Abstract
To address the limitations of traditional wheat quality breeding, this study developed a Wheat Quality Molecular Marker Selection System (QMMS) by integrating key genetic loci controlling core quality traits: grain protein content (GPC), grain hardness (GH), and high-molecular-weight glutenin subunits (HMW-GS). The QMMS comprises three KASP markers (Kgpc-2B, Kgpc-2D, Kgpc-4A) and two duplex KASP (dKASP) markers (Pin-ab, Glu-AD), enabling cost-effective (≈5 CNY per sample) and high-throughput genotyping. Systematic validation was conducted using four panels of materials: representative varieties, breeding nursery materials, regional trial materials from the Middle and Lower Reaches of the Yangtze River, and advanced lines from four cooperative institutions. Results showed that (1) the QMMS accurately distinguished quality types of representative varieties: strong-gluten varieties carried five or more strong-gluten–favorable alleles, while weak-gluten varieties harbored five or more weak-gluten favorable alleles; (2) in breeding nursery materials, quality traits increased significantly with the number of aggregated strong-gluten favorable alleles, and 48.15% of strong-gluten candidates met strong- and medium-strong-gluten standards; (3) in regional trial materials, 15.25% (36/236) and 1.69% (4/236) of lines carried ≥5 strong-gluten and weak-gluten favorable alleles, with low utilization of Kgpc-2D and Pina/Pinb favorable alleles (<30%); and (4) the QMMS screened 273 strong-gluten and 27 weak-gluten candidates for cooperative institutions, matching their breeding focuses. In conclusion, the QMMS provides reliable technical support for precise and efficient wheat quality breeding.
1. Introduction
Wheat, one of the most important food crops globally, has its quality improvement not only as a core component of ensuring food security but also as a critical support for meeting the diversified demands of the food processing industry and enhancing the added value of agricultural products [1]. Currently, wheat quality breeding primarily relies on phenotypic evaluation, supplemented only by a limited number of functional markers associated with grain hardness and high-molecular-weight glutenin subunits (HMW-GS) for assisted selection [2,3]. Molecular Marker-Assisted Selection (MAS) selects target traits based on genotypes. It can be conducted in the early generations, significantly shortening the breeding cycle and reducing the cost of field phenotypic identification. Moreover, it is not affected by environmental factors and enables the pyramiding of multiple target traits through the simultaneous detection of multiple markers. This study aims to establish a systematic and efficient Wheat Quality Molecular Marker Selection System (QMMS), facilitating precision breeding in quality improvement.
Grain protein content (GPC) is a core indicator for evaluating wheat nutritional and processing quality, and its level directly affects the nutritional value of wheat and processing properties of flour [4]. Although GPC is significantly influenced by environmental factors and exhibits substantial variation across different years and locations [5,6], its moderately high heritability provides feasibility for genetic improvement [7,8]. To date, researchers have identified genetic loci controlling GPC on all wheat chromosomes [5,7,9]. However, most studies remain in the initial mapping stage, and reports on the evaluation of locus effects and their breeding applications are relatively scarce, limiting their practical value in molecular breeding.
Grain hardness (GH) is another key trait determining wheat processing quality, directly affecting flour water absorption, processing efficiency, and end-product quality [10]. This trait is mainly regulated by the Pina and Pinb genes located on the short arm of chromosome 5D, which encode highly seed-specific proteins rich in cysteine and tryptophan. When both Pina and Pinb carry wild-type allelic variants, wheat grains exhibit a “soft” phenotype; when either gene undergoes mutation, the grains become “hard”. Among these mutations, Pina-D1b and Pinb-D1b are the most common types in nature [11].
HMW-GS are core factors influencing wheat processing quality and dough rheological properties [12]. Their encoding genes are located at three complex loci (Glu-A1, Glu-B1, and Glu-D1) on the long arms of chromosomes 1A, 1B, and 1D, respectively [13]. In genetic mapping studies of traits related to dough rheological properties and solvent retention capacity, numerous quantitative trait loci (QTL) have been detected on chromosomes 1A, 1B, and 1D. Notably, these QTL are either close to or overlapping with the Glu-A1, Glu-B1, and Glu-D1 loci, further confirming the critical role of HMW-GS in regulating flour quality [14,15,16].
The National Wheat Variety Approval Standards of China (2024 Revision) explicitly classifies wheat into four types (strong-gluten, medium-strong-gluten, medium-gluten, and weak-gluten wheat) based on core indicators, including GPC, flour wet gluten content (WG), water absorption (WA), and dough stability time (ST). This standard provides a scientific basis for the end-use positioning of wheat varieties and the assessment of their market value. However, most wheat quality traits are typical quantitative traits, regulated by multiple genes synergistically and easily affected by environmental factors. These characteristics result in limitations of traditional phenotypic selection, such as long breeding cycles, low accuracy, and high detection costs, which severely impede the breeding efficiency of high-quality and specialized wheat. Our research team has previously successfully established a molecular marker selection system for GPC and developed duplex KASP (dKASP) markers targeting GH and HMW-GS [17,18]. Building on these preliminary achievements, this study further integrates the key genetic loci of core quality traits of GPC, GH, and HMW-GS to establish an efficient QMMS. The aim is to provide technical support for precise wheat quality breeding and facilitate the efficient development of high-quality wheat varieties.
