Abstract
Climate change and livestock expansion have affected forage supply in the dry tropics. Therefore, optimizing planting methods adapted to adverse tropical environments is essential for establishment and yield. The objective of this study was to evaluate the effect of different planting methods on the establishment rate, morphology, yield, and nutritional composition of Cuba OM-22 under the soil and climate conditions of the dry tropics of Peru, using a block design with four replicates and five methods for propagation by cuttings. The S4 (two-node cuttings, 25 cm in length; horizontal position 180°, parallel to the soil surface; fully buried at 8 cm depth; no spacing between cuttings along the furrow) method offered the best balance between yield and quality, with higher establishment rate (55.93%), height (182.15 cm; higher than S1 and S5), and more tillers (surpassing S1 and S2 by 16.97% and 18.86%). In addition, it obtained good green forage yields (137.43 t ha−1) and was better than all planting methods in dry matter yield (37.45 t ha−1). In nutritional composition, S4 ranked among the highest averages for nitrogen-free extract (NFE) (43.22%) and ash (11.06%). However, protein, crude fiber, and fat content did not differ between methods. On the other hand, planting methods showed negative correlations between the number of tillers and ash content (p = 0.006; r = −0.79), ash and NFE (p = 0.000; r = −0.92), and protein with crude fiber (p = 0.029; r = −0.68). These findings highlight S4 as a key strategy for optimizing establishment, yield, and quality in Cuba OM-22 in the dry tropics.
1. Introduction
The global livestock sector has grown at an annual rate close to 3.81%, outpacing the increase in crops and other agricultural products [1,2]. This dynamism has driven demand for beef market, whose production is expected to increase by around 14% in the coming years [3]. In Peru, livestock accounts for 11.4% of the gross value of national production and has grown by 6.2% over the last decade, with particular relevance in the Amazonas region, where cattle and raw milk contribute more than 90% of the subsector’s value [4].
Ruminant feeding relies mainly on pastures, which constitute the largest source of dry matter consumed annually in tropical and subtropical regions [5]. Their contribution can reach up to 84% during the dry season and 58% in the rainy season [6]. However, forage production in the tropics faces constraints associated with high temperatures, low soil fertility, and marked seasonality, factors that reduce forage yield and quality and, consequently, livestock productivity [7,8]. In more severe settings such as Africa, limited forage availability not only causes weight loss in cattle but also nutritional disorders, weakened immune systems, and greater susceptibility to infections and parasites [9,10]. In Peru, the impact of climate change in the tropics has left a strong imprint on the Andes and the Amazon, reducing the supply of water resources and favoring pests and diseases that affect forage crops [11,12,13,14]. This increases dependence on imported supplements, raises production costs, and heightens the economic vulnerability of smallholders [15,16,17].
In this context, the introduction of improved forage germplasm emerges as a strategy to address these challenges, allowing up to a 2.6-fold increase in biomass production, with grasses showing the greatest gains compared with other forage types [18]. Interspecific hybrids between elephant grass (Pennisetum purpureum Schumach.) and pearl millet (Pennisetum glaucum L.) are a viable alternative due to their good adaptability and productivity, with use as fresh forage and silage [19,20]. Among the most notable hybrids is Cuba OM-22, typically used as a cut-and-carry grass; it shows drought tolerance, produces good biomass, and has high protein content under low soil fertility with short cutting intervals [21].
In tropical grasses, reproductive limitations such as low pollen viability, short pollen longevity, and, in some cases, limited stigma receptivity reduce the availability of viable seed [22]. In parallel, some forage grasses (Pennisetum ciliare) exhibit apomixis; when present, it can produce clonal seed, although its expression and agronomic usefulness vary among species and hybrids [23]. In the absence of apomixis, sexual reproduction entails segregation in the progeny and therefore loss of genetic uniformity relative to the parental material [24]. In perennial grasses, the propagation method plays a crucial role in establishment (rhizomes, stolons, tillers, and stem cuttings), with the advantage of providing genetically identical plants and, often, faster initial ground cover than direct seeding; however, success depends on operational factors such as cutting length, number of nodes, and orientation of the cutting with respect to the soil surface [25,26,27].
Studies conducted on Cuba OM-22 report a yield of 6.7 to 11.3 t ha−1 of dry matter per cut, with nitrogen applications between 50 and 150 kg N ha−1 [28]. Regarding its nutritive quality, during the dry season 7.44% crude protein and 36.2% crude fiber have been reported; in that study all treatments received 50 kg N ha−1 [29]. However, the establishment rate of Cuba OM-22 plants during establishment is determined by environmental and edaphic factors, temperature, radiation, moisture, and substrate type that directly affect rooting, given that its multiplication is clonal via stem cuttings [30,31]. There are studies showing that the number of nodes per cutting influences the rooting rate and dry matter, with variation among grass varieties [32]. Likewise, Fanindi et al. [33] reported that the length of the cuttings selected for planting influences the number of tillers and fresh weight per plant. Nevertheless, much of the available evidence comes from geographical and climatic contexts other than the Peruvian dry tropics, as well as from different management regimes.
Evidence on how planting methods using stem cuttings influence establishment, field yield, and nutritional composition of Cuba OM-22 is limited. Most available studies come from different edaphoclimatic contexts, species and management regimes, without integrated evaluations of the effect of different planting methods on agronomic and nutritional parameters, focusing mainly on establishment rate and yield. Therefore, this study was designed to fill this gap, generating a comparative scientific basis through data collection that addresses this question and contributes to optimizing local livestock systems under low-precipitation scenarios through the selection of planting methods. The objective was to evaluate the effect of five planting methods using stem cuttings on the establishment, morphology, yield, and nutritional composition of Cuba OM-22 under edaphoclimatic conditions of the Peruvian dry tropics. We hypothesized that, in this context, at least one of the five planting methods using stem cuttings of Cuba OM-22 would show significantly higher values of establishment, morphological vigor, yield, and nutritional quality compared with the other methods. The null hypothesis states that there are no differences among methods. Accordingly, this study generates applied evidence specific to the dry tropics to guide the choice of planting method in pasture establishment programs and improve forage productivity and quality in livestock systems under low rainfall conditions.
2. Materials and Methods
2.1. Description of the Research Area
The research was carried out at the Estación Experimental Agraria Amazonas, Centro Experimental Huarangopampa del Instituto Nacional de Innovación Agraria (INIA), located in the district of Milagro, province of Utcubamba, Amazonas region, Peru. The area was located at an altitude of 396 m above sea level, at latitude 5° 39′ 48″ S and longitude 78° 32′ 8″ W. Meteorological data were recorded using a Vantage Pro2-Davis (Davis Instruments Corp., Hayward, CA, USA) weather station during the experimental period from April to July 2023. As shown in Figure 1, the following parameters were monitored: temperature (maximum: 31.52 ± 2.02 °C; minimum: 22.47 ± 0.92 °C) (Figure 1a), relative humidity (79.83 ± 5.77%), and precipitation (2.97 ± 7.67 mm day−1) (Figure 1b). Prior to experiment initiation, soil sampling was carried out in accordance with the standards of soil study DS No. 013-2010-AG [34]. Soil subsamples were taken from the entire experimental area (3200 m2) at 12 points distributed in a zigzag pattern. The surface of each sampling point was cleaned, and a shovel was inserted to a depth of 32 cm; the subsamples were mixed and homogenized, and a 1 kg composite sample was obtained using the quartering method [35]. The samples were then coded and transferred to the Laboratorio de Investigación de Suelos y Aguas (LABISAG), accredited by the Instituto Nacional de Calidad, Ministerio de Producción, according to standard NTP-ISO/IEC 17025:2017 [36] of the Universidad Nacional Toribio Rodríguez de Mendoza del Amazonas, where a sandy clay loam textural class was obtained (sand 51%, silt 16%, and clay 33%). In addition, it was determined the pH (7.5), electrical conductivity (4 dS m−1), organic matter content (1.88%), carbon (1.09%), nitrogen (0.09%), phosphorus (7.35 ppm), and potassium (219.81 ppm) were determined. All analyses were performed following the Bazán methodology [37].
