Monitoring of Root-Knot Nematodes (Meloidogyne spp.) in Croatia (2022–2024): Occurrence, Distribution and Species Identification
Abstract
1. Introduction
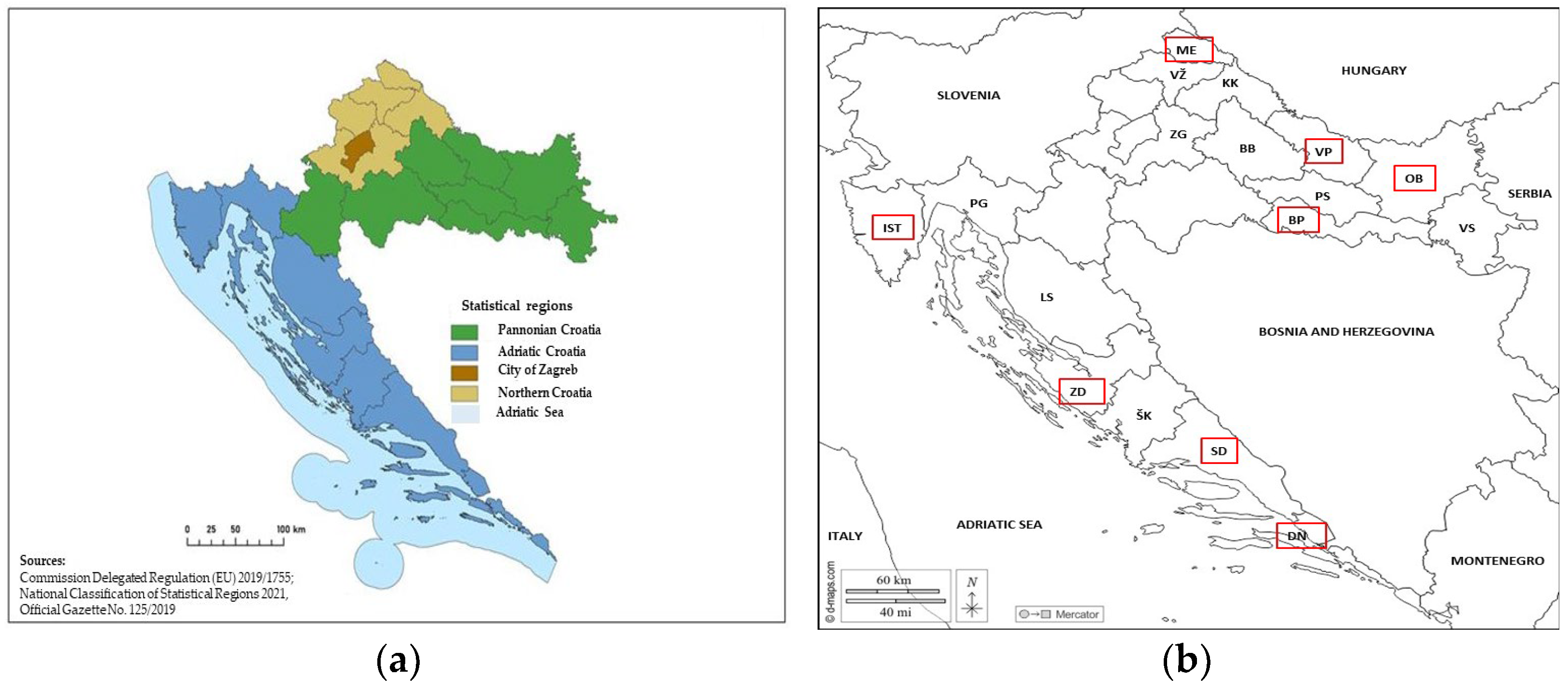
2. Materials and Methods
2.1. Sampling and Nematode Extraction
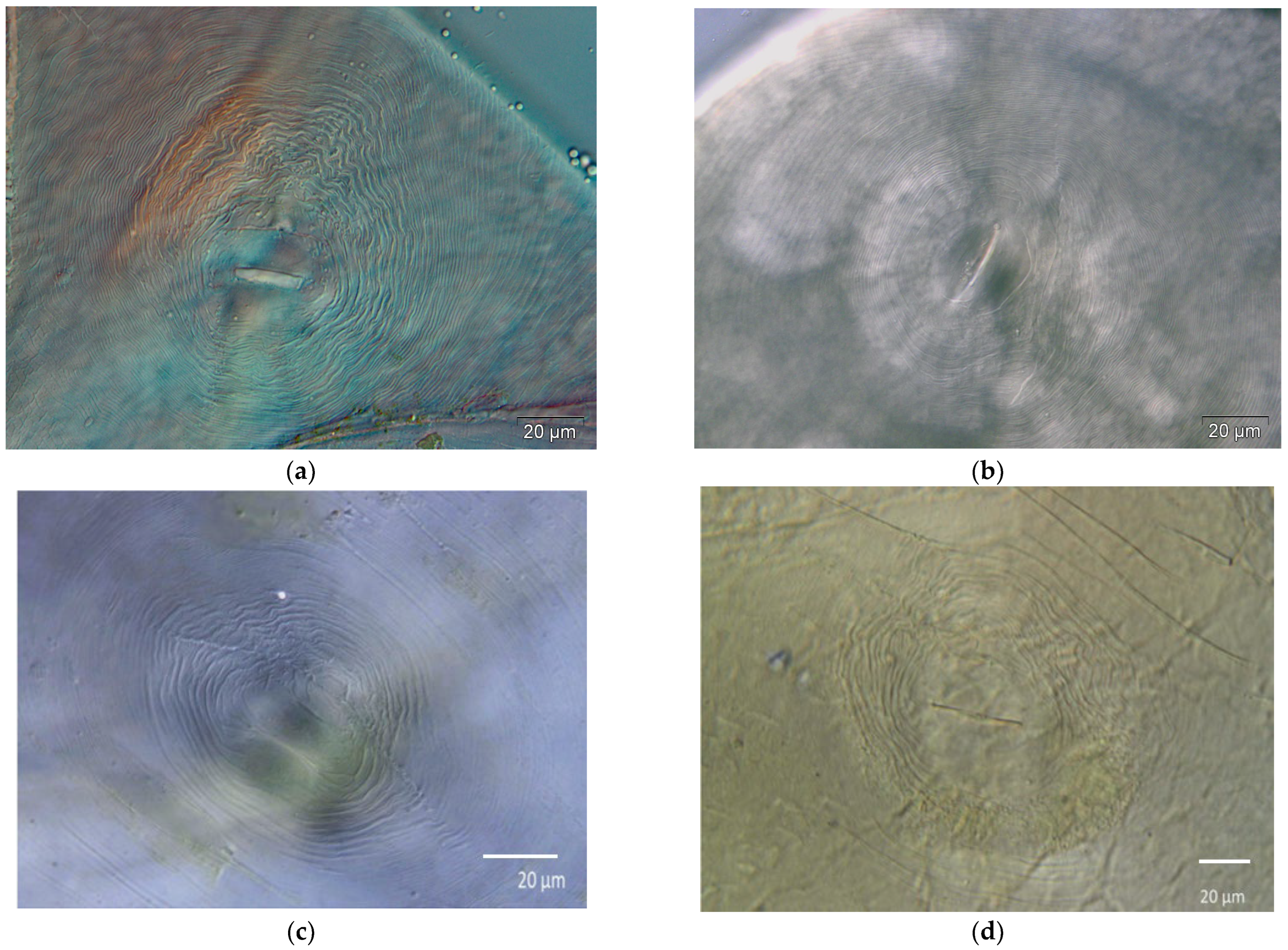
2.2. Morphological Analysis
2.3. DNA Extraction and Molecular Identification
2.4. Bioassay of Meloidogyne Populations
2.5. Statistical Analyses
3. Results
3.1. Detection of Meloidogyne spp.
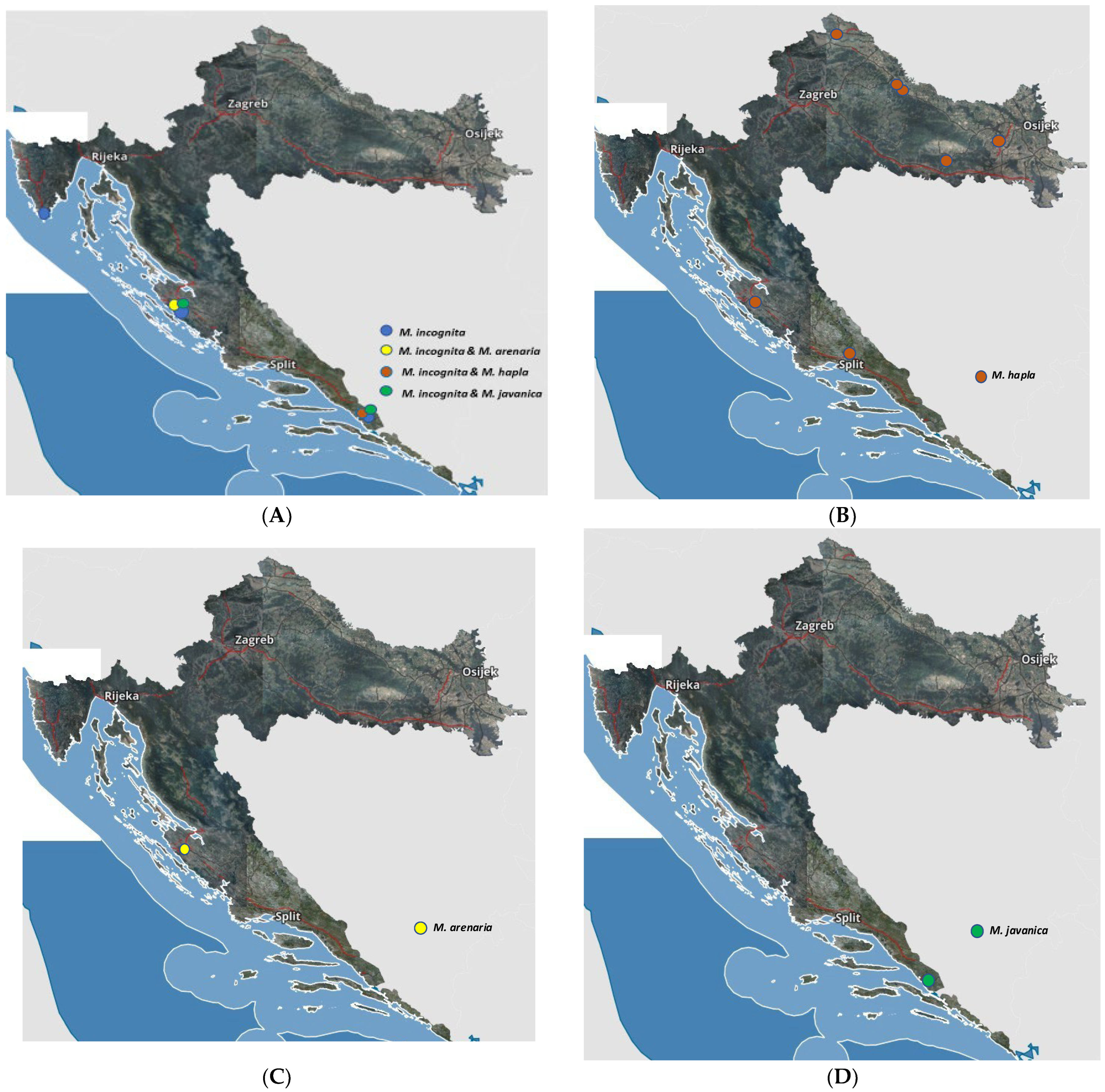
3.2. Species Identification of Meloidogyne spp.
3.3. Distribution of Meloidogyne Species
3.4. Host Plant Associations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RKN | Root knot nematode |
| USD | United States Dollar |
| BLAST | Basic Local Alignment Search Tool |
| NCBI | National Center for Biotechnology |
| TAF | Triethanolamine–formaldehyde |
| IPM | Integrated pest management |
| mtDNA | Mitochondrial DNA |
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne Species—A Diverse Group of Novel and Important Plant Parasites. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; Oxon (CABI): Wallingford, UK, 2009; pp. 1–17. [Google Scholar]