2. Materials and Methods
2.1. Experimental Materials
The experimental materials used in this study included four panels, with detailed information as follows:
Representative varieties (lines): Forty accessions were derived from the long-term collection of domestic and international registered wheat varieties and advanced lines by the Wheat Genetic Breeding Team of Jiangsu Academy of Agricultural Sciences (JAAS) (Supplementary Table S1). These materials were used for genotypic validation of QMMS and analysis of variety-genotype correspondence.
Breeding nursery materials: A total of 360 F4 generation and 85 F7 generation breeding materials were collected from the breeding nursery of the Wheat Genetic Breeding Team, JAAS. During cultivation, the water and fertilizer management regime was consistent with local field production practices. After normal maturity and harvest, 1000 g grains were randomly sampled from each plot for subsequent quality trait determination.
Regional trial materials: Two hundred and thirty-six accessions included regional trial materials from the Middle and Lower Reaches of the Yangtze River and Jiangsu Provincial Regional Trial. Only fresh leaves at the seedling stage were collected for genomic DNA extraction and genotypic identification.
Advanced lines from cooperative institutions: Advanced line materials were collected from four cooperative institutions, including Yangzhou University (Yangzhou, China, YZU: 276 lines), Jiangsu Lixiahe Institute of Agricultural Sciences (Yangzhou, China, LXH: 184 lines), Zhenjiang Institute of Agricultural Sciences in the Hilly Area of Jiangsu Province (Zhenjiang, China, ZJ: 184 lines), and Jiangsu Ruihua Agricultural Technology Co., Ltd. (Huaian, China, RH: 184 lines). These materials were mainly used for screening high-quality candidate lines.
2.2. Construction of QMMS and Genotype Analysis
2.2.1. Composition of QMMS
Seven core genetic loci associated with wheat quality were selected to construct the QMMS, including three GPC-controlling loci (Kgpc-2B, Kgpc-2D, Kgpc-4A), two major GH regulating genes (Pina and Pinb), and two HMW-GS encoding loci (Glu-A1, Glu-D1) (Figure 1). The system consisted of 3 KASP markers (Kgpc-2B, Kgpc-2D, and Kgpc-4A) [18] and 2 dKASP markers, where “Pin-ab” targeted Pina/Pinb and “Glu-AD” targeted Glu-A1/Glu-D1 [17] (Supplementary Table S2).
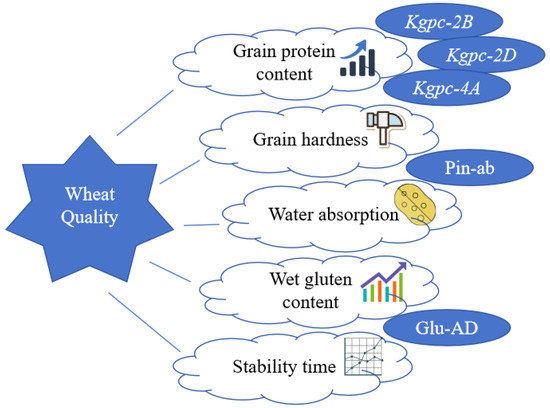
Figure 1.
Schematic diagram of the molecular marker selection system for wheat quality.
2.2.2. Genomic DNA Extraction
Genomic DNA was extracted from young wheat leaves using the CTAB method [19]. The concentration and purity of DNA were determined using a Nanodrop 2000 micro-spectrophotometer (Thermo Scientific, Waltham, MA, USA) to ensure DNA quality. The original DNA solution was diluted to 20 ng·μL−1 with ultrapure water and stored at −20 °C for subsequent use.
2.2.3. KASP Reaction and Genotype Determination
All the KASP primers were synthesized by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). The standard FAM (5′ GAAGGTGACCAAGTTCATGCT 3′) and HEX (5′ GAAGGTCGGAGTCAACGGATT 3′) tails were added to the 5′ end of the two allele-specific primers (F1/F2), respectively. The KASP assays were performed in 384-well PCR plates in a 5-μL reaction system, which comprised 2.5 μL KASP 2× Reaction Mix (LGC, Teddington, UK), 0.07 μL assay mix, and 2.43 μL 20 ng·μL−1 genomic DNA. 100 μL assay mix consisted of 12 μL each allele-specific forward primer (100 μM), 30 μL reverse primer (100 μM), and 46 μL ddH2O. The PCR program was as follows: 94 °C for 15 min; 10 touchdown cycles of 94 °C for 20 s, 61–55 °C for 60 s (decreasing by 0.6 °C per cycle); and 26 cycles of 94 °C for 20 s and 55 °C for 60 s. The PCR reactions were conducted in a Hydrocycler16 water-bath PCR instrument (LGC Genomics, Beverly, MA, USA), and the PCR fluorescence was detected using a PHERAstar microplate reader (BMG LABTECH, Ortenberg, Baden-Württemberg, Germany). KlusterCaller software (LGC Genomics, Beverly, MA, USA) was used to analyze the data.