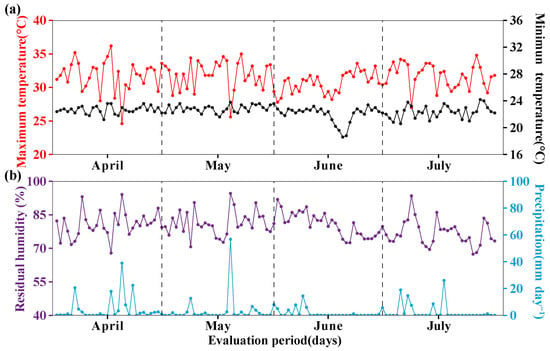
Figure 1.
Meteorological data records. (a) Maximum and minimum temperature, (b) residual humidity and precipitation.
2.2. Experimental Design
This study used a randomized complete block design (RCBD) with four blocks (replicates). The planting method factor (S) had five levels: S1, S2, S3, S4, and S5. Each block contained one plot per method, resulting in five plots per block and 20 experimental units in total (experimental unit = 120 m2 plot). For establishment rate, plant height, and number of tillers, the effect of planting methods and days after planting (D) was considered, while for yield and nutritional composition, only the planting method factor (S) was considered. Planting methods are detailed in Table 1.

Table 1.
Description of Cuba OM-22 planting methods.
2.3. Establishment of the Experimental Area
Primary tillage consisted of one pass with a disc plow at a working depth of 30 cm. Secondary tillage consisted of two transverse passes with an off-center disc harrow to break up clods and homogenize the surface layer, followed by final leveling with a leveling harrow. All tillage was carried out with adequate soil moisture (close to field capacity) to minimize compaction. Plot layout and furrow construction were performed manually using stakes, string, and hand tools. The trial was structured into experimental units of 120 m2 (12 m × 10 m) each, with a distance of 2 m between plots and 2 m wide access roads around the area. Eleven furrows were established per experimental unit, each 11 m long, with a distance of 80 cm between furrows. The cuttings were selected from the lower and middle third of vigorous mother plants approximately 160 days old. The plant material was provided by the agrostological garden of the Instituto Nacional de Innovación Agraria (INIA). The length of the cuttings with one node was 14 cm, those with two nodes was 25 cm, and the cuttings with nine nodes was 170 cm. All cuttings had a diameter of 3.5 ± 0.2 cm. The irrigation system was gravity-fed, with two scheduled irrigations per week until field capacity was reached, with water distribution through secondary channels. For weed control, manual weeding was performed after each week, following evaluation to avoid nutrient competition with weeds. This study did not use fertilization in any of the planting methods. The harvest was carried out 75 days after planting, at a cutting height of 8 cm above ground level, using a sickle and a machete. Next, yield and nutritional composition were evaluated.
2.4. Evaluation of Indicators
2.4.1. Establishment Rate
Establishment rate was evaluated in the field, where the number of cuttings per furrow (11 linear meters) was counted, evaluating 4 furrows per experimental unit, considering as a living plant any cutting with at least one rooted shoot and green leaf tissue. The evaluations were carried out every 15 days until 60 days after planting, and establishment rate was calculated by date using the following equation:
2.4.2. Plant Height and Number of Tillers
Plant height was evaluated by selecting 10 plants at random, then following the methodology of Vásquez et al. [38], where the height from the base of the stem to the tip of the highest secondary leaf was measured using a flexometer Bahco (SNA Europe SAS, Éragny-sur-Oise, Francia).
To evaluate the number of tillers, the number of tillers per plant was counted manually in the field. Data were collected every 15 days until 75 days after planting.
2.4.3. Yield
To evaluate green forage yield, a 1 m2 frame was randomly placed in each experimental unit. At 75 days, the forage was cut 8 cm above the ground and the biomass collected in the quadrant was weighed on an electronic scale (Coretto, NC-30K), then the weight per m2 was extrapolated to tons of green forage per hectare. To determine the percentage of dry matter, 100 g of green forage per experimental unit was weighed and dried in a forced-air oven at 60 °C for up to 72 h or until constant weight. The percentage of dry matter was then obtained as the ratio of dry weight to fresh weight of the subsample, expressed in percentage. The dry matter yield per hectare was calculated by multiplying the green forage yield by the percentage of dry matter.
2.4.4. Nutritional Composition
The nutritional composition analysis of ash, crude fat, protein, crude fiber, and nitrogen-free extract (NFE) was performed at the Laboratory de Nutrición Animal y Bromatología (LABNUT) de la Universidad Nacional Toribio Rodríguez de Mendoza del Amazonas. The samples arrived at the laboratory in properly labeled Kraft paper bags. The material was cut with scissors into 5–10 cm segments, collecting between 250 and 500 g of chopped sample per unit. Each sample was transferred to new, pre-weighed Kraft paper bags. The fresh weight was then recorded on a gram scale and dried in a forced-air oven at 60 °C for 48 h until a constant weight was reached. After this process, the dry weight was recorded and the material was ground in a hammer mill (2 mm screen), collecting the material in glass jars. The samples were stored in labeled Ziploc bags for nutritional analysis.
Crude protein was determined by the AOAC 928.08 method [30] with sulfuric acid digestion, ammonia distillation, and titration with HCl; the measured nitrogen was converted to protein (N × 6.25). Crude fiber was obtained using the ANKOM method 7, using an ANKOM200 analyzer and F57 bags. 1 g of sample (dry matter basis) was weighed per bag. The bags were subjected to successive acid and alkaline digestions with intermediate rinses, followed by rinsing with acetone and pre-drying. They were then dried in an oven at 105 °C for 2 h, cooled in a desiccator, and weighed; they were subsequently calcined in a muffle furnace at 650 °C and weighed again. The % crude fiber was calculated as the ash-corrected insoluble residue, according to the equation in Method 7; a bag blank correction was applied. Ash was quantified gravimetrically according to AOAC 942.05 [39], after incineration in a muffle furnace (Thermo Fisher Scientific, Waltham, MA, USA). 2 g of sample were weighed into porcelain crucibles, which were incinerated at 550 °C for 5 h until a constant weight was obtained. Crude fat was determined by Soxhlet extraction using petroleum ether as the solvent, in accordance with AOAC method No. 920.39 [39]. Samples with high moisture content were dried at 65 °C, then ground and sieved (1 mm). Approximately 3 g of dry sample was weighed into tared cellulose or filter paper cartridges. The aluminum containers were dried at 103 ± 2 °C for 30 min, cooled in a desiccator, and weighed. Fifty to 60 mL of solvent was added to each container, and extraction was initiated for 3 h at 120 °C. The solvent carried away the lipids, which were deposited by evaporation and cyclic recovery. Finally, the vessels were dried again at 103 ± 2 °C for 2–3 h, cooled in a desiccator, and weighed. The nitrogen-free extract (NFE) was estimated by difference under the Weende proximal system, following AOAC procedures. After quantifying moisture, ash, crude fiber, crude fat, and crude protein, the NFE was calculated as: NFE (%) = 100 − (% moisture + % ash + % crude fiber + % crude fat + % crude protein).
2.5. Statistical Analysis
The experiment was conducted under a randomized completely block design (RCBD) with a confidence level of 95%. For each variable, the model assumptions were verified using the Shapiro–Wilk (normality) and Bartlett (homogeneity of variances) tests with functions from the R base package (stats) [40]. When assumptions were met, a two-way ANOVA was fitted, followed by Tukey’s multiple comparisons using the agricolae package [41]. When assumptions were not met, data were log-transformed before analysis. In addition, a Pearson correlation analysis was performed to explore relationships between agronomic and nutritional variables. The analysis was developed using the corrplot package [42]. Graphical visualizations were performed with the ggplot2 package [43]; all processing was performed in Rstudio software version 4.5.0 for Windows.