- Karssen, G.; Wesemael, W.; Moens, M. Root-knot nematodes. In Plant Nematology, 2nd ed.; Perry, R.N., Moens, M., Eds.; Oxon (CABI): Wallingford, UK, 2013; pp. 73–108. [Google Scholar]
- Singh, S.; Singh, B.; Singh, A.B. Nematodes: A Threat to Sustainability of Agriculture. Procedia Environ. Sci. 2015, 29, 215–216. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P. Soil-Borne Nematodes: Impact in Agriculture and Livestock and Sustainable Strategies of Prevention and Control with Special Reference to the Use of Nematode Natural Enemies. Pathogens 2022, 11, 640. [Google Scholar] [CrossRef]
- Gerič Stare, B.; Strajnar, P.; Sušić, N.; Urek, G.; Širca, S. Reported populations of Meloidogyne ethiopica in Europe identified as Meloidogyne luci. Plant Dis. 2017, 101, 1627–1632. [Google Scholar] [CrossRef]
- Santos, D.; Correia, A.; Abrantes, I.; Maleita, C. New Hosts and Records in Portugal for the Root-Knot Nematode Meloidogyne luci. J. Nematol. 2019, 51, e2019-03. [Google Scholar] [CrossRef] [PubMed]
- Širca, S.; Gerič Stare, B.; Strajnar, P.; Knapič, M.; Žibrat, U.; Folcher, L.; Fabrice, O.; Buisson, A.; Chappe, A.M.; Inacio, M.L.; et al. Global Warming and Distribution of Root-Knot Nematode Species of the Tropical Group (MeloTrop). Report of the Euphresco Project, 2021, 2016-A-199, 1-39. Available online: https://zenodo.org/record/5171594 (accessed on 11 May 2022).
- Bebber, D.P.; Holmes, T.; Gurr, S.J. The global spread of crop pests and pathogens. Glob. Ecol. Biogeogr. 2014, 23, 1398–1407. [Google Scholar] [CrossRef]
- Bačić, J.; Pavlović, M.; Kušić-Tišma, J.; Širca, S.; Theuerschuh, M.; Gerič Stare, B. First Report of the Root-Knot Nematode Meloidogyne luci on Tomato in Serbia. Plant Dis. 2023, 107, 2554. [Google Scholar] [CrossRef]
- Rusinque, L.; Camacho, M.J.; Serra, C.; Nobrega, F.; Inacio, M.L. Root-knot nematode assessment: Species identification, distribution, and new host records in Portugal. Front. Plant Sci. 2023, 14, 1230968. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Regulation (EU) 2019/2072 of 28 November 2019 Establishing Uniform Conditions for the Implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as Regards Protective Measures Against Pests of Plants, and Repealing Commission Regulation (EC) No 690/2008 and Amending Commission Implementing Regulation (EU) 2018/2019; L 319/1; Official Journal of the European Union: Luxembourg, 2019. [Google Scholar]
- Official Journal of the European Union. Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on Health Technology Assessment and Amending Directive 2011/24/EUEU 2021/2285; L 458/1; Official Journal of the European Union: Luxembourg, 2021; Volume 64. [Google Scholar]
- European and Mediterranean Plant Protection Organization. EPPO A1 List of Pests Recommended for Regulation as Quarantine Pests. Version 2024-09. 2024. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list (accessed on 19 June 2025).
- European and Mediterranean Plant Protection Organization. EPPO A2 List of Pests Recommended for Regulation as Quarantine Pests. Version 2024-09. 2024. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed on 19 June 2025).
