Bacteriophages as a Sustainable Tool for Plant Disease Management: Benefits and Challenges
Abstract
1. Introduction: The Need for Sustainable Plant Disease Management
2. Benefits of Using Bacteriophages as Biological Control Agents
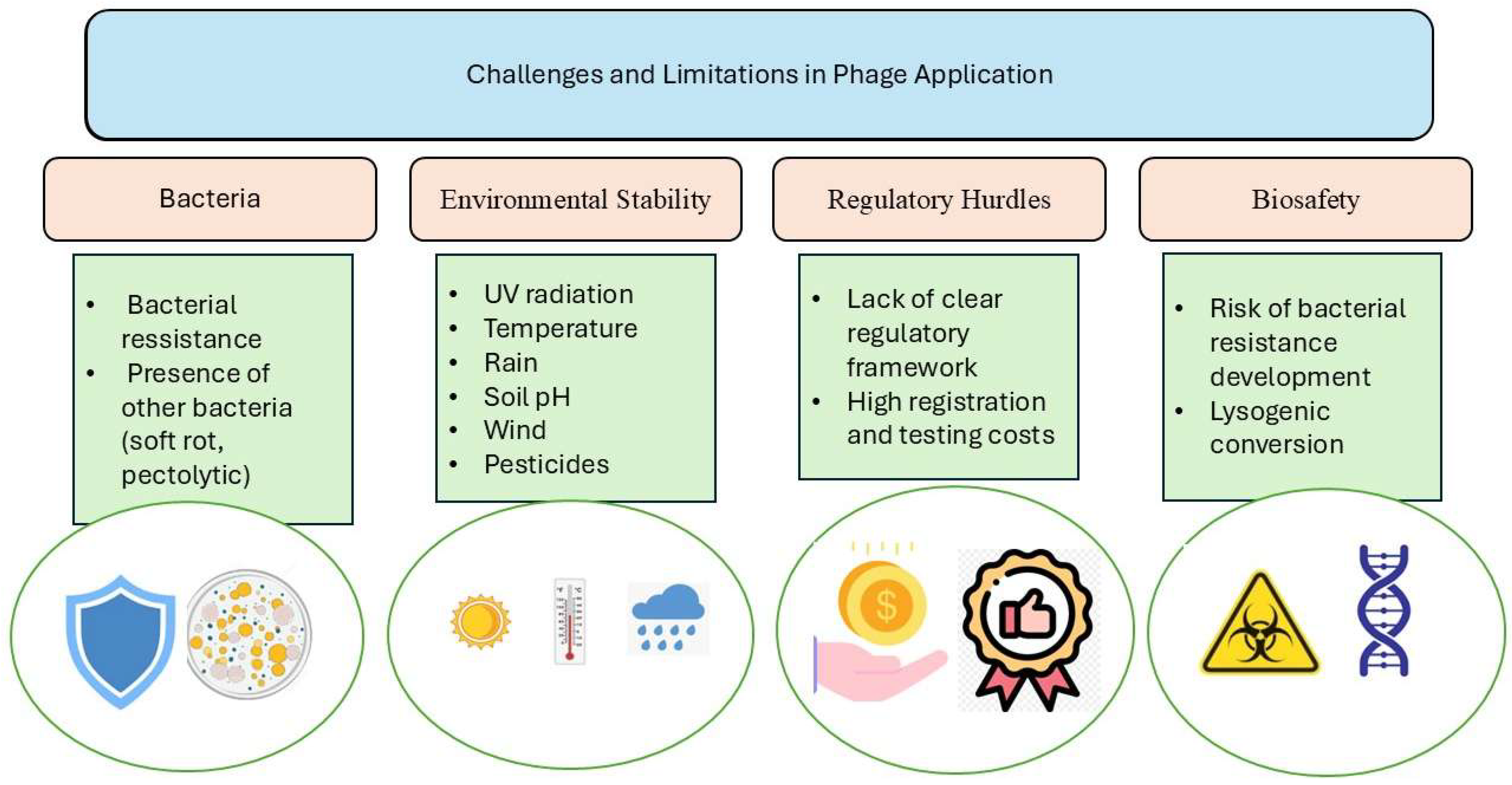
3. Challenges and Limitations in Phage Application
3.1. Internal Bacterial Defense Mechanisms Against Phages
3.1.1. Restriction–Modification System (RM)
3.1.2. Inhibition of Capsule Production and Lysogenization
3.1.3. Inhibition of Phage Adsorption
3.1.4. Blocking DNA Injection
3.1.5. Abortive Infection
3.1.6. Toxin–Antitoxin System
3.1.7. Bacteriophage Assembly Interference
3.2. Environmental Stability
3.3. Regulatory Hurdles and Biosafety
4. Bacterial Plant Diseases Addressable by Bacteriophages
| Disease | Pathogen | Plant | Method | Outcome | Source |
|---|---|---|---|---|---|
| Citrus Diseases | Xanthomonas axonopodis pv. citri | Grapefruit (Duncan cultivar) | Evening foliar application before infection | 59% reduction in disease severity | [72] |
| Tomato Diseases | Ralstonia solanacearum | Tomato (Oogata-Fukuju cultivar) | Plant soaking pre-infection | Prevention of wilting symptoms with phage ΦRSL1 | [74] |
| Potato Diseases | Streptomyces scabies | Potato (Kennebeck cultivar) | Tuber immersion | Significant reduction in lesion number and surface area | [73,74] |
| Leek Diseases | Pseudomonas syringae pv. porri | Leek | Field trial with foliar application | Reduced lesion length and improved storage life | [70] |
| Lettuce Diseases | Pectobacterium carotovorum subsp. carotovorum | Lettuce | Foliar spraying post-infection | Over 80% of seedlings showed no disease symptoms | [69] |
| Potato Diseases | Pectolytic bacteria | Potato | Application in storage | Reduction in bacterial concentrations in the warehouse | [57] |
