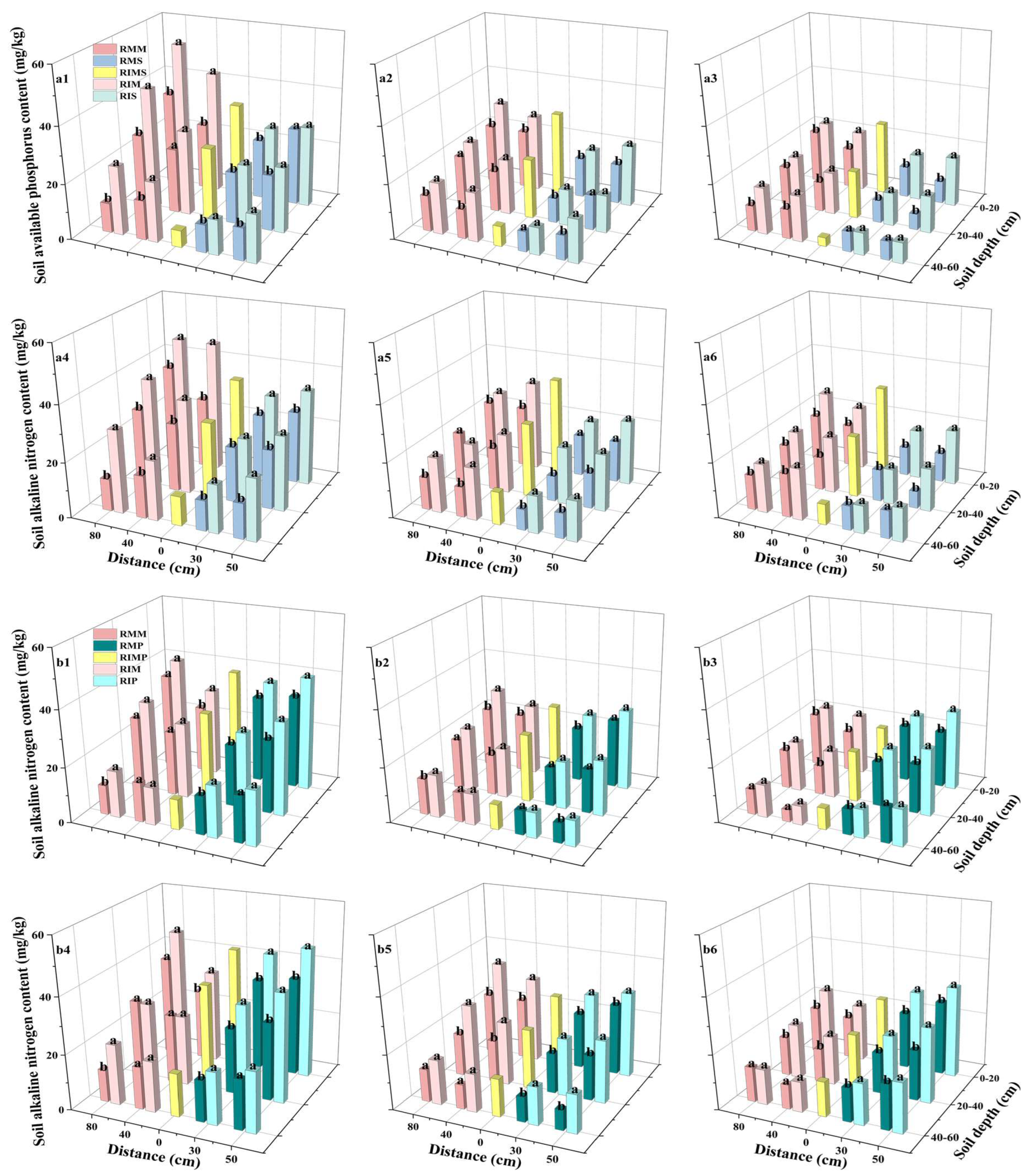
3.5. Soil Available Phosphorus (AP) Content
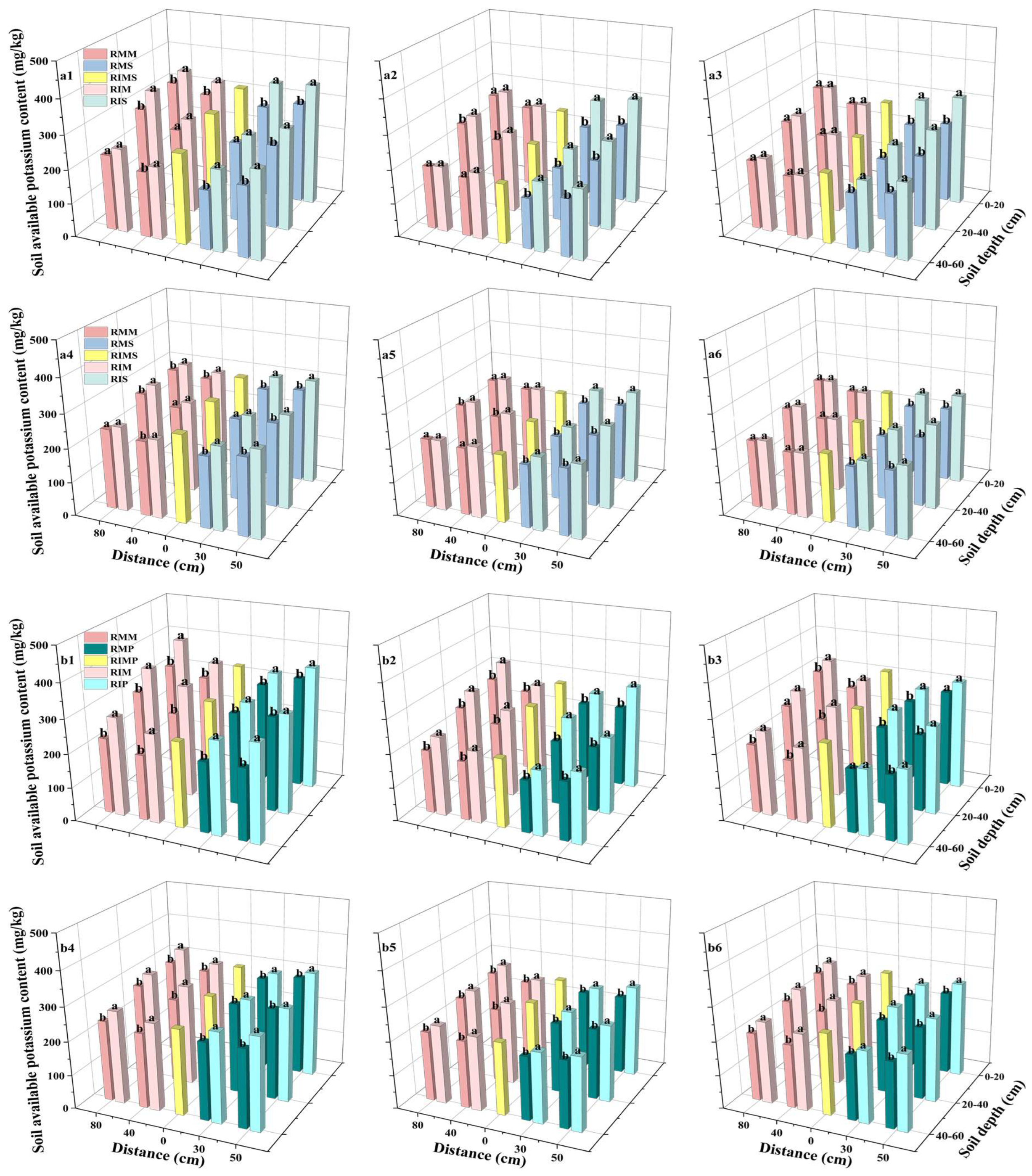
3.6. Soil Available Potassium (AK) Content
3.7. Yield, Yield Components, and Land Equivalent Ratio (LER)
4. Discussion
4.1. Intercropping Suppressed the Plant Height of Maize Grown with Peanuts, While Simultaneously Reducing the Stem Diameter of Maize, Soybeans, and Peanuts Within Each System
4.2. Intercropping Significantly Increased Maize Total Dry Weight (p < 0.05), Whilst Simultaneously Significantly Suppressing the Total Dry Weight Accumulation of Soybeans (p < 0.01) and Peanuts (p < 0.001)
4.3. Soil Alkaline-Hydrolyzable Nitrogen Content in Intercropped Maize, Soybean, and Peanut Fields Gradually Increased from T1 to T2, Then Decreased from T2 to T3
4.4. The Readily Available Phosphorus Content in Soil Decreased Progressively for Maize and Soybean Intercropping Systems. For Peanut Intercropping, Readily Available Phosphorus Levels Declined Significantly Between Treatment Periods T1 and T2, Before Showing a Slight Recovery Between T2 and T3
4.5. In Intercropped Maize, Soybean, and Peanut Plots, Soil Available Potassium Content Exhibited a V-Shaped Trend Across Growth Stages, Initially Decreasing Before Subsequently Increasing
4.6. Both Intercropping Systems Demonstrate Yield Advantages
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Gao, F.; Li, Z.; Yang, J.; Zhou, X. Effects of Different Maize-Soybean Intercropping Row Ratios on Soybean Photosynthetic Characteristics and Yield. Sichuan Agric. Univ. 2023, 41, 820–825. [Google Scholar] [CrossRef]
- Dai, J.; Qiu, W.; Wang, T.; Nakanishi, H.; Zuo, Y. From Leguminosae/Gramineae intercropping systems to see benefits of intercropping on iron nutrition. Front. Plant Sci. 2019, 10, 605. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Xu, G.; Qin, Y.; Zhang, S. Soil Fertilizer and Agricultural Sustainable Development. Ahnui. Agric. Sci. Bull. 2008, 21. 045. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Zhang, F.; Li, X.; Yang, S.; Rengel, Z. Wheat/maize or wheat/soybean strip intercropping: I. Yield advantage and interspecific interactions on nutrients. Field Crops Res. 2001, 71, 123–137. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, Z.; Zhou, Y.; Ma, Q.; Shen, S. Crop yield and fertilizer contribution under different fertilization systems. Chin. J. Ecol. Agric. 2007, 15, 54–58. [Google Scholar]