- Bačić, J.; Gerič Stare, B.; Strajnar, P.; Širca, S.; Urek, G. First report of a highly damaged potato crop from Serbia caused by Meloidogyne incognita. Plant Dis. 2016, 100, 1021–1022. [Google Scholar] [CrossRef]
- Bačić, J.; Kušić, J.; Strajnar, P.; Gerič Stare, B.; Širca, S. First Report of Meloidogyne arenaria on Calla (Zantedeschia aethiopica) in Serbia. In Proceedings of the Abstract Book of 7th International Congress of Nematology, Antibes Juan Les Pins, France, 1–6 May 2022; p. 527. [Google Scholar]
- Širca, S.; Urek, G.; Karssen, G. The incidence of the root-knot nematode Meloidogyne incognita and Meloidogyne hapla in Slovenia. Acta Agric. Slov. 2004, 83, 15–22. [Google Scholar] [CrossRef]
- Statista. Croatia: Share of Economic Sectors in the Gross Domestic Product (GDP) from 2013 to 2023. Statista. 2023. Available online: https://www.statista.com/statistics/348751/share-of-economic-sectors-in-the-gdp-in-croatia/ (accessed on 19 June 2025).
- Croatian Bureau of Statistics. Agricultural Production, 2023, Statistical Reports 1727, Croatian Bureau of Statistics, 2024. Available online: https://podaci.dzs.hr/media/pb2dl2w1/si-1727-poljoprivredna-proizvodnja-u-2023.pdf (accessed on 19 June 2025).
- Croatian Bureau of Statistics. The NUTS classification in Croatia. Croatian Bureau of Statistics. 2023. Available online: https://dzs.gov.hr/highlighted-themes/prostorne-klasifikacije-i-subnacionalne-statistike-2-694/the-nuts-classification-in-croatia/699?utm (accessed on 19 June 2025).
- Anonymous. Godišnje Izvješće o Stanju Poljoprivrede u 2023. Godini. Ministry of Agriculture of the Republic of Croatia, Directorate for Agricultural Policy, EU and International Cooperation. 2024. Available online: https://poljoprivreda.gov.hr/UserDocsImages/dokumenti/poljoprivredna_politika/zeleno_izvjesce/2024_11_05%20Zeleno%20izvje%C5%A1%C4%87e%202023%20(1).pdf (accessed on 19 June 2025).
- Rehak Biondić, T.; Puškarić, J.; Gerič Stare, B.; Brmež, M. The status of root-knot nematodes of the Meloidogyne Genus in Croatia, with a special reference to the quarantine species. Poljoprivreda 2023, 29, 27–34. [Google Scholar] [CrossRef]
- Rehak Biondić, T.; Milanović, J.; Poje, I.; Gerič Stare, B.; Vrandečić, K.; Brmež, M. Preliminary results of root-knot nematodes monitoring in Croatia over two years (2022–2023). In Proceedings of the 16th Slovenian Conference on Plant Protection with International Participation, Bohinjska Bistrica, Slovenia, 5–6 March 2024; pp. 536–542. [Google Scholar]
- Oštrec, L.j. Parazitske nematode (Nemathelminthes, Nematoda) na duhaništima Podravine i njihov značaj za proizvodnju duhana. Poljopr. Znan. Smotra 1988, 3–4, 237–255. [Google Scholar]
- Oštrec, L.j. Vrste korijenovih nematoda (Meloidogyne spp.) u obalnom području Hrvatske. Glasnik Zaštite Bilja 1992, 9, 249–254. [Google Scholar]
- Majić, I.; Raspudić, E.; Nježić, B.; Kanižai Šarić, G.; Sarajlić, A. Važnost plodoreda i bionematocida usuzbijanju Meloidogyne hapla i Paratylenchus bukowinensis u mrkvi i peršinu. Glas. Biljn. Zaštite 2017, 17, 394–403. [Google Scholar]