| Potato Diseases | Dickeya solani | Potato | T4-like phage cocktails | Reduction in the incidence and severity of soft rot | [66] |
| Potato Diseases | Pectobacterium atrosepticum | Potato | Application before storage | Prevention of rotting | [67] |
| Potato Diseases | Pectobacterium carotovorum, Dickeya solani | Potato | Phage cocktail application | Up to 80% reduction in soft rot severity | [60] |
| Potato Diseases | Pectobacterium spp. | Potato | Phage cocktail application | Reduction un bacterial growth and tissue maceration | [68] |
| Diseases of Potatoes, Tomatoes, Peppers | Ralstonia solanacearum | Potatoes, tomatoes, peppers | Application in stored products | Reduction in bacterial wilt | [75] |
| Tomato Diseases | Ralstonia solanacearum | Tomato | Phage application in the rhizosphere | Reduction in wilting symptoms | [76] |
| Product Spoilage | Staphylococcus aureus, Bacillus sp., Lactobacillus sp., Streptococcus sp., E. coli, Klebsiella sp., Enterococcus faecalis | Various vegetables and fruits (tomatoes, grapes, mushrooms, green pepper, lettuce, spinach, apples) | Phage application | Reduction in spoilage bacteria, extended shelf life | [78] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzo, D.M.; Lichtveld, M.; Mazet, J.A.K.; Togami, E.; Miller, S.A. Plant Health and Its Effects on Food Safety and Security in a One Health Framework: Four Case Studies. One Health Outlook 2021, 3, 6. [Google Scholar] [CrossRef]
- Gai, Y.; Wang, H. Plant Disease: A Growing Threat to Global Food Security. Agronomy 2024, 14, 1615. [Google Scholar] [CrossRef]
- Gauthier, N.; Leonberger, K.; Smith, H. Postharvest Disease Losses in Fruit & Vegetable Crops. Available online: https://plantpathology.mgcafe.uky.edu/files/ppfs-gen-24.pdf (accessed on 7 August 2025).