- Li, S.; Shen, C. Research progress on regulation of nitrate nitrogen metabolism and utilization in crops. J. Nanjing Agric. Univ. 2022, 45, 8. [Google Scholar] [CrossRef]
- Yang, X.; Fu, Y.; Kang, Q.; Zhang, Z.; Yuan, S. Study on the molecular mechanism of nitrogen interaction in plant leaf development. In Proceedings of the First Conference on Frontiers in Plant Science, Nanjing, China, 18 July 2022. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S.; Lv, Y.; Wang, Y.; Wang, L. Chlorophyll fluorescence reflects the nitrogen nutrition status of maize leaves under long-term nitrogen deficiency. In Proceedings of the 20th Annual Conference of the Chinese Crop Science Society, Changsha, China, 1 November 2023. [Google Scholar] [CrossRef]
- Li, S. Occurrence form and environmental significance of phosphorus in mountain soil at different altitudes in Shennongjia. J. Huazhong Agric. Univ. 2015. [Google Scholar]
- Wu, Y. Study on the Epigenetic Mechanism of Maize Seedling Roots under Nitrogen Deficiency and Re-nitrogen Treatment. J. Northwest A F Univ. 2012. [Google Scholar]
- Zhang, D.; Che, Z.; Liu, J.; Yang, J. Research progress on the response and mechanism of maize to nitrogen forms. J. Plant Nutr. Fertil. 2024, 30, 1020–1031. [Google Scholar] [CrossRef]
- Ma, H.; Yu, X.; Yu, Q.; Wu, H.; Zhang, H.; Pang, J.; Gao, Y. Maize/alfalfa intercropping enhances yield and phosphorus acquisition. Field Crops Res. 2023, 303, 109136. [Google Scholar] [CrossRef]
- Li, C. Effects of Coniferous and Broadleaf Mixed Transformation on Nitrogen and Phosphorus Fractions and Their Transformation in Rhizosphere Soil of Chinese Fir Plantation. Master’s Thesis, Guangxi University, China, 2024. [Google Scholar] [CrossRef]
- Liao, D.; Zhang, C.; Lambers, H.; Zhang, F. Changes in soil phosphorus fractions in response to long-term phosphate fertilization under sole cropping and intercropping of maize and faba bean on a calcareous soil. Plant Soil 2021, 463, 589–600. [Google Scholar] [CrossRef]