2.3. Determination of Quality Traits
Wheat grains were subjected to 1 month of after-ripening at room temperature before quality trait determination. All traits were measured following standard methods, as detailed below: GPC was determined using a DA7200 near-infrared grain analyzer (Perten Instruments, Hägersten, Sweden); GH was measured according to GB/T 21304-2007 [20] using a SKCS 4100 Single Kernel Characteristic System analyzer (Perten Instruments, Hägersten, Sweden); flour milling was conducted following NY/T 1094.5-2006 [21] using a Quadrumat Junior small-scale experimental mill (Brabender, Duisburg, Germany) to prepare standard flour. WG was determined in accordance with GB/T 5506.2-2024 [22] using a GM2200 gluten analyzer (Perten Instruments, Hägersten, Sweden). Farinograph parameters, including WA and ST, were measured based on GB/T 14614-2019 [23] using a JFZD farinograph (Dongfang Fude Instrument, Beijing, China). Wheat quality classification was performed strictly according to the National Wheat Variety Approval Standards (2024 Revision) [24] (Supplementary Table S3).
2.4. Data Analysis
Microsoft Excel 2016 was used for statistical analysis of phenotypic data, including calculation of the distribution frequency of different genotypes, as well as phenotypic means and standard deviations. Additionally, the association patterns between genotypic and phenotypic data were preliminarily explored through two methods: trend charting and proportion statistics. IBM SPSS 19.0 was employed to perform independent samples t-tests, verifying the significance of phenotypic differences between groups. The ggpubr 0.6.0 package in R was utilized to generate professional statistical charts, enabling visual presentation of inter-group phenotypic differences.
3. Results
3.1. Genotyping Analysis in Representative Varieties with QMMS
The genotyping results of the representative varieties showed a good correspondence with the QMMS established in this study (Supplementary Table S1): Elite strong-gluten varieties, including Xinmai 26, Xinong 979, Zhengmai 366, and Yannong 19, carried five favorable alleles for strong-gluten wheat of the QMMS; Varieties such as Bainong 4199, Aikang 58, Yangmai 158, Zhoumai 18, and Zhongmai 175 each carried GPC-increased alleles at two or more GPC-controlling loci, and their GPC all met the criteria for strong-gluten wheat (https://www.a-seed.cn/ (accessed on 28 August 2025)), but the lack of 5 + 10 subunit encoded by Glu-D1 resulted in ST failing to meet the strong-gluten wheat standard. Typical weak-gluten varieties, including Ningmai 9, Yangmai 13, and Yangmai 20, carried five favorable alleles for weak-gluten. All these results demonstrated the system’s efficacy in discriminating varietal quality types.
3.2. Evaluation and Validation of the QMMS in Breeding Nursery Materials
The QMMS was used to conduct quality-based genotypic identification of 360 F4 generation breeding materials and 85 F7 generation yield evaluation nursery materials. From these, materials carrying different numbers of favorable alleles for strong- or weak-gluten wheat were screened for subsequent phenotypic evaluation, including 26 F4 lines and 25 F7 lines. Key quality traits, including GPC, GH, WA, WG, and ST, were measured for the screened materials (Supplementary Table S4). The results showed that all tested quality values exhibited a consistent and significant upward trend as the number of aggregated favorable alleles for strong-gluten wheat increased (Figure 2).
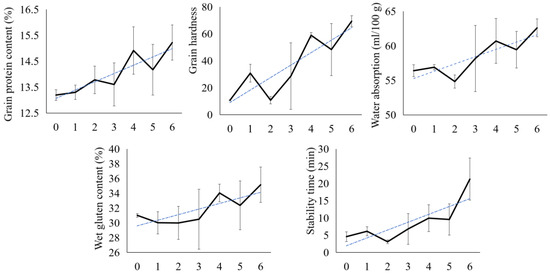
Figure 2.
Variation trend of quality traits with the number of favorable alleles in the breeding nursery materials. The solid lines represent the changes in the actual values of the corresponding indicators, and the dashed lines indicate their changing trends. The numbers on the x-axis represent the number of favorable alleles for strong-gluten wheat.
Based on the genotypes and actual quality traits of representative varieties, accumulating five or more favorable alleles enables the preliminary screening of high-quality materials. In the breeding nursery materials, 27 materials with 5 or more favorable alleles for strong-gluten wheat were classified as the strong-gluten wheat candidate group, and 6 materials with 5 or more favorable alleles for weak-gluten wheat were classified as the weak-gluten candidate group. Highly significant differences (p < 0.01) were observed between the two groups in all tested quality traits; significant differences in grain appearance were also observed (Figure 3 and Figure 4). Within the strong-gluten wheat candidate group, 7 lines (25.93%) met the national approval standards for strong-gluten wheat, and 13 lines (48.15%) met the standards for medium-strong-gluten wheat. In contrast, none of the six materials in the weak-gluten candidate group met the approval standards for weak-gluten wheat. However, all quality values of the weak-gluten candidates were significantly lower than those of the strong-gluten candidates (p < 0.05), confirming their potential as weak-gluten germplasm. These candidate lines will be prioritized for further monitoring of their agronomic traits and disease resistance in subsequent trials, serving as high-quality reserves for wheat regional trial.