- Baloch, S.B.; Ali, S.; Bernas, J.; Konvalina, P.; Naveed, M.; Baloch, F.B.; Jamali, Z.H.; Lošák, T.; Roubík, H.; Ghafoor, A.; et al. Crop Residue Management for Soil Health and Environmental Sustainability: A Comprehensive Review. J. Soil Sci. Plant Nutr. 2025, 25, 7808–7828. [Google Scholar] [CrossRef]
- Shahbazi, F.; Shahbazi, S.; Zare, D. Losses in Agricultural Produce: Causes and Effects on Food Security. Food Energy Secur. 2025, 14, e70086. [Google Scholar] [CrossRef]
- Ranveer, S.A.; Dasriya, V.; Ahmad, M.F.; Dhillon, H.S.; Samtiya, M.; Shama, E.; Anand, T.; Dhewa, T.; Chaudhary, V.; Chaudhary, P.; et al. Positive and Negative Aspects of Bacteriophages and Their Immense Role in the Food Chain. npj Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef]
- Xu, Y. Phage and Phage Lysins: New Era of Bio-Preservatives and Food Safety Agents. J. Food Sci. 2021, 86, 3349–3373. [Google Scholar] [CrossRef]
- Khan, F.M.; Chen, J.H.; Zhang, R.; Liu, B. A Comprehensive Review of the Applications of Bacteriophage-Derived Endolysins for Foodborne Bacterial Pathogens and Food Safety: Recent Advances, Challenges, and Future Perspective. Front. Microbiol. 2023, 14, 1259210. [Google Scholar] [CrossRef]
- Siyanbola, K.; Ejiohuo, O.; Ade-adekunle, O.; Adekunle, F.; Onyeaka, H.; Furr, C.-L.; Hodges, F.; Carvalho, P.; Elijah Kolawole, D.O. Bacteriophages: Sustainable and Effective Solution for Climate-Resilient Agriculture. Sustain. Microbiol. 2024, 1, qvae025. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000; ISBN 9780198506980. [Google Scholar]
- Nawaz, A.; Zafar, S.; Shahzadi, M.; Bukhari, S.M.A.U.S.; Khan, N.; Shah, A.A.; Badshah, M.; Khan, S. Bacteriophages: An Overview of the Control Strategies against Phytopathogens. Egypt. J. Biol. Pest Control 2023, 33, 108. [Google Scholar] [CrossRef]
- Halawa, E.M. Challenges of Bacteriophages Application in Controlling Bacterial Plant Diseases and How to Overcome Them. J. Genet. Eng. Biotechnol. 2023, 21, 98. [Google Scholar] [CrossRef]
- Baliyan, N.; Dhiman, S.; Dheeman, S.; Vishnoi, V.K.; Kumar, S.; Maheshwari, D.K. Bacteriophage Cocktails as Antibacterial Agents in Crop Protection. Environ. Sustain. 2022, 5, 305–311. [Google Scholar] [CrossRef]
- Elfadadny, A.; Ragab, R.F.; Abou Shehata, M.A.; Elfadadny, M.R.; Farag, A.; Abd El-Aziz, A.H.; Khalifa, H.O. Exploring Bacteriophage Applications in Medicine and Beyond. Acta Microbiol. Hell. 2024, 69, 167–179. [Google Scholar] [CrossRef]
- Nakayinga, R.; Makumi, A.; Tumuhaise, V.; Tinzaara, W. Xanthomonas Bacteriophages: A Review of Their Biology and Biocontrol Applications in Agriculture. BMC Microbiol. 2021, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Petrzik, K.; Brázdová, S.; Krawczyk, K. Novel Viruses That Lyse Plant and Human Strains of Kosakonia cowanii. Viruses 2021, 13, 1418. [Google Scholar] [CrossRef]
- Torkashvand, N.; Kamyab, H.; Aarabi, P.; Shahverdi, A.R.; Torshizi, M.A.K.; Khoshayand, M.R.; Sepehrizadeh, Z. Evaluating the Effectiveness and Safety of a Novel Phage Cocktail as a Biocontrol of Salmonella in Biofilm, Food Products, and Broiler Chicken. Front. Microbiol. 2024, 15, 1505805. [Google Scholar] [CrossRef]
- Połaska, M.; Sokołowska, B. Review Bacteriophages—A New Hope or a Huge Problem in the Food Industry. AIMS Microbiol. 2019, 5, 324–347. [Google Scholar] [CrossRef]