- Khashi u Rahman, M.; Wang, X.; Gao, D.; Zhou, X.; Wu, F. Root exudates increase phosphorus availability in the tomato/potato onion intercropping system. Plant Soil 2021, 464, 45–62. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Han, C.; Yang, D.; Yang, J.; Cade-Menun, B.J.; Sui, P. Maize-soybean intercropping facilitates chemical and microbial transformations of phosphorus fractions in a calcareous soil. Front. Microbiol. 2022, 13, 1028969. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Jia, X.; Shi, J.; Wei, M.; Teng, W. Research progress on effects of intercropping on crops, soils and microorganisms. Jiangsu Agric. Sci. 2024, 52, 18–30. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.; Kumar, V.; Shackira, A.; Puthur, J.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Li, N.; Zhang, J. Discussion on Extraction of Potassium from Plant Ash. Agric. Technol. 2016, 36, 2. [Google Scholar] [CrossRef]
- McGivern, B.; Cronin, D.; Ellenbogen, J.; Borton, M.; Knutson, E.; Freire-Zapata, V.; Wrighton, K. Microbial polyphenol metabolism is part of the thawing permafrost carbon cycle. Nat. Microbiol. 2024, 9, 1454–1466. [Google Scholar] [CrossRef]
- Lv, Y. Study on Phosphorus Balance and Soil Phosphorus Availability in Paddy-upland Rotation System. J. China Agric. Univ. 2016. [Google Scholar]
- Li, Y.; Sun, J.; Yu, C.; Zhang, F.; Li, L. Effects of nitrogen application rate and faba bean/maize intercropping on spatial and temporal distribution of soil inorganic nitrogen. J. Plant Nutr. Fertil. 2009, 15, 815–823. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Tang, L.; Zheng, Y.; Li, H.; Pan, H.; Zhu, D.; Wang, J.; Huang, S.; Qin, X.; et al. Dynamics of N acquisition and accumulation and its interspecific N competition in a wheat-faba bean intercropping system. Plant Nutr. Fertil. Sci. 2020, 26, 11. [Google Scholar] [CrossRef]
- Yu, R.; Yang, H.; Xing, Y.; Zhang, W.; Lambers, H.; Li, L. Belowground processes and sustainability in agroecosystems with intercropping. Plant Soil 2022, 476, 263–288. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Yang, M.; Liang, W.; Zhang, X. Intercropping with appropriate nitrogen reduction achieves the trade-off among soil biological health, soil multifunctionality, and crop productivity. Field Crops Res. 2025, 333, 110085. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Q.; Zhou, G.; Wu, W.; Xiu, X.; Wang, J. Intelligent Identification of Crop Agronomic Traits and Morphological Structure Phenotypes: A Review. Smart Agric. 2024, 6, 14. [Google Scholar] [CrossRef]