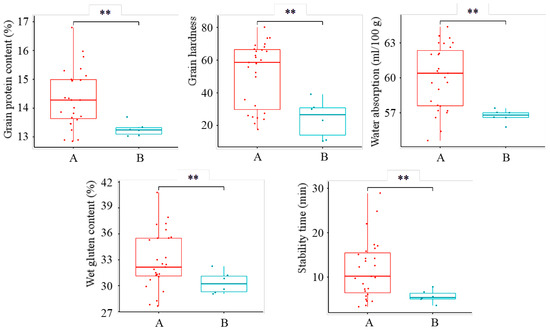
Figure 3.
Comparison of quality traits between the strong-gluten and weak-gluten wheat candidate groups. Panel A represents the strong-gluten candidate group; panel B represents the weak-gluten candidate group. ** indicates significant difference at p < 0.01.

Figure 4.
Grain appearance of the strong-gluten and weak-gluten candidate groups.
3.3. Genotype Identification of Regional Trial Materials
The QMMS was used to perform genotypic identification on 236 regional trial materials collected from the middle and lower reaches of the Yangtze River. The distribution characteristics of favorable alleles for strong-gluten wheat are summarized as follows (Table 1): The favorable alleles of the GPC-controlling locus (Kgpc-2D) and the major GH-regulating genes (Pina/Pinb) exhibited low distribution frequencies, with utilization rates of less than 30%. Among all tested lines, 36 accessions (15.25%) carried 5 or more favorable alleles for strong-gluten wheat; lines with 5 or more favorable alleles for weak-gluten wheat were even scarcer, accounting for only 1.69% (4 lines in total). This result is highly consistent with the current breeding trend of prioritizing the development of strong- and medium-strong-gluten wheat by the breeding institutions.

Table 1.
Distribution of favorable alleles in regional trial materials.
To further validate the system’s reliability, the genotypic identification results of this study were compared with public quality data from the Regional Wheat Trials (the National Agricultural Technology Extension and Service Center and Jiangsu Provincial Seed Management Station). Two lines carrying five favorable alleles for strong-gluten wheat were found to meet the approval standards for strong-gluten and medium-strong-gluten wheat in the 2025 National Regional Trial of the middle and lower reaches of the Yangtze River, respectively (Supplementary Table S5). Notably, one of these lines was the only accession in this set of regional trial materials that met the strict standards for strong-gluten wheat, highlighting the system’s efficiency in screening elite germplasm.
In the 2025 Jiangsu Province Wheat Regional Trial, 5 accessions carried at least 5 strong-gluten favorable alleles, of which 3 met the strong-gluten wheat standard and 2 met the medium-strong-gluten wheat standard (Supplementary Table S6). Among the 6 accessions that participated in the trial for two consecutive years (2024 and 2025), only two met the strong-gluten or medium-strong-gluten wheat standard in both years, and one of these two lines carried five strong-gluten favorable alleles (Supplementary Table S6).
3.4. Promotion and Application of the Wheat Quality Molecular Marker System
To strengthen quality-oriented wheat breeding in the middle and lower reaches of the Yangtze River, this study provided identification services using the QMMS to four local breeding entities, including YZU, LXH, ZJ, and RH (Table 2). The results showed that the four institutions screened out strong-gluten candidate materials of 66 (YZU), 39 (LXH), 84 (ZJ), and 84 (RH) accessions, respectively, and weak-gluten candidate materials of 12 (YZU), 13 (LXH), 1 (ZJ), and 1 (RH) accessions. This distribution of candidate materials was consistent with the current breeding focus of each institution. These results provided direct genotypic data support for the subsequent precise breeding of high-quality and specialized wheat varieties by the aforementioned institutions, helping them optimize parent selection and early-generation line screening.

Table 2.
Summary of high-quality candidate lines from four institutions.
4. Discussion
4.1. Advantages of QMMS in Addressing Limitations of Traditional Wheat Quality Breeding
China has made significant advances in wheat quality breeding [25,26], developing elite varieties like strong-gluten (Yannong 19, Zhengmai 366, etc.) and weak-gluten (Ningmai 9, Yangmai 13, etc.) wheat to meet diverse market demands. However, traditional breeding relies on phenotypic evaluation with inherent limitations—long cycles, high costs, and low efficiency. To address these challenges, this study successfully established a QMMS by systematically integrating key genetic regulatory loci controlling GPC, GH, and HMW-GS. This system offers remarkable cost advantage, with a detection cost of approximately 1 CNY per locus, resulting in a total cost of only 5 CNY per sample for genotyping, significantly lower than traditional phenotypic testing methods. In terms of selection efficiency, among the 27 strong-gluten candidate lines identified using this system, 13 (nearly 50%) met the national approval standards for strong- or medium-strong-gluten wheat. Given cost and efficiency, it is fully suitable as a routine quality breeding method. Furthermore, the system is fully based on the KASP genotyping platform, which enables rapid detection and high throughput, perfectly adapting to the large-scale genotyping needs of modern wheat breeding programs. Notably, the incorporation of dKASP marker technology further enhances its cost-effectiveness, providing robust technical support for the precision and efficiency of wheat quality breeding.