3. Results
3.1. Establishment Rate
The establishment rate of Cuba OM-22 (Figure 2) showed significant effects on the method of planting (S) and days of evaluation after planting (D); likewise, the interaction (S×D) was significant (p < 0.05). At 15 days after planting (DAP), methods S2 and S4 exhibited greater stability in the establishment rate up to 60 DAP. In contrast, S1 recorded a decrease at 30 DAP of 13.59% (from 54.68% to 47.25%), after which it remained stable, but with values significantly lower than S3 and S4. S3 started significantly low at 15 DAP, but showed an increase of 22.11% (from 43.50 to 53.12) at 30 DAP. Finally, the S5 method had the lowest establishment rates at the end of the evaluation period at 60 DAP, significantly lower than those of the other methods, indicating higher mortality rates with this planting method. The findings show that the planting method determines establishment rate in the field and, consequently, the number of plants established at harvest, with methods S2 (50.93%), S3 (54.68%), and S4 (55.93%) being the most outstanding, with a establishment rate of over 50%.
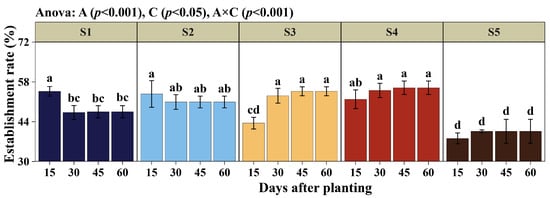
Figure 2.
Establishment rate. Planting methods (S): (2 nodes, length 25 cm, inclined at 45°, insertion depth 12 cm, spacing 50 cm), S2 (1 node, length 14 cm, inclined at 45°, insertion depth 12 cm, spacing 50 cm), S3 (2 nodes, length 25 cm, horizontal 180°, fully buried at 8 cm depth, spacing 50 cm), S4 (2 nodes, length 25 cm, horizontal 180°, fully buried at 8 cm depth, no spacing), and S5 (9 nodes, length 170 cm, horizontal 180°, fully buried at 8 cm depth, no spacing). Days of evaluation after planting (D): 15, 30, 45, and 60 days. Significant differences are indicated by different letters in vertical.
3.2. Plant Height and Number of Tillers
The morphological parameters of plant height and number of tillers of Cuba OM-22 (Figure 3) did not show significant effects on planting method (S), but did indicate variations according to the days of evaluation after planting (D). Similarly, the interaction (S × D) was significant (p < 0.05) for both morphological variables. At 75 days after planting (DAP), plant height did not differ significantly between S2 (182.15 cm), S3 (184.5 cm), and S4 (182.15 cm), with the highest means, with S4 exceeding S1 (178.89 cm) and S5 by 1.82% and 0.41% (181.40 cm), as shown in Figure 3a. On the other hand, in terms of the number of tillers at 75 DAP, S4 again had the highest averages with 23.51 tillers, being statistically higher than S1 (20.10 tillers) and S2 (19.78 tillers) by 16.97% and 18.86%; but did not differ from methods S3 (23.06 tillers) and S5 (22.81 tillers). Starting from 60 DAP, no changes were recorded in the number of tillers for any of the planting methods when analyzed individually (Figure 3b).
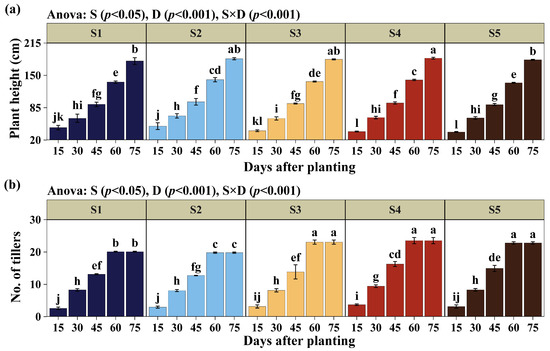
Figure 3.
Evaluation of plant height (a) and number of tillers (b). Planting methods (S): (2 nodes, length 25 cm, inclined at 45°, insertion depth 12 cm, spacing 50 cm), S2 (1 node, length 14 cm, inclined at 45°, insertion depth 12 cm, spacing 50 cm), S3 (2 nodes, length 25 cm, horizontal 180°, fully buried at 8 cm depth, spacing 50 cm), S4 (2 nodes, length 25 cm, horizontal 180°, fully buried at 8 cm depth, no spacing), and S5 (9 nodes, length 170 cm, horizontal 180°, fully buried at 8 cm depth, no spacing). Days of evaluation after planting (D): 15, 30, 45, and 60 days. Significant differences are indicated by different letters in vertical.
3.3. Yield
The dry matter content was higher with the S4 method (27.25%), exceeding S1 (26.70%), S2 (26.63%), and S5 (26.78%) by 2.06%, 2.33%, and 1.76%, respectively, with no differences compared to S3 (27.11%) (Figure 4a). In terms of green forage yield, S4 reached 137.43 t ha−1 and was higher than S1, S2, and S5 by 6.66%, 8.11%, and 3.92% (Figure 4b). Similarly, for dry matter yield, S4 recorded 37.45 t ha−1 and exceeded S1, S2, and S5 by 8.83%, 10.60%, and 5.73%, respectively (Figure 4c). Overall, S4 showed the best performance when combining dry matter content (%) and green forage and dry matter yields, surpassing S1, S2, and S5, confirming the planting method as a determining factor in the yields of Cuba OM-22.
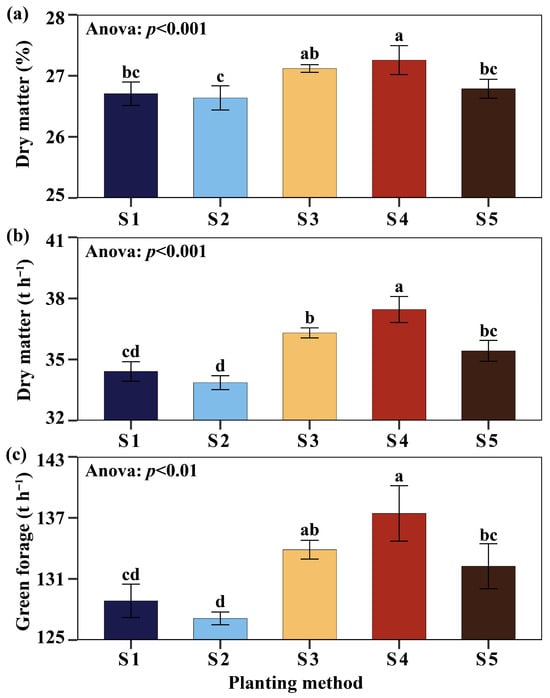
Figure 4.
Evaluation of dry matter content (a), dry matter (b), and green forage (c). Planting methods (S): (2 nodes, length 25 cm, inclined at 45°, insertion depth 12 cm, spacing 50 cm), S2 (1 node, length 14 cm, inclined at 45°, insertion depth 12 cm, spacing 50 cm), S3 (2 nodes, length 25 cm, horizontal 180°, fully buried at 8 cm depth, spacing 50 cm), S4 (2 nodes, length 25 cm, horizontal 180°, fully buried at 8 cm depth, no spacing), and S5 (9 nodes, length 170 cm, horizontal 180°, fully buried at 8 cm depth, no spacing). Significant differences are indicated by different letters in vertical.