- Xiang, D.; Song, Y.; Wu, Q.; Ma, C.; Zhao, J.; Wan, Y.; Zhao, G. Relationship between stem characteristics and lodging resistance of Tartary buckwheat (Fagopyrum tataricum). Plant Prod. Sci. 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, J.; Chen, L.; Li, W. Effects of maize row spacing on growth and interspecific competitiveness of soybean/maize intercropping crops. Soybean Sci. 2019, 38, 229–235. [Google Scholar]
- Raza, M.; Bin Khalid, M.; Zhang, X.; Feng, L.; Khan, I.; Hassan, M.; Yang, W. Effect of planting patterns on yield, nutrient accumulation and distribution in maize and soybean under relay intercropping systems. Sci. Rep. 2019, 9, 4947. [Google Scholar] [CrossRef]
- Liu, W.; Yong, T.; Liu, X.; Chen, P.; Dong, Q.; Xu, T.; Yang, W. Effects of reduced nitrogen application on nitrogen fixation and nitrogen absorption and utilization of soybean nodules in maize-soybean relay strip intercropping system. Soybean Sci. 2014, 33, 705–712. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, P.; Pang, T.; Du, Q.; Fu, Z.; Zhou, Y.; Yong, T. Effects of maize/legume intercropping patterns on dry matter accumulation, distribution and yield of crops. J. Sichuan Agric. Univ. 2017, 35, 484–490. [Google Scholar] [CrossRef]
- Jiao, N.; Wang, J.; Ma, C.; Zhang, C.; Guo, D.; Zhang, F.; Jensen, E. The importance of aboveground and belowground interspecific interactions in determining crop growth and advantages of peanut/maize intercropping. Crop J. 2021, 9, 1460–1469. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, P.; Du, Q.; Zhou, Y.; Ren, J.; Jin, F.; Yong, T. Effects of maize/soybean and maize/peanut intercropping systems on crops nitrogen uptake and nodulation nitrogen fixation. Chin. J. Eco-Agric. 2019, 27, 1183–1194. [Google Scholar] [CrossRef]
- Jiao, N.; Hou, L.; Ning, T.; Li, Z.; Li, Y.; Fu, G. Analysis on the advantages of maize-peanut intercropping nitrogen and phosphorus nutrition intercropping. Crops 2007, 4, 50–53. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, P.; Zhang, X.; Du, Q.; Zheng, B.; Yang, H.; Yong, T. Maize-legume intercropping achieves yield advantages by improving leaf functions and dry matter partition. BMC Plant Biol. 2023, 23, 438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Meng, L.; Li, Y.; Wang, X.; Ogundeji, A.; Li, X.; Li, S. Yield and nutrient uptake dissected through complementarity and selection effects in the maize/soybean intercropping. Food Energy Secur. 2021, 10, 379–393. [Google Scholar] [CrossRef]