- Artawinata, P.C.; Lorraine, S.; Waturangi, D.E. Isolation and Characterization of Bacteriophages from Soil against Food Spoilage and Foodborne Pathogenic Bacteria. Sci. Rep. 2023, 13, 9282. [Google Scholar] [CrossRef]
- Palma, M.; Qi, B. Advancing Phage Therapy: A Comprehensive Review of the Safety, Efficacy, and Future Prospects for the Targeted Treatment of Bacterial Infections. Infect. Dis. Rep. 2024, 16, 1127–1181. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Wang, X.; Liao, X.; Zhang, S.; Ding, T.; Ahn, J. Impact of Prophages on Gut Microbiota and Disease Associations. Microb. Pathog. 2025, 204, 107642. [Google Scholar] [CrossRef]
- Esfandiari, A.H.; Mobarezi, Z.; Abolbashari, S.; Meshkat, Z. Efficacy of Phage Therapy in Diabetic Foot Ulcers (DFUs): A Systematic Review. BMC Infect. Dis. 2025, 25, 819. [Google Scholar] [CrossRef]
- Faruk, O.; Jewel, Z.A.; Bairagi, S.; Rasheduzzaman, M.; Bagchi, H.; Tuha, A.S.M.; Hossain, I.; Bala, A.; Ali, S. Phage Treatment of Multidrug-Resistant Bacterial Infections in Humans, Animals, and Plants: The Current Status and Future Prospects. Infect. Med. 2025, 4, 100168. [Google Scholar] [CrossRef]
- Gliźniewicz, M.; Miłek, D.; Olszewska, P.; Czajkowski, A.; Serwin, N.; Cecerska-Heryć, E.; Dołęgowska, B.; Grygorcewicz, B. Advances in Bacteriophage-Mediated Strategies for Combating Polymicrobial Biofilms. Front. Microbiol. 2023, 14, 1320345. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Kiga, K.; Kondabagil, K.; Węgrzyn, A. Current and future directions in bacteriophage research for developing therapeutic innovations. Sci. Rep. 2024, 14, 24404. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, H.; Ryu, S. Bacteriophage and Endolysin Engineering for Biocontrol of Food Pathogens/Pathogens in the Food: Recent Advances and Future Trends. Crit. Rev. Food Sci. Nutr. 2023, 63, 8919–8938. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Annapure, U.S. Bacteriophage Biocontrol of Foodborne Pathogens. J. Food Sci. Technol. 2016, 53, 1355–1362. [Google Scholar] [CrossRef]
- Endersen, L.; Coffey, A. The use of bacteriophages for food safety. Curr. Opin. Food Sci. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Gildea, L.; Ayariga, J.A.; Robertson, B.K. Bacteriophages as Biocontrol Agents in Livestock Food Production. Microorganisms 2022, 10, 2126. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Carey-Smith, G.; Greening, G. Bacteriophages as Biocontrol Agents in Food. J. Food Prot. 2005, 68, 426–437. [Google Scholar] [CrossRef]
- Ishani, D.; Tina, S. Bacteriophages as Twenty-First Century Antibacterial Tools for Food and Medicine. J. Interdiscip. Multidiscip. Res. 2015, 2, 92–97. [Google Scholar]
- Coffey, B.; Mills, S.; Coffey, A.; McAuliffe, O.; Paul Ross, R. Phage and Their Lysins as Biocontrol Agents for Food Safety Applications. Annu. Rev. Food Sci. Technol. 2010, 1, 449–468. [Google Scholar] [CrossRef]
- Bisen, M.; Kharga, K.; Mehta, S.; Jabi, N.; Kumar, L. Bacteriophages in Nature: Recent Advances in Research Tools and Diverse Environmental and Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2024; Volume 31, ISBN 1135602432535. [Google Scholar]