In recent years, notable progress has been made in the cloning of quality loci, such as NAC-019 and NAC100 [27,28,29]. However, reports on their related breeding applications remain relatively limited. Given that the Glu-B1 locus is rich in subunit types and requires identification via multiple markers, it was not included in QMMS. Future studies may attempt to conduct targeted introgression of its elite subunit types (17 + 18 and 7OE) to enrich the existing QMMS [30,31].
For quantitative traits, pyramiding more loci usually works better. However, as the number of unlinked loci increases, the breeding population must be sufficiently large to obtain the target genotype. For example, when loci number reaches 5, the required population size needs to expand to nearly 5000 [32]. F2 enrichment can significantly reduce population size by increasing the frequency of selected alleles, making it highly suitable for multi-locus MAS [33].Combining QMMS with F2 enrichment can further reduce costs and improve efficiency in the breeding process.
4.2. QMMS-Guided Parental Selection for Strong-Gluten Wheat Breeding and Challenges in Weak-Gluten Wheat Improvement
Using widely cultivated varieties as parents for breeding is the most common practice in current wheat breeding. Clarifying their genotypic composition enables efficient trait combination and supports subsequent MAS breeding of specialized varieties. In QMMS established in this study, elite varieties such as Xinmai 26, Xinong 979, and Yannong 19 carry five favorable alleles for strong-gluten. This indicates that they possess the genetic basis to serve as elite strong-gluten parental lines. These varieties can thus be directly used in strong-gluten wheat breeding programs, and when combined with the QMMS here, will facilitate the precision improvement and efficient pyramiding of target quality traits. Meanwhile, varieties, including Bainong 4199, Aikang 58, Yangmai 158, Zhoumai 18, and Zhongmai 175, exhibit GPC levels that meet the standards for strong-gluten but lack the 5 + 10 subunit, resulting in suboptimal ST. Their quality can be optimized by crossing with wheat lines carrying 5 + 10 subunit, followed by directional screening using the QMMS to enhance ST.
Compared with strong- and medium-strong-gluten wheat materials, weak-gluten wheat candidates in breeding nurseries are extremely scarce, which was consistently observed across representative varieties, experimental materials, and breeding lines from collaborating institutions. Among 574 wheat varieties nationally approved in the southern part of the Huang-Huai Winter Wheat Region during 2011–2024, none were classified as weak-gluten [34]. Even in the middle and lower reaches of the Yangtze River, China’s dominant production area for weak-gluten wheat, only seven weak-gluten varieties were approved between 2013 and 2023 [35]. Currently, domestic weak-gluten wheat production meets merely ~10% of the national industrial demand, forcing China to rely heavily on imports from countries such as the United States and Australia. These facts underscore the urgent need to strengthen weak-gluten wheat breeding efforts. Notably, Lv et al. [36] screened four accessions of stable weak-gluten germplasm from the Chinese wheat mini-core collection, and their results showed high consistency with the identification outcomes of the molecular marker system developed in this study: Beijing 8 carried 6 favorable alleles for weak-gluten and Shijiazhuang 8 harbored 4; two low-protein accessions, Jinan 2 and Jinmai 47, each carried more than two alleles associated with reduced GPC. It further confirms the high selection efficiency of QMMS in breeding of weak-gluten wheat.
The middle and lower reaches of the Yangtze River is climatically suitable for medium- or weak-gluten wheat, yet the probability of meeting weak-gluten quality standards specifically for GPC was only 9.48% in Jiangsu Province [37]. A 2022 national survey (1377 samples) showed <1% compliance (12 samples met the weak-gluten criteria), confirming severe quality challenges [35]. A key factor contributing to this issue is the excessive nitrogen fertilizer application by farmers, which severely degrades the quality of weak-gluten wheat [38,39]. The seven weak-gluten candidate lines screened in this study, while exhibiting significantly lower quality indices than strong-gluten candidates, still failed to meet the weak-gluten approval standards, and suboptimal fertilizer management is likely a major contributor to this shortfall. Therefore, we propose two critical measures to address these challenges: (1) establishing dedicated weak-gluten wheat trial groups in regional breeding programs to prioritize weak-gluten trait selection; and (2) developing supporting production technical guidelines tailored for weak-gluten wheat.
4.3. Applicability and Future Optimization Directions of QMMS
This system was initially developed based on wheat germplasm resources from the middle and lower reaches of the Yangtze River [18]. However, genotypic analysis of representative varieties revealed that it also exhibits a certain degree of applicability to materials from other wheat-growing regions in China. Such cross-regional applicability may be attributed to two key factors: one is that quality-regulating loci show high genetic conservation across different regions, with limited haplotype variation [40], which reduces the risk of marker failure caused by differences in genetic background; the other is tight linkage between detection markers and functional loci, enabling accurate capture of haplotype differences at target loci while minimizing the interference of genetic recombination between markers and traits.