3.4. Nutritional Composition
The nutritional composition varied significantly depending on the planting method; however, the fat, protein, and crude fiber contents were not affected by the planting method. Fat content ranged from 1.34% to 1.77% among the different planting methods, while protein varied between 6.9% and 7.44%, and crude fiber between 30.9% and 32.67%. Although the results did not show significant variability, the highest relative protein averages were obtained in S4 with 7.44%, accompanied by the lowest relative fat content with 1.34% (Figure 5a–c). In terms of nitrogen-free extract (NFE), method S5 (44.62%) had the highest average, exceeding S1 (41.89%) and S2 (41.45%) by 6.52% and 7.65%, but did not differ from S3 (43.42%) and S4 (43.22%) (Figure 5d). On the other hand, in terms of ash content, method S1 (13.19%) had the featured value, but did not differ significantly from S2 (12.27%) and S4 (11.06%), but it was higher than S3 (10.56%) and S5 (9.36%) (Figure 5e).
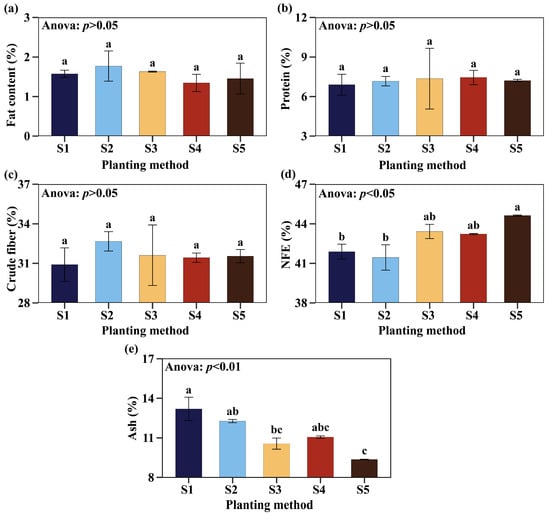
Figure 5.
Nutritional composition. Fat content (a), protein (b), crude fiber (c), nitrogen-free extract (NFE) (d), and ash (e). Planting methods (S): (2 nodes, length 25 cm, inclined at 45°, insertion depth 12 cm, spacing 50 cm), S2 (1 node, length 14 cm, inclined at 45°, insertion depth 12 cm, spacing 50 cm), S3 (2 nodes, length 25 cm, horizontal 180°, fully buried at 8 cm depth, spacing 50 cm), S4 (2 nodes, length 25 cm, horizontal 180°, fully buried at 8 cm depth, no spacing), and S5 (9 nodes, length 170 cm, horizontal 180°, fully buried at 8 cm depth, no spacing). Significant differences are indicated by different letters in vertical.
3.5. Correlation of Agronomic and Nutritional Parameters
The correlation analysis of the agronomic and nutritional parameters of Cuba OM-22 is presented in Figure 6, which shows 10 significant correlations between the variables studied, of which 3 were negative and 7 were positive. Among the negative correlations, the number of tillers and ash content (p = 0.006; r = −0.79), ash and nitrogen-free extract (NFE) (p = 0.000; r = −0.92), and protein with crude fiber (p = 0.029; r = −0.68). In addition, positive correlations were observed between number of tillers and dry matter content (p = 0.0195; r = 0.72), number of tillers and green forage (t ha−1) (p = 0.000; r = 0.92), number of tillers and dry matter (t ha−1) (p = 0.000; r = 0.90), number of tillers and nitrogen-free extract (NFE) (p = 0.005; r = 0.80), dry matter content and green forage (t ha−1) (p = 0.005; r = 0.80), dry matter content and dry matter (t ha−1) (p = 0.000; r = 0.89), green forage (t ha−1) and dry matter (t ha−1) (p = 0.000; r = 0.99).
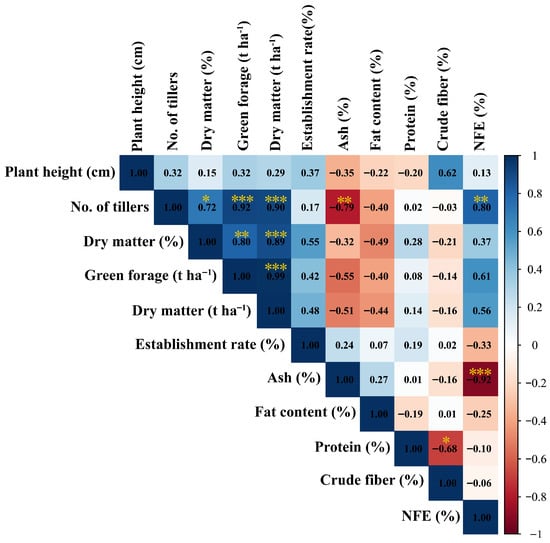
Figure 6.
Pearson correlation between the variables evaluated, both agronomic and forage quality. Asterisks show significant correlations: ∗, p < 0.05; ∗∗, p < 0.01; and ∗∗∗, p < 0.001.
4. Discussion
The evaluation of the effect of planting methods on the agronomic and nutritional parameters of Cuba OM-22 guides decision-making for the establishment of pastures under field conditions and provides applied evidence for management plans in the dry tropics affected by climate change.
Our results showed an establishment rate >50%, 60 days after planting, with planting methods S2, S3, and S4 standing out under limited water availability in open fields. These results are superior to those reported by du Toit [44], who reported an establishment rate of 39% in Eragrostis racemosa. However, they were lower than those described by Díaz-Páez et al. [45], who evaluated the establishment rate of cuttings in tropical Andean conditions and observed percentages of 81% and 64% in forage grasses. The difference in the establishment rate observed in Cuba OM-22 compared to other studies can be explained by genetic variability between species [46] and by the specific environmental conditions under which each trial was conducted. On the other hand, a limitation of our study, which probably contributed to the low establishment rate, was the low availability of water in the soil during planting, associated with the application of only two gravity irrigations per week. In addition, the amount of sand (51%) present in the soil may have influenced the low water retention capacity of the soil [47].
As a result, the lack of continuous hydration of the cuttings may have increased initial mortality [48]. This had a more drastic effect on the S5 planting method throughout the evaluation period, despite having been planted with a greater number of shoots and with the longest stake length (170 cm). This differs from the study by Pollock et al. [49], who noted that there is a higher probability of establishment rate in cuttings with greater length (50–100 cm) and diameter (>2.0 cm), due to greater availability of total carbohydrates for rooting, suggesting that results may depend on the water context. However, in our study, 25 cm cuttings with two nodes had a more stable establishment rate, supporting the idea that the planting method is decisive for the establishment of forage grasses such as Cuba OM-22 in tropical conditions. Consequently, standardizing the length and number of nodes of the vegetative material could reduce mortality in pastures established in the dry tropics. In addition, as a recommendation, it is important to consider reseeding to increase the plant population without significantly affecting the final biomass cover [50,51].
The superiority of S3 and S4 in plant height and number of tillers could be explained by the horizontal position (180°) in which they were planted. However, Nyiramvuyekure et al. [52] reported higher sprouting and establishment rates in cuttings placed vertically and at an inclined rather than horizontally. Similarly, Monteiro et al. [53] stated that cuttings planted at angles of 90° show positive results in plant height and dry mass of shoots, being significantly higher than those at 10 and 30° (with no effect on the number of shoots). However, under our dry tropical conditions, horizontal orientation produced more tillers and greater height. In contrast, S2 (1 node) had the lowest tillering, probably due to insufficient internal reserves and the redistribution of photosynthetic products (soluble sugars) to basal structures to sustain regrowth [54]. Likewise, despite having 9 nodes per cutting, S5 did not outperform S3 and S4; this behavior suggests that mainly the basal nodes contributed to establishment, while the middle nodes showed less vigor, limiting the total number of tillers per plant [55]. In grasses, auxin produced in the apical meristem inhibits the emergence of buds near the apex, so that more distant nodes are less suppressed [56]; in addition, strigolactones inhibit the emission of shoots near the apical region, while cytokinins promote their activation [57,58,59]. In summary, a greater number of nodes (9 nodes) does not guarantee more tillers; in certain cases, it may be associated with lower tiller production due to compensatory effects on plant growth and development [60].