- Raza, M.; Gul, H.; Wang, J.; Yasin, H.S.; Qin, R.; Khalid, M.; Yang, W. Land productivity and water use efficiency of maize-soybean strip intercropping systems in semi-arid areas: A case study in Punjab Province, Pakistan. J. Clean. Prod. 2021, 308, 127282. [Google Scholar] [CrossRef]
- Fang, P. Effects of maize-soybean intercropping on dry matter accumulation, yield and quality of vegetable soybean. J. Sichuan Agric. Univ. 2016. [Google Scholar]
- Ye, D.; Song, Y.; Yue, Y.; Yang, Y. Study on dry matter accumulation and transport characteristics of different varieties of soybean under maize-soybean intercropping. Shaanxi J. Agric. Sci. 2024, 70, 18–22. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Li, C.; Li, L.; Cheng, X.; Zhang, F. Effects of nitrogen application on agronomic traits and nodulation characteristics of faba bean in faba bean/maize intercropping system. Sci. Agric. Sin. 2009, 42, 3467–3474. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, B. Research of Intercropping Benefit of Crop Intercropping Systems. Chin. Agric. Sci. Bull. 2007, 11, 192–196. [Google Scholar] [CrossRef]
- Brewer, M.; Kanissery, R.; Strauss, S.; Kadyampakeni, D. Impact of cover cropping on temporal nutrient distribution and availability in the soil. Horticulturae 2023, 9, 1160. [Google Scholar] [CrossRef]
- Kosslak, R.; Bohlool, B.; Dowdle, S.; Sadowsky, M. Competition of Rhizobium japonicum strains in early stages of soybean nodulation. Appl. Environ. Microbiol. 1983, 46, 870–873. [Google Scholar] [CrossRef]
- Kang, G.; Wang, P.; Li, Y.; Wang, J.; Han, Q. Progress on mechanism on the phosphorus-efficient absorption and utilization in wheat (Triticum aestivum L.). J. Plant Nutr. Fertil. 2025, 31, 800–809. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, Y.; Ge, C. Molecular regulatory mechanisms of rice response to low phosphorus stress at seedling stage. J. Plant Nutr. Fertil. 2024, 30, 2230–2236. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, X.; Zhang, Z.; Yang, X.; Ai, C.; Wang, Z.; Xu, X. Revisiting potassium-induced impacts on crop production and soil fertility based on thirty-three Chinese long-term experiments. Field Crops Res. 2025, 322, 109732. [Google Scholar] [CrossRef]