Furthermore, the system enables high-throughput screening of quality breeding materials at a low cost. In the validation with F4/F7 generation breeding materials, its screening efficiency for strong- and medium-strong-gluten wheat reached nearly 50%, confirming its practical value for large-scale breeding programs. Future optimization of the system will focus on two directions to further enhance its precision and breeding utility: (1) Cloning of the three key loci controlling GPC, developing functional markers, and dissecting the genetic associations between these loci and other quality traits; and (2) incorporating the core quality loci of this system into a genomic selection model as fixed effects to enhance the selection efficiency for complex quality traits [41].
5. Conclusions
Integrated with prior research, this study developed an efficient, low-cost QMMS. The system employs three KASP and two dKASP markers to accurately genotype key loci for core wheat quality traits (GPC, GH, HMW-GS). Validation confirmed its reliability in breeding screening, and technical services based on QMMS provided critical genotypic data to other breeding institutions, supporting their targeted breeding of high-quality wheat varieties and parental optimization. This advancement facilitated precision breeding in the Yangtze River wheat region, laying a technical foundation for China’s sustainable high-quality wheat industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15112494/s1, Table S1: Genoyping results of representative varieties (lines). Table S2: Primers of the markers included in QMMS. Table S3: Key indices of wheat quality in national wheat variety approval standards (2024 Revision). Table S4 Genoyping and phenotyping results of the breeding nursery materials. Table S5: Quality Test Results for Group B in the 2025 National Regional Trial of the Middle and Lower Reaches of the Yangtze River. Table S6: Quality Test Results for Group B in the 2025 and 2024 Jiangsu Province Regional Trial.
Author Contributions
Conceptualization, P.J. and X.Z.; Methodology, X.F., L.W., C.L. and H.W.; Validation, L.W. and C.D.; Formal analysis, C.L. and Y.H.; Investigation, X.F.; Resources, H.W., Y.H., P.Z., C.D. and G.Y.; Data curation, P.J., X.F. and L.W.; Writing—original draft, P.J.; Writing—review & editing, X.Z.; Supervision, G.Y.; Funding acquisition, P.J., P.Z. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Zhongshan Biological Breeding Laboratory (ZSBBL-KY2023-02), Jiangsu key R & D plan (Modern Agriculture) (BE2022346), the International Scientific and Technological Cooperation Projects of Jiangsu Province (BZ2024043), and the China Agriculture Research System of MOF and MARA (CARS-03-57).
Data Availability Statement
The original data presented in this study are included in the supplementary material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to express our sincere gratitude to the colleagues from cooperative institutions for their assistance during the sample collection process: Hongxiang Ma and Yi Dai from Yangzhou University; Yong Zhang and Derong Gao from the Jiangsu Lixiahe Institute of Agricultural Sciences; Dongsheng Li and Jiajun Liu from the Zhenjiang Institute of Agricultural Sciences in the Hilly Area of Jiangsu Province; and Yongle Yang and Yangang Jin from Jiangsu Ruihua Agricultural Technology Co., Ltd.; We also thank Jiamin Shen from the Central Laboratory of Jiangsu Academy of Agricultural Sciences for her assistance in genotyping.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Brinton, J.; Uauy, C. A reductionist approach to dissecting grain weight and yield in wheat. J. Integr. Plant Biol. 2019, 61, 337–358. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhuang, Q.; Cheng, S.; Yu, Z.; Zhao, Z.; Liu, X. Wheat Production and Technology Improvement in China. J. Agric. 2018, 8, 99–106. [Google Scholar]
- He, Z.; Xia, X.; Chen, X.; Zhuang, Q. Progress of Wheat Breeding in China and the Future Perspective. Acta Agron. Sin. 2011, 37, 202–215. [Google Scholar] [CrossRef]
- Balyan, H.S.; Gupta, P.K.; Kumar, S.; Dhariwal, R.; Jaiswal, V.; Tyagi, S.; Agarwal, P.; Gahlaut, V.; Kumari, S. Genetic improvement of grain protein content and other health-related constituents of wheat grain. Plant Breed. 2013, 132, 446–457. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.-y.; Yan, G.-j.; Liu, C.-j. Identification of QTLs Conferring Agronomic and Quality Traits in Hexaploid Wheat. J. Integr. Agric. 2012, 11, 1399–1408. [Google Scholar] [CrossRef]