The S4 planting method, despite not using spacing between cuttings, achieved the highest yield of dry matter and green forage, which could be associated with a more efficient use of resources when managing two shoots, favoring a balance in the supply of water and nutrients, and mitigating competition between plants [61]. However, reducing spacing increases the number of plants per unit area, which consequently increases biomass yield, even if individual yield is not the most outstanding [62,63]. The advantage of this method is that it can help reduce labor costs for weeding, as reduced or zero spacing closes the canopy more quickly, reducing early weed competition and promoting growth and productivity [64,65].
Various studies on the establishment of the Tifton 85 bermudagrass (Cynodon spp.) cultivar have shown that high planting rates at a depth of 6 cm increase dry matter yield [66]. Similarly, in our study, the highest yield was observed with the S4 method, without spacing between planting units reflecting a higher planting density with higher performance when the entire plant was buried horizontally at a depth of 8 cm. In dwarf napier grass, studies have shown that completely covering stem cuttings with soil significantly increases plant emergence [67]. This evidence helps explain our findings, where the S4 planting method, with cuttings completely covered with soil, outperformed inclined planting with partial coverage, resulting in higher dry matter yield. On the other hand, the literature mentions that horizontal planting may experience a reduction in starch and phenolics, although these do not directly affect green forage and dry matter yield [68]. In our study, the S3 and S5 planting methods could demonstrate the opposite; however, our study did not evaluate biochemically, which constitutes a knowledge gap for future studies.
In relation to nutritional composition analyses, the highest ash averages ranged from 11.06 to 13.19 for S4, S2, and S1. The increase in ash content observed in these planting methods can be attributed to low precipitation. Some studies report that, during rainy seasons, ash tends to vary between 8.7% and 12.2%, while during periods of low precipitation, values vary between 7.6% and 10.8% [69]. Similar results were reported by Valqui et al. [70], who observed ash values ranging from 8.59% to 12.19% during the summer season. The nitrogen-free extract (NFE) showed higher content in planting methods S3, S4, and S5, which presented values above 43% without statistical differences. Different studies indicate that a high NFE content facilitates lactic fermentation, reduces pH, and limits the growth of undesirable microorganisms, improving silage preservation [71]. On the other hand, forages harvested for preservation with low NFE may require additives such as molasses to optimize fermentation and silage quality [72,73]. Nitrogen-free extract (NFE) alone does not define the preservation capacity of grass silage, but it is a fundamental component for efficient fermentation and good nutritional quality. An adequate NFE level promotes the preservation, energy value, and digestibility of silage, which is key in animal production based on pre-preserved forages [74]. While high NFE is positive, it must be balanced with other nutrients to avoid deficiencies or imbalances in the diet [75]. Overall, our S4 study showed a balance between yield and nutritional composition (NFE and ash), indicating that it is a viable method for establishing Cuba OM-22 in tropical conditions.
The number of tillers showed a positive and significant correlation with green forage (r = 0.92; p < 0.001), consistent with the findings of Aswini et al. [76], who demonstrated a strong association between these variables. On the other hand, a negative correlation was observed between ash content and nitrogen-free extract (NFE) (r = −0.92; p < 0.001), similar to that observed by Culqui et al. [77], who reported (r = −0.66; p = 0.00) for the same variables. However, these relationships may vary depending on the season (summer and winter), cutting times, and fertilization [71,78].
Among the limitations of this study are the absence of synthetic nitrogen fertilization and the evaluation in a single planting season. These restrictions limit the extrapolation of the results and raise questions about the performance of Cuba OM-22 under different nutrition regimes and establishment schedules. Given that N dosage is a key determinant of growth, future trials with N gradients could show additional increases in yield and quality, especially in cut-and-carry systems [79]. The results presented should be considered preliminary, but they provide a scientific basis for replications at different times, seasons, and cutting ages [80,81,82]. The implementation of these practices could contribute to improving the efficiency and sustainability of forage production in the dry tropics.
5. Conclusions
The results revealed that different planting methods had different impacts on agronomic and nutritional variables. The S4 planting method showed the best balance between forage yield and nutritional composition, with an establishment rate of 55.93%, greater plant height (182.15 cm) compared to S1 and S5, and a greater number of shoots that exceeded S1 and S2 by 16.97% and 18.86%, respectively. In addition, it obtained good green forage yields (137.43 t ha−1) and was better than all planting methods in dry matter yield (37.45 t ha−1). It also ranked among the top three averages for nitrogen-free extract (43.22%) and ash (11.06%). Based on protein, crude fiber, and fat content, no differences were found between planting methods. Our results indicate that the choice of planting method is key to optimizing the yield and nutritional composition of Cuba OM-22. However, validations are required in different geographical contexts, seasons, and species. Given that the study covered a single season and a single cut, longer-term evaluations are needed to support extrapolation to other areas and guide strategies to reduce feed costs and address establishment problems in the dry tropics.
Author Contributions
Conceptualization, G.A.-T. and J.L.M.; methodology, L.V.-V. and L.V.; software, M.A.A.-T. and J.Y.V.; validation, L.V., L.V.-V., M.A.A.-T. and G.A.-T.; formal analysis, G.A.-T. and J.L.M.; investigation, L.V.-V., L.G.B. and M.A.A.-T.; resources, L.V., H.V.V. and J.Y.V.; data curation, M.A.A.-T., L.G.B. and J.Y.V.; writing—original draft preparation, H.V.V. and J.L.M.; writing—review and editing, L.V., L.G.B. and J.Y.V.; visualization, L.G.B. and J.L.M.; supervision, H.V.V. and M.A.A.-T.; project administration, L.V.-V., G.A.-T. and J.Y.V.; funding acquisition, H.V.V. and J.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out and funded primarily by the Project CUI No. 2253484 “Creación del Servicio de un Laboratorio de Agrostología de la Universidad Nacional Toribio Rodríguez de Mendoza” which was funded by the Sistema Nacional de Inversión Pública (SNIP) of the Ministerio de Economía y Finanzas (MEF) of Peru. In addition, we had the support of the Vice-Rectorate for Research of the Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas—UNTRM.
Data Availability Statement
The original contributions presented in the study are included in this article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rout, P.K.; Behera, B.K. Sustainable Livestock Farming. In Sustainability in Ruminant Livestock: Management and Marketing; Springer: Singapore, 2021; pp. 137–170. [Google Scholar] [CrossRef]
- Ghahremaninejad, F.; Hoseini, E.; Jalali, S. The cultivation and domestication of wheat and barley in Iran: Brief review of a long history. Bot. Rev. 2021, 87, 1–22. [Google Scholar] [CrossRef]
- Ministerio de Desarrollo Agrario y Riego (MIDAGRI). La Producción Mundial y Nacional de Carne Vacuna y sus Perspectivas. Available online: https://cdn.www.gob.pe/uploads/document/file/7161113/5063792-nota-tecnica-n-024-la-producion-mundial-y-nacional-de-carne-vacuna-y-sus-perspectivas.pdf?v=1730415681 (accessed on 18 September 2025).
- Ministerio de Desarrollo Agrario y Riego (MIDAGRI). Perfil Competitivo de las Principales Especies y Productos Pecuarios. Available online: https://app.powerbi.com/view?r=eyJrIjoiYWM0MDIwYTktNTk3MS00OTc3LThiZTgtZjRmN2ZhMmZlNjVlIiwidCI6IjdmMDg0NjI3LTdmNDAtNDg3OS04OTE3LTk0Yjg2ZmQzNWYzZiJ9&pageName=ReportSection (accessed on 18 September 2025).