- Širca, S.; Urek, G.; Karssen, G. First report of the root-knot nematode Meloidogyne ethiopica on tomato in Slovenia. Plant Dis. 2004, 88, 680. [Google Scholar] [CrossRef] [PubMed]
- Gerič Stare, B.; Strajnar, P.; Širca, S.; Susič, N.; Urek, G. Record of a new location for tropical root knot nematode Meloidogyne luci in Slovenia. EPPO Bull. 2018, 48, 135–137. [Google Scholar] [CrossRef]
- Bačić, J.; Laličević, I.; Širca, S.; Theuerschuh, M.; Susič, N.; Gerič Stare, B. Occurrence and Distribution of Root-Knot Nematodes Meloidogyne spp. in Serbia. Agronomy 2025, 15, 372. [Google Scholar] [CrossRef]
- Hartman, R.M.; Sasser, J.N. Identification of Meloidogyne Species on the Basis of Different Host Test and Perineal Pattern Morphology. In An Advanced Treatise on Meloidogyne: Methodology; Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; Department of Plant Pathology, North Carolina State University: Raleigh, NC, USA, 1985; Volume 2, pp. 69–77. [Google Scholar]
- Esbenshade, R.; Triantapahyllou, A.C. Enzymatic Relationships and Evolution in the Genus Meloidogyne (Nematoda: Tylenchida). J. Nematol. 1987, 19, 8–18. [Google Scholar]
- Janssen, T.; Karssen, G.; Verhaeven, M.; Coyne, D.; Bert, W. Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype-based diagnostics and reveals evidence of recent reticulate evolution. Sci. Rep. 2016, 6, 22591. [Google Scholar] [CrossRef]
- Powers, T.O.; Harris, T.S. A polymerase chain reaction for the identification of five major Meloidogyne species. J. Nematol. 1993, 25, 1–6. [Google Scholar] [PubMed]
- Zijlstra, C.; Donkers-Venne, D.T.H.M.; Fargette, M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2000, 2, 847–885. [Google Scholar]
- Blok, V.C.; Powers, T.O. Biochemical and molecular identification. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CABI Publishing: Wallingford, UK, 2009; pp. 98–103. [Google Scholar]
- Adam, M.A.M.; Phillips, M.S.; Blok, V.C. Molecular diagnostic key for identification for single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.). Plant Pathol. 2007, 56, 190–197. [Google Scholar] [CrossRef]
- Gerič Stare, B.; Aydinli, G.; Devran, Z.; Mennan, S.; Strajnar, P.; Širca, S. Recognition of species belonging to Meloidogyne ethiopica group and development of a diagnostic method for its detection. Eur. J. Plant Pathol. 2019, 154, 621–633. [Google Scholar] [CrossRef]
- Janssen, T.; Karssen, G.; Topalović, O.; Coyne, D.; Bert, W. Integrative taxonomy of root-knot nematodes reveals multiple independent origins of mitotic parthenogenesis. PLoS ONE 2017, 12, e0172190. [Google Scholar] [CrossRef]
- Maleita, C.; Cardoso, J.M.S.; Rusinque, L.; Esteves, I.; Abrantes, I. Species-Specific Molecular Detection of the Root-Knot Nematode Meloidogyne luci. Biology 2021, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- NEM-EMERGE. An Integrated Set of Novel Approaches to Counter the Emergence and Proliferation of Invasive and Virulent Soil Borne Nematodes. Available online: https://nem-emerge.eu/project/work-packages (accessed on 19 June 2025).