- Sawa, T.; Moriyama, K.; Kinoshita, M. Current Status of Bacteriophage Therapy for Severe Bacterial Infections. J. Intensive Care 2024, 12, 44. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Application of Bacteriophages in the Agro-Food Sector: A Long Way toward Approval. Front. Cell. Infect. Microbiol. 2018, 8, 296. [Google Scholar] [CrossRef]
- Naureen, Z.; Dautaj, A.; Anpilogov, K.; Camilleri, G.; Dhuli, K.; Tanzi, B.; Maltese, P.E.; Cristofoli, F.; De Antoni, L.; Beccari, T.; et al. Bacteriophages Presence in Nature and Their Role in the Natural Selection of Bacterial Populations. Acta Biomed. 2020, 91, e2020024. [Google Scholar] [CrossRef]
- Xu, X.; Gu, P. Overview of Phage Defense Systems in Bacteria and Their Applications. Int. J. Mol. Sci. 2024, 25, 13316. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, T.; Sberro, H.; Weinstock, E.; Cohen, O.; Doron, S.; Charpak-Amikam, Y.; Afik, S.; Ofir, G.; Sorek, R. BREX Is a Novel Phage Resistance System Widespread in Microbial Genomes. EMBO J. 2015, 34, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, J.; Morozova, N.; Sierro, N.; Isaev, A.; Sinkunas, T.; Tsvetkova, K.; Matlashov, M.; Truncaite, L.; Morgan, R.D.; Ivanov, N.V.; et al. BREX System of Escherichia Coli Distinguishes Self from Non-Self by Methylation of a Specific DNA Site. Nucleic Acids Res. 2019, 47, 253–265. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.L.; Burbank, L.P. Natural Recombination among Type I Restriction-Modification Systems Creates Diverse Genomic Methylation Patterns among Xylella Fastidiosa Strains. Appl. Environ. Microbiol. 2023, 89, e01873-22. [Google Scholar] [CrossRef]
- Rendueles, O.; de Sousa, J.A.M.; Rocha, E.P.C. Competition between Lysogenic and Sensitive Bacteria Is Determined by the Fitness Costs of the Different Emerging Phage-Resistance Strategies. Elife 2023, 12, e83479. [Google Scholar] [CrossRef]
- Rudnicka, M.; Noszczyńska, M.; Malicka, M.; Kasperkiewicz, K.; Pawlik, M.; Piotrowska-Seget, Z. Outer Membrane Vesicles as Mediators of Plant–Bacterial Interactions. Front. Microbiol. 2022, 13, e83479. [Google Scholar] [CrossRef]
- Kering, K.K.; Kibii, B.J.; Wei, H. Biocontrol of Phytobacteria with Bacteriophage Cocktails. Pest Manag. Sci. 2019, 75, 1775–1781. [Google Scholar] [CrossRef]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, A.R.; Fortier, A.; Roush, C.; Lessing, A.J.; Bender, R.G.; Barahman, R.; Grant, R.; Chan, B.K.; Turner, P.E. Pleiotropy Complicates a Trade-off between Phage Resistance and Antibiotic Resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 11207–11216. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F.; Sorek, R. The Evolutionary Success of Regulated Cell Death in Bacterial Immunity. Curr. Opin. Microbiol. 2023, 74, 102312. [Google Scholar] [CrossRef] [PubMed]
- Teklemariam, A.D.; Al-Hindi, R.R.; Qadri, I.; Alharbi, M.G.; Ramadan, W.S.; Ayubu, J.; Al-Hejin, A.M.; Hakim, R.F.; Hakim, F.F.; Hakim, R.F.; et al. The Battle between Bacteria and Bacteriophages: A Conundrum to Their Immune System. Antibiotics 2023, 12, 381. [Google Scholar] [CrossRef]
- van Houte, S.; Buckling, A.; Westra, E.R. Evolutionary Ecology of Prokaryotic Immune Mechanisms. Microbiol. Mol. Biol. Rev. 2016, 80, 745–763. [Google Scholar] [CrossRef]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, M.T. Considerations for Using Bacteriophages for Plant Disease Control. Bacteriophage 2012, 2, e23857. [Google Scholar] [CrossRef]