- Islam, M.R.; Garcia, S.C.; Islam, M.A.; Bashar, M.K.; Roy, A.; Roy, B.K.; Sarker, N.R.; Clark, C.E.F. Ruminant Production from Napier Grass (Pennisetum purpureum Schum): A Review. Animals 2024, 14, 467. [Google Scholar] [CrossRef] [PubMed]
- Salas-Reyes, I.G.; Estrada-Flores, J.G.; Arriaga-Jordán, C.M.; García-Martínez, A.; Castro-Montoya, J.; Albarrán-Portillo, B. Productive performance of lactating Brown Swiss cows grazing on an agrosilvopastoral system in a dry tropical region in central Mexico: Contribution of grass, herbaceous and woody species. Agroforest. Syst. 2023, 97, 223–233. [Google Scholar] [CrossRef]
- Islam, M.R.; Garcia, S.C.; Sarker, N.R.; Islam, M.A.; Clark, C.E.F. Napier grass (Pennisetum purpureum Schum) management strategies for dairy and meat production in the tropics and subtropics: Yield and nutritive value. Front. Plant Sci. 2023, 14, 1269976. [Google Scholar] [CrossRef] [PubMed]
- Spain, J.M. Forage potential of allic soils of the humid lowland tropics of Latin America. In Tropical Forage in Livestock Production Systems, Proceedings of the Annual Meetings of the American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Las Vegas, NV, USA, November 1973; Doll, E.C., Mott, G.O., Eds.; American Society of Agronomy: Madison WI, USA; pp. 1–8.
- Slayi, M.; Zhou, L.; Dzvene, A.R.; Mpanyaro, Z. Drivers and Consequences of Land Degradation on Livestock Productivity in Sub-Saharan Africa: A Systematic Literature Review. Land 2024, 13, 1402. [Google Scholar] [CrossRef]
- Alimi, N.; Assani, A.S.; Sanni Worogo, H.; Baco, N.M.; Traoré, I.A. Livestock feed resources used as alternatives during feed shortages and their impact on the environment and ruminant performance in West Africa: A systematic review. Front. Vet. Sci. 2024, 11, 1352235. [Google Scholar] [CrossRef]
- Seehaus, T.; Malz, P.; Sommer, C.; Lippl, S.; Cochachin, A.; Braun, M. Changes of the tropical glaciers throughout Peru between 2000 and 2016: Mass balance and area fluctuations. Cryosphere 2019, 13, 2537–2556. [Google Scholar] [CrossRef]
- Tito, R.; Vasconcelos, H.L.; Feeley, K.J. Global climate change increases risk of crop yield losses and food insecurity in the tropical Andes. Glob. Chang. Biol. 2018, 24, e592–e602. [Google Scholar] [CrossRef]
- Gomes, V.C.; Meirelles, P.R.L.; Costa, C.; Barros, J.S.; Castilhos, A.M.; Souza, D.M.; Pariz, C.M. Production and quality of corn silage with forage and pigeon peas in a crop–livestock system. Semin. Ciências Agrárias 2021, 42, 861–876. [Google Scholar] [CrossRef]
- Cañete, D.C.; Alvarez, T.S. Commercialization of green corn-based silage production for dairy in Cagayan Valley: Profitability and viability assessment. Univers. J. Agric. Res. 2021, 9, 79–90. [Google Scholar] [CrossRef]
- Thomasz, E.; Pérez-Franco, I.; García-García, A. The Economic Impact of Climate Risk on Extensive Livestock: The Case of Lamb Production in Extremadura, Spain. Sustainability 2020, 12, 7254. [Google Scholar] [CrossRef]
- Alvarez-García, W.; Muñoz-Vílchez, Y.; Figueroa, D.; Estrada, R.; Quilcate, C. A review of sustainable cattle genetic improvement in the Peruvian Highlands. Vet. Anim. Sci. 2025, 27, 100427. [Google Scholar] [CrossRef]
- Gilardino, A.; Quispe, I.; Pacheco, M.; Bartl, K. Comparison of different methods for consideration of multifunctionality of Peruvian dairy cattle in Life Cycle Assessment. Livest. Sci. 2020, 240, 104151. [Google Scholar] [CrossRef]
- Paul, B.K.; Koge, J.; Maass, B.L.; Notenbaert, A.; Peters, M.; Groot, J.C.J.; Tittonell, P. Tropical forage technologies can deliver multiple benefits in Sub-Saharan Africa: A meta-analysis. Agron. Sustain. Dev. 2020, 40, 22. [Google Scholar] [CrossRef]
- Jin, Y.; Luo, J.; Yang, Y.; Jia, J.; Sun, M.; Wang, X.; Khan, I.; Huang, D.; Huang, L. The evolution and expansion of RWP-RK gene family improve the heat adaptability of elephant grass (Pennisetum purpureum Schum.). BMC Genom. 2023, 24, 510. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.D.; Azevedo, A.L.; Pereira, A.V.; Paula, C.M.; Campos, J.M.; Lédo, F.J.; Santos, V.B. DNA elimination in embryogenic development of Pennisetum glaucum × Pennisetum purpureum (Poaceae) hybrids. Genet. Mol. Res. 2013, 12, 4817–4826. [Google Scholar] [CrossRef]
- Maldonado-Peralta, M.Á.; Rojas-García, A.R.; Sánchez-Santillán, P.; Bottini-Luzardo, M.B.; Torres-Salado, N.; Ventura-Ríos, J.; Joaquín-Cancino, S.; Luna-Guerrero, M.J. Análisis de crecimiento del pasto Cuba OM-22 (Pennisetum purpureum × Pennisetum glaucum) en el trópico seco. Agro Product. 2019, 12, 17–22. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, J.; Mu, X.; Lin, J. Pollen dispersion, pollen viability and pistil receptivity in Leymus chinensis. Ann. Bot. 2004, 93, 295–301. [Google Scholar] [CrossRef]
- Fisher, W.D.; Bashaw, E.C.; Holt, E.C. Evidence for apomixis in Pennisetum ciliare and Cenchrus setigerus. Agron. J. 1954, 46, 401–404. [Google Scholar] [CrossRef]
- Barrett, S.C.H. Influences of clonality on plant sexual reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 8859–8866. [Google Scholar] [CrossRef]
- Khosa, J.; Bellinazzo, F.; Kamenetsky Goldstein, R.; Macknight, R.; Immink, R.G.H. Phosphatidylethanolamine-binding proteins: The conductors of dual reproduction in plants with vegetative storage organs. J. Exp. Bot. 2021, 72, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- López-Corona, B.E.; Mondaca-Fernández, I.; Gortáres-Moroyoqui, P.; Meza-Montenegro, M.M.; de Jesús Balderas-Cortés, B.; Ruiz-Alvarado, C.; Rueda-Puente, E.O. Rooting of plant cuttings of Salicornia bigelovii (Torr.) by chitosan as a bioproduct of marine origin. Rev. Terra Latinoam. 2019, 37, 361–369. [Google Scholar] [CrossRef]
- Knoll, J.E.; Anderson, W.F. Vegetative propagation of napiergrass and energycane for biomass production in the southeastern United States. Agron. J. 2012, 104, 518–522. [Google Scholar] [CrossRef]
- Cerdas-Ramírez, R.; Vidal-Vega, E.; Vargas-Rojas, J.C. Productividad del pasto Cuba OM-22 (Pennisetum purpureum × Pennisetum glaucum) con distintas dosis de fertilización nitrogenada. InterSedes 2021, 22, 136–161. [Google Scholar] [CrossRef]
- Martínez, R.O.; González, C. Evaluation of varieties and hybrids of elephant grass Pennisetum purpureum and Pennisetum purpureum × Pennisetum glaucum for forage production. Cuba. J. Agric. Sci. 2017, 51, 477–487. Available online: https://cjascience.com/index.php/CJAS/article/view/749/771 (accessed on 18 October 2025).
- Miranda-Leyva, M.; Ayala-Yera, J.R.; Diez-Núñez, J. Evaluación agroproductiva de ‘Cuba OM-22’ (Pennisetum purpureum × Pennisetum glaucum) en un suelo pardo grisáceo ócrico durante el período poco lluvioso en Las Tunas. Obs. Econ. Latinoam. 2012, 167. Available online: https://ideas.repec.org/a/erv/observ/y2012i16724.html (accessed on 26 October 2025).