- Hunt, D.J.; Handoo, Z.A. Taxonomy Identification and Principal Species. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; Oxon (CABI): Wallingford, UK, 2009; pp. 55–97. [Google Scholar]
- Carneiro, R.M.D.G.; Lima, F.S.O.; Correia, V.R. Methods and Tools Currently Used for the Identification of Plant Parasitic Nematodes. In Nematology—Concepts, Diagnosis and Control, Chapter 2; Shah, M.M., Mahamood, M., Eds.; InTechOpen: Rijeka, Croatia, 2017; pp. 19–52. [Google Scholar] [CrossRef]
- OEPP/EPPO. National regulatory control systems PM 9/17 (1) Meloidogyne chitwoodi and Meloidogyne fallax. EPPO Bull. 2013, 43, 527–533. [Google Scholar] [CrossRef]
- Cobb, N.A. Estimating the Nema Populations of Soil; USDA Technical Circular: St. Louis, MO, USA, 1918; p. 48. [Google Scholar]
- OEPP/EPPO. Diagnostics PM 7/119 (1) Nematode extraction. OEPP EPPO Bull. 2013, 43, 471–495. [Google Scholar] [CrossRef]
- Taylor, A.L.; Sasser, J.N. Biology, Identification, and Control of Root-Knot Nematodes (Meloidogyne Species); Department of Plant Pathology, North Carolina State University: Raleigh, NC, USA; United States Agency for International Development: Washington, DC, USA, 1978. [Google Scholar]
- Jepson, S.B. The use of second-stage juvenile tails as an aid in the identification of Meloidogyne species. Nematology 1983, 29, 11–28. [Google Scholar] [CrossRef]
- Hirschmann, H. The genus Meloidogyne and morphological characters differentiating its species. In An Advanced Treatise on Meloidogyne; Biology and Control; Sasser, J.N., Carter, C.C., Eds.; North Carolina State University Graphics: Raleigh, NC, USA, 1985; Volume 1, pp. 79–93. [Google Scholar]
- Eisenback, J.D.; Hunt, D.J. General morphology. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CAB International: Wallingford, UK, 2009; pp. 18–54. [Google Scholar]
- Karssen, G. The Plant-Parasitic Nematode Genus Meloidogyne Goldi, 1892 (Tylenchida) in Europe; Brill: Leiden, The Netherlands, 2002; p. 160. [Google Scholar]
- Wishart, J.; Phillips, M.S.; Blok, V.C. Ribosomal intergenic spacer: A polymerase chain reaction diagnostic for Meloidogyne chitwoodi, M. fallax, and M. hapla. Phytopathology 2002, 92, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI–BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Aydınlı, G.; Mennan, S. Identification of root-knot nematodes (Meloidogyne spp.) from greenhouses in the Middle Black Sea Region of Turkey. Turk. J. Zool. 2016, 40, 675–685. [Google Scholar] [CrossRef]
- Aydınlı, G. Detection of the root-knot nematode Meloidogyne luci Carneiro et al., 2014 (Tylenchida: Meloidogynidae) in vegetable fields of Samsun Province, Turkey. Turkiye Entomoloji Derg. 2018, 42, 229–237. [Google Scholar] [CrossRef]
- Lunt, D.H.; Kumar, S.; Koutsovoulos, G.; Blaxter, M.L. The complex hybrid origins of the root knot nematodes revealed through comparative genomics. PeerJ 2014, 2, e356. [Google Scholar] [CrossRef]
- Conceição, I.L.; Tzortzakakis, E.A.; Gomes, P.; Abrantes, I.; Da Cunha, M.J. Detection of the root-knot nematode Meloidogyne ethiopica in Greece. Eur. J. Plant Pathol. 2012, 134, 451–457. [Google Scholar] [CrossRef]
- Maleita, C.; Esteves, I.; Cardoso, J.M.S.; Cunha, M.J.; Carneiro, R.M.D.G.; Abrantes, I. Meloidogyne luci, a new root--knot nematode parasitizing potato in Portugal. Plant Pathol. 2018, 67, 366–376. [Google Scholar] [CrossRef]