- Prasad, M.; Kumar, N.; Kulwal, P.; Röder, M.; Balyan, H.; Dhaliwal, H.; Gupta, P. QTL analysis for grain protein content using SSR markers and validation studies using NILs in bread wheat. Theor. Appl. Genet. 2003, 106, 659–667. [Google Scholar] [CrossRef]
- Fatiukha, A.; Filler, N.; Lupo, I.; Lidzbarsky, G.; Klymiuk, V.; Korol, A.; Pozniak, C.; Fahima, T.; Krugman, T. Grain protein content and thousand kernel weight QTLs identified in a durum × wild emmer wheat mapping population tested in five environments. Theor. Appl. Genet. 2020, 133, 119–131. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, J.L.; Elias, E.M.; Kianian, S.F. Mapping genes for grain protein concentration and grain yield on chromosome 5B of Triticum turgidum (L.) var. dicoccoides. Euphytica 2004, 139, 217–225. [Google Scholar] [CrossRef]
- Kunert, A.; Naz, A.A.; Dedeck, O.; Pillen, K.; Léon, J. AB-QTL analysis in winter wheat: I. Synthetic hexaploid wheat (T. turgidum ssp. dicoccoides × T. tauschii) as a source of favourable alleles for milling and baking quality traits. Theor. Appl. Genet. 2007, 115, 683–695. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Zhao, W.; Wang, Y.; Qiao, L.; Wu, B.; Zhao, J.; Zheng, X.; Wang, J.; Zheng, J. Genome-wide association study of grain hardness and novel Puroindoline alleles in common wheat. Mol. Breed. 2022, 42, 40. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.F. Puroindolines: The molecular genetic basis of wheat grain hardness. Plant Mol. Biol. 2002, 48, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jondiko, T.O.; Tilley, M.; Awika, J.M. Effect of high molecular weight glutenin subunit composition in common wheat on dough properties and steamed bread quality. J. Sci. Food Agric. 2014, 94, 2801–2806. [Google Scholar] [CrossRef]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Würschum, T.; Leiser, W.L.; Kazman, E.; Longin, C.F.H. Genetic control of protein content and sedimentation volume in European winter wheat cultivars. Theor. Appl. Genet. 2016, 129, 1685–1696. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Liu, S.; Mai, S.; Qin, Y.; Wang, S.; Zhou, Z.; Yang, K.; Huang, X.; Deng, Y.; et al. Identification and validation of quantitative trait loci for seven quality-related traits in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2025, 138, 57. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.-e.; Ye, X.-l.; Li, Y.; Shi, B.-x.; Guo, Z.; Dai, S.-f.; Ma, J.; Liu, Z.-h.; Jiang, Y.-f.; Li, W.; et al. Identification and validation of novel loci associated with wheat quality through a genome-wide association study. J. Integr. Agric. 2022, 21, 3131–3147. [Google Scholar] [CrossRef]
- Jiang, P.; Fan, X.; Zhang, G.; Wu, L.; He, Y.; Li, C.; Zhang, X. Cost-effective duplex Kompetitive Allele Specific PCR markers for homologous genes facilitating wheat breeding. BMC Plant Biol. 2023, 23, 119. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, P.; Wu, L.; He, Y.; Li, C.; Ma, H.; Zhang, X. Linkage and association mapping and Kompetitive allele-specific PCR marker development for improving grain protein content in wheat. Theor. Appl. Genet. 2021, 134, 3563–3575. [Google Scholar] [CrossRef]
- Hill-Ambroz, K.L.; Brown-Guedira, G.L.; Fellers, J.P. Modified Rapid DNA Extraction Protocol for High Throughput Microsatellite Analysis in Wheat. Crop Sci. 2002, 42, 2088–2091. [Google Scholar] [CrossRef]
- GB/T 21304-2007; Determination of Wheat Hardness—Hardness Index Method. State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2007.
- NY/T 1094.5-2006; Wheat Experimental Milling—Quadruplex Milling Method. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2006.
- GB/T 5506.2-2024; Wheat and Wheat Flour-Gluten Content-Part 2: Determination of Wet Gluten and Gluten Index by Mechanical Means. State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2024.
- GB/T 14614-2019; Inspection of Grain and Oils-Doughs Rheological Properties Determination of Wheat Flour-Farinograph Test. Standardization State Administration for Market Regulation and Administration of China: Beijing, China, 2019.
- National Wheat Variety Approval Standards (2024 Revision); National Crop Variety Approval Committee: Beijing, China, 2024.
- Yuan, Q.; Zhao, Y.; Zhang, Z.; Zhen, S.; Wang, J.; Zhang, F.; Chen, L.; Liu, D.; Zhou, Y. Analysis of Quality and Breeding Strategies of Nationally Approved Strong-Gluten and Medium-Strong-Gluten Wheat Varieties in the Huang-Huai Wheat Region from 2020 to 2024. Crops 2025. Available online: https://link.cnki.net/urlid/11.1808.S.20250423.1103.016 (accessed on 23 April 2025).