- Mayo-Muñoz, D.; Smith, L.M.; Garcia-Doval, C.; Malone, L.M.; Harding, K.R.; Jackson, S.A.; Hampton, H.G.; Fagerlund, R.D.; Gumy, L.F.; Fineran, P.C. Type III CRISPR-Cas Provides Resistance against Nucleus-Forming Jumbo Phages via Abortive Infection. Mol. Cell 2022, 82, 4471–4486.e9. [Google Scholar] [CrossRef]
- Leroux, M.; Laub, M.T. Toxin-Antitoxin Systems as Phage Defense Elements. Annu. Rev. Microbiol. 2022, 76, 21–43. [Google Scholar] [CrossRef]
- Lin, J.; Guo, Y.; Yao, J.; Tang, K.; Wang, X. Applications of Toxin-Antitoxin Systems in Synthetic Biology. Eng. Microbiol. 2023, 3, 100069. [Google Scholar] [CrossRef]
- Singh, G.; Yadav, M.; Ghosh, C.; Rathore, J.S. Bacterial Toxin-Antitoxin Modules: Classification, Functions, and Association with Persistence. Curr. Res. Microb. Sci. 2021, 2, 100047. [Google Scholar] [CrossRef]
- Aguilar-Marcelino, L.; Mendoza-de-Gives, P.; Al-Ani, L.K.T.; López-Arellano, M.E.; Gómez-Rodríguez, O.; Villar-Luna, E.; Reyes-Guerrero, D.E. Using Molecular Techniques Applied to Beneficial Microorganisms as Biotechnological Tools for Controlling Agricultural Plant Pathogens and Pest. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Amsterdam, Netherlands, 2020; pp. 333–349. [Google Scholar] [CrossRef]
- Ibarra-Chávez, R.; Brady, A.; Chen, J.; Penadés, J.R.; Haag, A.F. Phage-Inducible Chromosomal Islands Promote Genetic Variability by Blocking Phage Reproduction and Protecting Transductants from Phage Lysis. PLoS Genet. 2022, 18, e1010146. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.; Cando-Dumancela, C.; Liddicoat, C.; Dresken, R.; Damen, R.H.; Edwards, R.A.; Ramesh, S.A.; Breed, M.F. Ecological Phage Therapy: Can Bacteriophages Help Rapidly Restore the Soil Microbiome? Ecol. Evol. 2024, 14, e70185. [Google Scholar] [CrossRef] [PubMed]
- Bugaeva, E.N.; Voronina, M.V.; Vasiliev, D.M.; Lukianova, A.A.; Landyshev, N.N.; Ignatov, A.N.; Miroshnikov, K.A. Use of a Specific Phage Cocktail for Soft Rot Control on Ware Potatoes: A Case Study. Viruses 2021, 13, 1095. [Google Scholar] [CrossRef]
- Korniienko, N.; Kharina, A.; Budzanivska, I.; Burketová, L.; Kalachova, T. Phages of Phytopathogenic Bacteria: High Potential, but Challenging Application. Plant Prot. Sci. 2022, 58, 89–91. [Google Scholar] [CrossRef]
- Sauvageau, D.; Cooper, D.G. Two-Stage, Self-Cycling Process for the Production of Bacteriophages. Microb. Cell Fact. 2010, 9, 81. [Google Scholar] [CrossRef]
- Zaczek, M.; Weber-Dabrowska, B.; Górski, A. Phages in the Global Fruit and Vegetable Industry. J. Appl. Microbiol. 2015, 118, 537–556. [Google Scholar] [CrossRef]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The Influence of External Factors on Bacteriophages-Review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and Antimicrobial Properties of Essential Oils from Four Wild Taxa of Lamiaceae Family Growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Janisiewicz, W.J. Biological Control of Postharvest Diseases: Hurdles, Successes and Prospects. Acta Hortic. 2013, 1001, 273–284. [Google Scholar] [CrossRef]