- Zhang, W.; Fornara, D.; Yang, H.; Yu, R.; Callaway, R.; Li, L. Plant litter strengthens positive biodiversity–ecosystem functioning relationships over time. Trends Ecol. Evol. 2023, 38, 473–484. [Google Scholar] [CrossRef]
- Li, C.; Stomph, T.; Makowski, D.; Li, H.; Zhang, C.; Zhang, F.; Werf, D. The productive performance of intercropping. Proc. Natl. Acad. Sci. USA 2023, 120, e2201886120. [Google Scholar] [CrossRef]
- Li, X.; Fan, C.; Xu, Q.; Hou, J.; Wang, H.; Liu, Y.; Han, W. Effects of different maize and soybean planting patterns on crop yield, economic benefits and soil nutrients. Chin. Agric. Sci. Bull. 2025, 41, 22–28. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, L.; Zhang, D.; Mao, S.; Hu, F.; Yin, W.; Chao, Q. The response of maize yield sustainability to intercropping green manure under different irrigation amounts. Acta Agron. Sin. 2025, 41, 22–28. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, J.; Li, L.; Li, W. Effects of changing maize row spacing on yield of pea/maize intercropping system. Chin. J. Eco-Agric. 2012, 20, 1451–1456. [Google Scholar] [CrossRef]

| Soil Depth (cm) | Organic Matter (g/kg) | Total Nitrogen (g/kg) | Alkali-Hydrolyzable Nitrogen (mg/kg) | Phosphorus (mg/kg) | Rapid-Acting Potassium (mg/kg) |
|---|---|---|---|---|---|
| 0–20 | 23.33 ± 2.59 | 1.29 ± 0.30 | 64.40 ± 3.12 | 23.73 ± 1.00 | 207.59 ± 8.27 |
| 20–40 | 17.19 ± 1.04 | 0.90 ± 0.15 | 52.33 ± 8.15 | 19.79 ± 1.64 | 161.26 ± 7.10 |
| 40–60 | 11.68 ± 1.34 | 0.44 ± 0.10 | 35.79 ± 4.47 | 14.39 ± 0.69 | 122.62 ± 8.10 |
| Year | Treatment | Number of Rows Per Ear/Number of Effective Pods | Grain Number Per Panicle/Grain Number Per Plant | Thousand Grain Weight (g) | Yield (kg·hm−2) | LER |
|---|---|---|---|---|---|---|
| 2024 | MM | 15.00 ± 1.05 a | 600.93 ± 52.81 a | 334.17 ± 4.11 a | 13,783.01 ± 169.53 a | |
| MS | 32.60 ± 2.41 a | 79.00 ± 22.63 a | 242.40 ± 9.57 a | 2680.96 ± 105.84 a | ||
| IM | 14.80 ± 1.03 a | 611.33 ± 42.80 a | 354.50 ± 13.36 a | 10,409.31 ± 392.37 b | 1.12 ± 0.02 | |
| IS | 26.20 ± 1.48 b | 73.40 ± 12.76 a | 207.30 ± 7.90 b | 973.82 ± 37.12 b | ||
| 2025 | MM | 14.90 ± 1.52 a | 628.80 ± 73.17 a | 319.70 ± 6.44 b | 12,489.19 ± 101.70 a | |
| MS | 32.00 ± 1.87 a | 83.80 ± 21.15 a | 234.87 ± 13.55 a | 2755.47 ± 158.98 a | ||