- Rusinque, L.; Nobrega, F.; Cordeiro, L.; Serra, C.; Inácio, M.L. First detection of Meloidogyne luci (Nematoda: Meloidogynidae) parasitizing potato in the Azores, Portugal. Plants 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]



| Type of the Monitoring | 2022 | 2023 | 2024 | Total (2022–2024) |
|---|---|---|---|---|
| National survey of RKNs on potato | 37 | 50 | 52 | 139 |
| Early warning programme of RKNs on vegetables | 12 | 30 | 29 | 71 |
| Total per year | 49 | 80 | 81 | 210 |
| Primer Name | Target Species/ Group of Species | Primer Sequences (5′-3′) | Expected Fragment Length (bp) | References |
|---|---|---|---|---|
| C2F3 Mt575 | tropical Meloidogyne group | 5′-GGTCAATGTTCAGAAATTTGTGG-3′ | 621 | [34,38] |
| 5′-AGAACTTAAACTCTAAATAAC-3′ | ||||
| Me309 Me549R | M. ethiopica group | 5′-CTAATTTGGGTGAATTT-3′ | 241 | [38] |
| 5′-AATCAAAATCTTCTCCT-3′ | ||||
| JMV1 JMV2 JMV hapla JMV tropical | M. hapla M. chitwoodi M. fallax M. incognita | 5′-GGATGGCGTGCTTTCAAC-3′ | 440 540 670 615 | [52] |
| 5′-TTTCCCCTTATGATGTTTACCC-3′ | ||||
| 5′-AAAAATCCCCTCGAAAAATCCACC-3′ | ||||
| 5′-GCKGGTAATTAAGCTGTCA-3′ | ||||
| Finc Rinc | M. incognita | 5′-CTCTGCCCAATGAGCTGTCC-3′ | 1200 | [35] |
| 5′-CTCTGCCCTCACATTAGG-3′ | ||||
| Far Rar | M. arenaria | 5′-TCGGCGATAGAGGTAAATGAC-3′ | 420 | [35] |
| 5′-TCGGCGATAGACACTACAACT-3′ | ||||
| Fjav Rjav | M. javanica | 5′-GGTGCGCGATTGAACTGAGC-3′ | 670 | [35] |
| 5′-CAGGCCCTTCAGTGGAACTATAC-3′ | ||||
| NAD5F2 NAD5R1 | Meloidogyne spp. | 5′-TATTTTTTGTTTGAGATATATTAG-3′ | 610 | [33] |
| 5′-CGTGAATCTTGATTTTCCATTTTT-3′ | ||||
| C2F3 1108 | M. incognita and M. javanica M. arenaria M. hapla | 5′-GGTCAATGTTCAGAAATTTGTGG-3′ | 1700 1100 520 | [34] |
| 5′-TACCTTTGACCAATCACGCT-3′ |
| Type of the Monitoring | 2022 | 2023 | 2024 | Total Positive Detections (2022–2024) |
|---|---|---|---|---|
| National survey of RKNs on potato | 1 (2.7%) | 4 (8.0%) | 8 (15.4%) | 13 (9.4%) |
| Early warning programme of RKNs on vegetables | 7 (58.3%) | 24 (80.0%) | 17 (58.6%) | 48 (67.6%) |
| Total per year | 8 (16.3%) | 28 (35.0%) | 25 (31.3%) | 61 (29.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehak Biondić, T.; Milanović, J.; Poje, I.; Popović, L.; Brmež, M.; Gerič Stare, B. Monitoring of Root-Knot Nematodes (Meloidogyne spp.) in Croatia (2022–2024): Occurrence, Distribution and Species Identification. Agronomy 2025, 15, 2492. https://doi.org/10.3390/agronomy15112492
Rehak Biondić T, Milanović J, Poje I, Popović L, Brmež M, Gerič Stare B. Monitoring of Root-Knot Nematodes (Meloidogyne spp.) in Croatia (2022–2024): Occurrence, Distribution and Species Identification. Agronomy. 2025; 15(11):2492. https://doi.org/10.3390/agronomy15112492
Chicago/Turabian StyleRehak Biondić, Tamara, Jasna Milanović, Ivan Poje, Luka Popović, Mirjana Brmež, and Barbara Gerič Stare. 2025. "Monitoring of Root-Knot Nematodes (Meloidogyne spp.) in Croatia (2022–2024): Occurrence, Distribution and Species Identification" Agronomy 15, no. 11: 2492. https://doi.org/10.3390/agronomy15112492
APA StyleRehak Biondić, T., Milanović, J., Poje, I., Popović, L., Brmež, M., & Gerič Stare, B. (2025). Monitoring of Root-Knot Nematodes (Meloidogyne spp.) in Croatia (2022–2024): Occurrence, Distribution and Species Identification. Agronomy, 15(11), 2492. https://doi.org/10.3390/agronomy15112492