- Ly-Chatain, M.H. The Factors Affecting Effectiveness of Treatment in Phages Therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Stefani, E.; Obradović, A.; Gašić, K.; Altin, I.; Nagy, I.K.; Kovács, T. Bacteriophage-Mediated Control of Phytopathogenic Xanthomonads: A Promising Green Solution for the Future. Microorganisms 2021, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.J.; de Proft, M.; Kropinski, A.M.; Noben, J.P.; Maes, M.; Lavigne, R. T4-Related Bacteriophage LIMEstone Isolates for the Control of Soft Rot on Potato Caused by “Dickeya Solani”. PLoS ONE 2012, 7, e33227. [Google Scholar] [CrossRef] [PubMed]
- Kmoch, M.; Vacek, J.; Loubová, V.; Petrzik, K.; Brázdová, S.; Ševčík, R. Potential of Limestonevirus Bacteriophages for Ecological Control of Dickeya Solani Causing Bacterial Potato Blackleg. Agriculture 2024, 14, 497. [Google Scholar] [CrossRef]
- Zaczek-Moczydłowska, M.A.; Young, G.K.; Trudgett, J.; Plahe, C.; Fleming, C.C.; Campbell, K.; O’Hanlon, R. Phage Cocktail Containing Podoviridae and Myoviridae Bacteriophages Inhibits the Growth of Pectobacterium spp. Under in Vitro and in Vivo Conditions. PLoS ONE 2020, 15, e0230842. [Google Scholar] [CrossRef]
- Lim, J.A.; Jee, S.; Lee, D.H.; Roh, E.; Jung, K.; Oh, C.; Heu, S. Biocontrol of Pectobacterium carotovorum Subsp. Carotovorum Using Bacteriophage PP1. J. Microbiol. Biotechnol. 2013, 23, 1147–1153. [Google Scholar] [CrossRef]
- Rombouts, S.; Volckaert, A.; Venneman, S.; Declercq, B.; Vandenheuvel, D.; Allonsius, C.N.; Van Malderghem, C.; Jang, H.B.; Briers, Y.; Noben, J.P.; et al. Characterization of Novel Bacteriophages for Biocontrol of Bacterial Blight in Leek Caused by Pseudomonas syringae Pv. porri. Front. Microbiol. 2016, 7, 279. [Google Scholar] [CrossRef]
- Ranjani, P.; Gowthami, Y.; Gnanamanickam, S.S.; Palani, P. Bacteriophages: A New Weapon for the Control of Bacterial Blight Disease in Rice Caused by Xanthomonas oryzae. Microbiol. Biotechnol. Lett. 2018, 46, 346–359. [Google Scholar] [CrossRef]
- Balogh, B.; Canteros, B.I.; Stall, R.E.; Jones, J.B. Control of Citrus Canker and Citrus Bacterial Spot with Bacteriophages. Plant Dis. 2008, 92, 1048–1052. [Google Scholar] [CrossRef]
- McNeil, D.L.; Romero, S.; Kandula, J.; Stark, C.; Stewart, A.; Larsen, S. Bacteriophages a Potential Biocontrol Agent against Walnut Blight (Xanthomonas campestris Pv Juglandis). N. Zeal. Plant Prot. 2001, 54, 220–224. [Google Scholar] [CrossRef]
- Fujiwara, A.; Fujisawa, M.; Hamasaki, R.; Kawasaki, T.; Fujie, M.; Yamada, T. Biocontrol of Ralstonia Solanacearum by Treatment with Lytic Bacteriophages. Appl. Environ. Microbiol. 2011, 77, 4155–4162. [Google Scholar] [CrossRef]
- Dong, L. Phage Soil Additives: A Frontier in Agricultural Health. Theor. Nat. Sci. 2024, 37, 103–108. [Google Scholar] [CrossRef]
- Kizheva, Y.; Eftimova, M.; Rangelov, R.; Micheva, N.; Urshev, Z.; Rasheva, I.; Hristova, P. Broad Host Range Bacteriophages Found in Rhizosphere Soil of a Healthy Tomato Plant in Bulgaria. Heliyon 2021, 7, e07084. [Google Scholar] [CrossRef]
- Ke, D.; Luo, J.; Liu, P.; Shou, L.; Ijaz, M.; Ahmed, T.; Shahid, M.S.; An, Q.; Mustać, I.; Ondrasek, G.; et al. Advancements in Bacteriophages for the Fire Blight Pathogen Erwinia Amylovora. Viruses 2024, 16, 1619. [Google Scholar] [CrossRef]
- Saleh, F.R. Bacteriophage as Bio-Preserving to Enhance Shelf Life of Fruit and Vegetables. Plant Arch. 2020, 20, 359–362. [Google Scholar]
| Defence Mechanism | Description | Examples | Trade-Offs/Limitations |
|---|---|---|---|