- Druege, U. Overcoming Physiological Bottlenecks of Leaf Vitality and Root Development in Cuttings: A Systemic Perspective. Front. Plant Sci. 2020, 11, 907. [Google Scholar] [CrossRef]
- Ramadhan, A.; Njunie, M.N.; Lewa, K.K. Effect of planting material and variety on productivity and survival of Napier grass (Pennisetum purpureum Schumach.) in the coastal lowlands of Kenya. East. Afr. Agric. For. J. 2015, 81, 40–45. [Google Scholar] [CrossRef]
- Fanindi, A.; Sutedi, E.; Sajimin; Herdiawan, I.; Harmini; Pamungkas, F.A.; Kusumaningrum, D.A.; Baehaki; Karya; Setiawan, A. Productivity of elephant grass pakchong (Pennisetum purpureum cv. Pakchong) and elephant grass taiwan (Pennisetum purpureum cv. Taiwan) cultivated based on different stem cutting sizes. IOP Conf. Ser. Earth Environ. Sci. 2024, 1362, 012027. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Riego (MINAGRI). Decreto Supremo N° 013-2010-AG: Reglamento para la Ejecución de Levantamiento de Suelos. Available online: https://www.midagri.gob.pe/portal/decreto-supremo/ds-2010/4804-decreto-supremo-no-013-2010-ag (accessed on 26 October 2025).
- Oliva-Cruz, M.; Cabañas-López, J.R.; Altamirano-Tantalean, M.A.; Juarez-Contreras, L.; Vigo, C.N. Agronomic Behavior of Peanut (Arachis hypogaea L.) Cultivars under Three Planting Densities in the Northeast of Peru. Agronomy 2024, 14, 1905. [Google Scholar] [CrossRef]
- NTP-ISO/IEC 17025:2017. Requisitos Generales Para la Competencia de los Laboratorios de Ensayo y Calibración, 3rd ed.; Instituto Nacional de Calidad (INACAL): Lima, Peru, 2017. [Google Scholar]
- Bazán, R. Manual de Procedimientos de los Análisis de Suelos y Agua con Fines de Riego. Available online: https://repositorio.inia.gob.pe/server/api/core/bitstreams/55bde890-0de8-4f7b-8d15-8b39ea07cd26/content (accessed on 18 September 2025).
- Vásquez, H.V.; Valqui, L.; Bobadilla, L.G.; Meseth, E.; Trigoso, M.J.; Zagaceta, L.H.; Valqui-Valqui, L.; Saravia-Navarro, D.; Barboza, E.; Maicelo, J.L. Agronomic and Nutritional Evaluation of INIA 910—Kumymarca Ryegrass (Lolium multiflorum Lam.): An Alternative for Sustainable Forage Production in Department of Amazonas (NW Peru). Agronomy 2025, 15, 100. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. (Eds.) Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005; Available online: https://www.researchgate.net/publication/292783651_AOAC_2005 (accessed on 18 September 2025).
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 18 September 2025).
- de Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 18 September 2025).
- Wei, T.; Simko, V. corrplot: Visualization of a Correlation Matrix, R package version 0.92; 2021. Available online: https://github.com/taiyun/corrplot (accessed on 18 September 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 18 September 2025).
- du Toit, J.C. Early survival and growth of vegetatively propagated indigenous grasses in a clear-felled timber plantation in KwaZulu-Natal, South Africa. Afr. J. Range Forage Sci. 2009, 26, 97–101. [Google Scholar] [CrossRef]
- Díaz-Páez, M.; Werden, L.K.; Zahawi, R.A.; Usuga, J.; Polanía, J. Vegetative propagation of native tree species: An alternative restoration strategy for the tropical Andes. Restor. Ecol. 2021, 30, e13611. [Google Scholar] [CrossRef]
- Tambong, J.D. Branch Cutting Propagation of Different Bamboo Species Through Varying Levels of Alpha Naphthalene Acetic Acid Supplementation. Int. J. Res. Rev. 2023, 10, 393–399. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Majrashi, M.A.; Ibrahim, H.M. Improvement of physical properties and water retention in sandy soils through the synergistic use of natural clay deposits and wheat straw. Sustainability 2024, 16, 46. [Google Scholar] [CrossRef]
- Lo, Y.-N. Root initiation of Shorea macrophylla cuttings: Effects of node position, growth regulators and misting regime. For. Ecol. Manag. 1985, 12, 43–52. [Google Scholar] [CrossRef]
- Pollock, A.; Grant, K.R.; Schoonmaker, A. Size influences on the survival of willow cuttings under operational field conditions. Ecol. Evol. 2025, 15, e70835. [Google Scholar] [CrossRef]
- Liu, Z.; Lan, J.; Li, W.; Ma, H. Reseeding improved soil and plant characteristics of degraded alfalfa (Medicago sativa) grassland in loess hilly plateau region, China. Ecol. Eng. 2023, 190, 106933. [Google Scholar] [CrossRef]
- Li, D.; Li, S.; Chen, H.; Wu, J. Reseeding promotes plant biomass by improving microbial community stability and soil fertility in a degraded subalpine grassland. Geoderma 2025, 453, 117160. [Google Scholar] [CrossRef]
- Nyiramvuyekure, V.; Obwoyere, G.O.; Inoti, S.K. The influence of planting orientation and ecotype on sprouting and survival of stem cuttings of African teak (Milicia excelsa (Welw.)) in Kenya. Open Access Res. J. Sci. Technol. 2022, 6, 001–008. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Leite, M.B.; Wink, C.; Durlo, M.A. Influência do ângulo de plantio sobre a brotação e o enraizamento de estacas de Phyllanthus sellowianus (Klotzsch) Müll. Arg. Cienc. Florest. 2010, 20, 523–532. [Google Scholar] [CrossRef]
- Decruyenaere, J.G.; Holt, J.S. Seasonality of clonal propagation in giant reed. Weed Sci. 2001, 49, 760–767. [Google Scholar] [CrossRef]
- Boersma, N.N.; Heaton, E.A. Effects of temperature, illumination and node position on stem propagation of Miscanthus × giganteus. GCB Bioenergy 2012, 4, 680–687. [Google Scholar] [CrossRef]
- Thimann, K.V.; Skoog, F. Studies on the growth hormone of plants: III. The inhibiting action of the growth substance on bud development. Proc. Natl. Acad. Sci. USA 1933, 19, 714–716. [Google Scholar] [CrossRef]
- Minakuchi, K.; Kameoka, H.; Yasuno, N.; Umehara, M.; Luo, L.; Kobayashi, K.; Hanada, A.; Ueno, K.; Asami, T.; Yamaguchi, S.; et al. FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol. 2010, 51, 1127–1135. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, T.-T.; Ma, S.-S.; Jiang, D.-J.; Bie, X.-M.; Sui, N.; Zhang, X.-S.; Wang, F. TaD27-B gene controls the tiller number in hexaploid wheat. Plant Biotechnol. J. 2020, 18, 513–525. [Google Scholar] [CrossRef]
- Chaves Gurgel, A.L.; dos Santos Difante, G.; Marques Costa, C.; Emerenciano Neto, J.V.; Tonhão, G.H.; Vinhas Ítavo, L.C.; Alce Miyake, A.W. Establishment of tropical forage grasses in the Cerrado biome. Rev. Mex. Cienc. Pecu. 2022, 13, 674–689. [Google Scholar] [CrossRef]