- Zhang, X.; Lu, C.; Jiang, W.; Zhang, Y.; Lv, G.; Wu, H.; Wang, C.; Li, M.; Wu, S.; Gao, D. Quality selection indices and parent combination principle of weak-gluten wheat. Acta Agron. Sin. 2023, 49, 1282–1291. [Google Scholar] [CrossRef]
- Cao, S.; Liu, B.; Wang, D.; Rasheed, A.; Xie, L.; Xia, X.; He, Z. Orchestrating seed storage protein and starch accumulation toward overcoming yield–quality trade-off in cereal crops. J. Integr. Plant Biol. 2024, 66, 468–483. [Google Scholar] [CrossRef]
- Li, J.; Xie, L.; Tian, X.; Liu, S.; Xu, D.; Jin, H.; Song, J.; Dong, Y.; Zhao, D.; Li, G.; et al. TaNAC100 acts as an integrator of seed protein and starch synthesis exerting pleiotropic effects on agronomic traits in wheat. Plant J. 2021, 108, 829–840. [Google Scholar] [CrossRef]
- Gao, Y.; An, K.; Guo, W.; Chen, Y.; Zhang, R.; Zhang, X.; Chang, S.; Rossi, V.; Jin, F.; Cao, X.; et al. The endosperm-specific transcription factor TaNAC019 regulates glutenin and starch accumulation and its elite allele improves wheat grain quality. Plant Cell 2021, 33, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Ragupathy, R.; Naeem, H.A.; Reimer, E.; Lukow, O.M.; Sapirstein, H.D.; Cloutier, S. Evolutionary origin of the segmental duplication encompassing the wheat GLU-B1 locus encoding the overexpressed Bx7 (Bx7OE) high molecular weight glutenin subunit. Theor. Appl. Genet. 2008, 116, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, K.-M.; Kang, C.-S.; Yoon, M.; Jang, K.-C.; Choi, C. Development of PCR-based markers for identification of wheat HMW glutenin Glu-1Bx and Glu-1By alleles. BMC Plant Biol. 2024, 24, 395. [Google Scholar] [CrossRef]
- Wang, J.; Chapman, S.; Bonnett, D.; Rebetzke, G.; Crouch, J. Application of Population Genetic Theory and Simulation Models to Efficiently Pyramid Multiple Genes via Marker-Assisted Selection. Crop Sci. 2007, 47, 582–590. [Google Scholar] [CrossRef]
- Bonnett, D.; Rebetzke, G.; Spielmeyer, W. Strategies for efficient implementation of molecular markers in wheat breeding. Mol. Breed. 2005, 15, 75–85. [Google Scholar] [CrossRef]
- Ge, D.; Wang, Y.; Wang, C.; Chen, C.; Liu, X.; Deng, P.; Li, T.; Wang, H.; Zheng, X.; Yang, H.; et al. Analysis on Yield, Quality and Disease Resistance of Wheat Varieties in the Southern Huang Huai Winter Wheat Region from 2011 to 2024. J. Triticeae Crops 2025. Available online: https://link.cnki.net/urlid/61.1359.s.20250704.1646.004 (accessed on 7 July 2025).
- Zhou, Y.; Li, F.; Peng, S.; Wang, D.; Man, J. Current Status and Development Strategies of China’s Weak-Gluten Wheat Industry. J. Huazhong Agric. Univ. 2025, 44, 145–157. [Google Scholar]
- Lv, G.; Zhang, B.; Zhang, X.; Cheng, S. Screening for Weak Gluten Resources from Chinese Mini-core Collections Germplasms. Chin. Agric. Sci. Bull. 2008, 24, 260–263. [Google Scholar]
- Xia, S.; Wang, F.; Wang, L.; Zhou, Q.; Cai, J.; Wang, X.; Huang, M.; Dai, T.; Jiang, D. Study on the Adaptability of Wheat Reaching the Protein Content Standard of Soft Wheat in Jiangsu Province. Sci. Agric. Sin. 2020, 53, 4992–5004. [Google Scholar]
- Wu, X.; Li, C.; Tang, Y.; Liu, Y.; Li, B.; Fan, G.; Xiong, T. Effect of nitrogen management modes on grain yield, nitrogen use efficiency and light use ef ficiency of wheat. Chin. J. Appl. Ecol. 2017, 28, 1889–1898. [Google Scholar]
- Zhou, W.; Li, W.; Li, H.; Zhang, S.; Yong, Y.; Zheng, C.; Gao, X.; Cai, Y.; Xu, Q.; Yan, S. Effects of Nitrogen Topdressing and Density Interaction on Yield, Quality and Nitrogen Utilization of Weak Gluten Wheat. J. Triticeae Crops 2024, 44, 1456–1466. [Google Scholar]
- Xiao, J.; Liu, B.; Yao, Y.; Guo, Z.; Jia, H.; Kong, L.; Zhang, A.; Ma, W.; Ni, Z.; Xu, S.; et al. Wheat genomic study for genetic improvement of traits in China. Sci. China Life Sci. 2022, 65, 1718–1775. [Google Scholar] [CrossRef] [PubMed]
- Arruda, M.P.; Lipka, A.E.; Brown, P.J.; Krill, A.M.; Thurber, C.; Brown-Guedira, G.; Dong, Y.; Foresman, B.J.; Kolb, F.L. Comparing genomic selection and marker-assisted selection for Fusarium head blight resistance in wheat (Triticum aestivum L.). Mol. Breed. 2016, 36, 84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).