| Inhibition of Capsule Production and Lysogenization | Bacteria inhibit capsule biosynthesis to survive under high phage pressure. | Mutations inhibiting capsule biosynthesis; fine-tuning capsule production. | High fitness cost due to the risk of prophage induction. |
| Inhibition of Phage Adsorption | Preventing phage attachment to the bacterial cell membrane. | Biofilms, OMVs, surface receptor modifications, protein mimics. | Increased susceptibility to antibiotics. |
| Blocking DNA Injection | The Superinfection Exclusion (SIE) system prevents phage DNA injection. | SIE proteins integrated into the bacterial membrane; interactions with type IV pili. | Hinders long-term adaptation of viral populations. |
| Restriction–Modification System | Internal defense systems activated to target the phage or eliminate the infected host. | Sensor and effector systems. | Protects remaining cellular population from viral particles. |
| Bacteriophage Exclusion (BREX) | Allows phage DNA injection but prevents its replication through methylation modifications. | Six-gene cassette in Bacillus cereus. | Novel mechanism without cleaving phage DNA. |
| Defence Island System Associated with Restriction–Modification (DISARM) | Prevents phage DNA replication through methylation modifications. | Similar to BREX system. | Does not cleave phage DNA. |
| CRISPR -Cas Systems | Captures phage DNA segments and incorporates them into the bacterial genome as spacers to recognize and destroy phage DNA upon subsequent infections. | Class 1 (types I, III, IV) and Class 2 (types II, V, VI) systems. | Phages can evolve to evade the system; bacteria seldom acquire multiple spacers. |
| Abortive Infection (Abi) | Induces controlled cell death or dormancy in the infected bacterial cell before the phage can complete its replication cycle. | CBASS, Pycsar, Thoeris systems; RADA system. | Preserves the surrounding microbial community; benefits related cells. |
| Toxin–Antitoxin System | Genetic elements that inhibit host cell growth unless restrained by corresponding antitoxins. | Type I-VIII systems; various toxins and antitoxins. | Functions remain controversial; diverse mechanisms. |
| Bacteriophage Assembly Interference | Disrupts the assembly of bacteriophages, preventing their successful replication. | Phage-induced chromosomal islands (PICIs). | Alters phage capsid size; depends on helper phages. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, A.; Sadowska, K.; Zenelt, W.; Krawczyk, K. Bacteriophages as a Sustainable Tool for Plant Disease Management: Benefits and Challenges. Agronomy 2025, 15, 2507. https://doi.org/10.3390/agronomy15112507
Hoffmann A, Sadowska K, Zenelt W, Krawczyk K. Bacteriophages as a Sustainable Tool for Plant Disease Management: Benefits and Challenges. Agronomy. 2025; 15(11):2507. https://doi.org/10.3390/agronomy15112507
Chicago/Turabian StyleHoffmann, Anna, Katarzyna Sadowska, Weronika Zenelt, and Krzysztof Krawczyk. 2025. "Bacteriophages as a Sustainable Tool for Plant Disease Management: Benefits and Challenges" Agronomy 15, no. 11: 2507. https://doi.org/10.3390/agronomy15112507
APA StyleHoffmann, A., Sadowska, K., Zenelt, W., & Krawczyk, K. (2025). Bacteriophages as a Sustainable Tool for Plant Disease Management: Benefits and Challenges. Agronomy, 15(11), 2507. https://doi.org/10.3390/agronomy15112507