| IM | 15.10 ± 1.29 a | 607.33 ± 51.50 a | 348.203 ± 15.38 a | 10,154.05 ± 448.61 b | 1.17 ± 0.06 | |
| IS | 26.80 ± 2.17 b | 74.80 ± 12.19 a | 204.77 ± 7.90 b | 980.26 ± 37.82 b |
| Year | Treatment | Number of Rows Per Ear/Number of Pods Per Plant | Grain Number Per Panicle/Full Fruit Number Per Plant | Thousand Kernel Weight (g) | Yield (kg·hm−2) | LER |
|---|---|---|---|---|---|---|
| 2024 | MM | 15.00 ± 1.05 a | 600.93 ± 52.81 a | 334.17 ± 4.11 a | 13,783.01 ± 169.53 a | |
| MS | 23.00 ± 2.39 a | 19.00 ± 1.41 a | 725.65 ± 44.62 a | 5066.88 ± 311.59 a | ||
| IM | 15.00 ± 1.41 a | 625.47 ± 76.12 a | 341.37 ± 4.45 a | 9745.58 ± 127.04 b | 1.05 ± 0.05 | |
| IS | 22.63 ± 1.69 a | 15.50 ± 1.07 b | 662.48 ± 26.90 a | 1725.10 ± 70.05 b | ||
| 2025 | MM | 14.90 ± 1.52 a | 628.80 ± 73.17 a | 319.70 ± 6.44 b | 12,489.19 ± 101.70 a | |
| MS | 25.13 ± 1.55 a | 21.50 ± 2.45 a | 756.71 ± 32.47 a | 5409.58 ± 232.10 a | ||
| IM | 15.00 ± 1.15 a | 593.53 ± 64.37 a | 352.40 ± 11.68 a | 9443.77 ± 168.69 b | 1.07 ± 0.02 | |
| IS | 22.38 ± 2.00 b | 15.88 ± 1.81 b | 662.30 ± 27.95 b | 1682.26 ± 71.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhao, Y.; Li, G.; Shen, L.; Wei, W.; Li, Z.; Tuerti, T.; Zhang, W. The Effects of Maize–Soybean and Maize–Peanut Intercropping on the Spatiotemporal Distribution of Soil Nutrients and Crop Growth. Agronomy 2025, 15, 2527. https://doi.org/10.3390/agronomy15112527
Zhang W, Zhao Y, Li G, Shen L, Wei W, Li Z, Tuerti T, Zhang W. The Effects of Maize–Soybean and Maize–Peanut Intercropping on the Spatiotemporal Distribution of Soil Nutrients and Crop Growth. Agronomy. 2025; 15(11):2527. https://doi.org/10.3390/agronomy15112527
Chicago/Turabian StyleZhang, Wenwen, Yitong Zhao, Guoyu Li, Lei Shen, Wenwen Wei, Zhe Li, Tayir Tuerti, and Wei Zhang. 2025. "The Effects of Maize–Soybean and Maize–Peanut Intercropping on the Spatiotemporal Distribution of Soil Nutrients and Crop Growth" Agronomy 15, no. 11: 2527. https://doi.org/10.3390/agronomy15112527
APA StyleZhang, W., Zhao, Y., Li, G., Shen, L., Wei, W., Li, Z., Tuerti, T., & Zhang, W. (2025). The Effects of Maize–Soybean and Maize–Peanut Intercropping on the Spatiotemporal Distribution of Soil Nutrients and Crop Growth. Agronomy, 15(11), 2527. https://doi.org/10.3390/agronomy15112527