- Cárdenas-Aquino, M.d.R.; Camas-Reyes, A.; Valencia-Lozano, E.; López-Sánchez, L.; Martínez-Antonio, A.; Cabrera-Ponce, J.L. The cytokinins BAP and 2-iP modulate different molecular mechanisms on shoot proliferation and root development in lemongrass (Cymbopogon citratus). Plants 2023, 12, 3637. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, G.; Zhao, J.; Chu, H.; Lin, W.; Zhang, D.; Wang, Z.; Liang, W. Dwarf Tiller1, a WUSCHEL-related homeobox transcription factor, is required for tiller growth in rice. PLoS Genet. 2014, 10, e1004154. [Google Scholar] [CrossRef] [PubMed]
- Stape, J.L.; Silva, C.R.; Binkley, D. Spacing and geometric layout effects on the productivity of clonal Eucalyptus plantations. Trees For. People 2022, 8, 100235. [Google Scholar] [CrossRef]
- Porfirio, M.D.; Neres, M.A.; Führ, C.A.; da Silva, T.H.; Guimarães, I.C.S.B. Effects of row spacing and planting density of forage sorghum on dry matter yield, morphologic parameters, nutritive value, and predicted milk yield of dairy cows. Res. Soc. Dev. 2021, 10, e36101119374. [Google Scholar] [CrossRef]
- Noland, R.; Dowdy, M.; Harris, G. Maize row spacing and seeding rate informed by space-per-plant geometry. Agronomy 2025, 15, 374. [Google Scholar] [CrossRef]
- Olson, N.A.; Trostle, C.; Meyer, R.; Hulke, B.S. Canopy closure, yield, and quality under heterogeneous plant spacing in sunflower. Agron. J. 2024, 116, 2275–2283. [Google Scholar] [CrossRef]
- Baseggio, M.; Newman, Y.; Sollenberger, L.E.; Fraisse, C.; Obreza, T. Planting Rate and Depth Effects on Tifton 85 Bermudagrass Establishment Using Rhizomes. Crop Sci. 2015, 55, 1338–1345. [Google Scholar] [CrossRef]
- Fukagawa, S.; Ishii, Y. Grassland establishment of dwarf napiergrass (Pennisetum purpureum Schumach.) by planting of cuttings in the winter season. Agronomy 2018, 8, 12. [Google Scholar] [CrossRef]
- Sivakumar, S.D.; Sridharan, N.; Babu, C. Effect of planting materials and sett treatment on establishment and yield of Bajra Napier hybrid grass CO (BN) 5. Madras Agric. J. 2022, 109, 28–32. [Google Scholar] [CrossRef]
- Iglesias-Gómez, J.M.; Domínguez-Escudero, J.M.A.; Wencomo-Cárdenas, H.B.; Olivera-Castro, Y.; Toral-Pérez, O.C.; Milera-Rodríguez, M.d.l.C. Comportamiento agronómico y nutricional de especies mejoradas en un sistema de pastoreo racional Voisin, en Panamá. Pastos y Forrajes 2022, 45, eE10. Available online: https://www.redalyc.org/journal/2691/269173684010/ (accessed on 19 September 2025).
- Valqui, L.; Saucedo-Uriarte, J.A.; Altamirano-Tantalean, M.A.; Bobadilla, L.G.; Portocarrero Villegas, S.M.; Bardales, W.; Frias, H.; Zagaceta Llanca, L.H.; Valqui-Valqui, L.; Puerta-Chavez, L.J.; et al. Influence of tree species on soil physicochemical composition, macrofauna, and forage production. J. Agric. Food Res. 2025, 23, 102220. [Google Scholar] [CrossRef]
- Li, Y.; Du, S.; Sun, L.; Cheng, Q.; Hao, J.; Lu, Q.; Ge, G.; Wang, Z.; Jia, Y. Effects of lactic acid bacteria and molasses additives on dynamic fermentation quality and microbial community of native grass silage. Front. Microbiol. 2022, 13, 830121. [Google Scholar] [CrossRef]
- Ayandiran, S.K.; Odeyinka, S.M.; Amoo, A.F.; Ojo, I.F.; Ogunmola, Y.E.; Olakunle, T.M.; Oloidi, F.F. Effect of ensiling elephant grass with molasses on the nutritive value and in vitro digestibility. Niger. J. Anim. Prod. 2024, 1585–1588. [Google Scholar] [CrossRef]
- Musa, A.R.; Garba, Y. Nutritive value of untreated and molasses-urea treated Typha (Typha domingensis) silage. FUDMA J. Agric. Agric. Technol. 2022, 8, 70–76. [Google Scholar] [CrossRef]
- Duran, N.N.; Elfaki, M.O.A.; Kılıç, Ü. Determination of the feed value, digestibility, and in vitro gas production of high-moisture corn grain silage. World J. Adv. Res. Rev. 2024, 24, 1995–2000. [Google Scholar] [CrossRef]
- Gordon, C.H.; Derbyshire, J.C.; Wiseman, H.G.; Jacobson, W.C. Variations in initial composition of orchardgrass as related to silage composition and feeding value. J. Dairy. Sci. 1964, 47, 987–992. [Google Scholar] [CrossRef]
- Aswini, M.S.; Ganesan, K.N.; Ezhilarasi, T. Correlation between green fodder yield and fodder quality traits in hybrids of pearl millet [Pennisetum glaucum (L.) R. Br.]. Int. J. Plant Soil. Sci. 2023, 35, 1975–1983. [Google Scholar] [CrossRef]
- Culqui, L.; Huaman-Pilco, Á.F.; Juarez-Contreras, L.; Vigo, C.N.; Goñas, M.; Pariente-Mondragón, E.; Maicelo-Quintana, J.L.; Oliva-Cruz, M. Nutritional potential of native shrub species for cattle feeding in northeastern Peru. Rangel. Ecol. Manag. 2025, 98, 600–608. [Google Scholar] [CrossRef]
- Vásquez, H.V.; Valqui, L.; Valqui-Valqui, L.; Bobadilla, L.G.; Reyna, M.; Maravi, C.; Pajares, N.; Altamirano-Tantalean, M.A. Influence of nitrogen fertilization and cutting dynamics on the yield and nutritional composition of white clover (Trifolium repens L.). Plants 2025, 14, 2765. [Google Scholar] [CrossRef]
- Beltran Barriga, P.A.; Corrêa de Lima, R.; Brugnara Soares, A.; Simioni Assmann, T.; Canaza Cayo, A.W. Intensidad de pastoreo y fertilización nitrogenada sobre la altura de Lolium multiflorum Lam. en un sistema de integración agricultura-ganadería. Agron. Costarric. 2020, 44, 127–137. [Google Scholar] [CrossRef]
- González Marcillo, R.L.; Castro Guamàn, W.E.; Guerrero Pincay, A.E.; Vera Zambrano, P.A.; Ortiz Naveda, N.R.; Guamàn Rivera, S.A. Assessment of Guinea Grass Panicum maximum under Silvopastoral Systems in Combination with Two Management Systems in Orellana Province, Ecuador. Agriculture 2021, 11, 117. [Google Scholar] [CrossRef]
- Oliva-Cruz, M.; Altamirano-Tantalean, M.A.; Chuquizuta-Torres, R.; Oliva-Cruz, C.; Maicelo-Quintana, J.L.; Leiva-Espinoza, S.T.; Culqui, L.; Mendez-Fasabi, L.D.; Rojas Ventura, H.M.; Corazon-Guivin, M.A.; et al. Isolation and Characterization of Native Isolates of Metarhizium sp. as a Biocontrol Agent of Hypothenemus hampei in Rodríguez de Mendoza Province—Peru. Agronomy 2024, 14, 1341. [Google Scholar] [CrossRef]
- Zeballos-Cabana, J.C.; Carrasco-Chilon, W.L.; Vásquez-Pérez, H.V. Efecto de zonas agroecológicas y condición de siembra sobre altura de planta y rendimiento en avena forrajera en la región Puno, Perú. Tecnol. En. Marcha 2023, 36, 89–96. Available online: https://www.redalyc.org/articulo.oa?id=699877376012 (accessed on 18 October 2025). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).